Abstract
Objectives
Tumor-associated antigens (TAAs) are frequently overexpressed in several cancer types. The aim of this study was to investigate the expression of TAAs in breast cancer.
Material and methods
A total of 250 selected invasive breast cancers including 50 estrogen receptor (ER)-positive (Luminal B like), 50 triple-negative (TN), 50 ER-positive lobular type, 50 ER- and progesterone receptor (PgR)-positive (Luminal A like) and 50 cerbB2-positive breast cancers, were assessed for New York esophageal squamous cell carcinoma-1 (NY-ESO-1), Wilms tumor antigen (WT-1) and PReferentially expressed Antigen of MElanoma (PRAME) antigen expression by immunohistochemistry (IHC).
Results
A significantly higher expression of cancer testis (CT)-antigens NY-ESO-1 and WT-1 antigen was detected in TN breast cancers compared with ER-positive tumors. NY-ESO-1 overexpression (score 2 + and 3+) assessed by monoclonal and polyclonal antibodies was detected in 9 (18%) TN cancers as compared to 2 (4%) ER-positive tumors (p = 0.002). WT1 over-expression (score 2 + and 3+) was confirmed in 27 (54%) TN tumor samples as compared to 6 (12%) ER-positive (p < 0.0001). PRAME over-expression (score 2 + and 3+) was detected in 8 (16%) HER2 positive tumor samples as compared to no TN and ER-positive cancers (p = 0.0021).
Conclusions
NY-ESO-1 and WT1 antigens are overexpressed in TN breast cancers. Because of the limited therapeutic options for this patient subgroup, CT antigen-based vaccines might prove to be useful for patients with this phenotype of breast cancer.
Keywords: Tumor-associated antigens, Cancer testis antigens, Breast cancer, WT1, PRAME, NY-ESO-1, Immunotherapy
Highlights
-
•
Tumor-associated antigens are frequently overexpressed in several cancer types, being also associated with poorer patients’ survival outcomes.
-
•
Our study confirmed that NY-ESO-1 and WT1 antigens are higher expressed in triple-negative than in other breast cancer subtypes.
-
•
Given the limited therapeutic options for triple-negative breast cancer patients, the assessment of WT1 and NY-ESO-1 antigens expression in breast cancer tissue at surgery may allow to identify patients potentially candidate to adjuvant peptide vaccines, alone or in combination with other systemic therapies.
1. Introduction
Research on tumor-associated antigen (TAA) peptides has identified a large collection of peptide epitopes that have been and are being used for the vaccination of cancer patients [1]. The use of peptide-based vaccines offers several potential advantages, such as the simplicity of peptide administration in the clinical setting, the possibility of treating only those patients whose tumors overexpress the antigens, and the availability of in vitro or ex vivo assays that can assess patients’ immune response to vaccine epitopes.
Over the last twenty years, the implementation of novel methodologies such as next generation sequencing and bioinformatics tools led to the identification of several TAAs. Among all recognized TAAs, a great interest has been focused on cancer testis (CT) and differentiation antigens, which are frequently downregulated in somatic adult tissues, while become aberrantly re-expressed in various malignancies [[2], [3], [4]]. These features render these antigens as appealing targets to generate anti-cancer vaccines and other types of immunotherapy. Moreover, several works suggest the association between TAAs expression and poorer outcomes across a broad spectrum of solid tumors, as well as a higher prevalence in undifferentiated and advanced-stage cancers [[5], [6], [7], [8], [9]]. As a result, intense research efforts have been directed toward the possible use of TAAs in the development of therapeutic vaccines due to their potent immunogenicity [10]. Several clinical trials with vaccines containing TAAs, such as New York esophageal squamous cell carcinoma-1 (NY-ESO-1), Wilms tumor antigen (WT-1) and PReferentially expressed Antigen of MElanoma (PRAME), accrued or are actually accruing patients with melanoma, lung, ovarian, and breast cancers [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. However, only few studies have investigated the expression of these TAAs in breast cancer and, in particular, across breast cancer subtypes [[20], [21], [22], [23], [24]].
The aim of this study was to assess the immunoreactivity for the TAAs NY-ESO-1, PRAME and WT-1 in a large series of breast cancer tumor samples classified, according to immunophenotype, in triple negative (TN), Luminal B-like, lobular type, human epidermal growth factor receptor 2 (HER2)-positive, and estrogen receptor (ER)- and progesterone receptor (PgR)-positive (Luminal A-like) human breast cancers.
2. Material and methods
2.1. Study population
Demographic, clinical, and pathological data of consecutive early breast cancer patients who underwent surgery at the European Institute of Oncology (Milan, Italy) between June 1995 and July 2002 were collected from the institutional database. Tumor types were classified according to the World Health Organization Histological Classification of Breast Tumors, as modified by Rosen and Obermann [25]. Tumor grading was assessed according to Elston and Ellis criteria [23].
A total of 250 cases of invasive breast cancer were selected and categorized, according to ER, PgR, HER2 status, and Ki67 labelling index, in: Luminal B like, defined as ER-positive with Ki67 > 14% (n = 50); Luminal A like, defined as ER- and/or PgR-positive more than 50% (N = 50); lobular histology (n = 50); HER2-positive, defined as any ER/PgR status and HER2+ (n = 50); and TN, defined as the lack of ER, PgR, and HER2 (n = 50).
All cases were examined for NY-ESO-1, PRAME and WT1 expression by immunohistochemistry (IHC).
2.2. Immunohistochemistry
ER, PgR status, Ki-67 labelling index (determined with the MIB1 monoclonal antibody) were assessed as previously reported [24,25]. HER2 IHC expression was evaluated using a 1/400 dilution of a polyclonal antiserum (Dako, Glostrup, Denmark). All tumors with equivocal (IHC 2+) results were tested for gene amplification by fluorescence in situ hybridization (FISH; Vysis PathVysion; Abbott, Chicago, IL), according to the international guidelines [26].
We defined as ER-positive tumors those showing ER and PgR expression in ≥50% neoplastic cells. Triple negative tumors were characterized by lack of immunoreactivity for ER and PgR, and by a negative (by both IHC and FISH) HER2 status. Slides were hybridized with probes to LSI HER-2/neu and CEP17.
NY-ESO-1 (monoclonal antibody E978 provided by Ludwig Institute for Cancer Research, at a working dilution of 1:200, and polyclonal antibody 195 provided by GSK, at a working dilution of 1:4000), WT1 (DakoCytomation, monoclonal antibody 6F–H2, at a working dilution of 1:200) and PRAME (Abcam, policlonal antibody ab32185, at a working dilution of 1:1600) expression has been investigated by IHC on whole tissue sections. Tissue specimens were dewaxed and heated in an antigen retrieval solution [EDTA buffer (1 mM, pH 8.0)] at 99 °C for 15 min (NY-ESO-1 monoclonal antibody) or 30 min (NY-ESO-1 polyclonal antibody, WT1 and PRAME). The sections were then incubated with the antibodies overnight at 4 °C (NY-ESO-1 monoclonal antibody and PRAME) or for 30 min at room temperature (NY-ESO-1 polyclonal antibody and WT1). The DAKO EnVision Mouse was used as a detection system and diaminobenzidine tetrahydrochloride as a chromogen. Sections of normal human testis were used as positive control for NY-ESO-1 and PRAME reactions; vascular endothelium was used as positive inner control for WT1.
2.3. Scoring
NY-ESO-1, PRAME, and WT-1 immunohistochemical results were scored using a semiquantitative scoring system similar to the previously described immunohistochemical-score [27]. This method considers both the percentage of immune-reactive cells and the staining intensity. The percentage of positive cells is then multiplied by the intensity of staining (1+, 2+, or 3+), and the final score ranges from 0 (no staining) to 300 (diffuse and strong immunostaining of all the tumor cells).
2.4. Statistical methods
Antigen expression (presence versus absence) among groups was evaluated using Fisher’s exact test (two proportions) or a chi-square test (more than two proportions). Different cut-offs of expression (i.e. 1+, 2 + and 3+) were considered to define presence of antigen expression. Kruskal-Wallis test was used for comparison of expression scores among different groups. P-values less than 0.05 were considered as statistically significant. All tests were two-sided.
3. Results
3.1. Pathological characteristics
From June 1995 to July 2002, a total of 4000 pT1-3 pN0-3 M0 early breast cancer patients were included in the institutional database. Among this patient population, a total of 250 cases of invasive breast cancer were identified. Tumor stage was pT1a-c for 174 patients, pT2 for 67 patients 3 and pT3-4 for 9 patients. Nodal involvement was present in 84 patients, while 166 were staged as pN0/pNX.
According to the histotype, 200 tumors were classified as invasive ductal and 132 displayed a Ki67 labelling index ≥20%. Lobular histology was identified in 50 cases. Baseline pathological characteristics of all breast tumors are listed in Table 1.
Table 1.
Baseline pathological characteristics, according to group.
| Luminal A-like |
HER-2 positive |
Lobular |
Luminal B-like |
Triple Negative |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| All samples | 50 | 100 | 50 | 100 | 50 | 100 | 50 | 100 | 50 | 100 |
| Histotype | ||||||||||
| Ductal | 49 | 98 | 42 | 84 | 0 | 0 | 39 | 78 | 35 | 70 |
| Lobular | 0 | 0 | 0 | 0 | 50 | 100 | 0 | 0 | 0 | 0 |
| Others | 1 | 2 | 8 | 16 | 0 | 0 | 11 | 22 | 15 | 30 |
| Grade | ||||||||||
| 1 | 8 | 16 | 5 | 10 | 10 | 20 | 1 | 2 | 1 | 2 |
| 2 | 24 | 48 | 18 | 36 | 40 | 80 | 16 | 32 | 6 | 12 |
| 3 | 17 | 34 | 26 | 52 | 0 | 0 | 30 | 60 | 42 | 84 |
| Unknown | 1 | 2 | 1 | 2 | 0 | 0 | 3 | 6 | 1 | 2 |
| pT | ||||||||||
| 1 | 40 | 80 | 32 | 64 | 39 | 78 | 31 | 62 | 32 | 64 |
| 2 | 10 | 20 | 14 | 28 | 11 | 22 | 15 | 30 | 17 | 34 |
| 3-4 | 0 | 0 | 4 | 8 | 0 | 0 | 4 | 8 | 1 | 2 |
| pN | ||||||||||
| 0 | 25 | 50 | 31 | 62 | 33 | 66 | 25 | 50 | 34 | 68 |
| 1 | 16 | 32 | 8 | 16 | 5 | 10 | 14 | 28 | 8 | 16 |
| 2-3 | 8 | 16 | 8 | 16 | 3 | 6 | 7 | 14 | 7 | 14 |
| x | 1 | 2 | 3 | 6 | 9 | 18 | 4 | 8 | 1 | 2 |
| ER | ||||||||||
| 0 | 0 | 0 | 18 | 36 | 1 | 2 | 10 | 20 | 50 | 100 |
| 1-49 | 0 | 0 | 7 | 14 | 3 | 6 | 10 | 20 | 0 | 0 |
| ≥50 | 50 | 100 | 25 | 50 | 46 | 92 | 30 | 60 | 0 | 0 |
| PgR | ||||||||||
| 0 | 0 | 0 | 21 | 42 | 16 | 32 | 30 | 60 | 50 | 100 |
| 1-49 | 0 | 0 | 19 | 38 | 12 | 24 | 14 | 28 | 0 | 0 |
| ≥50 | 50 | 100 | 10 | 20 | 22 | 44 | 6 | 12 | 0 | 0 |
| Ki-67 | ||||||||||
| <20% | 28 | 56 | 12 | 24 | 42 | 84 | 9 | 18 | 5 | 10 |
| ≥20% | 22 | 44 | 38 | 76 | 8 | 16 | 41 | 82 | 45 | 90 |
3.2. Expression of NY-ESO-1, WT1 and PRAME in breast cancer samples
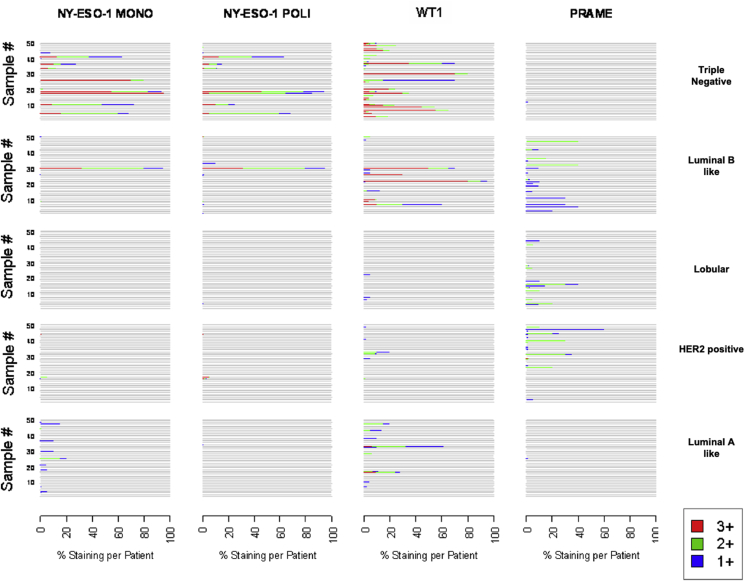
All samples were examined for NY-ESO-1 (mono and polyclonal antibody), WT1 and PRAME expression by IHC (Fig. 1, Fig. 2, Fig. 3, Fig. 4). A heterogeneous staining pattern was present within specific tumor samples, ranging from 1 + to 3+. In Fig. 5, the visual scale shows intensity (red for 3+, green for 2 + and blue for 1+) and percentage of staining for each one of the tumor samples. The expression of all antigens in the different subpopulations of breast cancer are summarized in Table 2.
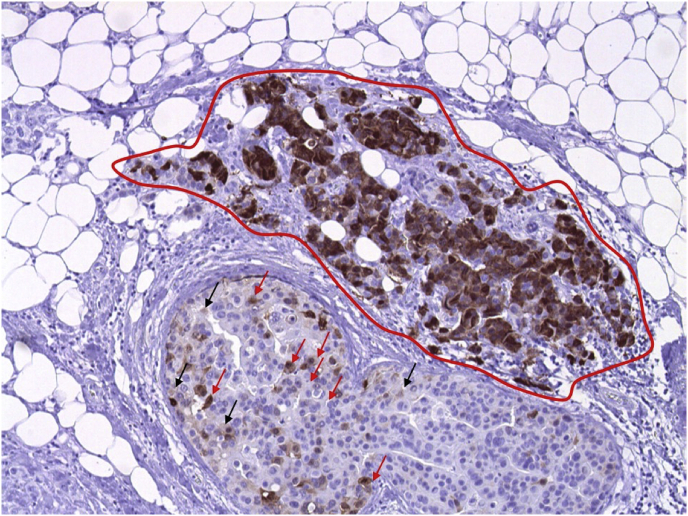
Fig. 1.
Example of assessing H-score in a case of breast cancer tested for NY-ESO-1. In this picture, about half of the neoplastic cells is stained with the brown chromogen and the remaining half of the cells is blue (no staining). Cells with strongly stained nuclei (3 + positivity) are circumscribed by the red line and these cells represent about 95% of all the colored cells in the picture (95% of 50% = 47,5%); the remaining 2,5% of colored cells are cells with moderately stained nuclei (2 + positivity), marked with the red arrows, and cells with weakly stained nuclei (1 + positivity), marked with black arrows. The percentage of 2 + cells is about 2% and the percentage of 1 + cells are 0,5%. The H-score is 147, generated adding the percentage of cells for the respective intensity of staining, as follows: (50 × 0) + (0,5 × 1) + (2 × 2) + (47,5 × 3) = 147. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
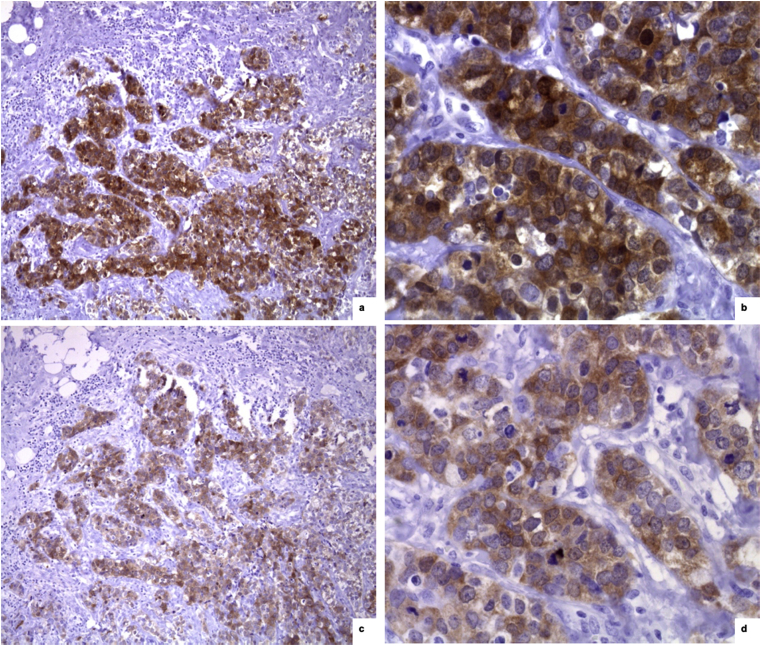
Fig. 2.
Comparison between mouse monoclonal NY-ESO-1 and rabbit policlonal NY-ESO-1 immunohistochemical expression in a case of triple negative infiltrating ductal carcinoma. a,b) Mouse monoclonal NY-ESO-1 positive staining [x 10 (a) e x 40 (b)]. c,d) Rabbit policlonal NY-ESO-1 positive staining [x 10 (c) e x 40 (d)].
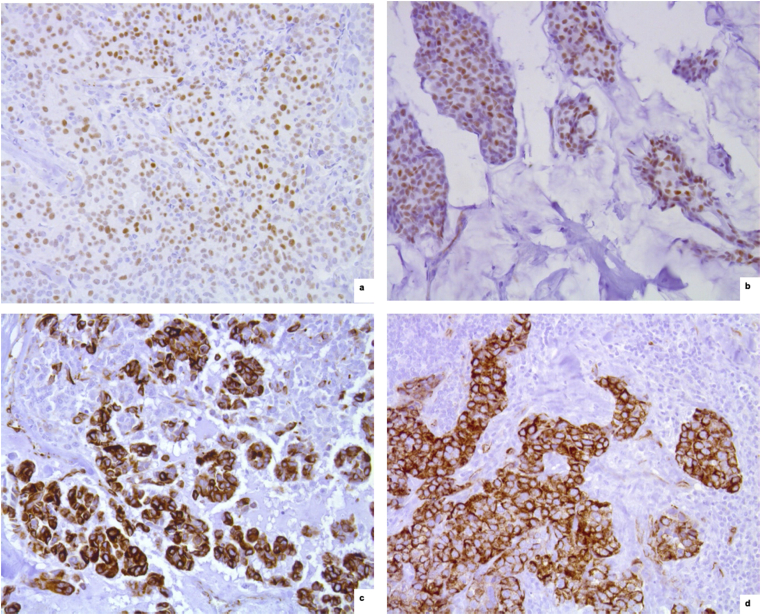
Fig. 3.
WT1 immunohistochemical expression. a,b) Nuclear staining in two cases of endocrine-responsive infiltrating ductal carcinoma (x 20, anti-WT1). c,d) Citoplasmic staining in a case of triple-negative infiltrating ductal carcinoma (c) and in a medullary carcinoma of moderately endocrine-responsive group (d) (x 20, anti-WT1).
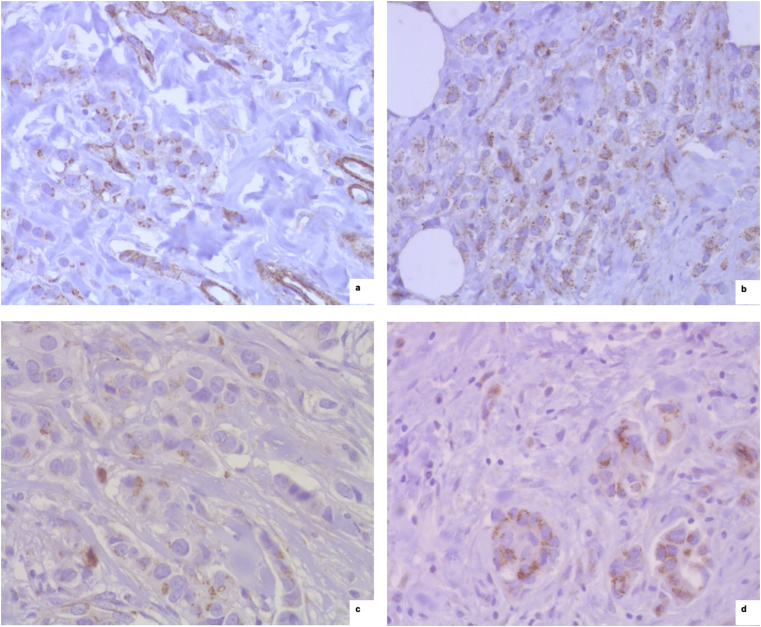
Fig. 4.
PRAME immunohistochemical expression. a) PRAME positive staining in a lobular carcinoma (x 40, anti-PRAME). b) PRAME positive staining in a carcinoma of moderately endocrine-responsive group (x 40, anti-PRAME). c,d) PRAME positive staining in two cases of breast carcinoma with HER2 overexpression (x 40, anti-PRAME).
Fig. 5.
Observed individual NY-ESO-1 MONO, NY-ESO-1 POLI, WT1 and PRAME antigen expression and percent staining, according to group.
Table 2.
MONO NY-ESO-1, POLI NY-ESO-1, WT1 and PRAME antigen expression, according to group.
| Antigen | Expression | Luminal A-like |
Her2-positive |
Lobular |
Luminal B-like |
Triple Negative |
p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |||
| NY-ESO-1 | ≥1+ | 11 | 22 | 3 | 6 | 0 | 0 | 4 | 8 | 11 | 22 | 0.007 |
| MONO | ≥2+ | 2 | 4 | 2 | 4 | 0 | 0 | 2 | 4 | 9 | 18 | 0.002 |
| 3+ | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 2 | 8 | 16 | <0.0001 | |
| Scorea | 5 (1, 35) | 3 (1, 10) | – | 2 (1207) | 114 (1285) | 0.04 | ||||||
| NY-ESO-1 | ≥1+ | 1 | 2 | 3 | 6 | 1 | 2 | 8 | 16 | 10 | 20 | 0.0023 |
| POLI | ≥2+ | 0 | 0 | 3 | 6 | 0 | 0 | 3 | 6 | 9 | 18 | 0.0007 |
| 3+ | 0 | 0 | 3 | 6 | 0 | 0 | 2 | 4 | 8 | 16 | 0.0015 | |
| Scorea | 1 (1, 1) | 9 (3, 17) | 1 (1, 1) | 2 (1, 207) | 44 (1, 217) | 0.11 | ||||||
| WT1 | ≥1+ | 10 | 20 | 6 | 12 | 3 | 6 | 12 | 24 | 28 | 56 | <0.0001 |
| ≥2+ | 6 | 12 | 3 | 6 | 0 | 0 | 8 | 16 | 27 | 54 | <0.0001 | |
| 3+ | 2 | 4 | 0 | 0 | 0 | 0 | 6 | 12 | 20 | 40 | <0.0001 | |
| Scorea | 11 (2, 99) | 3 (1, 30) | 5 (2, 5) | 12 (1, 265) | 21 (1230) | 0.049 | ||||||
| PRAME | ≥1+ | 1 | 2 | 17 | 34 | 12 | 24 | 16 | 32 | 1 | 2 | <0.0001 |
| ≥2+ | 0 | 0 | 8 | 16 | 8 | 16 | 5 | 10 | 0 | 0 | 0.0021 | |
| 3+ | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0.41 | |
| Scorea | 1 (1, 1) | 2 (1, 65) | 10 (3, 70) | 13 (1, 80) | 1 (1, 1) | 0.15 | ||||||
Median (minimum, maximum): computed only on patient with expression ≥1+.
NY-ESO-1 over-expression (score ≥2+) by monoclonal antibodies was documented in 9 (18%) TN tumors, but only in 2 (4%) ER-positive (p = 0.002). Similar results were obtained by means polyclonal antibodies against NY-ESO-1 with a higher expression in TN than in ER-positive tumors (18% vs 0%; p = 0.0007).
WT1 expression (score ≥2+) was detected in 27 (54%) TN cancers, while only in 6 (12%) and 3 (6%) cases of ER-positive and HER2-positive, respectively (p < 0.0001). Conversely, PRAME was overexpressed (score ≥2+) in 8 (16%) HER2-positive tumor samples, while it was not expressed in TN and ER-positive tumors (p = 0.0021).
Overall, no associations were found between pathological features of disease and NY-ESO-1, WT1, and PRAME over-expression (score ≥2+) (data not showed).
4. Discussion
Our study results showed that TN breast cancers frequently display a higher expression of WT1 and NY-ESO-1 antigens, corroborating the results of other studies that demonstrated a reduced expression of these antigens in ER-positive breast cancers as compared to TN tumors [[18], [19], [20], [21], [22],28,29]. Conversely, in our work we did not find significant differences in terms of PRAME expression in TN tumors as compared to other breast cancer subtypes, as suggested in previous studies [30]. Such discrepancy can be partially explained by the use of monoclonal antibodies that bind different epitopes of PRAME and WT1 antigens.
DNA microarray profiling have led to the identification of invasive breast cancer subgroups with common molecular features and to the recognition of breast cancer as a heterogeneous entity [[31], [32], [33], [34], [35]]. Choice of systemic therapies for breast cancer patients includes the identification of potential targets based on genetic signature and/or IHC [36]. In this respect, the TN subtype is characterized by a higher expression of the proliferation cluster of genes [31] with an elevated number of potential targets for novel therapies, included epidermal growth factor receptor (EGFR) overexpression and upregulation in endothelial growth factors [37]. However, disappointing results have been obtained by targeting these molecules/pathways in the context of clinical trials [38]. Interestingly, some authors revealed that human cells lacking BRCA1, including TN breast cancer cells, may be sensitive to drugs that cause double-strand breaks in DNA [39] (e.g. alkylating agents) and, more recently, biological agents such as poly (ADP-ribose) polymerase inhibitors (PARP-inhibitors) [[39], [40], [41]]. These preclinical and clinical evidences paved the way for implementing personalized therapies also in the TN subtype.
The identification of clinical characteristics and biomarkers associated with resistance to standard therapies represents a crucial step in the development of the most effective therapeutic approaches. Properly selecting patients who most likely benefit from a given therapy might allow to better understand patient populations and clinical setting in which investigate new therapeutic strategies. Considering breast cancer, the neoadjuvant studies represent excellent platforms to perform biomarker analysis studies and to test new drugs in selected patient populations. Indeed, TN tumors display higher rates of pathological complete response (pCR) after neoadjuvant chemotherapy compared to the hormone receptor-positive subset [42]. However, several studies demonstrated that the 5-year disease-free survival (DFS) of TN tumors is significantly worse than all other breast cancer subtypes [42,43]. Importantly, patients with ER-positive residual tumors present with better survival compared to patients with ER-negative tumors not achieving a pCR [43]. These scant survival outcomes are also due to the limited therapeutic options currently available for TN breast cancer patients who do not achieve pCR after neoadjuvant chemotherapy. In this setting, only capecitabine monotherapy demonstrated activity, according to the CREATE-X trial results [44], and it is currently the only recommended therapy for these patients in the clinical practice. In this context, the implementation of new treatments in the post-neoadjuvant setting is a topic of a great interest. Our results are of clinical relevance for such subpopulation of patients, specifically in the adjuvant and post-neoadjuvant settings of treatment. In our hypothesis, patients with TN breast cancer and residual disease after preoperative chemotherapy represent the ideal setting to test the efficacy of a vaccination strategy. To date, vaccines for breast cancer have been mainly investigated in end-stage diseases.
By contrast, ever-growing evidences suggest that immunotherapy might be most effective when administered in patients with early phase tumors and minimal burden of disease, also considering the smaller number of immune escape mechanisms adopted by tumor cells in these settings. To date, several clinical trials testing vaccines against antigens, including MUC1, CEA, and HER2, have been completed [45]. In addition, other vaccines including members of the MAGE and ESO families have been tested in clinical trials for patients with melanoma and lung cancer with controversial results [46,47]. Despite CT antigens potentially offer the opportunity for fostering vaccine development and therapy for a broad spectrum of cancers, other determinants involved in anti-tumor immune response have to be considered when we approach to design new trials investigating therapeutic cancer vaccines. These factors included the presence of an immune-suppressive tumor microenvironment such as the upregulation of negative immune checkpoint molecules on the tumor cells and tumor-associated immune cells as well as the presence of negative regulatory cells (T regulatory cells, myeloid-derived suppressor cells, tumor-associated macrophages etc …). As showed in an ovarian carcinoma preclinical model, NY-ESO-1-specific/CD8+ tumor-infiltrating lymphocytes frequently upregulate the negative immune checkpoint molecules programmed of death-1 (PD-1) and lymphocyte-activation gene 3 (LAG-3) as immune-escape mechanism [48]. In addition, given the recent approval of checkpoint inhibitors-based immunotherapy in the metastatic setting of TN breast cancer [49], we can speculate that some patients might benefit from the combination of a CT-based peptide vaccine and an immune checkpoint inhibitor. This hypothesis is currently being tested in some clinical trials.
5. Conclusions
Our results demonstrated the overexpression of WT1 and NY-ESO-1 antigen in a group of patients with TN breast cancer for whom therapeutic options are limited. Exploring the expression of WT1 and NY-ESO-1 antigens in breast cancer tissue at surgery may allow to identify patients potentially candidate to adjuvant peptide vaccines, alone or in combination with other systemic therapies.
Author contributions
GC and GV conceived and designed the work. AM and CC provided materials, collected and assembled data. GV and MG performed immunohistochemistry experiments. JL, VB and FFL, as part of the collaboration with GSK, provided insights on the design of the experiments and advised on the tools to use for the immunohistochemistry. VB performed the statistical analysis. All authors discussed the data and wrote the paper. All authors read and accepted the final version of the manuscript.
Funding
This study was supported by GSK via the provision of antibodies for the immunohistochemistry staining.
Compliance with ethical standards
Declaration of interest
GC received honoraria for speaker, consultancy or advisory role from Roche, Pfizer, Novartis, Seattle Genetics, Lilly, Ellipsis. JL, VB, FFL are/were employees of the GSK groups of companies in the course of the study; they hold shares in the GSK group of companies. The other authors declare that they have no conflict of interest.
Informed consent
All authors declare that the work has been carried out according to The Code of Ethics of the World Medical Association (Declaration of Helsinki). The local Ethical Committee approved this research protocol. Patients’ informed consent and authorization were obtained for using their tumor tissues for research purposes.
Availability of data and materials
The dataset analyzed during the current study is available from the corresponding author on reasonable request.
Research involving human and animal participants
This article does not contain any studies with human participants performed by any of the authors.
Acknowledgements
None.
References
- 1.Coulie P.G., Van den Eynde B.J., van der Bruggen P., Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 2.Simpson A.J., Caballero O.L., Jungbluth A., Chen Y.T., Old L.J. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 3.Almeida L.G., Sakabe N.J., deOliveira A.R., Silva M.C., Mundstein A.S., Cohen T. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 2009;37:D816–D819. doi: 10.1093/nar/gkn673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann O., Caballero O.L., Stevenson B.J., Chen Y.T., Cohen T., Chua R. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci U S A. 2008;105:20422–20427. doi: 10.1073/pnas.0810777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scanlan M.J., Simpson A.J., Old L.J. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 6.Gure A.O., Chua R., Williamson B., Gonen M., Ferrera C.A., Gnjatic S. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11:8055–8062. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 7.Velazquez E.F., Jungbluth A.A., Yancovitz M., Gnjatic S., Adams S., O’Neill D. Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)--correlation with prognostic factors. Cancer Immun. 2007;7:11. [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade V.C., Vettore A.L., Felix R.S., Almeida M.S., Carvalho F., Oliveira J.S. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immun. 2008;8:2. [PMC free article] [PubMed] [Google Scholar]
- 9.Napoletano C., Bellati F., Tarquini E., Tomao F., Taurino F., Spagnoli G. MAGE-A and NY-ESO-1 expression in cervical cancer: prognostic factors and effects of chemotherapy. Am J Obstet Gynecol. 2008;198:99 e1–7. doi: 10.1016/j.ajog.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Hu Z., Ott P.A., Wu C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender A., Karbach J., Neumann A., Jager D., Al-Batran S.E., Atmaca A. Lud 00-009: phase 1 study of intensive course immunization with NY-ESO-1 peptides in HLA-A2 positive patients with NY-ESO-1-expressing cancer. Cancer Immun. 2007;7:16. [PMC free article] [PubMed] [Google Scholar]
- 12.Atanackovic D., Altorki N.K., Cao Y., Ritter E., Ferrara C.A., Ritter G. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci U S A. 2008;105:1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jager E., Karbach J., Gnjatic S., Neumann A., Bender A., Valmori D. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci U S A. 2006;103:14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Baren N., Bonnet M.C., Dreno B., Khammari A., Dorval T., Piperno-Neumann S. Tumoral and immunologic response after vaccination of melanoma patients with an ALVAC virus encoding MAGE antigens recognized by T cells. J Clin Oncol. 2005;23:9008–9021. doi: 10.1200/JCO.2005.08.375. [DOI] [PubMed] [Google Scholar]
- 15.Valmori D., Souleimanian N.E., Tosello V., Bhardwaj N., Adams S., O’Neill D. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci U S A. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odunsi K., Qian F., Matsuzaki J., Mhawech-Fauceglia P., Andrews C., Hoffman E.W. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:12837–12842. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis I.D., Chen W., Jackson H., Parente P., Shackleton M., Hopkins W. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci U S A. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theurillat J.P., Ingold F., Frei C., Zippelius A., Varga Z., Seifert B. NY-ESO-1 protein expression in primary breast carcinoma and metastases: correlation with CD8+ T-cell and CD79a+ plasmacytic/B-cell infiltration. Int J Cancer. 2007;120:2411–2417. doi: 10.1002/ijc.22376. [DOI] [PubMed] [Google Scholar]
- 19.Bandic D., Juretic A., Sarcevic B., Separovic V., Kujundzic-Tiljak M., Hudolin T. Expression and possible prognostic role of MAGE-A4, NY-ESO-1, and HER-2 antigens in women with relapsing invasive ductal breast cancer: retrospective immunohistochemical study. Croat Med J. 2006;47:32–41. [PMC free article] [PubMed] [Google Scholar]
- 20.Mischo A., Kubuschok B., Ertan K., Preuss K.D., Romeike B., Regitz E. Prospective study on the expression of cancer testis genes and antibody responses in 100 consecutive patients with primary breast cancer. Int J Cancer. 2006;118:696–703. doi: 10.1002/ijc.21352. [DOI] [PubMed] [Google Scholar]
- 21.Sugita Y., Wada H., Fujita S., Nakata T., Sato S., Noguchi Y. NY-ESO-1 expression and immunogenicity in malignant and benign breast tumors. Cancer Res. 2004;64:2199–2204. doi: 10.1158/0008-5472.can-03-3070. [DOI] [PubMed] [Google Scholar]
- 22.Grigoriadis A., Caballero O.L., Hoek K.S., da Silva L., Chen Y.T., Shin S.J. CT-X antigen expression in human breast cancer. Proc Natl Acad Sci U S A. 2009;106:13493–13498. doi: 10.1073/pnas.0906840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elston C.W., Ellis I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 24.Viale G., Regan M.M., Maiorano E., Mastropasqua M.G., Golouh R., Perin T. Chemoendocrine compared with endocrine adjuvant therapies for node-negative breast cancer: predictive value of centrally reviewed expression of estrogen and progesterone receptors--International Breast Cancer Study Group. J Clin Oncol. 2008;26:1404–1410. doi: 10.1200/JCO.2007.10.6393. [DOI] [PubMed] [Google Scholar]
- 25.Viale G., Regan M.M., Mastropasqua M.G., Maffini F., Maiorano E., Colleoni M. Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst. 2008;100:207–212. doi: 10.1093/jnci/djm289. [DOI] [PubMed] [Google Scholar]
- 26.Wolff A.C., Hammond M.E., Hicks D.G., Dowsett M., McShane L.M., Allison K.H. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domfeh A.B., Carley A.L., Striebel J.M., Karabakhtsian R.G., Florea A.V., McManus K. WT1 immunoreactivity in breast carcinoma: selective expression in pure and mixed mucinous subtypes. Mod Pathol. 2008;21:1217–1223. doi: 10.1038/modpathol.2008.69. [DOI] [PubMed] [Google Scholar]
- 28.Adams S., Greeder L., Reich E., Shao Y., Fosina D., Hanson N. Expression of cancer testis antigens in human BRCA-associated breast cancers: potential targets for immunoprevention? Cancer Immunol Immunother. 2011;60:999–1007. doi: 10.1007/s00262-011-1005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curigliano G., Viale G., Ghioni M., Jungbluth A.A., Bagnardi V., Spagnoli G.C. Cancer-testis antigen expression in triple-negative breast cancer. Ann Oncol. 2011;22:98–103. doi: 10.1093/annonc/mdq325. [DOI] [PubMed] [Google Scholar]
- 30.Tessari A., Pilla L., Silvia D., Duca M., Paolini B., Carcangiu M.L. Expression of NY-ESO-1, MAGE-A3, PRAME and WT1 in different subgroups of breast cancer: an indication to immunotherapy? Breast. 2018;42:68–73. doi: 10.1016/j.breast.2018.08.106. [DOI] [PubMed] [Google Scholar]
- 31.Sorlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorlie T., Tibshirani R., Parker J., Hastie T., Marron J.S., Nobel A. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yehiely F., Moyano J.V., Evans J.R., Nielsen T.O., Cryns V.L. Deconstructing the molecular portrait of basal-like breast cancer. Trends Mol Med. 2006;12:537–544. doi: 10.1016/j.molmed.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Rakha E.A., El-Sayed M.E., Green A.R., Lee A.H., Robertson J.F., Ellis I.O. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 35.Hugh J., Hanson J., Cheang M.C., Nielsen T.O., Perou C.M., Dumontet C. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168–1176. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curigliano G., Burstein H.J., Winer E.P., Gnant M., Dubsky P., Loibl S. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol. 2017;28:1700–1712. doi: 10.1093/annonc/mdx308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viale G., Rotmensz N., Maisonneuve P., Bottiglieri L., Montagna E., Luini A. Invasive ductal carcinoma of the breast with the "triple-negative" phenotype: prognostic implications of EGFR immunoreactivity. Breast Canc Res Treat. 2009;116:317–328. doi: 10.1007/s10549-008-0206-z. [DOI] [PubMed] [Google Scholar]
- 38.Gusterson B.A., Hunter K.D. Should we be surprised at the paucity of response to EGFR inhibitors? Lancet Oncol. 2009;10:522–527. doi: 10.1016/S1470-2045(09)70034-8. [DOI] [PubMed] [Google Scholar]
- 39.James C.R., Quinn J.E., Mullan P.B., Johnston P.G., Harkin D.P. BRCA1, a potential predictive biomarker in the treatment of breast cancer. The Oncologist. 2007;12:142–150. doi: 10.1634/theoncologist.12-2-142. [DOI] [PubMed] [Google Scholar]
- 40.Robson M., Im S.A., Senkus E., Xu B., Domchek S.M., Masuda N. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 41.Litton J.K., Rugo H.S., Ettl J., Hurvitz S.A., Goncalves A., Lee K.H. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 43.Guarneri V., Piacentini F., Ficarra G., Frassoldati A., D’Amico R., Giovannelli S. A prognostic model based on nodal status and Ki-67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Ann Oncol. 2009;20:1193–1198. doi: 10.1093/annonc/mdn761. [DOI] [PubMed] [Google Scholar]
- 44.Masuda N., Lee S.J., Ohtani S., Im Y.H., Lee E.S., Yokota I. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 45.Curigliano G., Spitaleri G., Dettori M., Locatelli M., Scarano E., Goldhirsch A. Vaccine immunotherapy in breast cancer treatment: promising, but still early. Expert Rev Anticancer Ther. 2007;7:1225–1241. doi: 10.1586/14737140.7.9.1225. [DOI] [PubMed] [Google Scholar]
- 46.Gjerstorff M.F., Andersen M.H., Ditzel H.J. Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget. 2015;6:15772–15787. doi: 10.18632/oncotarget.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vansteenkiste J.F., Cho B.C., Vanakesa T., De Pas T., Zielinski M., Kim M.S. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:822–835. doi: 10.1016/S1470-2045(16)00099-1. [DOI] [PubMed] [Google Scholar]
- 48.Matsuzaki J., Gnjatic S., Mhawech-Fauceglia P., Beck A., Miller A., Tsuji T. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marra A., Viale G., Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med. 2019;17:90. doi: 10.1186/s12916-019-1326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analyzed during the current study is available from the corresponding author on reasonable request.