Abstract
Background
Young age is a poor prognostic factor in early stage breast cancer (BC) but its value is less established in metastatic BC (MBC). We evaluated the impact of age at MBC diagnosis on overall survival (OS) across three age groups (<40, 40 to 60 and > 60 years(y)).
Methods
ESME MBC database is a national cohort, collecting retrospective data from 18 participating French cancer centers between January 01, 2008 and December 31, 2014.
Results
Among 14 403 women included, 1077 (7.5%), 6436 (44.7%) and 6890 (47.8%) pts were <40, 40–60 and > 60 y respectively. Pts <40 had significantly more aggressive presentations than other age groups: more frequent HER2+ (25.7 vs 15.3% in >60y) and triple negative subtypes (27.4 vs 14.6% in >60y), and more frequent visceral involvement (36.3 vs 29.8% in >60y). At a median follow-up of 48 months, median OS differed across age groups: 38.8, 38.4 and 35.6 months for pts <40, 40–60 and > 60y, respectively (p < 0.0001). Compared to pts <40y, older pts had a statistically significant higher risk of death (all causes of death included), although of limited clinical value (HR = 1.1, IC 95%:1.01–1.20). There was a significant trend for better OS in pts <40y with HER2+ and luminal diseases. A possible explanation is a greater use of anti-Her2 therapies as first-line treatments: 86.6, 81.9 and 74.9% for pts <40, 40–60 and > 60y, respectively (p < 0.0001).
Conclusion
Although young age seems associated with more aggressive presentations at diagnosis of MBC, it has no deleterious effect on OS in this large series.
Keywords: Breast cancer, Metastatic disease, Overall survival, Real-world data, Age
Highlights
-
•
Young age is a poor prognosis factor in early stage breast cancer.
-
•
Young age is associated with an aggressive presentation in metastatic breast cancer.
-
•
Young age had no impact on overall survivall in metastatic breast cancer.
-
•
Oppositely, older women (>60y) had a stightly poorer prognosis at the metastatic stage.
1. Introduction
Breast cancer (BC) is the first female cancer and the leading cause of cancer death in women in France [1] and worldwide [2]. Latest French data show an estimated annual mortality rate of 11 833 women (2017) [3], and interestingly, overall survival (OS) at 5 years differs among age groups: 90% before 45 years old (y), 92% between 45 and 55y, 89% between 55 and 65y, 87% between 65 and 75y, and 58% after75y [1]. In early stage BC, young age is a known poor prognostic factor [[4], [5], [6]]. Aggressive subtypes, like triple negative, are more frequent in young patients. However, young age has been found to be an independent prognostic factor in most studies [4,7], sometimes only in luminal subtypes [8,9].
In metastatic breast cancer (MBC), recognized poor prognostic factors are short metastasis-free interval, visceral involvement and crisis, negative hormone receptor (HoR) and particularly triple negative subtype, primary endocrine resistance for luminal subtype and number of metastatic sites [10]. However, the prognostic impact of age remains unclear in this clinical setting. Several retrospective series have unexpectedly suggested that older women had a poorer prognosis than women under 50 years of age [[11], [12], [13]]. Nonetheless, international guidelines state that age should not guide the treatment strategy and the intensity of treatment, especially to avoid overtreatment in young patients [10,14].
Real-world data are important assets as they provide data from large data sets with long follow-up which usefully complements data from randomized clinical trials. The Epidemiological Strategy and Medical Economics (ESME) program is an academic initiative launched in 2014 by UNICANCER, the French network of cancer centers, to report exhaustive, high quality and centralized real-life data on different solid tumors including MBC. It allowed building a database of more than 16 000 MBC cases. The ESME Research program included 3 types of cancer: MBC, ovarian and lung cancers. It involves 18 academic cancer centers managing together over one-third of all BC cases nationwide; for MBC, other citations are available [[15], [16], [17], [18]]. We used the ESME MBC database (NCT03275311) to evaluate the impact of age at MBC diagnosis on overall survival (OS).
2. Patients and methods
2.1. Study design
The ESME MBC database is a unique national cohort, collecting retrospective data using clinical trial-like methodology. It included all consecutive MBC patients (pts) who initiated at least one treatment in one of the 18 participating French cancer centers between January 01, 2008 and Decemeber 31, 2014. Follow-up data have been collected until October 11, 2016, death or date of latest news. Exclusion criteria were: patients treated for another cancer in the last 5 years before MBC diagnosis, with unknown ER/PR/Her2 status, or men (who will be analyzed in a separate study [19]).
We carried out a retrospective, comparative study to assess overall survival of MBC patients selected from the ESME MBC database among 3 age groups (<40, 40 to 60 and > 60 y).
The ESME research program is handled by R&D Unicancer in accordance with current best practice guidelines and rules (Good pharmacoepidemiology pratices). The program is monitored by an independent scientific committee who approved the present work. The ESME MBC database [20] was authorised by the French data protection authority ([Registration ID 1704113 and authorisation N°DE-2013.-117], NCT03275311). All data are exclusively obtained retrospectively. The present analysis was approved by an independent ethics committee (Comité de Protection des Personnes Sud-Est II-2015-79). Considering the retrospective design of the study, no informed consent was deemed necessary. Nevertheless, all patients were informed about the re-use of their electronically recorded data.
Subtypes were defined according to the first histology available (primary tumor), otherwise on the metastasis. HoR + status was defined as ER+/PR+; ER+/PR- or missing; ER- or missing/PR+. HoR positivity was defined if nuclear staining was strictly superior to 10% in immunochemistry. HER2+ status was established if HER2 was found 3+ in IHC or 2+ with amplified FISH or CISH. De novo MBC was defined by the diagnosis of a metastasis within 90 days of the primary tumor. Among de novo MBC, loco-regional treatment was defined as breast surgery (mastectomy or lumpectomy) and/or loco-regional radiotherapy (including breast±regional lymph nodes) within the first year of diagnosis. OS was defined as the time between the diagnosis of metastasis and the date of death (from any cause), or censored to the date of latest news.
2.2. Objectives
The primary endpoint of this study was the evaluation of the impact of age at MBC diagnosis on OS, among 3 age groups (<40, 40 to 60 and > 60 y). Main secondary endpoints were the description of MBC features in each age group and evaluation of OS by breast cancer subtype in each age group.
2.3. Statistical analysis
Clinical, pathological and treatment characteristics were described overall and across age groups by their distribution for categorical data, and their mean and median for continuous data. Comparisons between age groups were performed using Chi-squared tests or Fisher’s exact test for categorical data and non-parametric Wilcoxon’s test for continuous data. A p-value <0.05 was considered statistically significant.
Median follow-up was calculated using reverse Kaplan-Meier estimation.
For OS, survival curves were determined using the Kaplan-Meier method; survival medians were given with their 95% confidence interval (CI). Survival curves were compared using log-rank tests. Hazard-ratio with their 95% CI were computed using a univariate Cox model; all significant factors were included in a multivariate Cox model. Survival analysis were conducted in the whole population and in each subtype group.
3. Results
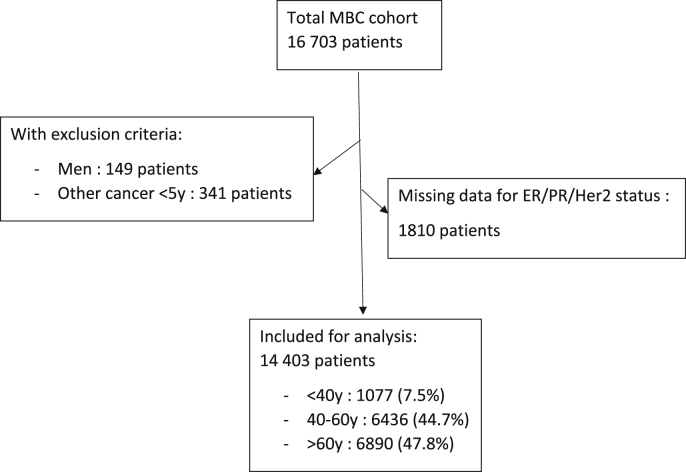
Among 16 703 included pts, 1810 had no information available in the database on tumor receptors (ER/PR/HER2) and 490 had at least one exclusion criterion (unknown age, men, other cancer in the last 5y), leaving 14 403 for analysis (Fig. 1).
Fig. 1.
Study flow diagram.
3.1. Patients <40y had more frequent aggressive features at both primary disease and first metastatic event
Data relative to TNM stage of the primary tumor were partly retrieved (Table 1). Tumors occurring in women aged <40y showed more aggressive features. Grade III tumors were recorded in 62.3%, 48.2% and 39.9% in patients <40, 40–60 and > 60 y respectively (p < 0.0001). HoR-/HER2-tumors were observed in 26.8%, 19.4% and 14.0% of patients <40, 40–60 and > 60 y respectively, and Her2+ subtype in 25.7%, 20.6% and 15.7% of patients <40, 40–60 and > 60 y respectively (p < 0.0001). Details of adjuvant treatments are described in Table 1.
Table 1.
Characteristics of primary tumor and adjuvant treatment: overall and across age groups(∗%/available data; ∗∗%/all patients).
| All N = 14403 |
Age at MBC |
P value between age groups | ||||||
|---|---|---|---|---|---|---|---|---|
| <40 N = 1077 |
40–60 years N = 6436 |
>60 years N = 6890 |
||||||
| N | N | % | N | % | N | % | ||
| Tumor size (cT) | <0.0001 | |||||||
| T0 | 164 | 4 | 0.7%∗ | 75 | 2.5%∗ | 85 | 2.8%∗ | |
| T1 | 1297 | 75 | 13.4%∗ | 548 | 17.9%∗ | 674 | 22.4%∗ | |
| T2 | 2389 | 210 | 37.6%∗ | 1119 | 36.6%∗ | 1060 | 35.3%∗ | |
| T3 | 1192 | 156 | 28.0%∗ | 617 | 20.2%∗ | 419 | 13.9%∗ | |
| T4 global | 1579 | 113 | 20.3%∗ | 698 | 22.8%∗ | 768 | 25.5%∗ | |
| Not avalaible | 7782 | 519 | 48.2%∗∗ | 3379 | 52.5%∗∗ | 3884 | 56.4%∗∗ | |
| Nodal status (cN) | <0.0001 | |||||||
| N0 | 2740 | 183 | 34.7%∗ | 1201 | 41.5%∗ | 1356 | 48.6%∗ | |
| N1 | 2513 | 242 | 45.9%∗ | 1235 | 42.6%∗ | 1036 | 37.1%∗ | |
| N2 | 575 | 63 | 12.0%∗ | 273 | 9.4%∗ | 239 | 8.6%∗ | |
| N3 | 386 | 39 | 7.4%∗ | 188 | 6.5%∗ | 159 | 5.7%∗ | |
| Not available | 8189 | 550 | 51.1%∗∗ | 3539 | 55.0%∗∗ | 4100 | 59.5%∗∗ | |
| Grade | <0.0001 | |||||||
| I | 915 | 26 | 2.6%∗ | 346 | 5.9%∗ | 543 | 8.8%∗ | |
| II | 6247 | 352 | 35.1%∗ | 2711 | 45.9%∗ | 3184 | 51.3%∗ | |
| III | 5947 | 624 | 62.3%∗ | 2847 | 48.2%∗ | 2476 | 39.9%∗ | |
| Not available | 1294 | 75 | 7.0%∗∗ | 532 | 8.3%∗∗ | 687 | 10.0%∗∗ | |
| Histological type | <0.0001 | |||||||
| Ductal | 11317 | 973 | 97.0%∗ | 5281 | 89.3%∗ | 5063 | 80.8%∗ | |
| Lobular | 1868 | 30 | 3.0%∗ | 636 | 10.7%∗ | 1202 | 19.2%∗ | |
| Other or Not available | 1218 | 74 | 6.9%∗∗ | 519 | 8.0%∗∗ | 625 | 9.0%∗∗ | |
| Subtypes | <0.0001 | |||||||
| HoR + Her2- | 8391 | 502 | 47.5%∗ | 3604 | 60.0%∗ | 4285 | 70.3%∗ | |
| HoR-Her2- | 2298 | 283 | 26.8%∗ | 1163 | 19.4%∗ | 852 | 14.0%∗ | |
| Her2+ | 2465 | 271 | 25.7%∗ | 1238 | 20.6%∗ | 956 | 15.7%∗ | |
| Not available | 1249 | 21 | 1.9%∗∗ | 431 | 6.7%∗∗ | 797 | 11.6%∗∗ | |
| Adjuvant chemotherapy | <.0001 | |||||||
| Yes | 7502 | 699 | 64.9%∗ | 3913 | 60.9%∗ | 2890 | 42.1%∗ | |
| No | 6870 | 378 | 35.1%∗ | 2515 | 39.1%∗ | 3977 | 57.9%∗ | |
| Not available | 31 | 0 | 0.0%∗∗ | 8 | 0.1%∗∗ | 23 | 0.3%∗∗ | |
| Adjuvant radiotherapy | 0.1309 | |||||||
| Yes | 8880 | 634 | 58.9%∗ | 3997 | 62.2%∗ | 4249 | 61.8%∗ | |
| No | 5493 | 441 | 40.9%∗ | 2429 | 37.8%∗ | 2623 | 38.2%∗ | |
| Not avalaible | 30 | 2 | 0.2%∗∗ | 10 | 0.2%∗∗ | 18 | 0.3%∗∗ | |
| Adjuvant endocrine therapy | <.0001 | |||||||
| Yes | 6695 | 380 | 35.3%∗ | 2838 | 44.1%∗ | 3477 | 50.5%∗ | |
| No | 7677 | 695 | 64.5%∗ | 3583 | 55.7%∗ | 3399 | 49.3%∗ | |
| Not avalaible | 31 | 2 | 0.2%∗∗ | 15 | 0.2%∗∗ | 14 | 0.2%∗∗ | |
At the onset of MBC, 1077 (7.5%), 6436 (44.7%) and 6890 (47.8%) pts were <40, 40–60 and > 60 y respectively. De novo metastatic disease occurred in 4124 patients (28.6%), more frequently in younger patients: 31.5%, 28.7 and 27.1% in patients <40, 40–60 and > 60 y respectively (p = 0.003). Younger patients had also a shorter time to first metastasis: 28.6% between 3 and 24 months versus 12.5% in patients >60y. Overall, median time to metastasis was shorter in younger patients: 18 months in <40y, 27 months in 40–60y and 42 months in >60y (p < 0.0001) which might be in line with subtype distribution.
Similarly, at metastatic disease onset, patients <40y also had significantly more aggressive presentations than other age groups (data are shown across age groups: <40, 40–60 and > 60 y respectively): more frequent visceral involvement (36.3%, 33.3% and 29.8%), more frequent HER2+ (26.6%, 21.2% and 16.1%), and HoR-/Her2- (25.3%, 17.7% and 12.1%) subtypes, (all p-value vs other age groups <0.0001) [Details are specified in Table 2].
Table 2.
Metastatic breast cancer characteristics according to age groups.
| All |
Age at MBC |
P value between age groups |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| <40 years |
[40–60] years |
>60 years |
|||||||
| N | % | N | % | N | % | N | % | ||
| Time to first metastasis | <0.0001 | ||||||||
| De novo (< 3 months) | 4058 | 28.2% | 339 | 31.5% | 1849 | 28.7% | 1870 | 27.1% | |
| [3–24[ months | 2284 | 15.9% | 308 | 28.6% | 1114 | 17.3% | 862 | 12.5% | |
| >= 24 months | 8035 | 55.8% | 429 | 39.8% | 3461 | 53.8% | 4145 | 60.2% | |
| NA | 26 | 0.2% | 1 | 0.1% | 12 | 0.2% | 13 | 0.2% | |
| Number of metastatic sites | 0.02 | ||||||||
| One site | 7976 | 55.4% | 572 | 53.1% | 3493 | 54.3% | 3911 | 56.8% | |
| Two sites | 3473 | 24.1% | 267 | 24.8% | 1586 | 24.6% | 1620 | 23.5% | |
| Three or more sites | 2954 | 20.5% | 238 | 22.1% | 1357 | 21.1% | 1359 | 19.7% | |
| Type of metastasis | <0.0001 | ||||||||
| Bone only | 8145 | 65.3% | 562 | 52.2% | 3587 | 55.7% | 3996 | 58.0% | |
| Visceral | 4584 | 15.6% | 391 | 36.3% | 2140 | 33.3% | 2053 | 29.8% | |
| Other (neither visceral nor bone) | 1674 | 19.1% | 124 | 11.5% | 709 | 11.0% | 841 | 12.2% | |
| Tumor subtype | <0.0001 | ||||||||
| HoR + HER2- | 9398 | 15.6% | 519 | 48.2% | 3934 | 61.1% | 4945 | 71.8% | |
| HoR-HER2- | 2247 | 19.1% | 272 | 25.3% | 1138 | 17.7% | 837 | 12.1% | |
| HER2+ | 2758 | 65.3% | 286 | 26.6% | 1364 | 21.2% | 1108 | 16.1% | |
| De novo MBC –treatment of primary tumor <1y from diagnosis | |||||||||
| Breast surgery | 691 | 17.0% | 90 | 26.5% | 374 | 20.5% | 227 | 12.1% | 0.001 |
| Loco-regional RT | 1333 | 32.8% | 173 | 51.0% | 705 | 38.1% | 455 | 24.3% | <0.0001 |
3.2. First line treatments for metastatic breast cancer
First-line treatments for MBC differed across age groups among all subtypes. In HoR+/HER2-younger patients received more frequently chemotherapy (80.5%, 60.8% and 47.4% in the 3 age groups respectively; p < 0.0001) and less endocrine therapy (70.1%, 75.6% and 81.3%; p < 0.0001). In Her2+ subtype, chemotherapy as well as anti-Her2 treatments were more frequently administered to younger patients: 80.5%, 68.5% and 47.4% across age groups for chemotherapy (p < 0.0001); 87.1%, 81.7% and 75% for anti-Her2 treatments (p < 0.0001). A similar trend was observed in HoR-/HER2-patients. Details are shown in Table 3.
Table 3.
First-line treatments according to tumor subtypes and age groups.
| All |
Age groups (years) |
P value between age groups |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| <40 |
40–60 |
>60 |
|||||||
| N |
% |
N |
% |
N |
% |
N |
% |
||
| HoR+/HER2 | |||||||||
| Chemotherapy | 5456 | 58.1% | 418 | 80.5% | 2693 | 68.5% | 2345 | 47.4% | <0.0001 |
| Endocrine therapy | 7358 | 78.3% | 364 | 70.1% | 2974 | 75.6% | 4020 | 81.3% | <0.0001 |
| Her2+ | |||||||||
| Chemotherapy | 2469 | 89.5% | 273 | 95.5% | 1257 | 92.2% | 939 | 84.7% | <0.0001 |
| Endocrine therapy | 1131 | 41.0% | 134 | 46.9% | 494 | 36.2% | 503 | 45.4% | <0.0001 |
| Anti-Her2 | 2195 | 79.6% | 249 | 87.1% | 1115 | 81.7% | 831 | 75.0% | <0.0001 |
| HoR-/HER2- | <0.0001 | ||||||||
| Chemotherapy | 2109 | 93.9% | 262 | 96.3% | 1092 | 96.0% | 755 | 90.2% | <0.0001 |
3.3. Loco-regional treatment of the primary tumor in de novo MBC
Among patients with de novo MBC, loco-regional treatment was more frequent in patients <40y: breast surgery (breast-conserving or mastectomy) was performed in 26.5% of patients <40y, 20.5% in the 40-60y group and 12.1% in >60y group; loco-regional radiotherapy was performed in 51% of patients <40y, 38.1% in the 40-60y group and 24.3% in the >60y group.
3.4. Overall survival differs after first metastatic event according to age and subtype
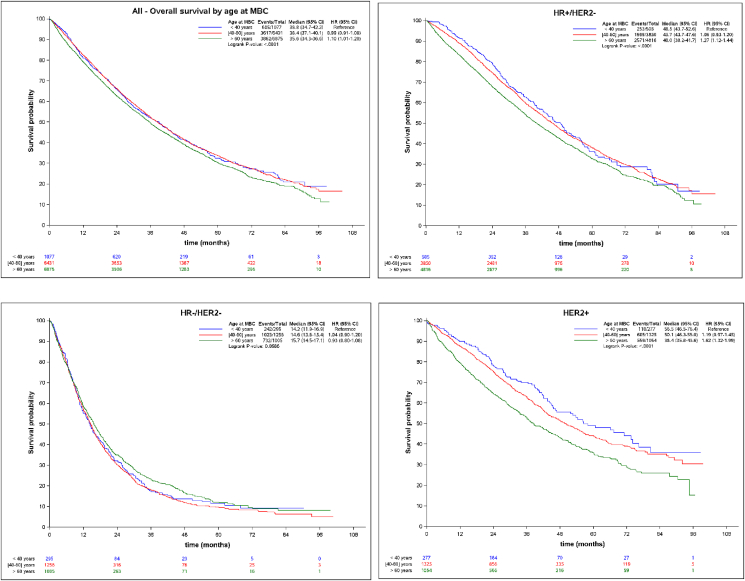
Median follow-up was 48 months. Median OS significantly differed across age groups, and was 38.8, 38.4 and 35.6 months for pts <40, 40–60 and > 60y, respectively (p < 0.0001). Compared to pts >60y, younger pts had a slightly significant lower risk of death (all causes of death included): HR = 0.91, CI 95% 0.83–0.99. This trend for a longer OS was confirmed in patients <40 in HoR+/HER2-and Her2+ subtypes, but not in HoR-/Her2- MBC [Fig. 2 and Table 4].
Fig. 2.
Overall survival according to age and tumor subtypes.
Table 4.
Uni- and multi-variate analysis of factors impacting on overall survival.
| Cox univariate |
Cox multivariate |
|||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age at MBC | ||||
| >60 years | 1 | <0.0001 | 1 | <0.0001 |
| [40–60] | 0.90 (0.86–0.94) | 0.84 (0.80–0.88) | ||
| <40 years | 0.91 (0.83–0.99) | 0.75 (0.69–0.82) | ||
| Time to MBC | ||||
| ≥24 months | 1 | <0.0001 | 1 | <0.0001 |
| [3–24[ months | 2.00 (1.89–2.12) | 1.88 (1.77–1.99) | ||
| De novo (<3 months) | 0.83 (0.79–0.88) | 0.84 (0.80–0.89) | ||
| Subtype | ||||
| HoR + HER2- | 1 | <0.0001 | 1 | <0.0001 |
| HoR-HER2- | 2.72 (2.58–2.88) | 2.48 (2.34–2.63) | ||
| HER2+ | 0.89 (0.83–0.94) | 0.87 (0.81–0.92) | ||
| Number of metastatic sites | ||||
| One site | 1 | <0.0001 | 1 | <0.0001 |
| Two sites | 1.40 (1.33–1.48) | 1.40 (1.32–1.48) | ||
| Three or more sites | 1.93 (1.82–2.03) | 1.99 (1.88–2.11) | ||
| Metastatic site | ||||
| Bone | 1 | <0.0001 | 1 | <0.0001 |
| Visceral | 1.19 (1.13–1.25) | 1.10 (1.05–1.16) | ||
| Other (neither visceral nor bone) | 0.80 (0.74–0.86) | 0.77 (0.71–0.84) | ||
Univariate analysis suggested that young age was a favorable prognostic factor in MBC. Multi-variate analysis confirmed that young age was an independent prognostic factor when controlling for other factors in patients with MBC. Indeed, compared to patients >60y, hazard ratio for death was 0.75 (95% CI 0.69–0.82) in patients <40y and 0.84 (95% CI 0.80–0.88) in patients aged 40-60y (p < 0.0001). Other expected prognostic factors were confirmed: longer time to MBC and de novo disease [HR for death 1.88 (95% CI 1.77–1.99) for patients relapsing between 3 and 24 months, 0.84 (95% CI 0.80–0.89) for de novo MBC, all compared to patients relapsing after 24 months (p < 0.0001)], subtype [HR 2.48 (95% CI 2.34–2.63) for the HoR-/HER2-subset, 0.87 (95% CI 0.81–0.92) for Her2+ compared to HoR + HER2- (p < 0.0001)], number of metastatic sites [HR 1.40 (95% CI 1.32–1.48) for 2 sites, 1.99 (95% CI 1.88–2.11) for 3 or more sites compared to one (p < 0.0001)], and type of metastatic sites [HR 1.10 (95% CI 1.05–1.16) for visceral involvement, 0.77 (95% CI 0.71–0.84) for neither visceral nor bone compared to bone only (p < 0.0001)]. Details are shown in Table 4.
4. Discussion
Our study analyzed real-world data on MBC across three commonly accepted age groups <40; 40–60 and > 60 years old. Our primary objectives were to describe metastatic breast cancer clinical characteristics according to age in order to evaluate its impact on overall survival, and to analyze the relationship between age and BC subtype at the metastatic stage.
This unique real-world database of a large cohort of patients with MBC demonstrated that young patients had not only, more aggressive presentations in primary tumors but also at the time of metastatic disease, most likely associated with subtype distribution. Indeed, patients under 40y exhibited more frequently three or more metastatic sites and visceral involvement, and had also more frequent HER2+ or triple negative subtypes (Table 2). These features had been identified as independent poor prognostic factors in a previous global report of the ESME MBC cohort [21]. Despite this poor risk presentation, and contrary to what is commonly observed in early breast cancer, OS was significantly longer in young patients in the present cohort: 38.8, 38.4 and 35.6 months for pts <40, 40–60 and > 60y, respectively (p < 0.0001). Specifically, OS was significantly better for young patients with HoR+/Her2-or Her2+ subtypes: HR comparing <40y to >60y was 1.27 (95%CI 1.12–1.44) and 1.62 (95%CI 1.32–1.99) respectively (Fig. 2). No difference was found in the triple negative subtype, where patients suffered a poor survival rate in all age groups. The multivariate analysis demonstrated that age <40y, HER2+ subtype and de novo metastatic disease were all independent favorable prognostic factors, along with disease limited to one site or neither visceral/nor bone sites (Table 4).
The independent favorable impact of young age on overall survival in metastatic breast cancer has been previously reported. One of the main ESME MBC report, which focused on overall survival time trends, showed that each incremental year of age was independently and significantly associated with a higher hazard ratio for death: HR 1.02 per additional year in the HoR+/HER2-and HER2+ subsets (p < 0.001) [21]. Similarly, in a large cohort of metastatic breast cancer from the SEER database, age under 50 was also found to be a favorable prognostic factor among 4932 patients including 850 patients (5%) under 50 years old [22]. Of note, aggressive phenotypes were also more frequent in younger patients in this cohort, again showing no negative impact on OS. On the contrary, overall and breast-cancer specific survivals were significantly better in younger patients compared to middle-aged patients (50–69 y): HR 0.77 (95%IC 0.68–0.87; p < 0.001) and HR 0.81 (95%IC 0.71–0.92; p = 0.002) respectively. In other cohorts as well, younger age was significantly associated with better prognosis, in uni- and multi-variate analysis [[11], [12], [13]].
A possible explanation for the difference in prognosis across age groups could be the less frequent use of chemotherapy. in older patients. Especially in Her2+ MBC chemotherapy as well as anti-Her2 treatments was less frequently used in older patients, possibly due to comorbidities, and could explain the negative impact on OS in patients >60y.
However, in HoR+/Her2-subtype, a dedicated analysis of the same ESME cohort showed that endocrine therapy as first line treatment was not associated with a significant impact on OS and PFS in the entire cohort [23] as well as in patients ≤45 years old [24]. Furthermore, young age is less frequently associated with co-morbidities and frailty. Conversely, older age may limit the possibility to prescribe and sustain treatments, and be associated with more frequent competitive causes of death [9], [25], [26].
For patients with de novo metastatic disease, a potential explanation for the impact of age on OS, is a higher rate of loco-regional treatments in younger patients. Loco-regional treatments are known to be more frequently performed in younger patients and to be associated with better outcomes [17,[27], [28], [29]]. Furthermore, recent prospective data also suggested that locoregional treatment of de novo MBC may improve long term prognosis [30].
This study has several limitations. Causes of death were mostly unknown (52.1% of the total cohort, probably due to the retrospective collection of data), and this hampers our interpretation of overall survival. This is particularly true in the elderly population, where underlying comorbidities and other medications may limit the use of cancer treatments, and/or favor more severe and potentially lethal adverse events, again limiting therapeutic options. Competitive causes of death in elderly patients may also blur the meaning of overall survival, and breast cancer specific survival might be a more suitable endpoint. Another important limitation is the absence of information on the biology of metastatic disease. It has been widely demonstrated that the biological subtype may differ between the primary tumor and the metastatic tissue, notably for HoR + breast cancer [31]. Of note, age did not impact overall survival in HoR-/HER2-patients, and triple negative disease is the less prone to subtype change. Finally, follow-up was collected until on October 2016, before wide use of novel therapeutic agents having shown positive impact on OS (e.g. CDK4/6 inhibitors). These targeted agents are widely prescribed also in elderly women, which may positively impact outcomes, warranting future real-life evaluations.
5. Conclusion
Although young age seems to be associated with a more aggressive presentation at diagnosis of MBC, it does not affect OS in this large serie. On the contrary, young age was associated with a better prognosis, particularly among HoR+/Her2-and Her2+ subtypes, possibly linked to a more frequent use of chemotherapy and anti-Her2 treatments. Further studies should question the possible under-treatment of older patients, and try tailoring treatments to compensate for poor prognosis.
Funding
This work was supported by R&D UNICANCER. The ESME MBC database is supported by an industrial consortium (Roche, Pfizer, AstraZeneca, MSD, Eisai and Daiichi Sankyo). Data collection, analysis and publications are totally managed by R&D UNICANCER independently of the industrial consortium.
Declaration of competing interest
Co-authors having declared no conflict of interests: Sophie Frank, Matthieu Carton, Coraline Dubot, Barbara Pistilli (GR), Audrey Mailliez (COL), Christelle Levy (CFB), Véronique D’Hondt (ICM), Marc Debled (IB), Thomas Vermeulin (CHB), Bruno Coudert (CGFL), Christophe Perrin (CEM), Anthony Gonçalves (IPC), Lionel Uwer (ICL), Jean-Marc Ferrero (CAL), Jean-Christophe Eymard (IJG), Thierry Petit (CPS), Marie-Ange Mouret-Reynier (CJP), Anne Patsouris (ICO PP), Tahar Guesmia (R&D Unicancer), Thomas Bachelot (CLB), Mathieu Robain (R&D Unicancer), Paul Cottu.
Mario campone: Advisory Board: Consulting fees to my Institut:Astra ZENECA, Novartis, Abbvie, Sanofi, Pfizer, Sandoz, ACCORD. Personal fees to Lilly, G1 Therapeutic Consultant: Fees to my Institute: Pierre Fabre Oncology; Sanofi; Novartis; Servier. Speaker bureau: Personnal fees Novartis, Lilly Travel: Pfizer, Novartis, Roche, Astra Zeneca.
Barbara Pistilli:P ersonal fees from AstraZeneca, Pfizer, Myriad, Pierre Fabre and non-financial support from Pfizer, Puma and Merus, Novartis outside the submitted work Florence Dalenc: Advisory Board: Novartis, Pfizer, Lilly Travel: Pfizer, Novartis, Roche, Astra Zeneca Paul Cottu: Advisory Board: Novartis, Pfizer, Lilly, Roche Travel: Pfizer, Novartis, Roche, Astra Zeneca.
Acknowledgements
We thank the 18 French Comprehensive Cancer Centers for providing the data and each ESME local coordinator for managing the project at the local level. Moreover, we thank the ESME Scientific Committee members for their ongoing support.
18 Participating French Comprehensive Cancer Centers (FCCC):; I. Curie, Paris/Saint-Cloud, G. Roussy, Villejuif, I. Cancérologie de l’Ouest, Angers/Nantes, C. F. Baclesse, Caen, ICM Montpellier, C. L. Bérard, Lyon, C. G-F Leclerc, Dijon, C. H. Becquerel, Rouen; I. C. Regaud, Toulouse; C. A. Lacassagne, Nice; Institut de Cancérologie de Lorraine, Nancy; C. E. Marquis, Rennes; I. Paoli-Calmettes, Marseille; C. J. Perrin, Clermont Ferrand; I. Bergonié, Bordeaux; C. P. Strauss, Strasbourg; I. J. Godinot, Reims; C. O. Lambret, Lille. We thank the 18 French Comprehensive Cancer Centers for providing the data and each ESME Contact for coordinating the project at the local level.
ESME central coordinating staff:
Head of Research and Development: Claire Labreveux.
Program director: Mathieu Robain.
Data Management team: Coralie Courtinard, Emilie Nguyen, Olivier Payen, Irwin Piot, Dominique Schwob and Olivier Villacroux.
Operational team: Michaël Chevrot, Daniel Couch, Patricia D’Agostino, Pascale Danglot, Cécilie Dufour, Tahar Guesmia, Christine Hamonou, Gaëtane Simon and Julie Tort.
Supporting clinical research associates: Elodie Kupfer and Toihiri Said.
Project Associate: Nathalie Bouyer.
Management assistant: Esméralda Pereira.
Software designers: Matou Diop, Blaise Fulpin, José Paredes and Alexandre Vanni.
ESME local coordinators:
Patrick Arveux, Thomas Bachelot, Stéphanie Delaine, Delphine Berchery, Etienne Brain, Mathias Breton, Loïc Campion, Emmanuel Chamorey, Marie-Paule Lebitasy, Valérie Dejean, Anne-Valérie Guizard, Anne Jaffré, Lilian Laborde, Carine Laurent, Agnès Loeb, Muriel Mons, Damien Parent, Geneviève Perrocheau, Marie-Ange Mouret-Reynier, Michel Velten.
Contributor Information
Sophie Frank, Email: sophie.frank@curie.fr.
Matthieu Carton, Email: matthieu.carton@curie.fr.
Coraline Dubot, Email: coraline.dubot@curie.fr.
Mario Campone, Email: Mario.Campone@ico.unicancer.fr.
Barbara Pistilli, Email: barbara.pistilli@gustaveroussy.fr.
Florence Dalenc, Email: dalenc.florence@iuct-oncopole.fr.
Audrey Mailliez, Email: a-mailliez@o-lambret.fr.
Christelle Levy, Email: c.levy@baclesse.unicancer.fr.
Véronique D’Hondt, Email: Veronique.Dhondt@icm.unicancer.fr.
Marc Debled, Email: M.Debled@bordeaux.unicancer.fr.
Thomas Vermeulin, Email: thomas.vermeulin@chb.unicancer.fr.
Bruno Coudert, Email: bcoudert@cgfl.fr.
Christophe Perrin, Email: c.perrin@rennes.unicancer.fr.
Anthony Gonçalves, Email: GONCALVESA@ipc.unicancer.fr.
Lionel Uwer, Email: l.uwer@nancy.unicancer.fr.
Jean-Marc Ferrero, Email: jean-marc.ferrero@nice.unicancer.fr.
Jean-Christophe Eymard, Email: jc.eymard@reims.unicancer.fr.
Thierry Petit, Email: tpetit@strasbourg.unicancer.fr.
Marie-Ange Mouret-Reynier, Email: Marie-Ange.MOURET-REYNIER@clermont.unicancer.fr.
Anne Patsouris, Email: Anne.patsouris@ico.unicancer.fr.
Tahar Guesmia, Email: t-guesmia@unicancer.fr.
Thomas Bachelot, Email: thomas.bachelot@lyon.unicancer.fr.
Mathieu Robain, Email: m-robain@unicancer.fr.
Paul Cottu, Email: paul.cottu@curie.fr.
References
- 1.Cowppli-Bony A., Uhry Z., Remontet L. February 2016. Survie des personnes atteintes de cancer en France métropolitaine 1989-2013 - etude à partir des registres des cancers du réseau Francim Partie 1 – tumeurs solides. [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.INCA Les cancers en France. https://www.e-cancer.fr/ressources/cancers_en_france/#page=65
- 4.Azim H.A., Michiels S., Bedard P.L. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Canc Res. 2012;18(5):1341–1351. doi: 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 5.Park Y.H., Lee S.J., Jung H.A. Prevalence and clinical outcomes of young breast cancer (YBC) patients according to intrinsic breast cancer subtypes: single institutional experience in Korea. Breast Edinb Scotl. 2015;24(3):213–217. doi: 10.1016/j.breast.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Murphy B.L., Day C.N., Hoskin T.L., Habermann E.B., Boughey J.C. Adolescents and young adults with breast cancer have more aggressive disease and treatment than patients in their forties. Ann Surg Oncol. 2019;26(12):3920–3930. doi: 10.1245/s10434-019-07653-9. [DOI] [PubMed] [Google Scholar]
- 7.Fredholm H., Eaker S., Frisell J., Holmberg L., Fredriksson I., Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PloS One. 2009;4(11) doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Partridge A.H., Hughes M.E., Warner E.T. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(27):3308–3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 9.Johansson A.L.V., Trewin C.B., Hjerkind K.V., Ellingjord-Dale M., Johannesen T.B., Ursin G. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int J Canc. 2019;144(6):1251–1261. doi: 10.1002/ijc.31950. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso F., Senkus E., Costa A. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(8):1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puente J., López-Tarruella S., Ruiz A. Practical prognostic index for patients with metastatic recurrent breast cancer: retrospective analysis of 2,322 patients from the GEICAM Spanish El Alamo Register. Breast Canc Res Treat. 2010;122(2):591–600. doi: 10.1007/s10549-009-0687-4. [DOI] [PubMed] [Google Scholar]
- 12.Largillier R., Ferrero J.-M., Doyen J. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol Off J Eur Soc Med Oncol. 2008;19(12):2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purushotham A., Shamil E., Cariati M. Age at diagnosis and distant metastasis in breast cancer--a surprising inverse relationship. Eur J Cancer Oxf Engl. 1990;50(10):1697–1705. doi: 10.1016/j.ejca.2014.04.002. 2014. [DOI] [PubMed] [Google Scholar]
- 14.Paluch-Shimon S., Pagani O., Partridge A.H. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3) Breast Edinb Scotl. 2017;35:203–217. doi: 10.1016/j.breast.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Delaloge S., Pérol D., Courtinard C. Paclitaxel plus bevacizumab or paclitaxel as first-line treatment for HER2-negative metastatic breast cancer in a multicenter national observational study. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(9):1725–1732. doi: 10.1093/annonc/mdw260. [DOI] [PubMed] [Google Scholar]
- 16.Cabel L., Carton M., Cheaib B. Oral etoposide in heavily pre-treated metastatic breast cancer: results from the ESME cohort and comparison with other chemotherapy regimens. Breast Canc Res Treat. 2019;173(2):397–406. doi: 10.1007/s10549-018-5017-2. [DOI] [PubMed] [Google Scholar]
- 17.Pons-Tostivint E., Kirova Y., Lusque A. Survival impact of locoregional treatment of the primary tumor in de novo metastatic breast cancers in a large multicentric cohort study: a propensity score-matched analysis. Ann Surg Oncol. 2019;26(2):356–365. doi: 10.1245/s10434-018-6831-9. [DOI] [PubMed] [Google Scholar]
- 18.Jacot W., Heudel P.-E., Fraisse J. Real-life activity of eribulin mesylate among metastatic breast cancer patients in the multicenter national observational ESME program. Int J Canc. May 2019 doi: 10.1002/ijc.32402. [DOI] [PubMed] [Google Scholar]
- 19.Sirieix J., Fraisse J., Mathoulin-Pelissier S. 294PD_PR - Management and outcome of metastatic breast cancer in men in the national multicenter observational ESME program. Ann Oncol. 2018;29 doi: 10.1093/annonc/mdy424.013. viii710. [DOI] [Google Scholar]
- 20.Pérol D., Robain M., Arveux P. The ongoing French metastatic breast cancer (MBC) cohort: the example-based methodology of the Epidemiological Strategy and Medical Economics (ESME) BMJ Open. 2019;9(2) doi: 10.1136/bmjopen-2018-023568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gobbini E., Ezzalfani M., Dieras V. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer Oxf Engl. 1990;96:17–24. doi: 10.1016/j.ejca.2018.03.015. 2018. [DOI] [PubMed] [Google Scholar]
- 22.Chen M.-T., Sun H.-F., Zhao Y. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci Rep. 2017;7(1):9254. doi: 10.1038/s41598-017-10166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacquet E., Lardy-Cléaud A., Pistilli B. Endocrine therapy or chemotherapy as first-line therapy in hormone receptor-positive HER2-negative metastatic breast cancer patients. Eur J Cancer Oxf Engl. 1990;95:93–101. doi: 10.1016/j.ejca.2018.03.013. 2018. [DOI] [PubMed] [Google Scholar]
- 24.Pistilli B., Lardy-Cléaud A., Jacquet E. vol. 28. Poster Present ESMO; 2017. https://www.esmo.org/content/download/117241/2057634/file/ESMO-2017-Abstract-Book.pdf (FICHE-YOUNG: FIrst-line treatment CHoicE in hormone receptor positive (HR+)/HER2- negative metastatic breast cancer patients (MBC) ≤ 45 years old. A large observational multicenter cohort survival analysis). 2017. [Google Scholar]
- 25.Derks M.G.M., van de Velde C.J.H., Giardiello D. Impact of comorbidities and age on cause-specific mortality in postmenopausal patients with breast cancer. The Oncologist. January. 2019 doi: 10.1634/theoncologist.2018-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dasgupta P., Aitken J.F., Pyke C., Baade P.D. Competing mortality risks among women aged 50-79 years when diagnosed with invasive breast cancer, Queensland, 1997-2012. Breast Edinb Scotl. 2018;41:113–119. doi: 10.1016/j.breast.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Warschkow R., Güller U., Tarantino I. Improved survival after primary tumor surgery in metastatic breast cancer: a propensity-adjusted, population-based SEER trend analysis. Ann Surg. 2016;263(6):1188–1198. doi: 10.1097/SLA.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 28.Sofi A.A., Mohamed I., Koumaya M., Kamaluddin Z. Local therapy in metastatic breast cancer is associated with improved survival. Am J Therapeut. 2013;20(5):487–492. doi: 10.1097/MJT.0b013e31822119c5. [DOI] [PubMed] [Google Scholar]
- 29.Lambertini M., Ferreira A.R., Di Meglio A. Patterns of care and clinical outcomes of HER2-positive metastatic breast cancer patients with newly diagnosed stage IV or recurrent disease undergoing first-line trastuzumab-based therapy: a multicenter retrospective cohort study. Clin Breast Canc. 2017;17(8):601–610. doi: 10.1016/j.clbc.2017.04.002. e2. [DOI] [PubMed] [Google Scholar]
- 30.Soran A., Ozmen V., Ozbas S. Randomized trial comparing resection of primary tumor with No surgery in stage IV breast cancer at presentation: protocol MF07-01. Ann Surg Oncol. 2018;25(11):3141–3149. doi: 10.1245/s10434-018-6494-6. [DOI] [PubMed] [Google Scholar]
- 31.Schrijver W.A.M.E., Suijkerbuijk K.P.M., van Gils C.H., van der Wall E., Moelans C.B., van Diest P.J. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(6):568–580. doi: 10.1093/jnci/djx273. [DOI] [PubMed] [Google Scholar]