Abstract
Introduction
Tumor-infiltrating lymphocytes (TILs) might be associated with host-cell mediated immunity, which could be partly reflected by peripheral blood cell counts. In addition, lymphocyte-predominant breast cancer (LPBC), which was defined as tumors having high TIL levels, showed a favorable prognosis among triple-negative breast cancer or HER2-positive breast cancer. We aimed to investigate whether peripheral blood cell counts are associated with LPBC.
Methods
We evaluated the percentage of stromal TILs in breast cancer patients who underwent primary surgery, using the standardized methodology proposed by the international TIL Working Group. Lymphocyte-predominant breast cancer (LPBC) was defined as tumors having high TIL levels (≥50%). Peripheral blood cell counts including absolute neutrophil counts (ANC), absolute lymphocyte counts (ALC) and neutrophil-to-lymphocyte ratio (NLR) was obtained from pretreatment laboratory data.
Result
Of the 810 patients, 132 (16.3%) had LPBC, and 678 (83.7%) had non-LBPC. In a comparison of 3 markers of peripheral blood counts, LPBC had a significantly lower mean ANC than non-LPBC (3,304 vs. 3,564; P = 0.023), but the other means were not different. In multivariable analysis, each 1K increment in ANC corresponded to an odds ratio of 0.790 (95% CI, 0.642 to 0.971) for LPBC. In the ER-negative and high-Ki67 subgroups identified by interaction tests, significant inverse correlations between continuous ANC and TILs were noted.
Conclusion
Low peripheral ANC could be linked with LPBC, supporting the hypothesis that systemic immune cell counts might be associated with the tumor-immune microenvironment.
Keywords: Tumor-infiltrating lymphocyte, Absolute neutrophil count, Lymphocyte-predominant breast cancer, TIL
Highlights
-
•
Tumor immune factors might be affected systemic immune cells.
-
•
We found the relationship between ANC and TIL count.
-
•
Increment in ANC corresponded to a less chance for LPBC.
-
•
In the subgroups, inverse correlations between two markers were noted.
Introduction
Leukocytes, or white blood cells (WBCs), are involved in both immune and inflammatory processes. There are two primary types of leukocytes: granular- and agranular-. Granular leukocytes include eosinophils, neutrophils, and basophils, while agranular leukocytes, which lack cytoplasmic granules, include monocytes and lymphocytes. In general, leukocytes play a major role in the immune response to macromolecules released from abnormal cells and microorganisms.
Increasing evidence has shown that blood cell counts or ratio, such as the absolute neutrophil counts (ANC), absolute lymphocyte counts (ALC), and neutrophil-to-lymphocyte ratio (NLR), are independent prognostic and predictive markers in several cancers [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]]. Moreover, NLR could be addressed as a biomarker for the degree of natural and adaptive immune response against tumor [[1], [2], [3],11,[15], [16], [17]].
While these leukocyte parameters are systemic biomarkers of host immunity, tumor-infiltrating lymphocytes (TILs) are tissue-based biomarkers for adaptive immune response. TILs, which are directly measured in malignant tissues, have been highlighted as a biomarker for predicting treatment response to chemotherapy in patients with breast cancer [1,4,[15], [16], [17], [18], [19]]. In addition, lymphocyte-predominant breast cancer (LPBC), which was defined as tumors having high TIL levels, showed a favorable prognosis among triple-negative breast cancer or HER2-positive breast cancer.
The level of TILs is directly influenced by the host immune system because it is a product of tumor-host immune interactions. Thus, it is worthwhile to explore the relationship between TILs and host immunity. Among the various assessing methods, peripheral blood cell count is the easiest non-invasiveness and repetition tool for assessing host immunity function. However, the relationship between TILs and blood cell counts in patients with breast cancer is unclear.
Here, we aimed to evaluate the association between LPBC and blood cell counts including ALC, ANC, and NLR in treatment-naïve breast cancer. After we found a significant association between ANC and LPBC, we further explored a linear correlation between ANC and TIL levels.
Material and methods
Patients
From August 2016 to July 2018, we enrolled patients with invasive breast cancer who underwent primary breast surgery without preoperative chemotherapy in Gangnam Severance Breast Cancer Center. Those who have (i) a history of transfusion within 3 months, (ii) active infection, (iii) acute/chronic inflammatory disease, and (iv) autoimmune disease were excluded. Clinical data including age at the time of surgery, histologic grade (HG), tumor size, lymph node status, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor-2 (HER2) status, and Ki-67 labelling index were collected from the medical database. Peripheral blood cell counts were used to determine the ANC, ALC, and NLR. The TNM stage was classified based on the American Joint Committee on Cancer, 7th edition, and tumor grade was determined using the modified Scarf-Bloomer–Richardson grading system.
Assessment of TIL scores and definition of LPBC
The TIL score was evaluated as previously described [20,21]. A pathologist (YJC) blinded to the clinical information reviewed the histologic features using hematoxylin and eosin (H&E) staining. Briefly, the TIL level was evaluated in treatment-naïve surgical specimens and was scored according to the standardized methodology proposed by the international TIL Working Group [18]. Stromal TILs were evaluated according to the guideline. The tumor area, defined by invasive tumor cells, was identified. All mononuclear cells, including lymphocytes and plasma cells but not polymorphonuclear leukocytes, were counted. The area outside the tumor border, around the intraductal component, and normal lobules were excluded. Within the tumor border, areas showing crush artifacts and necrosis were also excluded. For each case, the average TIL was counted on a representative section of the whole tumor, and the average score was reported as a percentage.
In this study, lymphocyte-predominant breast cancer (LPBC) was defined as tumors having high TIL levels (≥50%). The cut-off point of 50% was set according to previous studies using treatment-naïve surgical specimen [15,22].
Immunohistochemistry and interpretation
ER (1:100 clone 6F11; Novocastra, Newcastle upon Tyne, UK), PR (clone 16; Novocastra), HER2 (4B5 rabbit monoclonal antibody; Ventana Medical Systems, Tucson, AZ, USA), and Ki-67 (MIB-1; Dako, Glostrup, Denmark) were stained using formalin fixed paraffin-embedded tissue sections as previously described [23,24]. ER positivity and PR expression on immunohistochemistry were defined according to the modified Allred system. In our study, ER and PR positivity were defined as an Allred score ≥3. HER2 staining was analyzed according to the American Society of Clinical Oncology/College of American Pathologists guidelines [25]. HER2 immunostaining was considered positive when strong membranous staining (3+) was observed, whereas 0 and 1 + were regarded as negative. The cases showing 2 + HER2 expression were further evaluated for HER2 amplification via silver in situ hybridization. Nuclear positivity of Ki-67 staining was evaluated, and the percentage of positive tumor cell (range 0%–100%) was reported as the Ki-67 labeling index. For the breast cancer subtyping, the following definitions were used: i) luminal/HER2(−): ER positive and/or PR positive, and HER2 negative; ii) HER2(+): HER2 positive regardless of ER and PR status; iii) TNBC: ER negative, PR negative, and HER2 negative.
Complete blood count examination
Blood cell counts including hemoglobin, platelet, WBC, ANC, ALC, neutrophil percentage, lymphocyte percentage, and NLR were obtained from pretreatment laboratory data. Blood samples were collected during the treatment-naïve state after diagnosis. WBC counts were performed at the Department of Laboratory Medicine, Gangnam Severance Hospital using an automated counting machine (Sysmex XN-series; Kobe, Japan). The Wright or Mary-Grinewald-Giemsa technique was used to determine the differential count; briefly, a drop of blood was thinly spread over a glass-slide, air dried, and stained with Romanowsky stain. WBC differentiation was commonly performed using automated fractionation machines, but it was also performed manually in cases of morphologic abnormalities. The neutrophil and lymphocyte counts were calculated as the total WBC multiplied by the percentage of each. Meanwhile, NLR was calculated as ANC divided by ALC.
Statistical analysis
Categorical variables were compared using Chi-square test or Fisher’s exact test. Student’s t-test was used to compare means. Clinical-pathologic factors associated with LPBC were analyzed using the logistic regression analysis. The variables showing a statistical significance in the univariate analysis were entered in the multivariable analysis.
To evaluate the ability of the ANC to predict LPBC, we determined the area under the curve (AUC) using receiver operating characteristic (ROC) curves with Delong method [26]. The ROC curves were constructed to assess the diagnostic performance of ANC and other clinical parameters, and the AUCs were calculated and compared. We also performed net reclassification improvement (NRI) and integrated discrimination improvement (IDI) to analyze the degree to which the addition of ANC to the reference model improved its predictive ability [27].
Pearson’s Correlation coefficient was calculated to measure the correlative value between continuous TIL levels and markers of peripheral blood cell counts. The interaction between TIL and ANC, and major pathological parameters was tested at a significance level of 0.10. All statistical tests were two-tailed, and p < 0.05 was considered statistically significant and 0.05 ≤ p < 0.2 was considered a trend toward significance to increase the sensitivity to detect potential selection bias. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and SPSS version 23.0 (SPSS, Chicago, IL, USA).
Results
Baseline characteristics
A total of 810 patients were included in this study. The median patient age was 51 (24–91) years for the all patients. The mean value and standard deviation of TIL in all patients were 23.95 ± 25.52. The mean values of ANC, ALC, and NLR were 3.60 (K/μL), 1.98 (K/μL), and 1.68, respectively. The patients were divided into the LPBC and non-LPBC groups according to the TIL level. In total, 132 (16.3%) patients had LPBC, and 678 (83.7%) patients had non-LPBC.
In Table 1, the patients with ductal carcinoma had a higher rate of LPBC compared to the patients with non-ductal carcinoma (P < 0.001). Also, the LPBC group had higher rates of high HG (P < 0.001), negative ER (P < 0.001), negative PR (P < 0.001), high Ki67 expression, and HER2 overexpression (P < 0.001). In addition, the rates of breast cancer subtypes were significantly different between the two groups; the LPBC group had higher proportions of HER2-positive and TNBC subtypes than the non-LPBC group (P < 0.001). Meanwhile, other characteristics including age and anatomical tumor burden such as T stage, N stage, and AJCC stage did not differ between two groups.
Table 1.
Baseline characteristics of two groups with or without lymphocyte-predominant breast cancer (LPBC).
| LPBC; n = 132 (%) | Non-LPBC; n = 678 (%) | p value | |
|---|---|---|---|
| Age (years) | 52.36 ± 10.75 | 52.50 ± 10.80 | 0.891 |
| Histology | 0.045 | ||
| IDC | 117 (88.6) | 552 (81.4) | |
| non-IDC | 15 (11.4) | 126 (18.6) | |
| ER | <0.001 | ||
| Positive | 66 (50) | 603 (88.9) | |
| Negative | 66 (50) | 75 (11.1) | |
| PR | <0.001 | ||
| Positive | 44 (33.3) | 530 (78.2) | |
| Negative | 88 (66.7) | 148 (21.8) | |
| HER2b,c | <0.001 | ||
| Positive | 64 (48.5) | 89 (13.1) | |
| Negative | 67 (50.8) | 589 (86.9) | |
| T stage | 0.220 | ||
| 1 | 87 (65.9) | 461 (68.0) | |
| 2 | 44 (33.3) | 196 (28.9) | |
| 3 | 1 (0.8) | 21 (3.1) | |
| N stage | 0.209 | ||
| 0 | 113 (85.6) | 529 (78.0) | |
| 1 | 17 (12.9) | 122 (18.0) | |
| 2 | 1 (0.8) | 19 (2.8) | |
| 3 | 1 (0.8) | 8 (1.2) | |
| TNM Stagea | 0.197 | ||
| I | 77 (58.3) | 391 (57.7) | |
| II | 53 (40.2) | 253 (37.3) | |
| III | 2 (1.5) | 34 (5.0) | |
| HGb | <0.001 | ||
| I | 2 (1.5) | 158 (23.3) | |
| II | 66 (50) | 441 (65.0) | |
| III | 49 (37.1) | 65 (9.6) | |
| Ki67b | <0.001 | ||
| <20% | 28 (21.2) | 489 (72.1) | |
| ≥20% | 101 (76.5) | 185 (27.3) | |
| Subtypes | <0.001 | ||
| Luminal/HER2(−) | 38 (28.8) | 548 (80.8) | |
| HER2(+) | 64 (48.5) | 89 (13.1) | |
| TNBC | 30 (22.7) | 41 (6.0) | |
| ANC (1000 cells/mm3) | 3.30 ± 0.99 | 3.65 ± 1.24 | 0.009 |
| ALC (1000 cells/mm3) | 1.95 ± 0.52 | 1.98 ± 0.61 | 0.526 |
| NLR | 1.42 ± 0.87 | 1.44 ± 1.04 | 0.812 |
IDC, invasive ductal carcinoma; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor-2; HG, histologic grade; TNBC, triple negative breast cancer; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; NLR, neutrophil to lymphocyte ratio.
AJCC stage was performed based on 7th edition.
Missing value.
HER-2 positive was defined by 3 + on immunohistochemistry or amplification on fluorescence in situ hybridization.
The LPBC group had lower mean of ANC
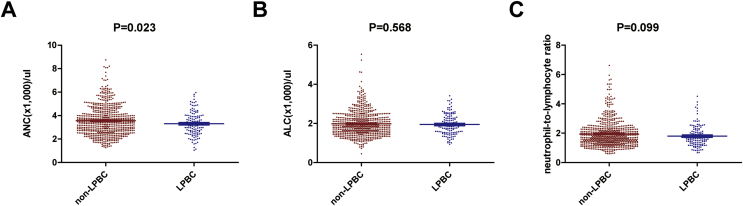
When we compared means of peripheral blood count between LPBC and non-LPBC, the mean ANC of the LPBC group was significantly lower than that of the non-LPBC (P = 0.023; Fig. 1A). However, means of ALC and NLR did not differ between two groups (Fig. 1B, P = 0.528; Fig. 1C, P = 0.099, respectively). Then, we performed further analyses on the relationship between ANC and LPBC.
Fig. 1.
Comparisons of peripheral blood markers according to LPBC. We compared means of peripheral blood count including absolute neutrophil count (ANC), absolute lymphocyte count (ALC), and neutrophil to lymphocyte ratio (NLR) between LPBC and non-LPBC (A) The mean ANC of the LPBC group was significantly higher than that of the non-LPBC (P = 0.023; Student’s t-test). (B&C) Means of ALC and NLR did not differ between two groups (P = 0.528 and P = 0.099, respectively; Student’s t-test).
Multivariable models for LPBC with ANC
To investigate an independent value of ANC to predicting LPBC, we performed multivariable analyses. First, in the univariate analysis, the significant variables were ANC, histology, subtypes, Ki-67 expression, HG, ER, PR, and HER2 using logistic regression analyses (Table 2). Because subtypes were reflected on ER, PR, and HER2 status, only subtype was included in the multivariable model to avoid collinearity of variables. In multivariable model, continuous ANC remained an independent variable associated with LPBC (OR: 0.790, 95% CI: 0.642–0.971, P = 0.025; Table 2), indicating that the probability of LPBC is reductive of 21.0% according to the increase of ANC as 1,000 unit.
Table 2.
The odds ratios (ORs) and 95% confidential intervals (CIs) to identify LPBC.
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| ORs (95% CIs) | p value | ORs (95% CIs) | p value | |
| ANC | 0.824 (0.697–0.975) | 0.024 | 0.790 (0.642–0.971) | 0.025 |
| ALC | 0.911 (0.662–1.254) | 0.568 | ||
| NLR | 0.980 (0.813–1.181) | 0.832 | ||
| Histology | 0.048 | 0.010 | ||
| IDC | Ref | Ref | ||
| non-IDC | 0.562 (0.317–0.994) | 0.145 (0.034–0.627) | ||
| Subtype | <0.001 | <0.001 | ||
| Luminal/HER2(−) | Ref | Ref | ||
| HER2(+) | 10.370 (6.549–16.422) | 3.120 (1.765–5.516) | ||
| TNBC | 10.552 (5.942–18.740) | 3.274 (1.589–6.745) | ||
| Ki67 | <0.001 | <0.001 | ||
| <20% | Ref | Ref | ||
| ≥20% | 9.535 (6.070–14.976) | 2.940 (1.657–5.217) | ||
| HG | <0.001 | 0.015 | ||
| I | Ref | Ref | ||
| II | 11.821 (2.862–48.823) | 4.648 (1.076–20.084) | ||
| III | 59.543 (14.065–252.066) | 8.228 (1.740–38.907) | ||
| T stage | 0.263 | |||
| I | Ref | |||
| II | 1.190 (0.798–1.773) | |||
| III | 0.252 (0.034–1.900) | |||
| N stage | 0.237 | |||
| 0 | Ref | |||
| I | 0.652 (0.378–1.127) | |||
| II | 0.246 (0.033–1.859) | |||
| III | 0.585 (0.072–4.725) | |||
| AJCC stage | 0.232 | |||
| I | Ref | |||
| II | 1.064 (0.725–1.562) | |||
| III | 0.299 (0.070–1.269) | |||
ANC, absolute neutrophil count; ALC, absolute lymphocyte count; ER, estrogen receptor; HER-2, human epidermal growth factor receptor-2; HG, histologic grade; IDC, invasive ductal carcinoma; PR, progesterone receptor; TNBC, triple negative breast cancer.
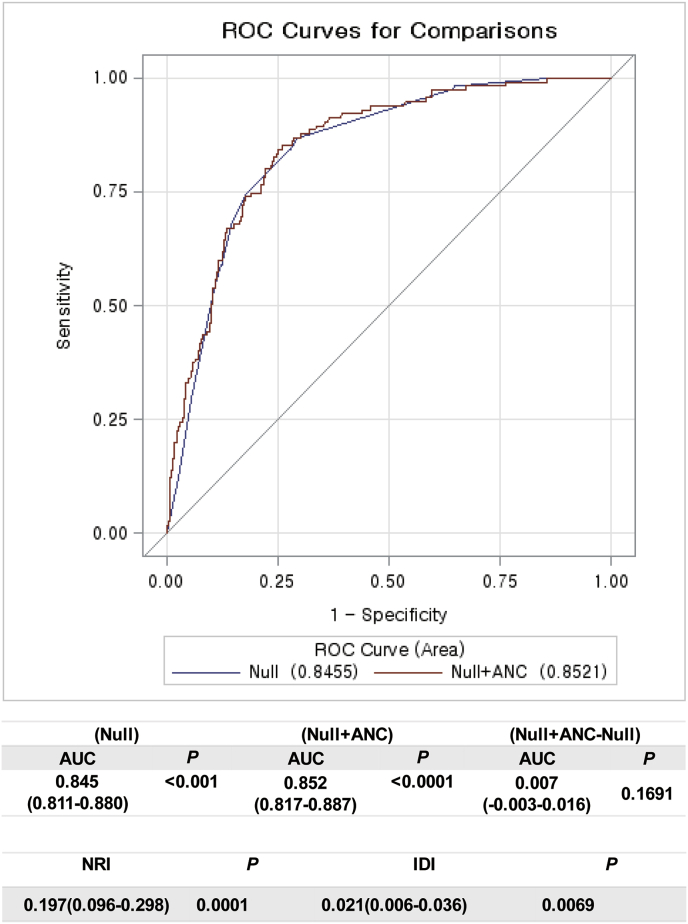
To evaluate an improvement of predicting ability of multivariable models with ANC, we compared the AUCs among the models using ROC curves with Delong method (Fig. 2). The AUC of the reference model was 0.845. When ANC was added to the model, the AUC of the ANC-added model improved as 0.852. The difference between two models was 0.007 (95% CI, −0.003-0.016) and showed a trend to significance (P = 0.1691, Fig. 2).
Fig. 2.
Comparisons of AUC to evaluate an improvement of predicting ability of multivariable models with Delong method The AUC of the reference model was 0.845. When ANC was added to the model, the AUC of the ANC-added model improved as 0.852. The difference between two models was 0.007 (95% CI, −0.003-0.016) and showed a trend to significance (P = 0.1691). Then the integrated discrimination improvement (IDI) and net reclassification improvement (NRI) were calculated. The diagnostic performance was significantly improved by adding ANC to the baseline model for predicting LPBC according to the IDI and NRI (NRI, P = 0.0001; IDI, P = 0.0069, respectively).
Then the IDI and NRI were calculated because they are indicators of improvements following reclassification in a nested model and can determine whether the predictive power of a combination of traditional factors is improved upon ANC inclusion. We found that the diagnostic performance was significantly improved by adding ANC to the baseline model for predicting LPBC according to the IDI and NRI (NRI, P = 0.0001; IDI, P = 0.0069, respectively, Fig. 2).
Correlation between TILs and ANC in the subgroups according to interaction effects
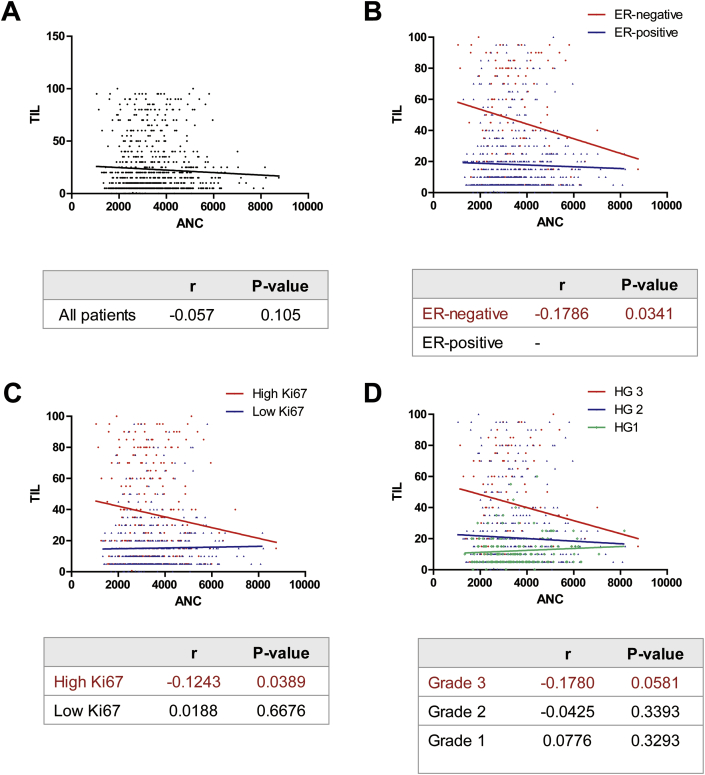
Next we evaluated the correlation between two continuous variables using Pearson’s correlation analysis. A trend for very weak inverse correlation was observed between TIL levels and ANC (Pearson’s r = −0.057, P = 0.105; Fig. 3A). Since TIL levels are largely affected by biological characteristics, we performed a linear regression model to investigate interaction effects in the correlation between TIL levels and ANC among the subgroups.
Fig. 3.
Pearson correlation analyses between TIL and ANC. (A) A trend for very weak inverse correlation was observed between TIL levels and ANC in all patients (Pearson’s r = −0.057, P = 0.105). The P-values of tests for an interaction effect were significant in relation to ER status, Ki67 expression, and HG (B) In the ER-negative subgroup, Pearson’s r was −0.1786 (P = 0.0341) (C) In the subgroup with high Ki67, Pearson’s r was −0.1243 (P = 0.0389) (D) In the subgroup with histologic grade 3, Pearson’s r was −0.1780 (P = 0.0581).
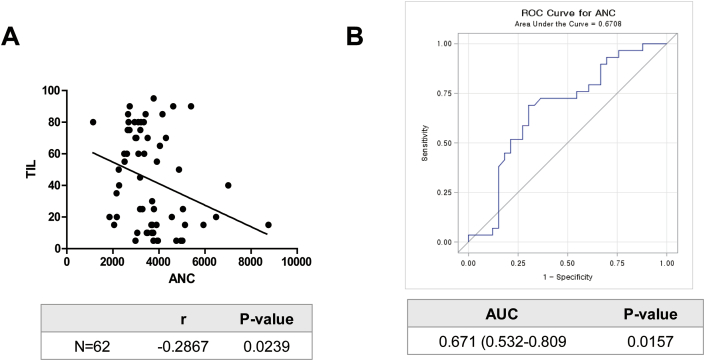
The effects of an interaction between TIL levels and ANC were tested according to the pathological factors at an alpha level of 0.10. The P-values of tests for an interaction effect between two markers were significant in relation to ER status, Ki67 expression, and HG (Table 3). Among the subgroups divided by these results, a significant inverse correlation between TIL levels and ANC was noticed in the group with negative ER or high Ki67 expression though the correlation was quite weak (Fig. 3B and C, respectively). Also, a trend for negative correlation between two markers was recognized in the group with HG of 3 (Fig. 3D). Furthermore, four-way interaction test among ANC, TIL levels, ER status, Ki67 expression, and HG showed a statistical significance. When these markers were combined, an inverse correlation between TIL and ANC was slightly increased in patients with ER-negative, high Ki67, and HG of 3 (Pearson’s r = −0.2867, P-value = 0.0239; Fig. 4A). Also, the AUC of ANC was 0.671 (0.532–0.809) in predicting TIL levels (P = 0.0157, Fig. 4B).
Table 3.
Linear regression analysis using interaction and subgroup analysis.
| Variables | P value of interaction | TIL |
|
|---|---|---|---|
| R | P value | ||
| Histology | 0.2638 | ||
| IDC | −0.0711 | 0.0661 | |
| non-IDC | 0.0418 | 0.6230 | |
| Subtype | 0.3815 | ||
| Luminal/HER2(−) | −0.0589 | 0.1544 | |
| HER2(+) | 0.0214 | 0.7930 | |
| TNBC | −0.1291 | 0.2835 | |
| Ki67 | 0.0115 | ||
| <20% | 0.0188 | 0.6676 | |
| ≥20% | −0.1243 | 0.0389 | |
| HG | 0.0876 | ||
| I | 0.0776 | 0.3293 | |
| II | −0.0425 | 0.3393 | |
| III | −0.1780 | 0.0581 | |
| T stage | 0.7353 | ||
| 1 | −0.0362 | 0.3976 | |
| 2 | −0.1020 | 0.1149 | |
| 3 | −0.0106 | 0.9627 | |
| N stage | 0.6718 | ||
| 0 | −0.0676 | 0.0871 | |
| 1 | −0.0046 | 0.9576 | |
| 2 | −0.1071 | 0.6533 | |
| 3 | 0.3858 | 0.3051 | |
| AJCC stage | 0.7380 | ||
| 1 | −0.0404 | 0.3832 | |
| 2 | −0.0885 | 0.1226 | |
| 3 | 0.0585 | 0.7347 | |
ER, estrogen receptor; HER-2, human epidermal growth factor receptor-2; HG, histologic grade; IDC, invasive ductal carcinoma; PR, progesterone receptor; TNBC, triple negative breast cancer.
Fig. 4.
Pearson correlation analysis and ROC curve between ANC and TILs in patients with ER-negative, high Ki67, and HG of 3 (n=62) (A) Pearson’s r was −0.2867 (P-value = 0.0239). (B) The AUC of ANC in predicting TIL levels was 0.671 (95% CI, 0.532–0.809; P = 0.0157).
Discussion
In this study, we investigated whether peripheral blood cell counts were associated with LPBC. We hypothesized that TILs were associated with blood cell counts, which reflect local and host immunity, respectively. We found a significantly reduced mean ANC in those with LPBC compared to those with non-LPBC. Moreover, continuous ANC was a significant predictive factor of LPBC, independent of the cancer subtypes and other related variables. The increase of ANC as 1K unit could be estimated as reduced by 21% in predicting LPBC. In addition, in the subgroups identified by interaction tests, we found a linear inverse correlation between TILs and ANC.
The negative correlation observed between high ANC and LPBC in our study is supported by the fact that neutrophils may act against the immune system via several mechanisms. Experimental data suggested that neutrophils could suppress the cytolytic activity of lymphocytes, natural killer cells, and activated T-cells when it is co-cultured with lymphocytes form normal healthy donor. Also, activated neutrophils have been reported to secrete myeloperoxidase, resulting in the suppression of lymphocyte function [28].
Moreover, tumor-associated neutrophils might influence local tumor immunity and tumor progression by regulating the tumor microenvironment. The enzymatic activity of neutrophils has been found to promote remodeling of the extracellular matrix, which results in the release of basic fibroblast growth factor and migration of either endothelial cells or tumor cells [28,29]. The modulated tumor microenvironment might contribute to tumor growth and acquisition of metastatic capability. Specifically, neutrophil-derived oncostatin M stimulates cancer cells to secrete vascular endothelial growth factor and increases invasiveness in breast cancer [30].
It is well known that LPBC was associated with the cancer subtypes [15,22,31]. The different rates of LPBC according to the subtypes was reproducible in our study; specifically, the rate of LPBC was higher in the HER2 and TNBC subtypes are than that in the luminal/HER2-negative subtype. Another notable finding was that the Ki-67 labelling index was correlated with LPBC, which may be explained by the fact that tumors with high Ki-67 labelling index were more frequent in the HER2 or TNBC subtypes. These findings provide evidence that our data is reliable.
Clinically, a previous study showed that high ANC could be a poor prognostic marker in patients with TNBC [5]. This finding is consistent with our result in that high ANC may negatively impact TILs while high TILs are associated with a good prognosis in this aggressive subset of breast cancer [32]. In this study, the association between NLR and TILs was not identified, although NLR is a well-known poor prognostic marker in various cancers including breast cancer [1,[6], [7], [8], [9], [10]]. The relationship between NLR and TILs warrants further research.
Interestingly, emerging evidence suggests that high ANC could be a negative predictor of response to immune checkpoint inhibitors (ICI) for cancer such as lung cancer and melanoma [11,33,34]. By contrast, high TILs are positive predictors of treatment response to ICI [35,36]. Contrasting responses to ICI according to high ANC and TILs indirectly support our results, but further studies are still needed to clearly establish the predictive value of TILs in the treatment response to ICI.
There is an another study evaluating the relationships between peripheral blood cell counts and CD8+ TILs, which are in line with an interest of our study [37]. Contrast to our study, they specifically addressed cytotoxic T-cells with CD8 using core-biopsy samples and found associations of CD8+ TILs with ALC and AMC. However, it is in line with our study that peripheral blood counts as systemic immune markers are linked with TILs as local one. Intensive studies are warranted regarding the relationships between immune element of tumor and those of host system using more specific labelling markers.
This study also has some limitations, including the absence of survival analyses due to short-term follow-up. Assessment of the clinical outcomes of the study population might have helped to specify the prognostic capability of these biomarkers. Another limitation is that neutrophil and lymphocyte counts may vary according to individual physiological and pathological processes such as infectious condition. The association between NLR and TILs in breast cancer warrants further research. Also, the tumor-infiltrating neutrophil should be identified and characterized by specific markers in relation to ANC or NLR in future study. Despite these limitations, we found relevant evidence showing specific correlations between LPBC and ANC, providing scientific insight on the host-tumor immune interaction in breast cancer.
Conclusion
Low peripheral ANC could be associated with LPBC, supporting the hypothesis that systemic immune cell counts might affect the tumor-immune microenvironment. A possible association between peripheral ANC and tumor-associated neutrophil reflecting the tumor microenvironment should be studied.
Funding
This research was supported by the Basic Science Research Program through the NRF, funded by the Ministry of Science, ICT, & Future Planning (NRF-2019R1C1C1002830); a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1520120).
Competing interests
The authors declare that they have no competing interest. Part of this study was presented in ESMO 2018.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on request.
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Institutional Review Boards of Gangnam Severance Hospital, Yonsei University, Seoul, Korea, and adhered to the tenets of the Declaration of Helsinki. Owing to the retrospective approach of this study, the need for informed consent was waived by the ethics committees.
Declaration of Competing Interest
The authors reports no conflicts of interest in this work.
Acknowledgements
We acknowledge all authors in our paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2019.09.013.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ethier J.L., Desautels D., Templeton A., Shah P.S., Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19:2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacdalan D.B., Lucero J.A., Sacdalan D.L. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. OncoTargets Ther. 2018;11:955–965. doi: 10.2147/OTT.S153290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labomascus S., Fughhi I., Bonomi P., Fidler M.J., Borgia J.A., Basu S. Neutrophil to lymphocyte ratio as predictive of prolonged progression free survival (PFS) and overall survival (OS) in patients with metastatic non-small cell lung cancer (NSCLC) treated with second-line PD-1 immune checkpoint inhibitors. J Clin Oncol. 2017;35 e14530-e. [Google Scholar]
- 4.Can C., Baseskioglu B., Yilmaz M., Colak E., Ozen A., Yenilmez A. Pretreatment parameters obtained from peripheral blood sample predicts invasiveness of bladder carcinoma. Urol Int. 2012;89:468–472. doi: 10.1159/000343278. [DOI] [PubMed] [Google Scholar]
- 5.Wariss B.R., de Souza Abrahao K., de Aguiar S.S., Bergmann A., Thuler L.C.S. Effectiveness of four inflammatory markers in predicting prognosis in 2374 women with breast cancer. Maturitas. 2017;101:51–56. doi: 10.1016/j.maturitas.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Beal E.W., Wei L., Ethun C.G., Black S.M., Dillhoff M., Salem A. Elevated NLR in gallbladder cancer and cholangiocarcinoma - making bad cancers even worse: results from the US Extrahepatic Biliary Malignancy Consortium. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2016;18:950–957. doi: 10.1016/j.hpb.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Q.T., Zhou L., Zeng W.J., Ma Q.Q., Wang W., Zhong M. Prognostic significance of neutrophil-to-lymphocyte ratio in ovarian cancer: a systematic review and meta-analysis of observational studies. Cell Physiol Biochem : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;41:2411–2418. doi: 10.1159/000475911. [DOI] [PubMed] [Google Scholar]
- 8.Marchioni M., Primiceri G., Ingrosso M., Filograna R., Castellan P., De Francesco P. The clinical use of the neutrophil to lymphocyte ratio (NLR) in urothelial cancer: a systematic review. Clin Genitourin Cancer. 2016;14:473–484. doi: 10.1016/j.clgc.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Mei Z., Shi L., Wang B., Yang J., Xiao Z., Du P. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1–13. doi: 10.1016/j.ctrv.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Yang J.J., Hu Z.G., Shi W.X., Deng T., He S.Q., Yuan S.G. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21:2807–2815. doi: 10.3748/wjg.v21.i9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zer A., Sung M.R., Walia P., Khoja L., Maganti M., Labbe C. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 Axis inhibitors in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2018;19:426–434.e1. doi: 10.1016/j.cllc.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe A., Harimoto N., Araki K., Kubo N., Igarashi T., Tsukagoshi M. Absolute neutrophil count predicts postoperative prognosis in mass-forming intrahepatic cholangiocarcinoma. Anticancer Res. 2019;39:941–947. doi: 10.21873/anticanres.13197. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Shawer O., Abu-Shawer M., Haimour A., Alhouri A., Alkhatib A.A., Abki M. Hematologic markers of distant metastases in gastric cancer. J Gastrointest Oncol. 2019;10:529–536. doi: 10.21037/jgo.2019.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong J., Chen X., Gao W., Zhu S., Wu J., Huang O. A high absolute lymphocyte count predicts a poor prognosis in HER-2- positive breast cancer patients treated with trastuzumab. Cancer Manag Res. 2019;11:3371–3379. doi: 10.2147/CMAR.S187233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieci M.V., Mathieu M.C., Guarneri V., Conte P., Delaloge S., Andre F. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol : official journal of the European Society for Medical Oncology. 2015;26:1698–1704. doi: 10.1093/annonc/mdv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutkin D.W., Shurin M.R. Clinical evaluation of systemic and local immune responses in cancer: time for integration. Cancer immunology, immunotherapy. CII. 2014;63:45–57. doi: 10.1007/s00262-013-1480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore M.M., Chua W., Charles K.A., Clarke S.J. Inflammation and cancer: causes and consequences. Clin Pharmacol Ther. 2010;87:504–508. doi: 10.1038/clpt.2009.254. [DOI] [PubMed] [Google Scholar]
- 18.Salgado R., Denkert C., Demaria S., Sirtaine N., Klauschen F., Pruneri G. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol : official journal of the European Society for Medical Oncology. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West N.R., Kost S.E., Martin S.D., Milne K., Deleeuw R.J., Nelson B.H. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Canc. 2013;108:155–162. doi: 10.1038/bjc.2012.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn S.G., Cha Y.J., Bae S.J., Yoon C., Lee H.W., Jeong J. Comparisons of tumor-infiltrating lymphocyte levels and the 21-gene recurrence score in ER-positive/HER2-negative breast cancer. BMC Canc. 2018;18:320. doi: 10.1186/s12885-018-4228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha Y.J., Ahn S.G., Bae S.J., Yoon C.I., Seo J., Jung W.H. Comparison of tumor-infiltrating lymphocytes of breast cancer in core needle biopsies and resected specimens: a retrospective analysis. Breast Canc Res Treat. 2018;171:295–302. doi: 10.1007/s10549-018-4842-7. [DOI] [PubMed] [Google Scholar]
- 22.Loi S., Sirtaine N., Piette F., Salgado R., Viale G., Van Eenoo F. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 23.Ahn S.G., Dong S.M., Oshima A., Kim W.H., Lee H.M., Lee S.A. LOXL2 expression is associated with invasiveness and negatively influences survival in breast cancer patients. Breast Canc Res Treat. 2013;141:89–99. doi: 10.1007/s10549-013-2662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey J.M., Clark G.M., Osborne C.K., Allred D.C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol : official journal of the American Society of Clinical Oncology. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 25.Wolff A.C., Hammond M.E., Schwartz J.N., Hagerty K.L., Allred D.C., Cote R.J. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 26.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 27.Pencina M.J., D’Agostino R.B., Sr., D’Agostino R.B., Jr., Vasan R.S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 28.De Larco J.E., Wuertz B.R., Furcht L.T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res : an official journal of the American Association for Cancer Research. 2004;10:4895–4900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- 29.el-Hag A., Clark R.A. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139:2406–2413. Baltimore, Md : 1950. [PubMed] [Google Scholar]
- 30.Queen M.M., Ryan R.E., Holzer R.G., Keller-Peck C.R., Jorcyk C.L. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 31.Denkert C., Loibl S., Noske A., Roller M., Muller B.M., Komor M. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 32.Loi S., Drubay D., Adams S., Pruneri G., Francis P.A., Lacroix-Triki M. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient Analysis of early-stage triple-negative breast cancers. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2019;37:559–569. doi: 10.1200/JCO.18.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okuhira H., Yamamoto Y., Inaba Y., Kunimoto K., Mikita N., Ikeda T. Prognostic factors of daily blood examination for advanced melanoma patients treated with nivolumab. Bioscience trends. 2018;12:412–418. doi: 10.5582/bst.2018.01158. [DOI] [PubMed] [Google Scholar]
- 34.Soda H., Ogawara D., Fukuda Y., Tomono H., Okuno D., Koga S. Thoracic cancer; 2018. Dynamics of blood neutrophil-related indices during nivolumab treatment may be associated with response to salvage chemotherapy for non-small cell lung cancer: a hypothesis-generating study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loi S., Adams S., Schmid P., Cortés J., Cescon D.W., Winer E.P. LBA13Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): results from KEYNOTE-086. Ann Oncol. 2017;28 [Google Scholar]
- 36.Tumeh P.C., Hellmann M.D., Hamid O., Tsai K.K., Loo K.L., Gubens M.A. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer immunology research. 2017;5:417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K.H., Kim E.Y., Yun J.S., Park Y.L., Do S.I., Chae S.W. The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Canc. 2018;18:938. doi: 10.1186/s12885-018-4832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on request.