Abstract
Purpose
Metaplastic breast cancer (MBC) is a rare, aggressive variant of breast cancer that has been associated with poor clinical outcomes, as has triple-negative breast (TNBC) cancer. Limited studies compare the clinical characteristics and prognosis of MBC to TNBC. This study uses a large, contemporary US cancer database to compare clinical characteristics and survival outcomes for patients with MBC to those with TNBC.
Methods
The National Cancer Database was queried for women with cT1-4N1-3M0 MBC or TNBC diagnosed between 2004 and 2013 and treated with definitive surgery. Chi-squared analysis was performed to determine differences between the cohorts. Kaplan-Meier curves compared overall survival (OS), and Cox regression determined patient factors associated with OS.
Results
Altogether, 55,847 patients met the inclusion criteria; 50,705 (90.8%) had TNBC and 5,142 (9.2%) had MBC. Most patients had no comorbid conditions (82%), N0 disease (71%), poorly differentiated histology (77%), received chemotherapy (87%), and received radiation therapy (60%). Amongst all patients, patients with TNBC disease were observed to have greater OS than those with MBC (5-year OS 72.0% vs 55.8%, p < 0.001). The greater observed OS for patients with TNBC persisted when controlling for stage and when comparing propensity score matched cohorts. On Cox regression, lower age, T1 status, N0 status, chemotherapy, TNBC disease, and radiation therapy (RT) were associated with improved OS.
Conclusions
MBC had an association with poorer OS compared to TNBC, while RT and chemotherapy receipt were associated with improved OS for patients regardless of stage. Further studies are needed to corroborate the conclusions herein.
Keywords: Breast cancer, Triple-negative, Metaplastic, Radiation therapy, Chemotherapy
Highlights
-
•
Metaplastic breast cancer is associated with poor clinical outcomes.
-
•
Metaplastic breast cancer associated with worse survival than patients with triple negative invasive ductal carcinoma.
-
•
Radiation therapy and chemotherapy associated with improved survival for patients with metaplastic breast cancer.
1. Introduction
Metaplastic breast cancer (MBC) is a rare histological variant of breast cancer, which is thought to be more aggressive than typical invasive ductal carcinoma [[1], [2], [3], [4]]. MBC is associated with high tumor grade, large tumor size, less advanced nodal involvement, and high rates of metastasis [[5], [6], [7], [8]]. Triple-negative breast cancers (TNBC) with invasive ductal carcinoma histology are also an aggressive form of breast cancer with absent estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression. This subtype, which comprises 12–17% of breast cancer cases, cannot be effectively treated with targeted therapies to the HER2 receptor or anti-estrogen treatment, and is associated with poorer outcomes than hormone receptor positive disease [9].
MBC patients usually have a triple-negative phenotype, but exhibit a different gene expression profile compared to those with invasive ductal breast carcinoma. These include higher expression of genes associated with an epithelial to mesenchymal transition (EMT) attributed to acquisition of migratory morphology and dissemination of malignancy, as well as downregulation of genes associated with a chemoresistant phenotype [1,10,11]. While there is limited data to guide management of metaplastic breast cancer due to its infrequency (0.25%–1%) [12,13], the available studies support aggressive treatment, including the use of adjuvant radiation therapy (RT) [3,7]. Few studies addressing the outcomes comparing TNBC with MBC have been undertaken. While these studies have suggested worse outcomes with MBC, they have been limited by small sample size and short follow up [4,5,14,15]. The purpose of our study is to further expand on prior studies by evaluating national practice patterns for patients with TNBC and MBC, compared clinical characteristics between these two cohorts of patients, and to determine long-term comparative outcomes using a large, contemporary US cancer database.
2. Materials and methods
This investigation analyzed patients from the National Cancer Database (NCDB), which is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The database consists of de-identified information regarding tumor characteristics, patient demographics, and patient survival for approximately 70% of the US population [[16], [17], [18], [19], [20]]. The NCDB contains information not included in the Surveillance, Epidemiology, and End Results database, including details regarding use of systemic therapy and radiation dose. The data used in this study were derived from a de-identified NCDB file. The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data by the investigators. As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
Inclusion criteria for this study were women with newly-diagnosed, T1-4N0-3M0 breast cancer with either MBC histology (International Classification of Disease [ICD]-0-3 codes 8560, 8562, 8570–8572, 8575, and 8980–8982) or TNBC with infiltrating ductal carcinoma histology (ICD-0-3 8500). In order for including, a complete record of clinical staging for T, N, and M stage was required. Cases with unknown information regarding chemotherapy, radiation, definitive surgical therapy, and vital status were excluded. The χ2 test analyzed categorical frequencies between groups non-parametrically. Multivariate logistic regression was used to determine characteristics associated to a greater extent with MBC as compared to TNBC. The Kaplan-Meier method was used for survival analysis, with comparisons between the groups made using the log-rank test. Overall survival (OS) was defined as the interval between the date of diagnosis and the date of death, or censored at last contact. Subset analysis was performed to compare OS between patients with either TNBC or MBC while stratifying patients by T stage and N stage. Subset analysis was also performed for estrogen receptor positive (ER+) MBC patients while stratifying patients based on receipt of hormonal therapy. Cox multivariable analysis was performed to determine factors associated with overall survival using variables that showed statistical significance on univariate analysis.
Due to imbalances between the arms, propensity score matching (PSM) was performed to compare survival outcomes between different groups. Statistically significant variables from multivariate Cox analysis were included for matching. These variables included age, race, Charlson Deyo score, insurance status, median income, year of diagnosis, T stage, N stage, grade, chemotherapy receipt, RT receipt, and HER2 status. Specifically, PSM balanced these variables through matching and provided a propensity score based on the probability of receiving treatment for the given variables [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30]]. Patients from the different groups were paired together based on the similarity of the propensity score. Patients were matched 1:1 without replacement and a caliper of 0.05 was used to ensure balance. Standardized mean differences were determined to check for large imbalances for each variable between the matched cohorts with a value of <0.1 reflecting a significant imbalance [31]. Data was analyzed using SPSS (IBM Corp. Version 24.0. Armonk, NY).
3. Results
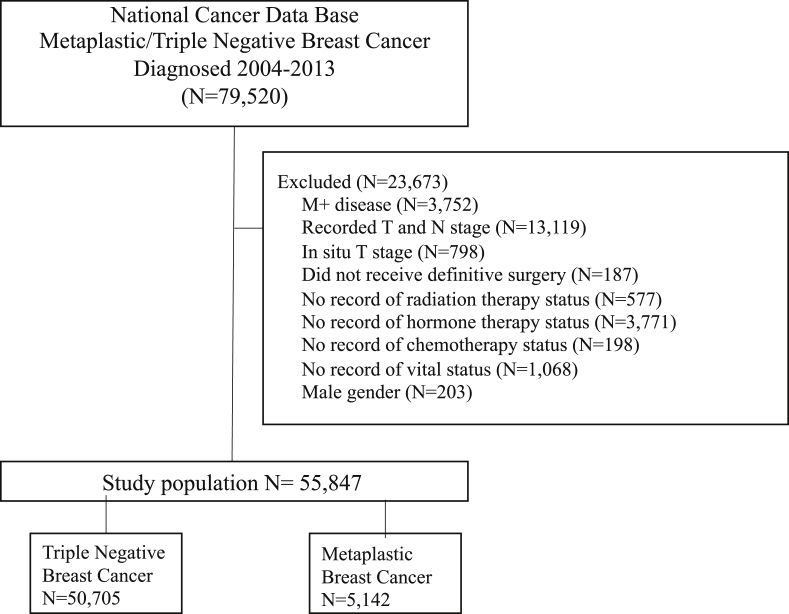
A complete flow diagram with inclusion criteria is provided in Fig. 1. In all, 55,847 patients met the inclusion criteria. Of these, 50,705 (90.8%) patients had TNBC and 5,142 (9.2%) had MBC. Table 1 shows the clinical and demographic characteristics for the patients included in the study. A greater proportion of patients with MBC compared to those with TNBC were over 65 years of age; had T2-4, N0 and well differentiated disease; and received treatment with mastectomy. MBC patients were less likely to have private insurance or receive treatment with either chemotherapy or RT. Of note, most patients were Caucasian, had pN0 disease discovered on pathology, poorly differentiated or anaplastic grade, and fewer comorbidities. 25.4% of MBC and 26.5% of TNBC patients received an axillary lymph node dissection (number of lymph nodes examined ≥ 10). HER2 status was not recorded in 51.9% of MBC patients compared to 2.3% of TNBC patients.
Fig. 1.
Patient selection diagram.
Table 1.
Baseline characteristics of patients in each of the cohorts.
| Characteristic | Intraductal Breast Cancer (N = 50,705) | Metastatic Breast Cancer (N = 5142) | P value |
|---|---|---|---|
| Age | |||
| ≤50 | 14,184 (28%) | 1153 (22.4%) | <0.001 |
| 51-64 | 19,729 (38.9%) | 1760 (34.2%) | |
| ≥65 | 16,792 (33.1%) | 2229 (43.3%) | |
| Race | |||
| White | 39,586 (78.1%) | 4198 (81.6%) | <0.001 |
| Black | 8677 (17.1%) | 709 (13.8%) | |
| Other | 2442 (4.8%) | 235 (4.6%) | |
| Charlson Deyo Score | |||
| 0 | 41,785 (82.4%) | 4186 (81.4%) | 0.102 |
| 1 | 7191 (14.2%) | 756 (14.7%) | |
| ≥2 | 1729 (3.4%) | 200 (3.9%) | |
| Insurance Status | |||
| Medicaid | 4450 (8.8%) | 359 (7.0%) | <0.001 |
| Private | 27,421 (54.1%) | 2427 (47.2%) | |
| Medicare | 16,302 (32.2%) | 2078 (40.4%) | |
| Not Insured | 1362 (2.7%) | 115 (2.2%) | |
| Other | 1170 (2.3%) | 163 (3.2%) | |
| Median Income | |||
| ≤ $62999 | 34,192 (67.4%) | 3421 (66.5%) | <0.001 |
| ≥ $63000 | 16,303 (32.2%) | 1659 (32.3%) | |
| Not recorded | 210 (0.4%) | 62 (1.2%) | |
| Facility Type | |||
| Academic | 20,410 (40.3%) | 2134 (41.5%) | 0.009 |
| Nonacademic | 27,371 (53.6%) | 2742 (53.3%) | |
| Not recorded | 3122 (6.2%) | 266 (5.2%) | |
| Year of Diagnosis | |||
| 2004–2008 | 1308 (2.6%) | 2077 (40.4%) | <0.001 |
| 2009–2013 | 49,397 (97.4%) | 3065 (59.6%) | |
| T stage | |||
| T1 | 28,839 (56.9%) | 1639 (31.9%) | <0.001 |
| T2 | 18,355 (3.62%) | 2490 (48.4%) | |
| T3 | 2530 (5.0%) | 736 (14.3%) | |
| T4 | 981 (1.9%) | 277 (5.4%) | |
| N stage | |||
| N0 | 35,750 (70.5%) | 4143 (80.6%) | <0.001 |
| N1 | 10,084 (19.9%) | 709 (13.7%) | |
| N2 | 3171 (6.3%) | 207 (4.0%) | |
| N3 | 1700 (3.4%) | 86 (1.7%) | |
| Grade | |||
| Well differentiated | 2476 (4.9%) | 698 (13.6%) | <0.001 |
| Moderately differentiated | 8006 (15.8%) | 600 (11.7%) | |
| Poorly differentiated/anaplastic | 39,426 (77.8%) | 3602 (70.1%) | |
| Not recorded | 797 (1.6%) | 242 (4.7%) | |
| Chemotherapy use | |||
| Yes | 44,365 (87.5%) | 4054 (78.8%) | <0.001 |
| No | 6340 (12.5%) | 1088 (21.2%) | |
| Hormonal therapy use | |||
| Yes | 2809 (5.5%) | 828 (16.1%) | <0.001 |
| No | 47,896 (94.5%) | 4314 (83.9%) | |
| Radiation therapy | |||
| Yes | 30,812 (60.8%) | 2698 (52.5%) | <0.001 |
| No | 19,893 (39.2%) | 2444 (47.5%) | |
| Surgery | |||
| Lumpectomy | 263,373 (52.0%) | 2247 (43.7%) | <0.001 |
| Mastectomy | 24,332 (48.0%) | 2895 (56.3%) | |
| ER status | |||
| Positive | 0 (0.0%) | 750 (14.6%) | <0.001 |
| Negative | 50,667 (99.9%) | 4187 (81.4%) | |
| Not reported | 38 (0.1%) | 205 (4.0%) | |
| PR status | |||
| Positive | 0 (0.0%) | 536 (10.4%) | <0.001 |
| Negative | 50,669 (99.9%) | 4389 (85.4%) | |
| Not reported | 36 (0.1%) | 217 (4.2%) | |
| HER2 status | |||
| Positive | 0 (0.0%) | 124 (2.4%) | <0.001 |
| Negative | 49,541 (97.7%) | 2349 (45.7%) | |
| Not reported | 1164 (2.3%) | 2669 (51.9%) | |
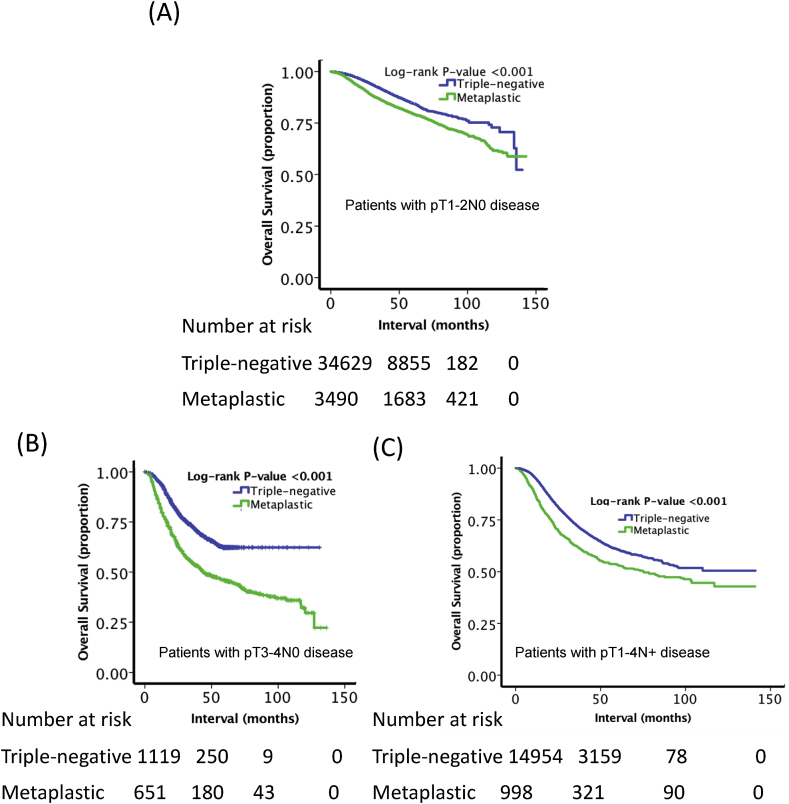
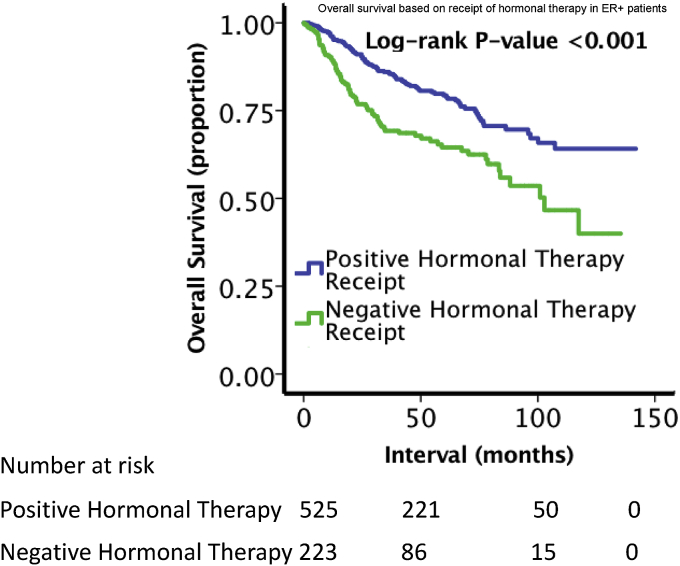
Table 2 shows the results of the multivariable logistic regression for characteristics associated with MBC histology. Older age, Caucasian ethnicity, higher median income, treatment at academic centers, diagnosis at earlier years, high T stage, low N stage, well-differentiated grade, mastectomy, and receipt of hormonal therapy were all factors associated with MBC histology (p < 0.05). The 5-year overall survival for patients with MBC was 55.8%, compared to 72.0% (p < 0.001) for those with TNBC (Fig. 2A). Due to imbalance between the groups, PSM was performed to determine OS in balanced cohorts. The groups were relatively evenly balanced in the matched cohorts (Supplemental Tables 1 and 2). As shown in Fig. 2B, after PSM matching, patients with TNBC remained associated with a greater OS (5-year OS 65.2% vs. 60.5%, p < 0.001). Due to differences in T and N stage, patients were compared following stratification based on stage. As shown in Fig. 3, when compared to patients with TNBC, poorer OS persisted for MBC patients based on the following subgroups: T1-T2N0 (5-year OS 63.8% vs. 79.7%, p < 0.001), T3-T4N0 (5-year OS 33.1% vs. 62.1%, p < 0.001), and T1-4N+ (5-year OS 42.7% vs. 56.0%, p < 0.001). As shown in Fig. 4, ER + MBC patients who received hormonal therapy showed improved OS compared to ER + MBC patients who did not receive hormonal therapy (5-year OS 63.4% vs 49.3%, p < 0.001). In total, 750 MBC patients had ER + status with 526 (70.1%) receiving hormonal therapy versus 224 (29.9%) who did not.
Table 2.
Characteristics showing association with MBC using multivariate logistic regression.
| Characteristic | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Age | |||
| ≤50 | 1 (reference) | ||
| 51-64 | 1.276 | 1.132–1.438 | <0.001 |
| ≥65 | 1.733 | 1.479–2.030 | <0.001 |
| Race | |||
| White | 1 (reference) | ||
| Black | 0.759 | 0.674–0.854 | <0.001 |
| Other | 0.958 | 0.787–1.165 | 0.666 |
| Charlson Deyo Score | |||
| 0 | 1 (reference) | ||
| 1 | 1.092 | 0.972–1.227 | 0.138 |
| ≥2 | 1.04 | 0.837–1.292 | 0.724 |
| Insurance Status | |||
| Medicaid | 1 (reference) | ||
| Private | 1.211 | 1.028–1.426 | 0.022 |
| Medicare | 1.248 | 1.025–1.518 | 0.318 |
| Not Insured | 0.852 | 0.621–1.167 | 0.001 |
| Other | 1.64 | 1.230–2.186 | 0.022 |
| Median Income | |||
| ≤ $62999 | 1 (reference) | ||
| ≥ $63000 | 1.216 | 1.112–1.330 | <0.001 |
| Not recorded | 1.784 | 1.110–2.865 | 0.017 |
| Facility Type | |||
| Academic | 1 (reference) | ||
| Nonacademic | 0.879 | 0.807–0.958 | 0.003 |
| Not recorded | 0.865 | 0.702–1.066 | 0.173 |
| Year of Diagnosis | |||
| 2004–2008 | 1 (reference) | ||
| 2009–2013 | 0.269 | 0.235–0.309 | <0.001 |
| T stage | |||
| T1 | 1 (reference) | ||
| T2 | 2.854 | 2.596–3.138 | <0.001 |
| T3 | 8.73 | 7.511–10.147 | <0.001 |
| T4 | 8.603 | 6.862–10.787 | <0.001 |
| N stage | |||
| N0 | 1 (reference) | ||
| N1 | 0.409 | 0.363–0.462 | <0.001 |
| N2 | 0.214 | 0.172–0.267 | <0.001 |
| N3 | 0.170 | 0.125–0.230 | <0.001 |
| Grade | |||
| Well differentiated | 1 (reference) | ||
| Moderately differentiated | 0.259 | 0.218–0.308 | <0.001 |
| Poorly differentiated/anaplastic | 0.295 | 0.258–0.337 | <0.001 |
| Not recorded | 1.073 | 0.835–1.378 | 0.584 |
| Chemotherapy use | |||
| Yes | 1 (reference) | ||
| No | 1.069 | 0.947–1.207 | 0.280 |
| Hormonal therapy use | |||
| Yes | 1 (reference) | ||
| No | 1.172 | 0.971–1.416 | 0.098 |
| Radiation therapy | |||
| Yes | 1 (reference) | ||
| No | 0.954 | 0.859–1.060 | 0.382 |
| Surgery | |||
| Lumpectomy | 1 (reference) | ||
| Mastectomy | 1.208 | 1.082–1.350 | <0.001 |
| ER status | |||
| Positive | 1 (reference) | ||
| Negative | 0.000 | 0.000–0.000 | 0.983 |
| Not reported | 0.000 | 0.000–0.000 | 0.983 |
| PR status | |||
| Positive | 1 (reference) | ||
| Negative | 0.000 | 0.000–0.000 | 0.986 |
| Not reported | 0.000 | 0.000–0.000 | 0.987 |
| HER2 status | |||
| Positive | 1 (reference) | ||
| Negative | 0.000 | 0.000–0.000 | 0.994 |
| Not reported | 0.000 | 0.000–0.000 | 0.994 |
Fig. 2.
Kaplan-Meier overall survival curves comparing the two cohorts in (A) all patients and (B) the propensity matched population.
Fig. 3.
Kaplan-Meier overall survival curves comparing the two cohorts in (A) patients with pT1-T2N0 status, (B) and patients with pT3-T4N0 status, and (C) patients with pT1-4N + status. MBC was associated with poorer OS when compared to TNBC (p < 0.001).
Fig. 4.
Kaplan-Meier overall survival curves comparing ER + MBC patients based on receipt of hormonal therapy.
Results of Cox univariate and multivariate analysis to determine factors associated with overall survival are displayed in Table 3. Several factors were associated with improved overall survival on univariate analysis, including TNBC, younger age, lower nodal disease burden, Charlson Deyo score of 0, private insurance, median income ≥ $63,000, receipt of treatment at academic centers, diagnosis from 2009 to 2013, T1 status, moderately-differentiated disease, chemotherapy use, RT use, hormonal therapy use, and treatment with lumpectomy (Table 3). On multivariable Cox regression analysis, factors associated with improved OS were TNBC disease, younger age, Charlson Deyo score 0, higher socioeconomic status, earlier year of diagnosis, earlier T and N stage of disease, chemotherapy use, hormonal therapy use, and RT use.
Table 3.
Univariate and Multivariate Cox regression analysis of factors predictive of overall survival for all patients.
| Characteristic | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% confidence interval | P value | Hazard ratio | 95% confidence interval | P value | |
| Triple-negative | 1 (reference) | 1 (reference) | ||||
| Metaplastic | 1.475 | 1.393–1.562 | <0.001 | 1.310 | 1.217–1.410 | <0.001 |
| Age | ||||||
| ≤50 (first) | 1 (reference) | 1 (reference) | ||||
| 51–64 | 0.950 | 0.899–1.004 | 0.068 | 1.046 | 0.984–1.111 | 0.148 |
| ≥65 | 1.733 | 1.647–1.824 | <0.001 | 1.427 | 1.319–1.545 | <0.001 |
| Race | ||||||
| White | 1 (reference) | 1 (reference) | ||||
| Black | 1.120 | 1.063–1.180 | <0.001 | 1.054 | 1.000–1.112 | 0.052 |
| Other | 0.836 | 0.752–0.929 | 0.001 | 0.882 | 0.794–0.980 | 0.020 |
| Charlson Deyo Score | ||||||
| 0 | 1 (reference) | 1 (reference) | ||||
| 1 | 1.453 | 1.377–1.532 | <0.001 | 1.242 | 1.176–1.311 | <0.001 |
| ≥2 | 2.482 | 2.285–2.695 | <0.001 | 1.859 | 1.709–2.021 | <0.001 |
| Insurance Status | ||||||
| Medicaid | 1 (reference) | 1 (reference) | ||||
| Private | 0.552 | 0.515–0.592 | <0.001 | 0.741 | 0.690–0.795 | <0.001 |
| Medicare | 1.117 | 1.043–1.197 | 0.002 | 0.983 | 0.900–1.073 | 0.700 |
| Not Insured | 0.931 | 0.817–1.060 | 0.280 | 0.99 | 0.869–1.127 | 0.878 |
| Other | 0.667 | 0.571–0.778 | <0.001 | 0.777 | 0.664–0.908 | 0.001 |
| Median Income | ||||||
| ≤ $62999 | 1 (reference) | 1 (reference) | ||||
| ≥ $63000 | 0.767 | 0.732–0.802 | <0.001 | 0.901 | 0.860–0.944 | <0.001 |
| Not recorded | 2.479 | 2.009–3.059 | <0.001 | 2.330 | 1.887–2.878 | <0.001 |
| Facility Type | ||||||
| Academic | 1 (reference) | 1 (reference) | ||||
| Nonacademic | 1.112 | 1.066–1.161 | <0.001 | 1.038 | 0.994–1.084 | 0.089 |
| Not recorded | 0.897 | 0.820–0.980 | 0.016 | 0.994 | 0.901–1.097 | 0.908 |
| Year of Diagnosis | ||||||
| 2004–2008 | 1 (reference) | 1 (reference) | ||||
| 2009–2013 | 0.817 | 0.763–0.874 | <0.001 | 1.104 | 1.019–1.195 | 0.015 |
| T stage | ||||||
| T1 | 1 (reference) | 1 (reference) | ||||
| T2 | 2.174 | 2.074–2.278 | <0.001 | 1.748 | 1.664–1.837 | <0.001 |
| T3 | 5.345 | 5.015–5.696 | <0.001 | 3.212 | 2.90–3.449 | <0.001 |
| T4 | 9.392 | 8.664–10.181 | <0.001 | 4.297 | 3.931–4.697 | <0.001 |
| N stage | ||||||
| N0 | 1 (reference) | 1 (reference) | ||||
| N1 | 2.043 | 1.945–2.146 | <0.001 | 1.900 | 1.804–2.001 | <0.001 |
| N2 | 4.259 | 4.008–4.525 | <0.001 | 3.473 | 3.249–3.714 | <0.001 |
| N3 | 6.829 | 6.376–7.315 | <0.001 | 5.142 | 4.766–5.547 | <0.001 |
| Grade | ||||||
| Well differentiated | 1 (reference) | 1 (reference) | ||||
| Moderately differentiated | 0.728 | 0.658–0.807 | <0.001 | 0.846 | 0.763–0.938 | 0.002 |
| Poorly differentiated/anaplastic | 0.949 | 0.869–1.035 | 0.238 | 1.031 | 0.943–1.127 | 0.501 |
| Not recorded | 0.530 | 0.433–0.648 | <0.001 | 0.636 | 0.519–0.779 | <0.001 |
| Chemotherapy use | ||||||
| Yes | 1 (reference) | 1 (reference) | ||||
| No | 1.371 | 1.298–1.447 | <0.001 | 1.527 | 1.438–1.621 | <0.001 |
| Hormonal therapy use | ||||||
| Yes | 1 (reference) | 1 (reference) | ||||
| No | 1.127 | 1.036–1.226 | 0.005 | 1.133 | 1.035–1.240 | 0.007 |
| Radiation therapy | ||||||
| Yes | 1 (reference) | 1 (reference) | ||||
| No | 1.445 | 1.387–1.504 | <0.001 | 1.475 | 1.405–1.549 | <0.001 |
| Surgery | ||||||
| Lumpectomy | 1 (reference) | 1 (reference) | ||||
| Mastectomy | 2.074 | 1.988–2.163 | <0.001 | 1.044 | 0.991–1.100 | 0.106 |
| ER status | ||||||
| Positive | 1 (reference) | 1 (reference) | ||||
| Negative | 0.841 | 0.726–0.976 | 0.022 | 1.050 | 0.872–1.265 | 0.608 |
| Not reported | 1.518 | 1.185–1.944 | 0.001 | 1.102 | 0.643–1.888 | 0.724 |
| PR status | ||||||
| Positive | 1 (reference) | 1 (reference) | ||||
| Negative | 0.944 | 0.788–1.131 | 0.535 | 1.191 | 0.961–1.476 | 0.111 |
| Not reported | 1.769 | 1.357–2.305 | <0.001 | 1.436 | 0.836–2.465 | 0.189 |
| HER2 status | ||||||
| Positive | 1 (reference) | |||||
| Negative | 0.985 | 0.628–1.545 | 0.946 | |||
| Not reported | 1.374 | 0.873–2.162 | 0.169 | |||
4. Discussion
The present study is the largest to date with over 5,000 MBC and 50,000 TNBC patients to compare the clinical characteristics, national practice patterns, and outcomes between patients with either TNBC or MBC. As has been demonstrated in previously published reports, MBC patients were found to have poorer OS compared to those with TNBC [4,5,14,15].
Additionally, MBC patients had different clinical and pathologic characteristics than TNBC patients, including a higher proportion of patients with well differentiated disease, more advanced T stage, and less advanced or similar N stage. This is concordant with a prior retrospective study comparing patients with MBC to patients with typical invasive breast ductal carcinoma [6], as well as smaller single institution studies comparing characteristics of patients with MBC to patients with TNBC [4,5]. While TNBC disease was associated with improved OS when comparing all patients, due to the differences in stage as well as grade between these two patient cohorts, it was important to compare patients in more balanced groups. Worse survival with MBC persisted when comparing the propensity matched cohorts, and also after stratifying patients by stage. These results are in line with previously published reports comparing outcomes for patients with TNBC disease to those with MBC. In a retrospective study of 46 MBC and 508 TNBC patients, El Zein et al. also employed matching (40 MBC and 40 TNBC) using a variety of covariates, and found that patients with MBC had worse disease-free survival and OS compared to patients with TNBC (p < 0.05) [5]. In another study by Lee et al. comparing 67 MBC and 520 TNBC patients, the 5-year OS rate for MBC patients was 53.7% compared to 84.6% for TNBC patients and 5-year disease-free survival (DFS) was 45.6% compared to 81.6% (p < 0.001 for all) [15]. After matching and stratifying by stage, significantly worse DFS and OS were noted only for stage II MBC versus TNBC patients. However, this study did not use one-to-one matching and had a relatively small sample size compared, limiting its power to draw conclusions. Additionally, there were temporal differences observed between TNBC and MBC patients, with patients with TNBC disease being diagnosed in more recent time periods. This is likely due to the lack of complete information regarding HER2 status in earlier time periods in the NCDB.
In general, the presence of nodal involvement has been considered to be the most important prognostic factor for OS and disease-free survival (DFS) in breast cancer [32,33]. Consistent with this statement, TNBC patients with nodal involvement in the present study had poorer OS compared to T3-T4N0 TNBC patients (5-year OS 56.0% vs 62.1%). However, T3-T4N0 MBC patients had poorer OS compared to MBC patients with node-positive disease (5-year OS 33.1% vs 42.7%). Likewise, El Zein et al. found that T4 MBC patients had a 40% 5- year DFS and 40% 5-year OS rate compared to a 78.9% 5-year DFS and 5-year 73.1% OS rate for patients with lower T stage disease [4]. Other large retrospective reviews of patients with MBC have demonstrated that tumor size/more advanced T stage are significant variables predicting for poor OS [34,35]. This suggests that unlike the majority of breast cancer cases, T stage may be a more robust prognosticator for MBC than nodal status.
The most common surgical procedure for MBC patients was mastectomy, in contrast to TNBC patients, who most frequently received surgical treatment with lumpectomy for TNBC patients. This may be due to the aggressive nature of MBC, which often presents at advanced T stages, warranting a more invasive approach [3,4]. Additionally, a smaller proportion of patients with MBC patients received chemotherapy than patients with TNBC patients. Previously published reports have suggested that patients with MBC have a poor response to chemotherapy without benefits in OS, distant metastasis, or local-regional recurrence [8]. MBC may also have a different molecular profile than invasive ductal carcinoma, making it less responsive to systemic therapy [1]. In one single-institution retrospective study with 55 Stage I-III MBC patients, 87% of patients received adjuvant chemotherapy and over 40% experienced distant metastasis [36]. Thus, the chemoresistance of MBC may contribute to the frequency of metastasis. In another single-institution retrospective study with 46 MBC patients, Chen et al. found a lack of response in those receiving anthracycline, vinorelbine, or cyclophosphamide-based chemotherapy and 90% of patients receiving neoadjuvant therapy had disease progression [37]. Others have found a complete response rate of only 10–17% following neoadjuvant chemotherapy in MBC patients [38,39].
In general, hormonal therapy is recommended for ER+ and/or progesterone-receptor positive (PR+) breast cancer patients due to its efficacy [40]. However, hormonal therapy is usually ineffective for MBC patients because the majority of the patients have triple-negative status [41,42]. Furthermore, a retrospective study by Paul Wright et al. utilizing the Surveillance, Epidemiology, and End Results Database did not find a survival benefit even in patients with positive hormone-receptor MBC tumors, although this study is limited due to a lack of information on receipt of hormonal-therapy [43]. In contrast, our study demonstrated that ER + MBC patients receiving hormonal therapy demonstrated improved OS compared to those who did not. This suggests that there may be subsets of MBC patients, in particular ER + patients, who may benefit from the use of hormonal therapy.
MBC tissue samples have been shown to express low levels of genes associated with cell-cell adhesion (claudin-low), but high levels of EMT and stem-cell like markers, such as elevated CD29/CD24 ratios [44]. CD24 expression has also been described as a prognostic feature in various malignancies, including sarcomas [45]. These distinct factors, in addition to mutations activating the phosphatidylinositol 3-kinase (PI3K/AKT) pathway may contribute to the chemoresistant profile of MBCs compared to TNBC [46,47]. Our group has previously demonstrated that a smaller percentage of patients with MBC received RT than patients with TNBC disease, despite use of RT having an association with improved OS [3]. Prior studies have also shown that RT may result in improved OS, local-regional recurrence rates, and disease-specific survival for MBC patients [7]. These studies suggest postoperative RT should be administered in patients with MBC following lumpectomy and following mastectomy in the setting of locally advanced or node positive disease.
There are several limitations to this study. As a retrospective study, there is potential for selection bias and imbalance between the cohorts. While PSM was conducted to minimize imbalance between the arms, there may be unmeasured cofounding covariates. Second, the NCDB does not keep track of certain information such as the reason why a patient received a particular treatment or exact agents used for treatment. It also does not provide information about important high-risk features, such as lymphovascular invasion or Ki-67 proliferative index. Third, there are epithelial and mixed types of MBC, each with different subtypes. The NCDB did not provide data regarding the various subtypes and there is a lack of a central pathologic review of the diagnoses, which may influence the conclusions herein [1,48]. While some did not find a significant difference in outcomes among these subtypes [4,8], other studies suggest the subtypes of MBC may have different prognoses and have different rates of achieving complete pathologic response following chemotherapy [39,49]. The presence of more than one metaplastic components may also be associated with poorer outcomes [39]. Finally, the NCDB also does not provide information on disease-free survival, disease-specific survival, or local recurrence rates of cancer. Despite these limitations, further prospective studies are needed to corroborate the findings highlighted in this study.
5. Conclusions
This is the largest study to date comparing clinical characteristics and outcomes of patients with MBC to patients with TNBC. MBC patients present more often with well differentiated disease, more advanced T stage, and less advanced or similar N stage than TNBC patients. A smaller percentage of MBC versus TNBC patients received RT, chemotherapy, and lumpectomy for treatments. While there may be a subset of ER + MBC patients who may respond to hormonal therapy, MBC patients, in general, have worse OS compared with TNBC patients. Further prospective studies are needed to corroborate our conclusions.
Disclaimers
This manuscript has never been presented/published before in any form. All authors declare that conflicts of interest do not exist.
Funding
There was no research support for this study.
Declaration of competing interest
The authors have no conflicts of interest. There was no funding for this study. Ethical approval was not required for the present manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2019.10.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Chi-Square analysis of baseline characteristics significant on multivariate Cox regression after propensity score matching.
Standardized mean differences for each covariate significant on multivariate Cox regression after propensity score matching.
References
- 1.Schwartz T.L., Mogal H., Papageorgiou C., Veerapong J., Hsueh E.C. Metaplastic breast cancer: histologic characteristics, prognostic factors and systemic treatment strategies. Exp Hematol Oncol. 2013;2:31. doi: 10.1186/2162-3619-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzanninis I.G., Kotteas E.A., Ntanasis-Stathopoulos I., Kontogianni P., Fotopoulos G. Management and outcomes in metaplastic breast cancer. Clin Breast Canc. 2016;16:437–443. doi: 10.1016/j.clbc.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Haque W., Verma V., Naik N., Butler E.B., Teh B.S. Metaplastic breast cancer: practice patterns, outcomes, and the role of radiotherapy. Ann Surg Oncol. 2018;25:928. doi: 10.1245/s10434-017-6316-2. [DOI] [PubMed] [Google Scholar]
- 4.El Zein D., Hughes M., Kumar S., Peng X., Oyasiji T., Jabbour H., Khoury T. Metaplastic carcinoma of the breast is more aggressive than triple-negative breast cancer: a study from a single institution and review of literature. Clin Breast Canc Aug. 2017;17(5):382–391. doi: 10.1016/j.clbc.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung S.Y., Kim H.Y., Nam B.H. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Canc Res Treat Apr. 2010;120(3):627–637. doi: 10.1007/s10549-010-0780-8. [DOI] [PubMed] [Google Scholar]
- 6.Pezzi C.M., Patel-Parekh L., Cole K. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. Jan. 2007;14(1):166–173. doi: 10.1245/s10434-006-9124-7. [DOI] [PubMed] [Google Scholar]
- 7.Tseng W.H., Martinez S.R. Metaplastic breast cancer: to radiate or not to radiate? Ann Surg Oncol. Jan. 2011;18(1):94–103. doi: 10.1245/s10434-010-1198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leyrer C.M., Berriochoa C.A., Agrawal S. Predictive factors on outcomes in metaplastic breast cancer. Breast Canc Res Treat. 2017 Oct;165(3):499–504. doi: 10.1007/s10549-017-4367-5. Epub 2017 Jul 8. [DOI] [PubMed] [Google Scholar]
- 9.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N Engl J Med Nov. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 10.Weigelt B., Kreike B., Reis-Filho J.S. Metaplastic breast carcinomas are basal-like breast cancers: a genomic profiling analysis. Breast Canc Res Treat. 2009;117:273–280. doi: 10.1007/s10549-008-0197-9. [DOI] [PubMed] [Google Scholar]
- 11.Lien H.C., Hsiao Y.H., Lin Y.S. Vol. 13. 2007. Molecular signatures of metaplastic carcinoma of the breast by large-scale transcriptional profiling: identification of genes potentially related to epithelial-mesenchymal transition; pp. 7859–7871. 26(57) [DOI] [PubMed] [Google Scholar]
- 12.Leddy R., Irshad A., Rumboldt T., Cluver A., Campbell A., Ackerman S. Review of metaplastic carcinoma of the breast: imaging findings and pathological features. J Clin Imag Sci. 2012;2(1):21. doi: 10.4103/2156-7514.95435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberman H.A. Metaplastic carcinoma of the breast: a clinicopathologic study of 29 patients. Am J Surg Pathol. 1987;11(12):918–929. doi: 10.1097/00000478-198712000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Aydiner A., Sen F., Tambas M. Vol. 94. 2015. Metaplastic breast carcinoma versus triple-negative breast cancer survival and response to treatment. 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H., Jung S.Y., Ro J.Y. Metaplastic breast cancer: clinicopathological features and its prognosis. J Clin Pathol May. 2012;65(5):441–446. doi: 10.1136/jclinpath-2011-200586. [DOI] [PubMed] [Google Scholar]
- 16.Bilimoria K., Stewart A., Winchester D. The national cancer data base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15 doi: 10.1245/s10434-007-9747-3. 683–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haque W., Verma V., Butler E.B. Management of pathologic node-positive disease following initial surgery for clinical T1-T2 N0 esophageal cancer: patterns of care and outcomes from the national cancer data base. Acta Oncol. 2017:1–8. doi: 10.1080/0284186X.2017.1409435. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Haque W., Verma V., Butler E.B. Patterns of care and outcomes of multi-agent versus single-agent chemotherapy as part of multimodal management of low grade glioma. J Neuro Oncol. 2017;133:369–375. doi: 10.1007/s11060-017-2443-7. [DOI] [PubMed] [Google Scholar]
- 19.Stahl J.M., Corso C.D., Verma V. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer. 2017;103:11–16. doi: 10.1016/j.lungcan.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 20.McMillan M.T., Ojerholm E., Verma V. Radiation treatment time and overall survival in locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;98:1142–1152. doi: 10.1016/j.ijrobp.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Bertsekas D.P., Tseng P. Relaxation methods for minimum cost ordinary and generalized network flow problems. Oper Res. 1988;36(1):93–114. [Google Scholar]
- 22.Hansen B.B. Full matching in an observational study of coaching for the SAT. J Am Stat Assoc. 2004;99:609–618. [Google Scholar]
- 23.Hansen B.B., Klopfer S.O. Optimal full matching and related designs via network flows. J Comput Graph Stat. 2006;15(3) [Google Scholar]
- 24.Hansen B.B., Bowers J. Covariate balance in simple, stratified and clustered comparative studies. Stat Sci. 2008;2:219–236. [Google Scholar]
- 25.Ho D.E., Imai K., King G. Matchit: nonparametric processing for parametric causal inference. J Stat Softw. 2011;42(8) [Google Scholar]
- 26.Ho D., Imai K., King G. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Political Anal. 2007;15:199–236. [Google Scholar]
- 27.Iacus S.M., King G., Porro G. CEM: software for coarsened exact matching. J Stat Softw. 2009;30:1–27. [Google Scholar]
- 28.Thoemmes F. Propensity score matching in SPSS. 2012. http://arxiv.org/abs/1201.6385 [cited 2018 Feb 14] Available from:
- 29.Bates D., Maechler M., Bolker B. lme4: linear mixed effects models using Eigen and S4. R package version 1.0-4. 2013. http://CRAN.R-project.org/package=lme4 Cited 2018 Feb 14. Available from:
- 30.Thoemmes F., Liao W. Storrs; Connecticut: 2013. Propensity score matching (with multi level data) using SPSS and R. Modern modeling methods conference. [Google Scholar]
- 31.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher B., Bauer M., Wickerham D.L. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52(9):1551–1557. doi: 10.1002/1097-0142(19831101)52:9<1551::aid-cncr2820520902>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgibbons P.L., LiVolsi V.A. Recommendations for handling radioactive specimens ob- tained by sentinel lymphadenectomy: surgical pathology committee of the College of American pathologists, and the association of directors of anatomic and surgical pathology. Am J Surg Pathol. 2000;24 doi: 10.1097/00000478-200011000-00012. 1549-155. [DOI] [PubMed] [Google Scholar]
- 34.McCart Reed A.E., Kalaw E., Nones K. Phenotypic and molecular dissection of metaplastic breast cancer and the prognostic implications. J Pathol. 2019;247:214–227. doi: 10.1002/path.5184. [DOI] [PubMed] [Google Scholar]
- 35.Takala S., Heikkila P., Nevanlinna H., Blomgvist C., Mattson J. Metaplastic carcinoma of the breast: prognosis and response to systemic treatment in metastatic disease. Breast J. 2019;25:418–424. doi: 10.1111/tbj.13234. [DOI] [PubMed] [Google Scholar]
- 36.Gwin K., Buell-Gutbrod R., Tretiakova M., Montag A. Epithelial-to-mesenchymal transition in metaplastic breast carcinomas with chondroid differentiation: expression of the E-cadherin repressor Snail. Appl Immunohistochem Mol Morphol. 2010;18(6):526–531. doi: 10.1097/PAI.0b013e3181e8d54b. [DOI] [PubMed] [Google Scholar]
- 37.Song Y., Liu X., Zhang G. Unique clinicopathological features of metaplastic breast carcinoma compared with invasive ductal carcinoma and poor prognostic indicators. World J Surg Oncol. 2013;11:129. doi: 10.1186/1477-7819-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen I.C., Lin C.H., Huang C.S. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Canc Res Treat. 2011;130:345–351. doi: 10.1007/s10549-011-1686-9. [DOI] [PubMed] [Google Scholar]
- 39.Hennessy B.T., Giordano S., Broglio K. Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol. 2006;17:605–613. doi: 10.1093/annonc/mdl006. [DOI] [PubMed] [Google Scholar]
- 40.Han M., Salamat A., Zhu L. Metaplastic breast carcinoma: a clinical-pathologic study of 97 cases with subset analysis of response to neoadjuvant chemotherapy. Mod Pathol. 2019 Feb 5 doi: 10.1038/s41379-019-0208-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Burstein H.J., Temin S., Anderson H. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused updated. J Clin Oncol. 2014;32(21):2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah D.R., Tseng W.H., Martinez S.R. Treatment options for metaplastic breast cancer. ISRN Oncol. 2012;2012 doi: 10.5402/2012/706162. 706162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzanninis I.G., Kotteas E.A., Ntanasis-Stathopoulos I. Management and outcomes in metaplastic breast cancer. Clin Breast Canc. 2016;16(6):437–443. doi: 10.1016/j.clbc.2016.06.002. Epub 2016 Jun 15. [DOI] [PubMed] [Google Scholar]
- 44.Paul Wright G., Davis A.T., Koehler T.J. Ann Surg Oncol. 2014 Oct;21(11):3497–3503. doi: 10.1245/s10434-014-3782-7. Epub 2014 May 17. [DOI] [PubMed] [Google Scholar]
- 45.Hennessy B.T., Gonzalez-Angulo A.M., Stemke-Hale K. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69(10):4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang J., Cai H., Lin L. Increased expression of CD24 is associated with tumor progression and prognosis in patients with suffering osteosarcoma. Clin Transl Oncol. 2013;15:541–547. doi: 10.1007/s12094-012-0961-5. Epub 2012 Nov 10. [DOI] [PubMed] [Google Scholar]
- 47.Piscuoglio S., CKY Nc, Geyer F.C. Genomic and transcriptomic heterogeneity in metaplastic carcinomas of the breast. NPJ Breast Canc. 2017;3:48. doi: 10.1038/s41523-017-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakhani S.R., Ellis S.I., Schnitt S.J., Tan P.H., van de Vijver M.T. fourth ed. IARC Press; Lyon: 2012. WHOClassification of tumors of the breast. [Google Scholar]
- 49.Tse G.M., Tan P.H., Putti T.C. Metaplastic carcinoma of the breast: a clinicopathological review. J Clin Pathol Oct. 2006;59(10):1079–1083. doi: 10.1136/jcp.2005.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chi-Square analysis of baseline characteristics significant on multivariate Cox regression after propensity score matching.
Standardized mean differences for each covariate significant on multivariate Cox regression after propensity score matching.