Abstract
Purpose
The Time to First Metastatic Recurrence (TFMR) could be considered as an indirect reflection of the tumour growth kinetics which plays an important role in cancer. Molecular subtypes such as expression of estrogen receptor are known predictive factors of TFMR. The CinéBreast study aimed to identify predictive factors of the time to TFMR.
Methods
The French Epidemiological Strategy and Medical Economics (ESME) Metastatic Breast Cancer (MBC) Database (NCT03275311) was used, which contains data from a cohort of metastatic breast cancer patients from 2008 to 2016 using retrospective data collection. It is a national multi-centre database. The impact of TFMR on overall survival (OS) since first metastasis was also evaluated.
Results
Among 16 702 patients recorded in the ESME MBC database, 10 595 had an initially localised breast cancer with hormone receptor (HR) and HER2 status available, with a metastatic recurrence. Median follow up was 56 months. Median TFMR was 59 months (<24: 20%, 24–60: 31%, 60–120: 25%, >120: 24%). HER2+ and TNBC were respectively 4 times and 12 times (p < 0.0001) more likely to have a recurrence within 2 years when compared to the luminal subgroup. Short TFMR and HR-/HER2-subtype significantly correlated with a poor OS in multivariate analysis.
Some patients with MBC (20% in HER2+, 10% in ER+/HER2-and <5% in the ER-/HER2-) were long-term survivors in all 3 subgroups.
Conclusions
In this large-scale real-life data study, patients with a TNBC metastatic recurrence had a shorter TFMR. Short TFMR significantly correlated with worse overall survival.
Keywords: Breast cancer, Metastatic recurrence, Growth rate, Real-life data
Highlights
-
•
ESME is a large-scale real-life database of 16 702 metastatic breast cancer patients.
-
•
A short time to first metastatic recurrence is associated with poor overall survival.
-
•
Triple-negative tumours were more likely to recur early than HR+ and HER2+ tumours.
1. Introduction
Despite considerable advances in the adjuvant setting, many patients treated [1] for local breast cancer (BC) will eventually have a recurrent disease that will lead to their death due to either distant metastases and/or locoregional inoperable relapse. Although triple-negative breast cancer (TNBC) recurrence are known to occur within the first 3 years [2], luminal breast cancer can recur after 10 years [1]. Several predictors of time to first metastatic recurrence (TFMR) have been reported from clinical trials [[3], [4], [5]] and retrospective cohorts [6] to be associated with prolonged TFMR, including hormone receptor expression, HER2 expression, a low Ki67, low grade according to Scarff-Bloom-Richardson scale and Elston-Ellis modifications, younger age, and stage at diagnosis. However, none of these factors have been investigated in a large high-quality real-life database, and the impact of the TFMR on the kinetics of the metastatic setting is poorly described. We hypothesised that the TFMR could be used as a proxy of the tumour growth rate [7,8], and could, therefore, be valuable information to predict the evolution of the metastatic recurrence.
Most available data come from randomised clinical trials with highly selected patients. The CinéBreast study population is a subpopulation from the Epidémio-Stratégie Médico-Economique (ESME) MBC (metastatic breast cancer) Database (NCT03275311), which is a large-scale population of patients in a real-life setting. Data were extracted from information systems, pharmacy record databases and patients’ electronic medical records collected retrospectively and processed according to quality control guidelines. The ESME MBC database is an academic programme led by Unicancer that aimed to centralise real-life metastatic breast cancer patients’ data. Patients partially or entirely treated in one of the 18 French Comprehensives Cancer Centers (FCCC), which are managing together over one-third of all BC cases nationwide, were selected. Delaloge et al. [9] previously reported the use of the ESME MBC database to investigate a potential benefit of bevacizumab added to paclitaxel in the treatment of luminal MBC in real-life setting.
We aimed at identifying factors associated with TFMR from a real-life database.
2. Methods
2.1. Study design
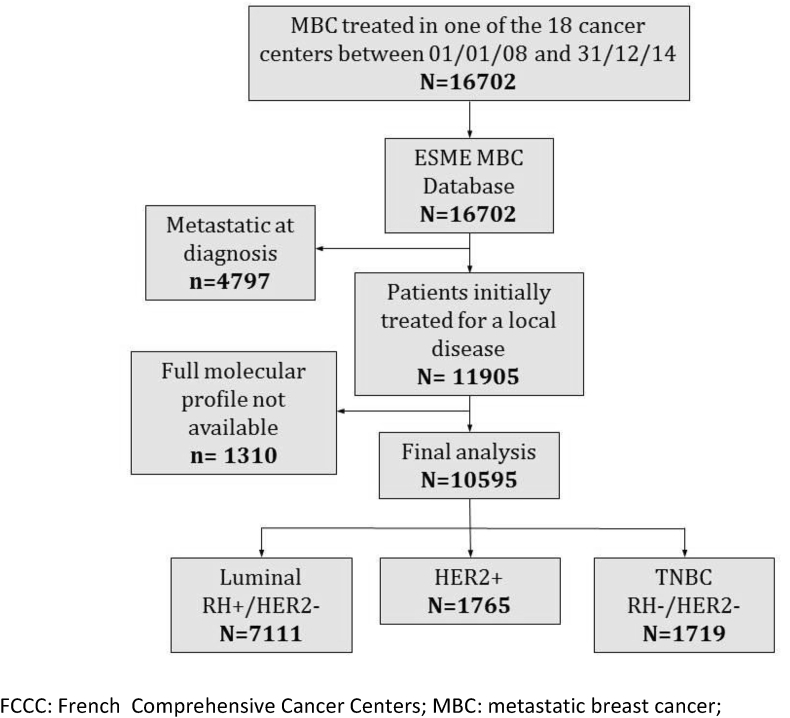
All patients over 18 years old with initial care (radiotherapy, chemotherapy, targeted therapy or hormonal therapy) for a MBC (either metastatic at the beginning or with metastatic recurrence) between the 1st of January 2008 and the 31st of December 2014, were recorded with longitudinal follow-up latest by 2016 for the last patient recruited. Patients were identified through hospitalisation records or pharmacy records and identified through anonymised individual data (a detailed description of the ESME MBC Cohort 2008–2016 is available online in appendix A). The CinéBreast study included all patients from the ESME MBC database which were not metastatic at diagnosis with a known hormonal receptor and HER2 status. The luminal status was defined as either ER+ and/or PR+ and HER2-, whereas HER2+ was defined as an expression score of 3 or amplified HER2/neu and either ER + or ER- and TNBC as non-luminal and HER2 negative (Fig. 1). Both ER and PR status were used to define the luminal status, and the nuclear receptor expression cut-off was 10% (cf. the management of hormone receptor status in appendix A). TFMR was defined as the time to first distant recurrence not amenable to curative treatment.
Fig. 1.
Study flow-chart.
French data protection authorities authorised the ESME MBC database (authorisation no. 1704113). Approval was obtained from an independent ethics committee, which waived the requirement for informed consent. The study was approved by the ESME Scientific Committee. The ESME Research Program was managed by R&D Unicancer according to the applicable best practice guidelines in epidemiology [10,11]. A more detailed description of the ESME MBC database is available online (appendix A).
The primary endpoint was the time to first metastatic recurrence studied as a class variable when categorised into four groups (<24, 24–60, 60–120 and ≥ 120 months). TFMR was investigated in the whole population and the 3 different molecular subgroups (luminal, HER2+ and TNBC). The influence of age at initial tumour diagnosis, age at first metastatic recurrence, the type of metastases (visceral, bone with or without non-visceral metastases and exclusively non-visceral metastases) and the number of metastatic sites were studied in the whole population and the 3 molecular subgroups. Secondary endpoints included the influence of TFMR on overall survival (OS) during the metastatic setting, which was analysed in the general population and the 3 molecular subgroups.
2.2. Statistical analysis
TFMR was analysed comparing the different subgroups, and odds ratio (OR) were performed using nominal polytomous logistic regression and adjusted to the characteristics of the initial local tumour. OS was estimated using Kaplan-Meyer method and survival curve generated. Univariate analysis of OS for each molecular subgroup was performed with log-rank tests. A multivariate analysis of OS was performed using a Cox model adjusted for prognostic factors of survival.
3. Results
3.1. Patient characteristics
Among 16 702 patients recorded in the ESME MBC database, 4797 patients (29%) were excluded due to the presence of metastases at diagnosis (or metastatic recurrence within the first 6 months), and 1310 additional patients (8%) were excluded because of unavailable information on ER and/or HER2 status (Fig. 1). 10 595 patients were analysed, median follow up was 56 months.
BC subtypes were as follows: 7111 patients (67%) had a luminal BC, 1765 patients (17%) HER2+ BC, and 1719 patients (16%) had TNBC (Table 1). Median age at diagnosis was 52 years old, with 40% of patients being 50 years old or younger, 48% being between 50 and 70 years old, and 12% over 70 years old. Median age at metastatic setting was 61 years old. Treatment of the primary cancer included chemotherapy in 7676 patients (72%), radiotherapy in 9322 patients (88%), endocrine therapy in 7000 patients (66%), and trastuzumab in 326 patients (3%) (Table 1).
Table 1.
Patient characteristics at diagnosis.
| n (%) | ||
|---|---|---|
| Molecular characteristics | ||
| ER+/HER2- | 7111 (67) | |
| HER2+ | 1765 (17) | |
| ER-/HER2- | 1719 (16) | |
| Sex | ||
| Female | 10,506 (99.2) | |
| Male | 89 (0.8) | |
| Age at diagnosis | ||
| <50 | 4274 (40) | |
| [50–70] | 5071 (48) | |
| >70 | 1250 (12) | |
| T stage | ||
| Unknown | 5684 (54) | |
| Tis (all type) | 20 (0.2) | |
| T1 | 759 (7) | |
| T2 | 1978 (19) | |
| T3 | 803 (8) | |
| T4 | 170 (2) | |
| N stage | ||
| Unknown | 5848 (55) | |
| N0 | 2400 (23) | |
| N1 | 1435 (14) | |
| N2 | 259 (2) | |
| N3 | 117 (1) | |
| Lymph node invasion | ||
| unknown | 852 (8.0) | |
| no | 5393 (50.9) | |
| yes | 4350 (41.0) | |
| Adjuvant treatments of the primary tumour | ||
| chemotherapy | 7676 (72) | |
| radiotherapy | 9322 (88) | |
| endocrine therapy | 7000 (66) | |
| oral or targeted therapies | 326 (3) | |
| Age at first metastatic recurrence | ||
| <50 | 2263 (21) | |
| [50–70] | 5616 (53) | |
| >70 | 2716 (26) | |
| Types of metastases | ||
| visceral | 5645 (53) | |
| non-visceral only | 3416 (32) | |
| bone1 | 4811 (45) | |
| Number of metastatic sites1 | ||
| 1 | 8327 (79) | |
| 2 | 1961 (18) | |
| 3 or more | 307 (3) | |
| TFMR (months) | ||
| <24 | 2066 (20) | |
| [24–60[ | 3309 (31) | |
| [60–120[ | 2659 (25) | |
| ≥120 | 2561 (24) | |
TFMR: time to first metastatic recurrence.
1: No visceral metastases.
2: Defined by the number of invaded organs (nodes, bone, liver, lung or other visceral).
3.2. Time to first metastatic recurrence
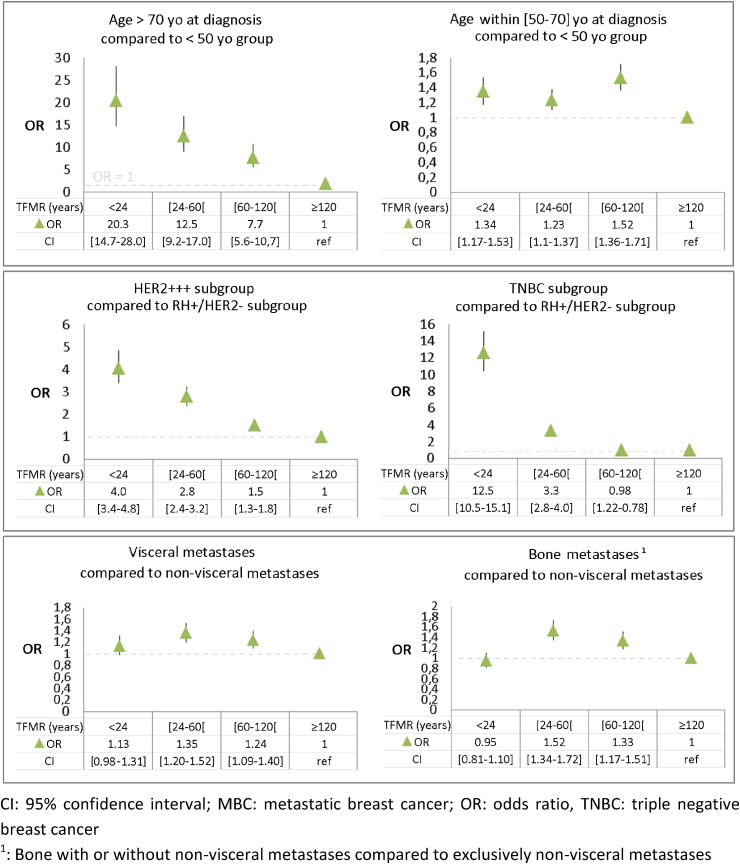
Median TFMR was 59 months. First metastatic recurrence occurred within 24 months in 20% of patients, between 24 and 60 months in 31% of patients, between 60 and 120 months in 25% of patients, and after 120 months in 24% of patients. TNBC and HER2+ patients were 12 and 4 times more likely respectively to have a recurrence within 2 years when compared to the luminal subgroup (Fig. 2). Only 192 out of 1719 (11%) of TNBC patients had a TFMR longer than 10 years (appendix B). The number of patients with a recurrence after 10 years (TFMR >120 months) was 1270 among 4274 (30%) patients under 50 years old, 1244 out of 5071 (24%) in the 50–70 years old group and 47 out of 1250 (4%) patients over 70 years old. The correlation of metastases site (visceral, bone or non-visceral) with short TFMR was significant (p < 0.0001) but with a low OR (Fig. 2 and Appendix B). Within subgroups of TFMR, bone metastasis was slightly more frequent compared to the group of other metastases for patients with relapse between 24 and 60 months (OR = 1.52, confidence interval 95% (CI95%) [1.34–1.72]) and between 60 and 120 months (OR = 1.33, CI95% [1.17–1.51]). The correlation of the site of metastasis was not significant in the short TFMR group (<24 months). The number of metastatic sites did not impact TFMR (p = 0.3). Patients over 70 years old at diagnosis were 20 times more likely (p < 0.0001) to have a recurrence within 2 years compared to patients under 50 years old (Fig. 2). In the luminal BC population, 27% of the population over 70 years old, 9% in the population under 50 and 10% in the population between 50 and 70 had a TFMR <2 years (Appendix C).
Fig. 2.
Odds ratio and 95% confidence interval of TFMR according to age at diagnosis, molecular subgroups and the type of metastasis at recurrence.
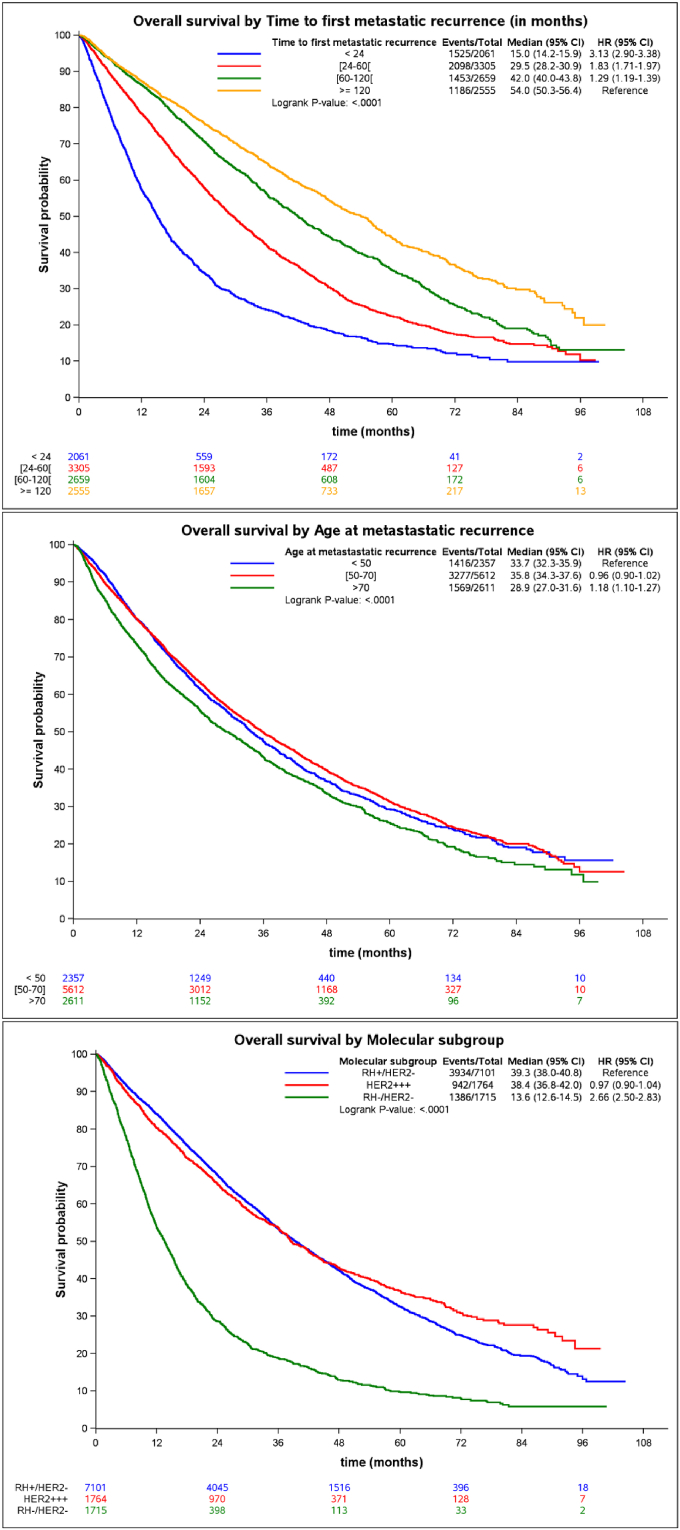
Short TFMR significantly correlated with a shorter OS (p < 0.0001) with a hazard ratio (HR) of 2.48 (95%CI 2.28; 2.69), 1.78 (1.66; 1.92) and 1.35 (1.25; 1.46) in the <24, 24–60, 60–120 months groups respectively when compared to the >120 months group (Table 2, Fig. 3). Median OS were 15.0 (95%CI 14.2; 15.9), 29.5 (28.2; 30.9), 42.0 (40.0; 43.8) and 54.0 months (50.3; 56.4) in the TFMR <24, 24–60, 60–120 and ≥ 120 groups, respectively. Molecular characteristics also correlated with the risk of death. HR were 0.97 (0.90; 1.04) and 2.66 (2.50; 2.83) for HER2+ and TNBC respectively when compared to the luminal group (Table 2). The median OS were 39, 38 and 14 months in the luminal, HER2+ and TNBC groups. The risk of death in the group of patients over 70 was 1.68 (p < 0.0001) and 1.27 in the group of patients between 50 and 70 when compared to the group of patients under 50 years old after adjustment for TMFR and molecular type.
Table 2.
Univariate and multivariate analysis of overall survival with hazard ratio.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Variables | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| TFMR (months) | ||||
| <24 | 3.13 (2.90; 3.38) | <0.0001 | 2.99 (2.75; 3.24) | <0.0001 |
| [24–60] | 1.83 (1.71; 1.97) | <0.0001 | 2.00 (1.86; 2.16) | <0.0001 |
| [60−120] | 1.29 (1.19; 1.39) | <0.0001 | 1.42 (1.32; 1.54) | <0.0001 |
| ≥120 (ref) | 1 | 1 | ||
| Molecular subtype | ||||
| ER+/HER2- (ref) | 1 | 1 | ||
| HER2+ | 0.97 (0.90; 1.04) | 0.38 | 0.86 (0.80; 0.92) | 0.008 |
| TNBC | 2.66 (2.50; 2.83) | <0.0001 | 2.14 (2.00; 2.28) | <0.0001 |
| Age at metastatic recurrence (years) | ||||
| <50 (ref) | 1 | 1 | ||
| [50–70] | 0.96 (0.90; 1.02) | 0.19 | 1.27 (1.19; 1.35) | <0.0001 |
| >70 | 1.18 (1.10; 1.27) | <0.0001 | 1.68 (1.56; 1.81) | <0.0001 |
TFMR: time to first metastatic recurrence; TNBC: triple negative breast cancer.
Fig. 3.
Overall survival according to time to first metastatic recurrence, the age at first metastatic recurrence and the 3 molecular subgroups.
Fewer patients over 70 years old received adjuvant therapy (35% versus 77%, p < 10−15), and adjuvant radiotherapy (79% versus 89%, p < 10−15). In contrast, older patients were more likely to receive adjuvant endocrine therapy (69% versus 66%, p = 0.01). Interestingly, patients who were diagnosed with metastatic breast cancer before 2010 had a poorer OS as compared to those who were diagnosed after 2010 (HR = 1.07 [CI95%: 1.02–1.13], p = 0.01).
4. Discussion
The ESME MBC database has exhaustively recorded patient data from 18 FCCC with a standardised screening procedure and a high level of quality control. The amount of data is therefore essential, with a total of 16 702 patients and a median follow-up of 48 months, which is the most prominent real-life cohort on MBC to our knowledge. However, even when they are large-scale and collected with a highly standardised methodology, retrospective data collection has limitations. For instance, 11% did not have a fully available molecular status, and this proportion would be much smaller in a prospective study. HER2 status was not performed systematically before 2005, and the missing status of hormone receptors was much lower (<2%).
Few studies have been done on high scale real-life data, and the difference of practice might make the datasets challenging to compare. Daniels et al. [12] recently reported real-life data for patients treated by trastuzumab with a median OS of 37.7 months between 2009 and 2015, which was slightly shorter than the median OS reported in the CLEOPATRA study (40.8 months) [13]. In a real-life set-up, Gamucci et al. [14] reported a median OS of 25 months (CI95% [17–33]) with comparable setting for TNBC patients in a multicentre design. There is a need for more real-life data collection that would allow a better comparison of therapeutic strategies in real-life practice through time and centers and a better MBC comprehension. Regierer and others [5] previously reported the influence of a metastasis-free interval ≤ 2 years on the outcome of trial patients. However, in the present analysis, we specifically focused on factors associated with TFMR and the influence of TFMR on MBC outcomes at recurrence in a real-life study. Patients over 70 years old at diagnosis were much more likely to have a recurrence within 2 years. This result might be explained by the fact that therapeutic strategies in the elderly are far less aggressive. Muss et al. reported the need not to undertreat patients over 65, with a significant difference of disease-free survival between the standard group, which was using standard chemotherapy (mostly doxorubicin plus cyclophosphamide) and the capecitabine group [15] (risk of recurrence or death had of 2.09, p < 0.001) and OS rate at 3 years were 91% versus 86% respectively). However, practitioners might be still reluctant to treat elderly people with standard guidelines in this fragile population. Also, in the luminal BC patients, they were approximately 3 times more patients in proportion in the group of patients over 70 than in the 2 other groups with a TFMR <2 years. Shorter relapse within the older population could be explained by less aggressive therapeutic strategies, with only 35% receiving chemotherapy compared to 77% in the younger population. Moreover, adherence to adjuvant endocrine therapy is a significant problem in delaying the TFMR [16]. The tolerance and the adherence in the elderly population are not as good, and this might explain the higher percentage of early metastatic relapse that is observed in the over 70 years old group. Furthermore, older patients have a higher risk of dying from another cause than cancer than younger patients, and this could also increase the proportion of patients that are observed with a short TFMR. However, the HR was close to 1, and the OS Kaplan-Meyer curves are similar when compared to the under 50 years old group which contrasts with the high risk of short-term recurrence. This could be explained by the higher rate of survival at 60 months. There was no significant influence of the metastases site for tumours with short relapse. The influence of metastases site was not significant or low, except for more bone metastasis in the population relapsing between 2 and 5 years. Bone metastases are the most frequent metastatic site for luminal BC. Patients with only bone metastases were shown to have a better outcome than the others in other cohorts [17].
Despite similar surgery for the 3 molecular subgroups, adjuvant treatments are often different [18]. Endocrine therapy given for 5 years remains a standard in Europe. In preclinical models [[19], [20], [21]], and in clinics [22] aromatase inhibitors and tamoxifen induce a decrease in tumour size and eventually a quiescence of micrometastasis that would subsequently lead to late metastases [1].
The poor prognosis of TNBC is well known, and few recent improvements have been made in the management of this disease. With numerous HER2 targeting agents (trastuzumab, lapatinib, neratinib, T-DM1, pertuzumab), the prognosis of HER2/neu amplified breast cancers has significantly improved in the last 20 years. The number of patients who received trastuzumab in the neoadjuvant/adjuvant setting in this study is low, which is explained by the fact that many patients have been treated before 2005; trastuzumab was not approved at that time. This may impact the conclusions drawn from our study in the HER2-positive subgroup. Another limitation of this analysis is that stage at disease presentation was missing for 50% of the patients. For this reason, stage could not be included in the multivariate analysis, although it is a known factor of early relapse. Patients diagnosed before 2010 had a worse OS, which might partly be explained by the therapeutic progresses made in the treatment of metastatic breast cancer over time, especially in HER2-positive (trastuzumab) and hormone receptor-positive (aromatase inhibitors) BC.
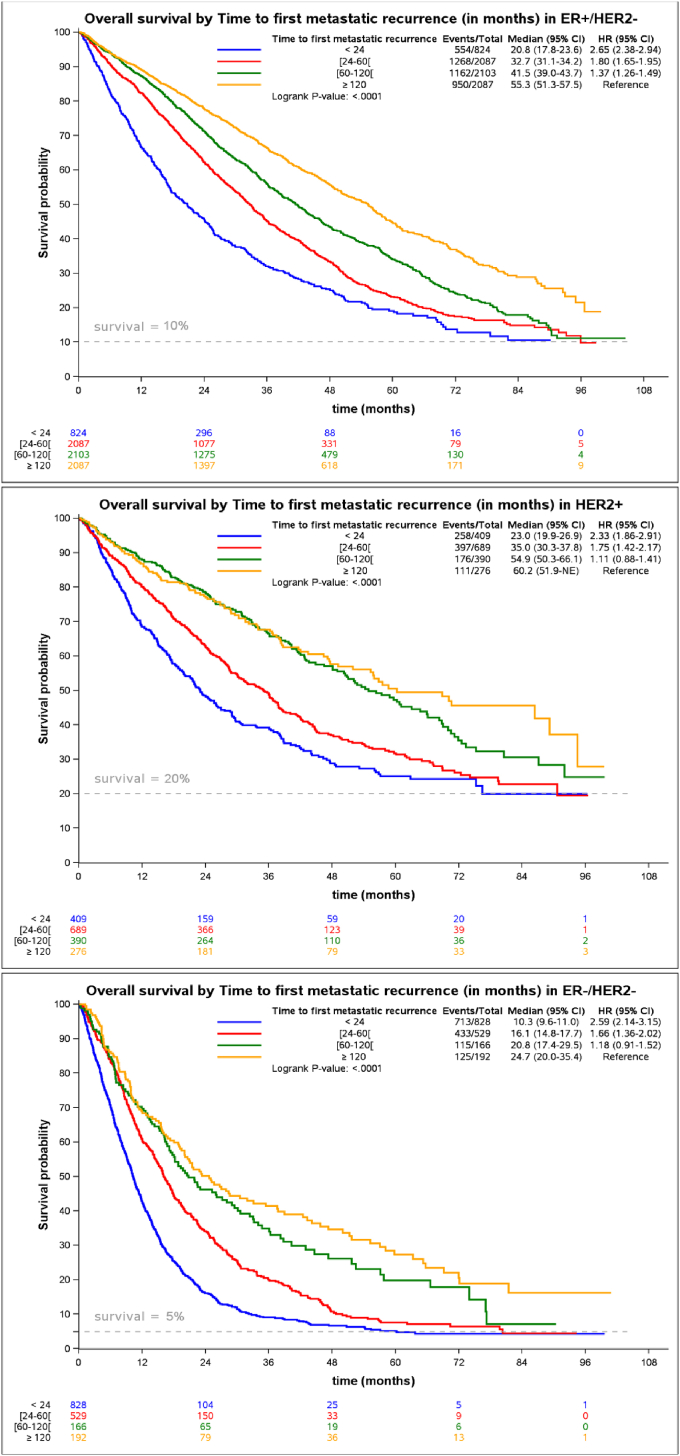
Although median OS was slightly shorter in the HER2+ group when compared to the luminal group, their survival is significantly better which could be explained by a better “tail effect”. This “tail effect” is mostly described in immuno-oncology [23] or for estimation of disease-free survival in adjuvant treatment. It could be graphically approached by an asymptote [24] which reflects the fraction of the population which is no longer at risk of recurrence (for the adjuvant setting) or death from the disease. Interestingly, the HER2+ subgroup seems to have an asymptote of survival around 0.20 which is higher than the 0.10 and much higher than the 0.05 that is observed respectively in the luminal and the TNBC groups (Fig. 4). Another intriguing observation is the fact that the asymptotes in the 3 survival curves of the different molecular subgroups do not seem to be dependent on the TFMR (<24, 24–60, 60–120, ≥120). For late recurrences, however (TFMR >120 months), the curves take longer to reach their asymptote, which is consistent with a better OS. There is a need for better statistical approaches to confirm the significance of such observations. Late observations of those patients could provide better insight and more robust conclusions about the long-term survivors of those different subgroups.
Fig. 4.
Impact of TFMR on overall survival within the 3 molecular subgroups.
5. Conclusions
We first reported the association of age and each molecular subgroup with the TFMR in a real-life database. Patients over 70 are 20 times more likely to have a TFMR lower than 2 years when compared to the group of under 50. Short TFMR is also highly predictive of OS, and the risk of death was 2.99 when compared to patients having a late recurrence (>10 years), which probably reflects that the first group has a more aggressive tumour that will be harder to treat in the metastatic setting. The Cinébreast study unexpectedly described a “tail effect” in a metastatic population which did not seem to be dependent of the TFMR but was different between the molecular subgroups, with a survival which was tending to an asymptote that would be 20% in HER2+, 10% in ER+/HER2-and <5% in the TNBC. Further studies, either with more mature data from the ESME database or from a more extensive and extended dataset would be needed to confirm this trend.
Funding
This work was supported by R&D UNICANCER. The ESME MBC database is supported by an industrial consortium (Roche, Pierre Fabre, Pfizer and AstraZeneca). No grant numbers apply. Data collection, analyses and publications are totally independent of the industrial funding partners of R&D UNICANCERESME.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Data from the ESME MBC database are fully anonymised prior to being made available to researchers, so data can not be traced back to the individual patient. Therefore, no informed consent was required from the included patients and there was no need for approval of the medical ethical committee.
Declaration of competing interest
TB reports grants, personal fees, and non-financial support from Roche, grants, personal fees, and non-financial support from Novartis, grants and personal fees from AstraZeneca, outside the submitted work. MC reports personal fees from Roche, during the conduct of the study; grants and personal fees from Novartis, personal fees from Astra Zeneca, personal fees from Menarini, outside the submitted work. WJ reports personal fees and non-financial support from Roche, outside the submitted work. AG reports grants, personal fees, and non-financial support from Roche, during the conduct of the study. TP reports non-financial support and other support from Roche, outside the submitted work. All other authors declare no competing interests.
Acknowledgments
We thank the 18 Participating French Comprehensive Cancer Centers (FCCC):; I. Curie, Paris/Saint-Cloud, G. Roussy, Villejuif, I. Cancérologie de l’Ouest, Angers/Nantes, C. F. Baclesse, Caen, ICM Montpellier, C. L. Bérard, Lyon, C. G-F Leclerc, Dijon, C. H. Becquerel, Rouen; I. C. Regaud, Toulouse; C. A. Lacassagne, Nice; Institut de Cancérologie de Lorraine, Nancy; C. E. Marquis, Rennes; I. Paoli-Calmettes, Marseille; C. J. Perrin, Clermont Ferrand; I. Bergonié, Bordeaux; C. P. Strauss, Strasbourg; I. J. Godinot, Reims; C. O. Lambret, Lille. We thank the 18 French Comprehensive Cancer Centers for providing the data and each ESME Contact for coordinating the project at the local level.
We thank the ESME Scientific Committee members for their ongoing support. ESME central coordinating staff: Head of Research and Development: Christian Cailliot. Program director: Mathieu Robain. Data Managers: Irwin Piot, Olivier Payen et Olivier Villacroux. Operational team: Coralie Courtinard, Michaël Chevrot, Tahar Guesmia, Pauline Jouany, Clémence Marciano and Gaëtane Simon. Project Associate: Nathalie Bouyer. Management assistant: Esméralda Pereira. Software designers: Alexandre Vanni and Baltazar Torres. ESME local coordinating Staff: Patrick Arveux, Thomas Bachelot, Jean-Pierre Bleuse, Delphine Berchery, Etienne Brain, Mathias Breton, Loïc Campion, Emmanuel Chamorey, Margot Cucchi, Valérie Dejean, Anne-Valérie Guizard, Anne Jaffré, Lilian Laborde, Carine Laurent, Agnès Loeb, Muriel Mons, Damien Parent, Geneviève Perrocheau, Marie-Ange Mouret-Reynier, Michel Velten.
We thank Nafisa Keynan for English grammar and spell check.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2019.10.004.
Appendix. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pan H., Gray R., Braybrooke J., Davies C., Taylor C., McGale P. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol Off J Eur Soc Med Oncol. 2012;23(Suppl 6):vi7–12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 3.Ellis M.J., Tao Y., Luo J., A’Hern R., Evans D.B., Bhatnagar A.S. Outcome prediction for estrogen receptor–positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. JNCI J Nat Cancer Inst. 2008;100:1380–1388. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuzick J., Dowsett M., Pineda S., Wale C., Salter J., Quinn E. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:4273–4278. doi: 10.1200/JCO.2010.31.2835. [DOI] [PubMed] [Google Scholar]
- 5.Regierer A.C., Wolters R., Ufen M.-P., Weigel A., Novopashenny I., Köhne C.H. An internally and externally validated prognostic score for metastatic breast cancer: analysis of 2269 patients. Ann Oncol Off J Eur Soc Med Oncol. 2014;25:633–638. doi: 10.1093/annonc/mdt539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobbezoo D.J.A., van Kampen R.J.W., Voogd A.C., Dercksen M.W., van den Berkmortel F., Smilde T.J. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Canc. 2015;112:1445–1451. doi: 10.1038/bjc.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Tourneau C., Servois V., Diéras V., Ollivier L., Tresca P., Paoletti X. Tumour growth kinetics assessment: added value to RECIST in cancer patients treated with molecularly targeted agents. Br J Canc. 2012;106:854–857. doi: 10.1038/bjc.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Tourneau C., Paoletti X., Coquan E., Sablin M.-P., Zoubir M., Tannock I.F. Critical evaluation of disease stabilization as a measure of activity of systemic therapy: lessons from trials with arms in which patients do not receive active treatment. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32:260–263. doi: 10.1200/JCO.2013.53.5518. [DOI] [PubMed] [Google Scholar]
- 9.Delaloge S., Pérol D., Courtinard C., Brain E., Asselain B., Bachelot T. Paclitaxel plus bevacizumab or paclitaxel as first-line treatment for HER2-negative metastatic breast cancer in a multicenter national observational study. Ann Oncol Off J Eur Soc Med Oncol. 2016;27:1725–1732. doi: 10.1093/annonc/mdw260. [DOI] [PubMed] [Google Scholar]
- 10.ISPE Guidelines for good pharmacoepidemiology practices (GPP) Pharmacoepidemiol Drug Saf. 2008;17:200–208. doi: 10.1002/pds.1471. [DOI] [PubMed] [Google Scholar]
- 11.Association des Epidémiologistes de Langue Française (ADELF) 2007. Déontologie et bonnes pratiques en épidémiologie, recommendations révisées. [Google Scholar]
- 12.Daniels B., Kiely B.E., Lord S.J., Houssami N., Lu C.Y., Ward R.L. Trastuzumab for metastatic breast cancer: real world outcomes from an Australian whole-of-population cohort (2001-2016) Breast Edinb Scotl. 2017;38:7–13. doi: 10.1016/j.breast.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Swain S.M., Baselga J., Kim S.-B., Ro J., Semiglazov V., Campone M. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamucci T., Mentuccia L., Natoli C., Sperduti I., Cassano A., Michelotti A. A real-world multicentre retrospective study of paclitaxel-bevacizumab and maintenance therapy as first-line for HER2-negative metastatic breast cancer. J Cell Physiol. 2017;232:1571–1578. doi: 10.1002/jcp.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muss H.B., Berry D.A., Cirrincione C.T., Theodoulou M., Mauer A.M., Kornblith A.B. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziller V., Kalder M., Albert U.-S., Holzhauer W., Ziller M., Wagner U. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol Off J Eur Soc Med Oncol. 2009;20:431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 17.Solomayer E.-F., Diel I.J., Meyberg G.C., Gollan Ch, Bastert G. Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Canc Res Treat. 2000;59:271–278. doi: 10.1023/A:1006308619659. [DOI] [PubMed] [Google Scholar]
- 18.Senkus E., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rutgers E. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2015;26(Suppl 5):v8–30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 19.Brodie A., Sabnis G., Macedo L. Xenograft models for aromatase inhibitor studies. J Steroid Biochem Mol Biol. 2007;106:119–124. doi: 10.1016/j.jsbmb.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Brodie A., Jelovac D., Macedo L., Sabnis G., Tilghman S., Goloubeva O. Therapeutic observations in MCF-7 aromatase xenografts. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11 884s–8s. [PubMed] [Google Scholar]
- 21.Macedo L.F., Sabnis G., Brodie A. Preclinical modeling of endocrine response and resistance: focus on aromatase inhibitors. Cancer. 2008;112:679–688. doi: 10.1002/cncr.23191. [DOI] [PubMed] [Google Scholar]
- 22.Smith I.E., Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 23.Harris S.J., Brown J., Lopez J., Yap T.A. Immuno-oncology combinations: raising the tail of the survival curve. Cancer Biol Med. 2016;13:171–193. doi: 10.20892/j.issn.2095-3941.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamel J.W., Bonadonna G., Valagussa P., Edwards M.J. Refined measurement of outcome for adjuvant breast carcinoma therapy. Cancer. 2003;97:1139–1146. doi: 10.1002/cncr.11171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.