Abstract
Introduction
We investigated whether a web-based cognitive training video game is an effective approach to improve cognitive decline in combination with our standard of care for rehabilitation of breast cancer (BC) patients.
Materials and methods
Self-selected BC patients between 18 and 71 years old complaining of disturbing cognitive impairment were studied. The patients received access to a web-based internet video game and online cognitive assessments (Aquasnap, Cambridge, MyCQ™). The early intervention group (n = 23) had a training program of 6 months of at least three times a week for a minimum of 60 min of game playing per week at home in addition to standard of care rehabilitation. The delayed intervention (n = 23) received standard of care for three months, followed by three months of similar MyCQ training. Outcome measures were the MyCQ (sub)scores and Activity of Daily Life (ADL), mood, subjective cognition and functional cognitive status measured by classic neuropsychological tests.
Results
At baseline the means for CFQ (a measure of self-reported cognitive failure), anxiety, PSQI and self-reflectiveness were beyond normal range in both groups. CFQ improved significantly better in the intervention group (p = 0.029). Combining the evolution over time in the entire population a significant improvement was seen for overall MyCQ score, level of fear, physical and emotional role limitation, and health change (all p < 0.05), but self-reflectivess deteriorated (p < 0.05)). Significant differences in the various MyCQ subtests over time were: improved speed in choice reaction time, visual memory recognition, N back 1 and 2, coding, trail making test B, improved accuracy of N back 1 and 2 (all p < 0.05).
Conclusion
A program of cognitive training improves cognitive functioning over time. “Aquasnap” has a beneficial effect on the perception of subjective cognitive functioning (CFQ) but the exact role of video gaming in this process remains uncertain.
Keywords: Breast cancer, Cognition, Cognitive impairment, Cognitive training, Video gaming
What is already known about this subject.
-
•
up to 75% of breast cancer patients have cognitive decline during treatment
-
•
cognitive impairment can persist for many years and can have a profound negative effect on quality of life (QOL) and may impair occupational, psychological and social functioning
-
•
cognitive failure is difficult to treat
What does this study add ?
-
•
CFQ (a measure of self-reported cognitive failure) improved significantly better in the intervention group starting with a web-based internet video game and online cognitive assessments (Aquasnap, Cambridge, MyCQ™).
-
•
Combining the evolution over time in the entire population a significant improvement was seen for overall MyCQ score, level of fear, physical and emotional role limitation, and health change (all p < 0.05), but self-reflectiveness deteriorated (p < 0.05)
-
•
Adherence to cognitive rehabilitation of programs is disappointing
How might it impact on clinical practice ?
-
•
More attention should be given to cognitive failure as cognitive training improves cognitive functioning over time
-
•
Computer gaming has a beneficial effect on the perception of subjective cognitive functioning (CFQ)
1. Introduction
Advances in early detection and treatment of breast cancer (BC) have greatly improved survival. Many cancer patients and survivors report cognitive dysfunction, also called cancer or cancer treatment related cognitive impairment (CRCI). This may be caused by toxicity of the systemic medication (“chemobrain”), radiotherapy, and treatment induced menopause, but may also be secondary to stress and depression after the cancer diagnosis [1,2]. Estimates of the prevalence of CRCI in this setting varies: approximately 40% of patients experience CRCI after diagnosis before any treatment, but up to 75% may have cognitive decline during treatment and up to 60% after completion of therapy [3]. CRCI can persist for months and sometimes years after treatment [2,[4], [5], [6]]. Although it is often mild to moderate in nature, CRCI can have a profound negative effect on quality of life (QOL) and may impair occupational, psychological and social functioning [4]. Medical interventions to treat CRCI have a poor track record, with respect to therapeutic response rates and adverse events [7]. It has been shown that web-based training can have a beneficial effect on cognition in healthy elderly patients, patients suffering from psychiatric conditions (such as schizophrenia, unipolar and bipolar depression, anxiety disorders …) and patients recovering from stroke alongside standard approach [8]. Recently the first studies emerged using this technology in cancer patients [9,10].
The rationale is based on the concept of neural plasticity which implies that active training of brain functions increases neural connectivity and brain function. Therefore brain training may prevent cognitive decline or enhance specific cognitive domain functions. In this pilot study we investigated whether an attractive web-based cognitive training video game is a feasible approach to treat cognitive decline in combination with our standard of care for rehabilitation of breast cancer patients (education, physical and psychological support). The game is dynamic, trains holistically and adjusts itself to the performance level of the patient. The assessment of cognitive function is performed with an online tool: MYCQ (My Clinical Cognition) [11].
2. Materials and methods
2.1. Participants
Participants were recruited between 2016 and 2018 in the Multidisciplinary Breast Clinic of the Antwerp University Hospital and via the media (radio, newspapers) bringing our study under attention to the general public. To be eligible for the study participants had to 1) have a history of breast cancer 2) have subjective complaints of cognitive impairment, 3) be in the age group of 18–71 years, 4) have access to the internet, 5) not receive any other cognitive therapy or online cognitive game playing. Exclusion criteria were: 1) brain metastasis or brain tumors, 2) severe neurological disorders impairing brain function (eg previous stroke, dementia, Parkinson, …) and 3) severe hearing or visual deficits. A total of 463 patients letters of invitation were sent to our patient population and 32 patients responded through external advertising. After a screening interview in the clinic 46 women were found eligible to participate in the study. Patient characteristics are given in Table 1. Formal written consent was obtained for all participants.
Table 1.
Demographics of the patient population according to treatment arm. Means (SD) or n (%) are reported. P-values are for the between group comparison of the considered variable and the used test is mentioned.
| Variable | Controls (n = 23) | Intervention (n = 23) | p-value |
|---|---|---|---|
| Age | 52.1 (9.1) | 51.5 (8.0) | 0.824 (t-test) |
| Education level | Secondary: 6 (26.1%) | 6 (26.1%) | 1.000 (Chi-square test) |
| College and university: 17 (73.9%) | 17 (73.9%) | ||
| Marital status | Living alone: 1 (6.7%) | 4 (26.7%) | 0.442 (Fisher’s exact test) |
| Living with children: 2 (13.3%) | 1 (6.7%) | ||
| Spouse and children: 6 (40.0%) | 7 (46.7%) | ||
| Partner: 6 (40.0%) | 3 (20.0%) | ||
| (n = 15) | (n = 15) | ||
| Chemo therapy type | |||
| None | 3 (13.0%) | 8 (34.8%) | |
| AC | 1 (4.3%) | 0 (0.0%) | |
| TAX + other | 0 (0.0%) | 1 (4.3%) | |
| TAX + HER | 4 (17.4%)2 (8.7%) | 1 (4.3%) | |
| TAX + HER + other | 9 (39.1 (4.3%) | 0 (0.0%) | |
| TAX + AC | 3 (13.0%) | 9 (39.1%) | |
| TAX + AC + other | 2 (8.7%) | ||
| TAX + AC + HER | 2 (8.7%) | ||
| Any chemo therapy (%) | 20 (87.0%) | 15 (65.2%) | 0.084 (Chi-square test) |
| Endocrine therapy | None: 7 (30.4%) | 3 (13.0%) | |
| AI: 11 (47.8%) | 11 (47.8%) | ||
| TAM: 4 (17.4%) | 7 (30.4%) | ||
| TAM + AI: 1 (4.3%) | 2 (8.7%) | ||
| Any endocrine therapy (%) | 16 (69.6%) | 20 (87.0%) | 0.153 (Chi-square test) |
| smokers (%) | 1 (4.3%) | 2 (8.7%) | 1.000 (Fisher’s exact test) |
| Alcohol: units per week | 0: 4 (17.4%) | 7 (30.4%) | 0.844 (Fisher’s exact test) |
| 1: 12 (52.2%) | 7 (30.4%) | ||
| 2: 1 (4.3%) | 1 (4.3%) | ||
| 3: 1 (4.3%) | 1 (4.3%) | ||
| 4: 3 (13.0%) | 2 (8.7%) | ||
| 5: 1 (4.3%) | 2 (8.7%) | ||
| 6: 0 (0.0%) | 1 (4.3%) | ||
| 7: 0 (0.0%) | 1 (4.3%) | ||
| 10: 1 (4.3%) | 1 (4.3%) | ||
| Previous psychological diagnosis (%) | 6 (27.3%) (n = 22) | 11 (47.8%) (n = 23) | 0.155 (Chi-square test) |
| Using psychotropics (%) | 7 (30.4%) | 5 (21.7%) | 0.502 (Chi-square test) |
| Tumorstage (T) (%) | 0: 0 (0.0%) | 1 (4.3%) | 0.023 (Fisher’s exact test) |
| 1: 8 (34.8%) | 15 (65.2%) | ||
| 2: 9 (39.1%) | 7 (30.4%) | ||
| 3: 5 (21.7%) | 0 (0.0%) | ||
| 4: 1 (4.3%) | 0 (0.0%) | ||
| Nodal stage (N) (%) | 0: 11 (47.8%) | 11 (47.8%) | 0.222 (Fisher’s exact test) |
| 1: 8 (34.8%) | 12 (52.2%) | ||
| 2: 2 (8.7%) | 0 (0.0%) | ||
| 3: 2 (8.7%) | 0 (0.0%) | ||
| Metastatic disease present (%) | 3 (13%) | 1 (4.3%) | 0.608 (Fisher’s exact test) |
| Menopause (%) | 21 (91.3%) | 20 (87.0%) | 1.000 (Fisher’s exact test) |
| Time between diagnosis and inclusion (months) | 29.0 (33.4) | 34.9 (41.1) | 0.991 (Mann Whitney test) |
| Time between end of chemo (except trastuzumab) and inclusion (months) | 21.2 (36.9) (n = 20) |
25.3 (30.7) (n = 15) |
0.317 (Mann Whitney test) |
TAX: taxanes; AC: antracycline/cyclophosphamide; HER: herceptine; TAM: tamoxifen; AI aromatase inhibitor.
2.2. Study design
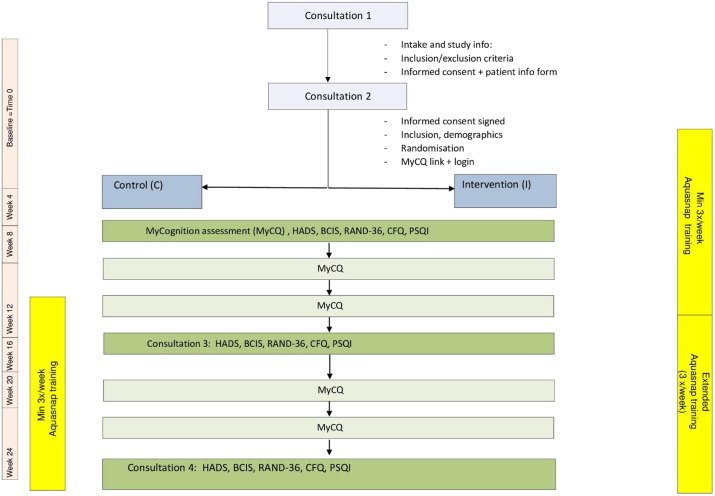
This is a single center phase-II randomized controlled pilot trial (RCT) with a crossover of the control group participants after 3 months. Approval for the study was obtained by the local ethical committee of the Antwerp University hospital (ID: B300201627683). Women were informed about the study in a session in the outpatient clinic by the principal investigator (AB). After a baseline assessment the participants were randomized using a web based system (Q-minim) in a 1:1 ratio to an intervention group (n = 23) or a waitlist control group (n = 23) (Fig. 1). Stratified randomization was used with stratification according to age (<45 years, 45–65 years, >65 years) and using minimization ensured a minimal imbalance between treatment groups.
Fig. 1.
Outline of the study design.
2.2.1. The waitlist control group
Patients received classic supportive care for 3 months and then started the Aquasnap training for a period of 3 months (n = 23).
2.2.2. The intervention group
Participants in the intervention group (n = 23) started web-based cognitive training using the Aquasnap videogame (MyCQ, Med by Mycognition Cambridge, UK) from months 0–3. The video gaming took place at home on the participants own computer of choice. The training consisted of game playing for three months: at least three times a week, for a minimum of 60 min or a total minimum of 12 h. Both groups had cognitive videogaming from months 3–6 (Fig. 1 flowchart). All patients included in the study received a standard base line psychological assessment and support by a female gynecologist with a special interest and education in psychosocial support (AB) and a breast nurse.
Patients in both groups had an online cognitive assessment (MyCQ) every 4 weeks [11]. Duration of this test is approximately 20 min. Total amount of tests is 7 (baseline being T0 and last evaluation at 6 months being T6). The test at baseline had to be performed twice in order to get used to online testing. Patients were assessed at the outpatient clinic by the principal investigator with a neuro-psychological survey at 3 different time points: at baseline, after 3 and 6 months. The total duration of this assessment was approximately 1 h: Hospital Anxiety and Depression Scale (HADS), RAND 36, BCIS (Beck Cognitive Insight Scale), CFQ (Cognitive Failure Questionnaire) and Pittsburgh Sleep Quality Indicator (PSQI). The questionnaires that were chosen are validated and were offered as a “lime-survey” free of charge in the outpatient clinic The primary outcome measure was the difference in the MyCQ™ score before and after the 3-months training program which is a compound composite score of measures in the following cognitive domains: executive function/attention, memory, language and visual perception/construction. Secondary outcome measures were: Activity of Daily Life (ADL), mood, subjective cognition, functional cognitive status measured by the above neuropsychological tests.
2.3. Supervision
During the study period the patients were seen on a 3 monthly basis by the principal investigator. If they had any problems or reflections on the cognitive training program they could contact the study team by email or telephone.
2.4. Intervention: the aquasnap online training program
AquaSnap (Cambridge, MyCQ™) is a cognitive training videogame developed to improve cognitive function. It targets the five cognitive domains (attention, working memory, episodic memory, executive function and processing speed) and is assessed by MyCQ. The game consists of fulfilling tasks and assignments as an underwater photographer (Figure supplement 1 and 2). Initially the difficulty levels are equal for all patients. Thereafter the program produces personalized training for every user, calibrating the amount of the training for each cognitive domain on the MyCQ individual scores. The higher a player’s score, the more training tasks they will complete for that domain. Based on the player’s MyCQ score AquaSnap encourages the player to undertake repetitive, and increasingly more challenging and complex tasks, that are embedded in a video game. The different tasks are designed to train a specific cognitive domain. Each cognitive domain is mainly trained by a particular loop, and some domains are trained across different tasks [11].
For each of the tests a measure of latency, i.e. the user’s average reaction time, and accuracy, i.e. the percentage of correct answers, is recorded. Then, the system automatically combines these measures in real-time according to a recipe set thereby producing a score for each domain in a scale from 1 to 100, setting the mean at 50. In addition to the five domains’ score, an overall MyCQ score is calculated as an average of the five domains. This provides an empirical measure of the overall individual cognitive fitness and discriminates which cognitive domains show greater strengths or weaknesses, to address a more targeted intervention.
It was recently shown that MyCQ subtests significantly correlated with the Cambridge Neuropsychological Automated Test Battery (CANTAB), one of the most well validated, reliable, and word-wide professionally adopted tools to assess cognition [11]. This reinforces the assumption, based on experts’ design, that MyCQ is a clinically valid tool to identify the cognitive constructs, which it aims to assess. The results of MyCQ have been normalized by age group based on the large amount of data collected by the system (almost 6000 assessments completed).
2.5. Neuropsychological testing
Patients were assessed at 3 different time points: at baseline, after 3 months (short-term effect) and after 6 months (long-term effect). All assessments were performed at the outpatient clinic by the principal investigator. The total duration of this assessment was approximately 1 h. The following tests were used:
The RAND 36 which is the Dutch equivalent of 36-item short-form (MOS SF-36) and was validated by Van der Zee and Sanderman [12]. The SF-36 was designed for use in clinical practice and research, to monitor medical care outcomes. It includes one multidimensional scale that assesses eight health concepts: 1) limitations in physical functioning; 2) limitations in social functioning; 3) limitations in usual role activities because of physical health problems; 4) limitations in usual role activities due to emotional problems; 5) mental health; 6) bodily pain; 7) general health perceptions; 8) health change. The closer the scale is to 100, the better.
The Hospital Anxiety and Depression Scale (HADS) is a self-assessment scale that has been found to be a reliable instrument for detecting states of depression and anxiety in the setting of an hospital medical outpatient clinic. The 14 items are divided into 7 items on anxiety and 7 on depressive mood. The anxiety and depressive subscales are also valid measures of severity of the emotional disorder. It detects emotional disorder in patients under investigation and treatment in medical and surgical departments. We used the validated Dutch version by Pouwer et al. [13]. Subscales distinguish 3 categories: 0–7 is normal, 8–10 possible anxiety or depression disorder, above 11 is indicative of anxiety or depression disorder.
The Beck Cognitive Insight Scale (BCIS) was developed to evaluate patients’ self-reflectiveness and their overconfidence in their interpretations of their experiences. A 15-item self-report questionnaire was subjected to a principle components analysis, yielding a 9-item self-reflectiveness subscale and a 6-item self-certainty subscale. A composite index of the BCIS reflecting cognitive insight was calculated by subtracting the score for the self-certainty scale from that of the self-reflectiveness scale [14]. A higher cognitive insight is not always correlated with better psychological functioning and using subscales separately is helpful. The norm of the subscale of self-reflectiveness is thought to be above the score of 14. The norm of self-certainty is thought to be below a score of 12. A good cognitive insight is reflected by a level above 5 or in some references above 8.
The CFQ or Broadbent Cognitive Failure Questionnaire is a measure of self-reported failures in perception, memory, and motor function. The score is reasonably stable over long periods and thus far it has not been found to change in persons exposed to life-stresses. However, it does frequently correlate with the number of current psychiatric symptoms reported by the same person. Subjects with a high CFQ are vulnerable to stress because self-attentional processing disrupts coping strategies. We used the Dutch variant by Ponds et al. to distinguish subgroups: normal 21–43, high score 44–54, very high >54, low score 10–21, very low <10 [15].
The Pittsburgh Sleep Quality Index (PSQI) is a self-rated questionnaire which assesses sleep quality and disturbances over a 1-month time interval. Nineteen individual items generate seven “component” scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The sum of scores for these seven components yields one global score. A global PSQI score can distinguish good and poor sleepers (PSQI> 5) [16].
3. Statistical analysis
Descriptive statistics are reported per treatment group, mean and standard deviation for the continuous variables and observed numbers with percentages for the categorical variables. Between group-comparisons for the variables measured at baseline are done with a t-test for continuous variables or Chi-square test for categorical variables (or respectively Mann Whitney test or Fisher’s exact test as appropriate). As primary outcome analysis a Mann-Whitney test was performed to compare the change in overall MyCQ score form the baseline to 3 months between intervention and control group. To explore whether the intervention had a different effect on the outcome over time a linear mixed model was fitted with treatment, time (as a categorical variable with values baseline, 3 and 6 months) and the interaction of treatment and time as fixed effects and subject as random effect. If the interaction was not significant a model with only time and group was considered to see if the outcome changed over time. For the MyCQ we considered the speed and accuracy for each of the 10 subtests, but corrected for multiple testing using the Holm correction for these 10 tests. Adjusted analysis where variables measured at baseline were added to the model (one by one), was considered for the main outcomes (MyCQ, CFQ and pain).
4. Results
4.1. Participant characteristics
The mean age of the 46 breast cancer patients was 51.8 years (range 32–71 years). The level of education was secondary school (26%), college or university (74%), the majority had a partner (73%) and they were all Kaukasian. Demographic data per treatment arm are given in Table 1. Only for tumor stage a significant difference between control and intervention group was noticed (p = 0.023). Median time since diagnosis was 29 months (range 3–174 months) in the control arm and 35 months (range 5–180 months) in the intervention group. At baseline the means for CFQ, anxiety, PSQI and self-reflectiveness were beyond normal range (Table 1 Supplement).
4.2. Primary outcome analysis
A Mann-Whitney test comparing the change in overall MyCQ score from baseline to 3 months between intervention and control group gave a non-significant result (p = 0.539).
4.3. Interaction between time and treatment arm
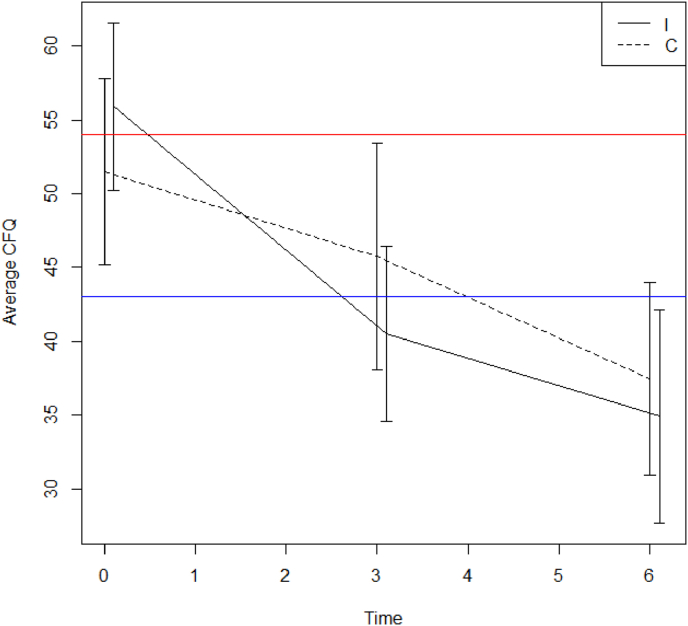
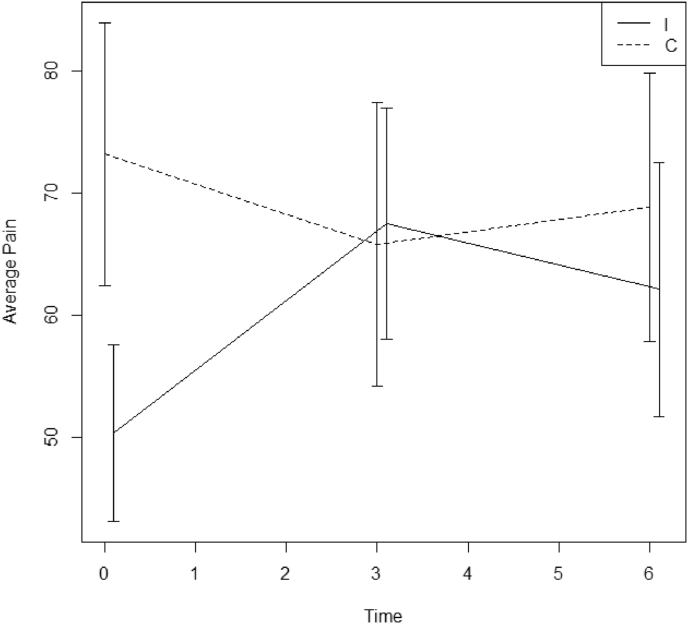
For each outcome a linear mixed model was fitted with time, treatment arm and the interaction between time and treatment arm as fixed effects and subject as random effect. Levels of significance of the interaction term between time and treatment group are given in Table 2. Only for CFQ and pain (RAND36) intervention and control group evolved differently over time (respectively p = 0.029 and 0.014). In the control group CFQ goes down by 5.6 units on average between month 0 and 3 (95% CI: −10.5, −0.7), compared to a significantly different decrease (p = 0.006) of 16.1 units (95% CI: −21.6,-10.5) in the intervention group (Fig. 2). Analyzing patients who have complete baseline and 3 month data, the interaction between time and treatment group remains significant for CFQ (p = 0.008). Initial pain levels at baseline are lower in the intervention group but at 3 and 6 months pain was comparable between two groups (Fig. 3). There was a pain reduction of 5.8 units between 0 and 3 months in the control group (95% CI: −14.8,3.0) and a significantly different increase (p = 0.003) of 14.9 units in the intervention group (95% CI: 4.9,25.0) at 3 months. No significant different evolutions over time were found for the overall MyCQ score (p = 0.588) or individual MyCQ tests after correction for multiple testing (Table 3).
Table 2.
P-values of the interaction term between time and treatment from the linear mixed model.
| Variable | p-value interaction |
|---|---|
| Overall _MyCQ | 0.588 |
| CFQ | 0.029 |
| Anxiety | 0.245 |
| Depression | 0.332 |
| Self reflectiveness | 0.776 |
| Self certainty | 0.477 |
| BCIS | 0.632 |
| PSQI | 0.538 |
| Limitations in physical functioning | 0.226 |
| Limitations in social functioning | 0.196 |
| Limitations in usual role activities because of physical health problems | 0.808 |
| limitations in usual role activities due to emotional problems | 0.972 |
| Mental health | 0.171 |
| Vitality | 0.076 |
| Pain | 0.014 |
| General health perception | 0.584 |
| Health change | 0.959 |
Fig. 2.
Mean Cognitive Failure Questionnaire score with 95% CI for the intervention (I) and control (C) group at time points 0, 3 and 6 months (blue line: normal CFQ levels in the general population; red line: upper limit demarking elevated from very elevated cognitive symptoms).
Fig. 3.
Mean Pain score with 95% CI for the intervention (I) and control (c) group at time points 0, 3 and 6 months.
Table 3.
Uncorrected and (for multiple testing) corrected p-values for the interaction between time and treatment arm from the linear mixed model.
| Mycq subtest | Uncorrected p-value | Corrected p-value | |
|---|---|---|---|
| Latency | Simple Reaction Time | 0.657 | 1 |
| Choice Reaction Time | 0.910 | 1 | |
| Go No Go reaction time | 0.945 | 1 | |
| Visual Memory Recognition | 0.012 | 0.121 | |
| Verbal Memory Recognition | 0.615 | 1 | |
| N-back 1 | 0.754 | 1 | |
| N-back 2 | 0.045 | 0.405 | |
| Coding | 0.397 | 1 | |
| Trail making test A | 0.690 | 1 | |
| Trail making test B | 0.295 | 1 | |
| Accuracy | Simple Reaction Time | 0.705 | 1 |
| Choice Reaction Time | 0.214 | 1 | |
| Go No Go reaction time | 0.302 | 1 | |
| Visual Memory Recognition | 0.431 | 1 | |
| Verbal Memory Recognition | 0.263 | 1 | |
| N-back 1 | 0.591 | 1 | |
| N-back 2 | 0.032 | 0.321 | |
| Coding | 0.552 | 1 | |
| Trail making test A (∗) | 0.255 | 1 | |
| Trail making test B | 0.400 | 1 |
(∗) only 5 non-zero values.
Adding the variables from Table 1 to the linear mixed model for the CFQ outcome does not change the estimates and all p-values for these variables are above 0.05 (also for tumorstage). For the outcome MyCQ the adjusted analysis doesn’t result in a significant interaction between time and treatment arm. For the outcome pain there are some significant factors. Adding a previous history of psychological diagnosis gives a significant p-value (0.019) but estimates of pain between baseline and 3 months are similar as in the model without psychological diagnosis. The same is seen for tumor stage which is borderline significant (p = 0.068). Adding marital status (p = 0.646) or time between end of chemo and inclusion (p = 0.382) results in a non-significant interaction term (p = 0.270 and 0.094 respectively).
4.4. Effect of time
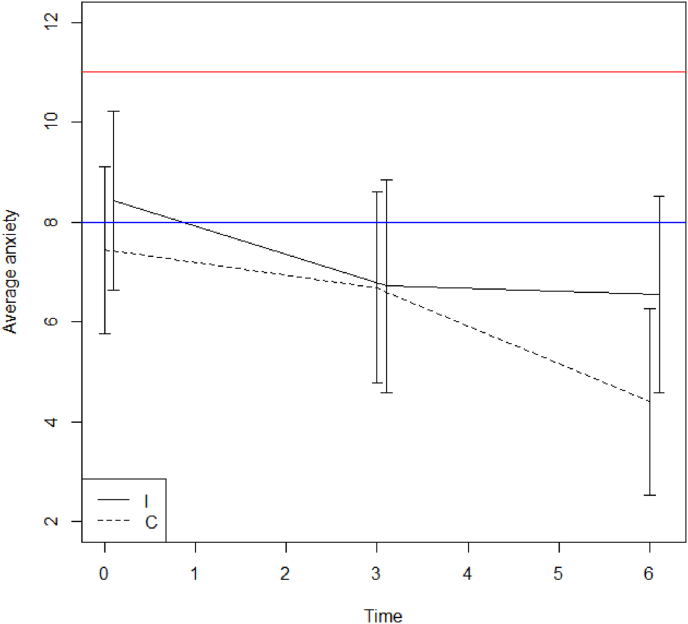
In case of a non-significant interaction between time and treatment group, a second model was fitted with time and treatment arm as fixed effects thereby combining the effect of both groups over time. P-values for the time effect are given in Table 4. A significant improvement was seen for overall MyCQ score, level of fear, physical and emotional role limitation, and health change (all p < 0.05), but self-reflectiveness deteriorated (p < 0.05) (Fig. 4, Fig. 5). Table 5 outlines the estimated differences for these parameters between baseline and 3 or 6 months respectively. In Table 6 p-values for the time effect for the various MyCQ subtests can be found, with and without correction for multiple testing.
Table 4.
P-values for time effect of cognitive and mental health parameters for the entire study population.
|
Variable |
p-value time |
|---|---|
| Overall _MyCQ | <0.001 |
| Anxiety | 0.031 |
| Depression | 0.266 |
| Self reflectiveness | 0.018 |
| Self certainty | 0.564 |
| BCIS | 0.319 |
| PSQI | 0.127 |
| limitations in physical functioning | 0.660 |
| limitations in social functioning | 0.064 |
| limitations in usual role activities because of physical health problems | 0.035 |
| limitations in usual role activities due to emotional problems | 0.036 |
| Mental health | 0.059 |
| Vitality | 0.052 |
| General health perception | 0.418 |
| Health change | 0.027 |
Fig. 4.
Mean Level of Anxiety score with 95% CI for the intervention (I) and control (C) group at time points 0, 3 and 6 months (0–7: no -; 8–10: possible -; 11–21: likely anxiety disorder).
Fig. 5.
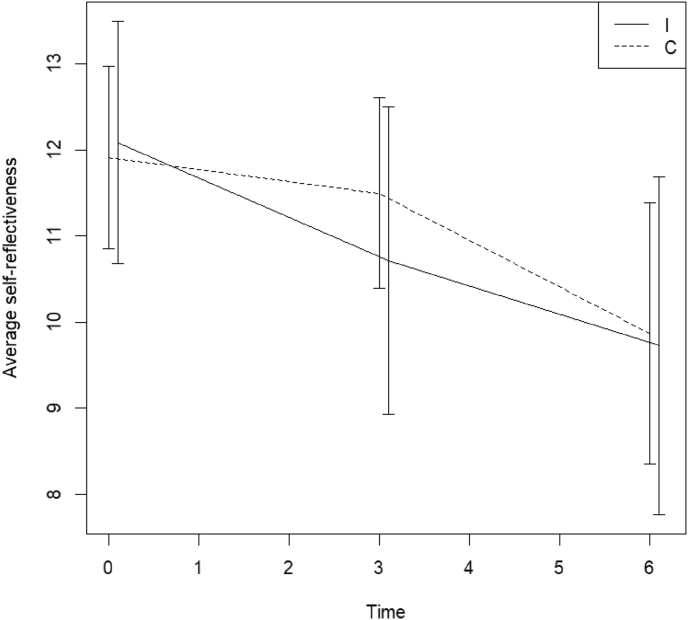
Mean level of self-reflectiveness score measured by the Beck Cognitive insight scale with 95% CI for the intervention (I) and control (C) group at time points 0, 3 and 6 months () (normal levels in an average population are above 14).
Table 5.
Estimated differences for the different outcomes between baseline and 3 or 6 months from the linear mixed model with time and arm.
| Variable | Change from baseline to 3 months | Change from baseline to 6 months |
|---|---|---|
| Overall_MyCQ | 10.07 [6.30,13.87] | 14.46 [9.84,19.24] |
| Anxiety | −1.00 [-2.10,0.08] | −1.60 [-2.83,-0.42] |
| Self reflectiveness | −0.82 [-2.09,0.43] | −2.05 [-3.46,-0.70] |
| Limitations in usual role activities because of physical health problems | 11.61 [-1.53,24.82] | 18.91 [4.62,33.46] |
| Limitations in usual role activities due to emotional problems | 20.41 [2.66,38.25] | 21.49 [2.37,40.83] |
| Health change | 12.82 [-0.53,25.85] | 18.75 [4.70,33.07] |
Table 6.
P-values for time effect of the various MyCQ subtests for the entire study population with and without correction for multiple testing.
| MyCQ subtest | Uncorrected p-value | Corrected p-value | |
|---|---|---|---|
| Latency | Simple Reaction Time | 0.017 | 0.068 |
| Choice Reaction Time | <0.001 | 0.001 | |
| Go No Go reaction time | 0.255 | 0.255 | |
| Visual Memory Recognition | <0.001 | 0.001 | |
| Verbal Memory Recognition | 0.033 | 0.1 | |
| N-back 1 | <0.001 | <0.001 | |
| N-back 2 | <0.001 | <0.001 | |
| Coding | 0.003 | 0.016 | |
| Trail making test A | 0.106 | 0.212 | |
| Trail making test B | <0.001 | <0.001 | |
| Accuracy | Simple Reaction Time | 0.298 | 1 |
| Choice Reaction Time | 0.152 | 0.911 | |
| Go No Go reaction time | 0.426 | 1 | |
| Visual Memory Recognition | 0.025 | 0.187 | |
| Verbal Memory Recognition | 0.023 | 0.187 | |
| N-back 1 | 0.002 | 0.016 | |
| N-back 2 | 0.002 | 0.016 | |
| Coding | 0.490 | 1 | |
| Trail making test A | 0.192 | 0.958 | |
| Trail making test B | 0.681 | 1 |
In Table 7 changes at 3 and 6 months were given for the variables with a significant evolution over time: choice reaction time, visual memory recognition, N back 1 and 2,coding, trail making test B, error percentage N back 1 and 2.
Table 7.
Changes of the MyCQ subtests of the entire population which altered significantly over time.
| MyCQ subtest | Change between baseline and 3 m | Change between baseline and 6 m | |
|---|---|---|---|
| Latency | Choice Reaction Time | −59.44 [-89.04,-29.06] | −77.53 [-114.10,-40.21] |
| Visual Memory recognition | −64.56 [-103.05,-27.62] | −103.88 [-150.89,-58.06] | |
| N back 1 | −223.47 [-292.26,-154.59] | −234.88 [-320.09,-150.29] | |
| N back 2 | −250.86 [-370.06,-130.35] | −405.22 [-552.50,-256.66] | |
| Coding | −215.40 [-363.12,-70.80] | −283.30 [-461.60,-105.57] | |
| Trail making test B | −344.44 [-479.79,-215.37] | −391.25 [-554.75,-231.21] | |
| Accuracy | N back 1 | −5.94 [-9.72,-2.17] | −6.90 [-11.57,-2.23] |
| N back 2 | −7.73 [-12.75,-2.67] | −10.65 [-17.10,-4.52] |
In the intervention group 7 patients played more than 40 min a week, 10 less than 40 min a week and 6 never played and for the control group these numbers respectively were 7,8 and 8.
As a considerable amount of patients dropped out before 3 months (primary outcome MyCQ at baseline n = 38, at 3 months n = 22 and at 6 months n = 13) we did a sensitivity analysis on the one hand comparing patients with data at 3 months versus patients with missing data at 3 months (no significant differences were found) and on the other hand the linear mixed models were refitted for only those patients who had data at baseline and 3 months. This also led to similar conclusions with a highly significant interaction between time and treatment for CFQ (p = 0.008) and similar estimates for change from baseline to 3 months (−5.5 [-10.1,-0.9] and −17.2 [-22.6, −11.8] in intervention and control group respectively).
5. Discussion
Cognitive impairment is a common phenomenon in a variety of clinical and psychological disorders. It can be detected in 4%–11% of healthy persons, but cross-sectional studies showed that it is present in up to 60% of women after breast cancer treatment [4]. Although a meta-analysis of 17 studies on cognitive function in breast cancer survivors treated with standard adjuvant chemotherapy suggested that the cognitive deficiencies in most patients are generally limited in magnitude, over the last decade it became clear that there is a subgroup of patients which appears to be particularly vulnerable to experience persisting severe cognitive changes [17,18]. It has been hypothesized that neurotoxic effects of the anticancer therapy (eg cytotoxic drugs), hormonal changes, immune dysregulation, inactivity, co-morbidities and certain medications (eg. benzodiazepines, corticosteroids) are the most important causal culprits [1]. However, recent evidence emerged that the “cognitive reserve” of a patient (determined by genetic predisposition, acquired coping mechanisms, previous psychological trauma) is a crucial factor involved [19]. As perceived cognitive function is often affected by fatigue, anxiety, pain and depression, and a practical golden standard test to assess cancer related cognitive impairment (CRCI) objectively is currently not available, research in this field is difficult and hampered by the use of complex and subjective questionnaires [1,20]. Therefore studies attempting to find cost-effective interventions to treat this common complaint are limited in number and size [21]. Up to now the effectivity of pharmacologic interventions has been disappointing with no drugs approved by the Food and Drug Administration (FDA) for this indication [22]. Several study designs have focused on cognitive training, cognitive behavioral training or a combination of both. The results for neuropsychological performance are mixed but most of these trials demonstrated a reduced cognitive impairment after these interventions [9,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]]. In a systematic review assessing 19 studies on cognitive rehabilitation for CRCI including 1124 patients, Fernandes et al. found improvements on at least one cognitive measure in all of them. Particularly objective improvements in memory were most commonly reported, followed by executive functions and processing speed [37]. However these studies have been heterogeneous with various patient selection criteria, cognitive training programs, statistical reporting and measured outcomes.
In the present pilot-study we assessed the efficacy of an online game to improve cognitive and psychosocial functioning in a self-selected population of breast cancer survivors. These patients complained of long lasting CRCI, were all Caucasian and mostly highly educated, and many received adjuvant chemotherapy. Interestingly the subjective perception of cognitive failure could not be objectively confirmed in all patients. Such inconsistency is common in studies on cognition and raises the question whether current neuropsychological tests are sufficiently sensitive to measure subtle functional defects [2,5,6,9]. In line with other studies, levels of anxiety, depression, pain, are high in our study population (Fig. 4) [9]. A recent paper by Van Dyk et al. (2018) showed that fatigue, pain and sleep correlated with several cognitive domains (regardless of treatment exposure) in 189 breast cancer survivors [2]. The interplay between these complaints is complex and difficult to entangle, but should be taken into account dealing with these patients in a holistic way. This can explain why cognitive rehabiliatation therapy can improve cognitive functioning by tackling emotional and sleep disorders. We could show that CFQ (a measure of self-reported failures in perception, memory, and motor function) and pain (RAND36) evolved significantly different for the intervention compared to the control group (respectively p = 0.029 and 0.014). The improvement of CFQ was higher in the intervention group. Initial pain levels at baseline were lower in the intervention group but at 3 and 6 months pain was comparable between two groups. We could not detect any other differences between the psycho-cognitive tests in both treatment arms. The good news is that we could demonstrate that even after a long time of CRCI improvement is possible. Combining the evolution over time in the entire study population a significant amelioration was seen for overall MyCQ score, level of fear, physical and emotional role limitation, and health change (all p < 0.05). The effect of the cognitive training, mental training by repeatedly filling in questionnaires, tender loving care, tackling sleeping disorders, physical rehabilitation, psychosocial support and may be some placebo effect probably explain these results. The role of MyCQ gaming in this process remains uncertain as the video game training did not have a significant effect on objective cognitive test results, although most participants claimed to feel subjectively better as measured by CFQ. This raises the question whether this effect is also transferable to other tasks aiming to train cognition, such as filling in cross-word puzzles or sudokus. So far reliable evidence is lacking that computerized tests improve the level of general cognitive functioning.
Another crucial factor involved is spontaneous recovery over time. In the study of Bray et which included patients 6–60 months after completing adjuvant chemotherapy no major improvement was seen after 6 months in the control group receiving only standard care [9]. However in a large longitudinal cohort study in China cognitive functions (particularly short-term, attention, executive function, and long-term memory) significantly improved among breast cancer survivors between 18 and 36 months after cancer diagnosis [38]. Breast cancer survivors who received neither chemotherapy nor tamoxifen showed no significant improvement in any of the tests, suggesting that their CRCI is mainly endogenous and not treatment induced. There is reliable evidence that cognitive deficits (particularly immediate and delayed verbal memory, processing speed, executive functioning and psychomotor speed) after breast cancer treatment can (partially) persist for decades [39,40]. In order to assess the effect of spontaneous improvement of CRCI in our study, the ideal design would have included a third group of patients randomly assigned to have minimal support. We regarded this as un-ethical in our patient population as cognitive impairment was their major complaint which needed to be addressed. We tried to solve this issue partially by having a waiting list group which started training after 3 months.
Adherence to the gaming was disappointing in our project. Six out of 23 patients in the intervention group and 8/23 in the control group never played, and the dropout rate was very high. Despite this we could show that there was a significant correlation between the intervention and CFQ in the intention to treat analysis and the analysis of complete date sets. Non-adherence is a common phenomenon in medicine with many patients not following their physicians advice to take medication, start a diet or change certain aspects of a life-style [41]. In the largest published randomized study evaluating a web-based cognitive rehabilitation program in 242 cancer survivors (self-reporting cognitive symptoms after chemotherapy) 14% of the patients in the intervention group never started using the program and the average total training time in the other patients in this group was 25.08 of the recommended 40 h [9]. Only 33 participants completed the program in the recommend 15 week timeframe [9]. Patient ideas, feelings and expectations about the proposed treatment and their motivation likely play a key role in treatment adherence, but recent evidence showed that also side effects of the medication, morbidity induced by the chronic disease, depression and socioeconomic background are involved [41]. This should be considered in the design of future trials but it remains a challenge to find strategies to improve this.
Our study is the first to assess cognitive insight in patients with CRCI. Beck cognitive insight scale is a score for self-reflectiveness minus score for self-certainty (Fig. 5). In an average population cognitive insight should be higher than 14 [14]. The majority of our participants demonstrated inadequate cognitive insight: respectively 73.9% (34/46) below 8 and 54.3% (25/46) below 5. This even became worse by the end of the study: 84.6% (22/26) below 8 and 65.4% (17/26) below 5. Our study population also demonstrated an abnormal low score on self-reflectiveness at onset and during training it also went down (Fig. 5). On average a higher self-reflectiveness in combination with an adequate amount of self-certainty is necessary to attain appropriate cognitive insight. Low self-reflectiveness demonstrates ones incapacity to reflect on ones thoughts and beliefs. This creates new therapeutic challenges and options since our participants had a normal level of self-certainty on average, making them susceptible to feedback.
The limitations of this study are sample size, low compliance and generalizability. The breast cancer survivors included in the study were selected on their personal perception suffering from cognitive dysfunction and is likely to be different from breast cancer survivors in general. Our small sample size limits power to detect statistically significant associations between the trajectory of cognitive training, and other psychological outcomes such as depression and anxiety. Standardized and objective measures of cognitive functioning would allow for a more better assessment of various domains of cognitive functioning, and should be implemented in other studies in this area.
Declaration of competing interest
AB received a research grant form MyCognition. The other authors do not have any conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.06.003.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Asher A., Myers J.S. The effect of cancer treatment on cognitive function. Clin Adv Hematol Oncol. 2015;13(7):1–10. [PubMed] [Google Scholar]
- 2.Van Dyk K., Bower J.E., Crespi C.M., Petersen L., Ganz P.A. Cognitive function following breast cancer treatment and associatoons with concurrent symptoms. NPJ Breast Cancer. 2018;4:25. doi: 10.1038/s41523-018-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray V.J., Dhillon H.M., Bell M.L., Kabourakis M., Fiero M.H., Yip D., Boyle F., Price M.A., Vardy J.L. Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J Clin Oncol. 2017;35(2):217–225. doi: 10.1200/JCO.2016.67.8201. [DOI] [PubMed] [Google Scholar]
- 4.Janelsins M.C., Kohli S., Mohile S.G., Usiki K., Ahles T.A., Morrow G.R. An update on cancer – and chemotherapy related cognitive dysfunction: current status. Semin Oncol. 2011;46(5):707–721. doi: 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janelsisns M.C., Heckler C.E., Peppone L.J., Ahles T.A., Mohile S.G., Mustian K.M., Palesh O., O’Mara A.M., Minasian L.M., Williams A.M., Magnuson A., Geer J., Dakhil S.R., Hopkins J.O., Morrow G.R. Longitudinal assessment of cancer-related cognitive impairment (CRCI) up to six months post chemotherapy with multiple cognitive testing methods in 943 breast cancer (BC) patients and controls. J Clin Oncol. 2017;35:10014. [Google Scholar]
- 6.Janelsisns M.C., Heckler C.E., Peppone L.J., Ahles T.A., Mohile S.G., Mustian K.M., Palesh O., O’Mara A.M., Minasian L.M., Williams A.M., Magnuson A., Geer J., Dakhil S.R., Hopkins J.O., Morrow G.R. Longitudinal trajectory and characterization of cancer related cognitive impairment in a nationwide cohort study. J Clin Oncol. 2018;36(32):3231–3239. doi: 10.1200/JCO.2018.78.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung N.C., Walker A.K., Dhillon H.M., Vardy J.L. Mechanisms and treatmenr of cancer and chemotherapy related cognitive impairment in survivors of non-CNSmalignancies. Oncology. 2018;32(12):591–598. [PubMed] [Google Scholar]
- 8.Kueider A.M., Parisi J.M., Gross A.L., Rebok J. Computerized cognitive training with older adults: a systematic review. PloS One. 2012;7(7) doi: 10.1371/journal.pone.0040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray V.J., Dhillon H.M., Bell M.L. Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J Clin Oncol. 2017;35(2):217–225. doi: 10.1200/JCO.2016.67.8201. [DOI] [PubMed] [Google Scholar]
- 10.Damholdt M.F., Mehlsen M., O’Toole M.S., Andreasen R.K., Pedersen A.D., Zachariae R. Web-based cognitive training for breast cancer survivors with cognitive complaints-a randomized controlled trial. Psycho Oncol. 2016 Nov;25(11):1293–1300. doi: 10.1002/pon.4058. Epub 2016 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domen A.C., van de Weijer S.C.F., Jaspers M.W., Denys D., Nieman D.H. The validation of a new online cognitive assessment tool: the MyCognition Quotient. Int J Methods Psychiatr Res. 2019 Sep;28(3) doi: 10.1002/mpr.1775. Epub 2019 Feb 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Zee K.I., Sanderman RHeyink J. De psychometrische kwaliteiten van de MOS 36-item Short Form Health Survey (SF-36) in een Nederlandse populatie (1993) Tijdschr Soc Gezondheidszorg (TSG) 1993:183–191. [Google Scholar]
- 13.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 14.Beck A.T., Baruch E., Balter J.M., Steer R.A., Warman D.M. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2014;68(2):319–329. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- 15.Ponds R., Van Boxtel M., Jolles J. De Cognitive Failure Questionnaire als maat voor subjectief cognitief functioneren. Tijdschr Neuropsychol. 2006;2:37–45. [Google Scholar]
- 16.Buysse B.D., Reynolds C.F., Monk T.H., Hoch C.C., Yeager A.L., Kupfer D.J. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh sleep quality index (PSQI) Sleep. 1991;14(4):331–338. [PubMed] [Google Scholar]
- 17.Jim H.S., Phillips K.M., Chait S. Meta-analysis of cognitive functioning in breats cancer survivors previously treated with standard low-dose chemotherapy. J Clin Oncol. 2012;30(29):3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodin G., Ahles T.A. Accumulating evidence for the effect of chemotherapy on cognition. J Clin Oncol. 2012;30(29):3568–3569. doi: 10.1200/JCO.2012.43.5776. [DOI] [PubMed] [Google Scholar]
- 19.Pullens M.J., De Vries J., Roukema J.A. Subjective dysfunction in breast cancer patients a systematic review. Psycho Oncol. 2010;19(11):1127–1138. doi: 10.1002/pon.1673. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network . 2015. NCCN clinical practice guidelines in oncology.http://www.nccn.org/professionals/physician-gls/f-guidelines.asp Survivorship vol. 1. [Google Scholar]
- 21.Wefel Jeffrey S. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 22.Mihuta M., Green H., Shum D. Efficacy of a web-based cognitive rehabilitation intervention for adult cancer survivors: a pilot study. Eur J Canc Care. 2018;27:1–11. doi: 10.1111/ecc.12805. [DOI] [PubMed] [Google Scholar]
- 23.Kesler S., Hadi Hosseini S.M., Heckler C. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Canc. 2013;13:299–306. doi: 10.1016/j.clbc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Ah D., Carpenter J.S., Saykin A. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Canc Res Treat. 2012;135:799–809. doi: 10.1007/s10549-012-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker H., Henneghan A.M., Volker D.L. A pilot study of a cognitive-behavioral intervention for breast cancer survivors. Oncol Nurs Forum. 2017;44:255–264. doi: 10.1188/17.ONF.255-264. [DOI] [PubMed] [Google Scholar]
- 26.Park J.H., Jung Y.S., Kim K.S. Effects of compensatory cognitive training intervention for breast cancer patients undergoing chemotherapy: a pilot study. Support Care Canc. 2017;25:1887–1896. doi: 10.1007/s00520-017-3589-8. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson R.J., Ahles T.A., Saykin A.J. Cognitive-behavioral management of chemotherapy-related cognitive change. Psycho Oncol. 2007;16:772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson R.J., Sigmon S.T., Pritchard A.J. A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction. Cancer. 2016;122:1782–1791. doi: 10.1002/cncr.29891. [DOI] [PubMed] [Google Scholar]
- 29.Ercoli L.M., Petersen L., Hunter A.M. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial. Psycho Oncol. 2015;24:1360–1367. doi: 10.1002/pon.3769. [DOI] [PubMed] [Google Scholar]
- 30.Green H.J., Tefay M., Mihuta M.E. Feasibility of small group cognitive rehabilitation in a clinical cancer setting. Psycho Oncol. 2018;27:1341–1343. doi: 10.1002/pon.4600. [DOI] [PubMed] [Google Scholar]
- 31.King S., Green H.J. Psychological intervention for improving cognitive function in cancer survivors: a literature review and randomized controlled trial. Front Oncol. 2015;5:72. doi: 10.3389/fonc.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johns S.A., Von Ah D., Brown L.F. Randomized controlled pilot trial of mindfulness-based stress reduction for breast and colorectal cancer survivors: effects on cancer-related cognitive impairment. J Cancer Surviv. 2016;10:437–448. doi: 10.1007/s11764-015-0494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston M.F., Hays R.D., Subramanian S.K. Patient education integrated with acupuncture for relief of cancer-related fatigue randomized controlled feasibility study. BMC Compl Alternative Med. 2011;11:49. doi: 10.1186/1472-6882-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartman S.J., Nelson S.H., Myers E. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory & motion study. Cancer. 2018;124(1):192–202. doi: 10.1002/cncr.30987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid-Arndt S.A., Matsuda S., Cox C.R. Tai Chi effects on neuropsychological, emotional, and physical functioning following cancer treatment: a pilot study. Compl Ther Clin Pract. 2012;18:26–30. doi: 10.1016/j.ctcp.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Oh B., Butow P.N., Mullan B.A. Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: a randomized controlled trial. Support Care Canc. 2012;20:1235–1242. doi: 10.1007/s00520-011-1209-6. [DOI] [PubMed] [Google Scholar]
- 37.Fernades J.H.A., Richard N.M., Edelstein K. Cognitive rehabilitation for cancer-related cognitive dysfunction: a systematic review. Support Care Canc. 2019;27(9):3253–3279. doi: 10.1007/s00520-019-04866-2. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y., Luo J., Boa P. Long-term cognitive function change among breast cancer survivors. Breast Canc Res Treat. 2014;146(3):599–609. doi: 10.1007/s10549-014-3044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koppelmans V., Breteler M.M., Boogerd W., Seynaeve C., Gundy C., Schagen S.B. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- 40.Yamada T.H., Denburg N.L., Beglinger L.J., Schultz S.K. Neuropsychological outcomes of older breast cancer survivors: cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci. 2010;22:48–50. doi: 10.1176/appi.neuropsych.22.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gast A., Mathes T. Medication adherence influencing factors – an updated overview of systematic reviews. Syst Rev. 2019;8:112. doi: 10.1186/s13643-019-1014-8. org/10.1186/s13643-019-1014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.