Abstract
Objectives
Randomized clinical trials do not include a population that truly reflects a real-world population, due to their inclusion and exclusion criteria. This leads to concerns about the applicability of these studies in a clinical practice. In the present study, we aim to describe the clinical and demographic characteristics, treatment patterns, and clinical outcomes in a population of patients with HER2-positive metastatic breast cancer who received pertuzumab and trastuzumab as first-line treatment in a real-world setting.
Methods
The database of the Danish Breast Cancer Group was used to assemble data on patients included in the period April 2013 to August 2017. The primary endpoints were overall survival (OS) and progression-free survival (PFS).
Results
A cohort of 291 patients with a median age of 58 years was registered. Hereof 112 (38%) patients with de novo disease (primary disseminated) and 179 (62%) with recurrence. The median follow-up for OS was 24.1 months. The median OS was 41.8 months (95% CI, 37.7 to NE) and the median PFS was 15.8 months (95% CI, 14.0 to 19.9). For de novo patients alone, the median OS was not reached whereas the median PFS was 17.9 months (95% CI, 14.3 to 27.3).
Hazard ratios for patients receiving vinorelbine showed comparable results as for the whole population.
Conclusion
This heterogeneous patient population in a real-world setting had a PFS comparable with what could be expected from the related randomized trial. The de novo patients had better OS and PFS as compared to patients with recurrence.
Keywords: Pertuzumab, Trastuzumab, Real-world
Highlights
-
•
Patients with HER2-positive metastatic breast cancer.
-
•
Pertuzumab and trastuzumab as first-line treatment in a real-world setting.
-
•
A cohort of 291 patients; 112 with de novo disease and 179 with recurrence.
-
•
Median OS; 41.8 months and median PFS; 15.8 months.
-
•
PFS comparable with what could be expected from the related randomized trial.
1. Introduction
Breast cancer is the most frequent cancer among women and the second most common cancer in the world. In 2018, an estimate of 2.1 million new breast cancers have been diagnosed. In developing countries, breast cancer is the most frequent cause of cancer death among women and the second most frequent cause of cancer death in more developed regions [1]. HER2-positive breast cancer is a subtype that overexpresses human epidermal growth factor receptor 2 (HER2) protein and its pathways. Approximately 25% of breast cancers are HER2-positive and overexpression is associated with poor prognosis [2]. For more than a decade HER2-targeted therapies have been the standard of care for HER2-positive metastatic breast cancer (mBC) [2]. Trastuzumab, a monoclonal antibody directed against the extracellular domain of HER2, has primarily been used in combination with a taxane. This is due to the risk of cardiotoxicity in trastuzumab combined with an anthracycline [3]. In previous prospective studies of first-line treatment with trastuzumab and chemotherapy in patients with HER2-positive mBC, the median overall survival varied from 25.1 months to 38.1 months [2,[4], [5], [6], [7]]. A randomized Nordic study (HERNATA) that investigated trastuzumab in combination with docetaxel or vinorelbine showed comparable time to progression and survival but increased toxicity with docetaxel [5]. In Denmark, combination therapy with vinorelbine and trastuzumab has therefore been considered as standard first-line treatment for HER2 positive mBC in patients who could tolerate chemotherapy.
In 2012, the first results from the CLEOPATRA study were published. Along with docetaxel, dual blocking of HER2 receptors with pertuzumab, a new HER2-binding antibody, and trastuzumab versus placebo and trastuzumab was tested [8]. An updated publication showed that first-line treatment with pertuzumab, trastuzumab, and docetaxel significantly increased median overall survival to 56.5 months (95% CI, 49.3 to NE) among patients with HER2-positive mBC compared to 40.8 (95% CI, 35.8 to 48.3) in the placebo arm of the study [9]. Based on the CLEOPATRA study, pertuzumab in combination with trastuzumab was approved as first-line therapy for HER2 mBC in Denmark, April 2013. Based on the results of the HERNATA trial the approval was given for combinational use not only with docetaxel but also with vinorelbine and other chemotherapeutic agents. The combination of vinorelbine as chemotherapy-backbone has not been investigated with the dual blockade of trastuzumab and pertuzumab in a randomized study.
In the present study, we aim to describe the clinical and demographic characteristics, treatment patterns, and clinical outcomes in a population of patients with HER2-positive mBC who received pertuzumab and trastuzumab in a real-world setting in Denmark.
2. Methods
2.1. Design
A retrospective observational study involving all Danish Departments of Oncology (12 sites) at university and public hospitals.
2.2. Patient selection
The study included all women (aged 18+) who started first-line treatment with trastuzumab and pertuzumab for either primary metastatic HER2-positive (stage IV) breast cancer (de novo) or recurrence of a HER2-positive breast cancer between April 2013 and August 2017.
2.3. Data sources
DBCG (Danish Breast Cancer Group): Data on demographic, diagnostic, therapeutic and follow-up on patients were collected prospectively in the nationwide, population-based clinical DBCG database. Remote data entry is accessible from all Danish hospital units involved in the diagnosis and treatment of breast cancer patients. All 12 oncological treatment sites in Denmark committed to, when pertuzumab, together with trastuzumab, was approved for metastatic breast cancer to report date of recurrence, site of recurrence, treatment (chemo backbone), start and end of treatment to DBCG.
Electronic chart review: Data extraction was conducted retrospectively by authors Thomas Christensen (TC) and Tobias Berg (TB). Chart review was completed for all patients and was controlled twice by TC and TB after the last follow up, August 2017.
Central population register: Data from the National Central Population Register (CPR) was linked to the DBCG database using the unique personal identification number assigned to all Danish citizens by the CPR. The CPR holds information on vital and emigration status on all Danish citizens. A complete follow-up until April 2018 was retrieved.
2.4. Ethical approval
The study was conducted as a quality control of the DBCG database and thus needed no further approval. Approval was obtained from the Danish Data Protection Agency (I-Suite nr.: 0539 and J. nr.:RH-2017-89).
2.5. Measures
The primary endpoints were OS and PFS for patients who received first-line therapy. The date of the initiation of pertuzumab was defined as the index date. OS was defined as time from index date until death of any cause. PFS was defined as time from index date to progression or death of any cause. If death without progression was registered within 90 days after the date of the last visit, it would be encountered an event.
Patients were divided into subgroups: de novo patients and patients with recurrent disease. The patients with recurrent disease were further subdivided into whether or not they had received adjuvant treatment with trastuzumab, and into time of recurrence relative to primary diagnosis (±16 months). The first subdivision as an attempt to elucidate the influence of adjuvant HER2-blockade on dual blockade efficiency in the metastatic setting, the second to mimic the study population in the CLEOPATRA trial, where patients who had received adjuvant/neoadjuvant chemotherapy with or without trastuzumab had to have had a disease-free interval of at least 12 months after the last dose of chemotherapy (with an estimated duration of approximately four months).
The Danish guidelines for HER2 expression recommend testing with immunohistochemistry (IHC) or in situ hybridization (ISH). The algorithm for HER2 testing recommends that pathologists start with IHC and analyse the reaction based on the ASCO guidelines. In the case of an IHC-HER2-2+, a supplementary ISH test is recommended to prove HER2 gene amplification. An IHC-HER2-3+ or a HER2/CEN17 ratio of 2 or more is considered HER2-positive [10].
2.6. Statistical analysis
Descriptive statistics were utilized to summarize demographics, clinical characteristics and treatment patterns. Associations between the characteristics of patients from the study cohort and the patients included in the CLEOPATRA trial were analyzed by χ2 and Fischer’s exact test, excluding unknowns. The Kaplan–Meier method was used to estimate PFS and OS. Subgroups for comparison were de novo patients as opposed to patients with recurrent disease. The latter was further subdivided into prior treatment with trastuzumab or not and into disease-free interval. The Cox proportional hazards regression model was used to assess unadjusted hazard ratios. The assumptions of proportional hazards were assessed by Schoenfeld residuals. P-values are two-sided.
Time on treatment was calculated from start to end of treatment with pertuzumab. Median time on treatment was calculated using the Kaplan-Meier method with events defined as the end of treatment and patients still on treatment were censored at the latest visit date.
All statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Patient characteristics
During the study period, 291 women with HER2-positive mBC received trastuzumab and pertuzumab as first-line treatment and were registered. Patient demographics and disease characteristics are shown in Table 1.
Table 1.
Characteristics of patients and tumors.
| Characteristics, 1. Line | Real life (Denmark) N = 291 (100%) |
|---|---|
| Age (years) | |
| <65 | 194 (67) |
| 65–74 | 67 (23) |
| 75 + | 30 (10) |
| Site of disease | |
| Visceral | 201 (69) |
| Non visceral | 90 (31) |
| Brain metastases | |
| Yes | 1 (0) |
| No | 290 (100) |
| HR status | |
| Positive | 184 (63) |
| Negative | 103 (36) |
| Unknown | 4 (1) |
| HER2 status IHC |
|
| 0/1+ | 0 (0) |
| 2+ | 37 (13) |
| 3+ | 236 (81) |
| Missing | 18 (6) |
| FISH/ISH/CISH | |
| Amplified | 65 (22) |
| Not amplified | 10 (4) |
| Missing/not performed | 216 (74) |
| Prior (neo)adjuvant therapy | |
| No | 112 (38) |
| Yes | 179 (62) |
| (Neo)Adjuvant therapy type | |
| Anthracyclines | 115 (40) |
| Hormone therapy | 97 (33) |
| Taxane | 107 (37) |
| Trastuzumab | 101 (35) |
The mean age was 58 years. The study included 112 (38%) de novo patients and 179 (62%) with recurrent disease of which 101 (56%) had received adjuvant treatment with trastuzumab. Visceral disease was present in 201 (69%) of the patients, and the most common sites of metastases were bone (25%), liver (18%), lymph node (18%) and lung/pleura (16%) (data not shown).
3.2. Treatment patterns
In total, 235 (81%) patients received pertuzumab and trastuzumab in combination with vinorelbine, 34 (12%) were treated with dual HER2-blockade in combination with a taxane and 20 (7%) patients received other combinations of chemotherapy as part of the first-line treatment. Two patients received pertuzumab and trastuzumab without any other antineoplastic treatment. In total, 51 (18%) patients received maintenance endocrine therapy as part of their first-line treatment in combination with pertuzumab and trastuzumab.
All patients received intravenous pertuzumab with a loading dose of 840 mg, and a maintenance dose of 420 mg every third week. Trastuzumab (600 mg) was given subcutaneously every third week.
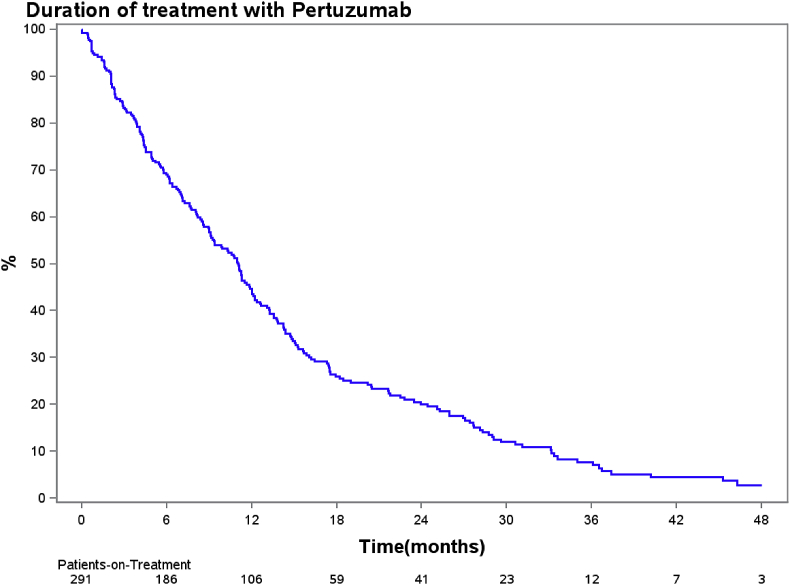
Forty-two (14%) patients received more than 20 months of treatment. Median time on dual blockade was 11.1 months (95% CI, 9.1–12 months). In total, 150 patients were treated for up to one year and 106 for more than a year, hereof 20 still on treatment. An additional 35 patients were still on treatment and treated for less than a year at the end of follow-up (Fig. 1).
Fig. 1.
– Kaplan-Meier chart of duration of treatment with pertuzumab and trastuzumab.
3.3. Clinical outcomes
3.3.1. Overall survival
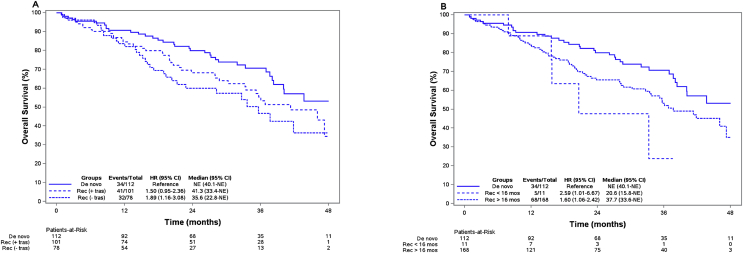
The median follow-up was 24.1 months. The median overall survival (mOS) for patients who received first-line treatment (N = 291) was 41.8 months (95% CI, 37.7-NE) and after 48 months 43% (95% CI, 34.7–53.7) of the patients were still alive. Fig. 2A shows overall survival for the 112 de novo patients, the 101 patients with recurrent breast cancer who received trastuzumab in the adjuvant setting, and the 78 patients who did not receive trastuzumab in the adjuvant setting. For the de novo patients the mOS was not reached (95% CI, 40.1-NE). The mOS for the patients with recurrent disease who received adjuvant trastuzumab was 41.3 months (95% CI, 33.4-NE) and mOS for patients who did not receive adjuvant trastuzumab was 35.6 months (95% CI, 22.8-NE). After 4 years the survival estimates were 53.2% (95% CI, 41.1–68.9), 34.5% (95% CI, 19.7–60.3) and 36.4% (95% CI, 22.5–58.8) for the three groups respectively. In a univariate analysis, hazard ratio for patients with recurrent disease was 1.50 (95% CI, 0.95–2.36) for those who received adjuvant trastuzumab and 1.89 (95% CI, 1.16–3.08) for those who did not receive adjuvant trastuzumab, using the group of de novo patients as reference.
Fig. 2.
A and B – Top: Kaplan-Meier estimates of overall survival for patients who did and did not receive adjuvant trastuzumab. Bottom: Kaplan–Meier estimates of overall survival for patients with recurrence within 16 months or later. Rec: recurrence, tras: Trastuzumab, mos: months, NE: not estimated.
A supplementary analysis of patients receiving vinorelbine showed comparable results with HR = 1.54 (95% CI, 0.93–2.55) and HR = 2.19 (95% CI, 1.27–3.80) for patients with recurrent disease who did and did not, respectively, receive adjuvant trastuzumab as compared to de novo patients. The groups of patients with other types of chemotherapeutic backbone were limited in numbers, not allowing for further analysis.
In total, 168 patients were treated with dual blockade after a disease-free interval of more than 16 months. The mOS for these patients was 37.7 months (95% CI, 33.6-NE). Only 11 patients, were treated with dual blockade after a disease-free interval of less than 16 months and they had a mOS of 20.6 months (95% CI, 15.8-NE) (Fig. 2B).
3.3.2. Progression-free survival
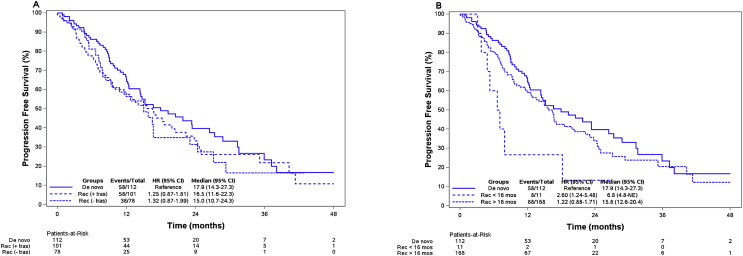
The median follow-up was 9.6 months. For all patients, the median progression-free survival (mPFS) was 15.8 months (95% CI, 14.0–19.9). For the 112 patients with de novo metastatic disease, the mPFS was 17.9 months (95% CI, 14.3–27.3). For patients with recurrent metastatic disease who had received adjuvant trastuzumab, the mPFS was 16.5 months (95% CI, 11.6–22.3). The mPFS for patients who had not received trastuzumab as adjuvant treatment was 15.0 months (95% CI, 10.7–24.3) (Fig. 3A). In a univariate analysis, hazard ratio was 1.25 (95% CI, 0.87–1.81) for patients with recurrent disease who received adjuvant trastuzumab and 1.32 (95% CI, 0.87–1.99) for patients with recurrent disease who did not receive adjuvant trastuzumab, using the group of novo patients as reference.
Fig. 3.
A and B Top: Kaplan-Meier estimates of progression-free survival for patients who did and did not receive adjuvant trastuzumab. Bottom: Kaplan–Meier estimates of progression-free survival for patients with recurrence within 16 months or later. Rec: recurrence, tras: Trastuzumab, mos: months, NE: not estimated.
A supplementary analysis of patients receiving vinorelbine showed comparable results with HR = 1.29 (95% CI, 0.86–1.93) and HR = 1.48 (95% CI, 0.93–2.36) for patients with recurrent disease who did and did not, respectively, receive adjuvant trastuzumab as compared to de novo patients.
The groups of patients with other types of chemotherapeutic backbone were limited in numbers, not allowing for further analysis.
The eleven patients treated with dual blockade after a disease-free interval of less than 16 months had a mPFS of 6.8 months (95% CI, 4.8-NE) (Fig. 3B).
4. Discussion
Trastuzumab and pertuzumab combined with taxane-chemotherapy have become standard of care for HER2-positive mBC in the first-line setting due to the results from the CLEOPATRA trial [9]. In the future the majority of patients with recurrent HER2-positive metastatic disease will have received adjuvant treatment with a taxane and other HER-2 targeted therapies and some patients can be expected to have developed resistance towards taxanes. Therefore, the combination of pertuzumab and trastuzumab with vinorelbine as the chemotherapy backbone may be attractive provided that the efficacy is comparable to taxane-containing therapy. In Denmark, the dual blockade was implemented, but with vinorelbine as chemotherapy backbone based on the results from the HERNATA trial.
In the HERNATA trial [5] patients were randomized between either docetaxel or vinorelbine combined with trastuzumab. In total, 97% of the patients had recurrent breast cancer, and all but four of the patients were naïve to taxanes. All patients but one was naïve to trastuzumab. The mOS was 35.7 months with docetaxel versus 38.8 months with vinorelbine (HR = 1.01; 95% CI, 0.71–1.42; P = 0.98). Median time to progression was 12.4 months with docetaxel vs 15.3 months with vinorelbine (HR = 0.94; 95% CI, 0.71–1.25; P = 0.67). In our study, the mOS was 41.8 months with dual blockade, but the two populations were even more different than patients enrolled in the CLEOPATRA study regarding expected treatment sensitivity.
In the VELVET cohort 1 trial the efficacy of pertuzumab, trastuzumab and vinorelbine were evaluated as first-line treatment of HER2-positive metastatic breast cancer [11]. The mPFS was 14.3 months (95% CI, 11.2–17.5; N: 89). The patient population differed from the CLEOPATRA study in several aspects; the VELVET population had no Asian patients, had a higher number of hormone receptor-positive patients and a higher frequency of patients with visceral disease, which according to the VELVET authors were part of the explanation for their differences in results. The same baseline characteristics are present in our study. We have few to none Asian patients and a higher grader of ER-positive patients.
In this real-world, national population-based study, we found a median PFS for the Danish cohort (15.8 months (95% CI, 14.0–19.9)) that was significantly better than the point estimate of 12.4 months in the control arm in the CLEOPATRA study (95% CI, 10.0–14.0) and comparable with the estimate of 18.7 months in the pertuzumab arm, considering the confidence interval [9]. Thus, with the data we have, we cannot rule out that the true median PFS for the Danish cohort could be the 18.7 from the pertuzumab-arm in the CLEOPATRA study.
Regarding OS, the upper 95% CI in the present study was not estimated (41.8 months (95% CI, 37.7 – NE)), however, the point estimate is low compared to the pertuzumab arm in the CLEOPATRA study (56.5 months (95% CI, 49.3-NE)) and only slightly above the control arm (40.8 months (95% CI, 35.8–48.3)) in the study [9]. There could be several reasons for this finding:
The age distribution in the Danish cohort was significantly different; 84% were under 65 years in the CLEOPATRA study vs 67% in the Danish cohort, and correspondingly 2% vs 10% in the group of 75 years of age and above (P < 0.0001), which affects OS.
Patients with de novo HER2-positive mBC had a better prognosis than patients with recurrent HER2-positive mBC (Fig. 2). In CLEOPATRA, 54% had de novo disease compared to 38% (P < 0.001) in the present study, which can explain part of the OS difference.
The data from the CLEOPATRA study was reported after a median observation time of 30 months and 50 months, where the latter showed a slightly higher median OS. For the Danish cohort, the median observation time was 24 months and based on the CLEOPATRA study, a longer follow-up could increase the mOS. In the CLEOPATRA trial, HER2 status was centrally confirmed and it has been shown that patients with centrally confirmed HER2-positive disease have a higher overall response rate and a longer mPFS and disease-free survival [12].
Median time on dual blockade was 11.1 months in our study (95% CI, 9.1–12.0) compared to 11.4 months (control arm) and 17.4 months (pertuzumab arm) in the CLEOPATRA study.
Time on a given treatment is often considered as a pseudo-marker for PFS. In this study, PFS of the Danish cohort is comparable with the CLEOPATRA study. The shorter duration of treatment in this real-world study might reflect that patients are less inclined to stay on treatment as opposed to a phase III trial. There may be several explanations for this difference. Clinical trials use RECIST criteria to determine disease progression where as this might not always be the case in clinical practice. One might also speculate whether clinicians and patients are more likely to end treatment earlier due to either toxicity or patient preferences than in a clinical trial.
The shorter time on treatment may be one of the reasons why patients in this study have a shorter OS compared to the CLEOPATRA study.
In a real-world study, the patient cohort will inherently be heterogeneous. The heterogeneity in our patient cohort is among others reflected in the previous adjuvant treatment (Table 1). The differences in adjuvant treatment will most likely impact the sensitivity of the patients in the recurrent setting.
Comparison of the groups with recurrent disease according to adjuvant trastuzumab should be done with precaution as the groups are likely different by other parameters.
Other groups have looked at local or regional tethered observational data regarding efficacy of dual HER2 blockade. A real-world study from 2017 found a mPFS of 16.9 months among 266 patients with HER2-positive mBC receiving first-line trastuzumab and pertuzumab [13]. The majority of patients (n = 249) received a taxane as the chemotherapy-backbone. The proportion of patients with de novo metastatic disease was higher than in our study - 49% vs. 38%, but the proportion of patients who received adjuvant trastuzumab was similar; 35% vs. 31%. An Italian study from 2017 found a mPFS of 27.8 months among 155 patients with HER2-positive mBC receiving trastuzumab, pertuzumab and a taxane in the first-line setting [14]. Almost half of the patients had de novo metastatic disease (48%) and most were trastuzumab naïve patients (24% received adjuvant trastuzumab). The median age was lower compared to our study - 52 years vs 58 years.
5. Conclusion
This is the first real-world study performed on a national basis in Denmark regarding a newly approved drug; pertuzumab. We were able to capture data from nearly 300 HER2-positive patients, who had received pertuzumab and trastuzumab with mainly vinorelbine as backbone and could demonstrate that the efficacy of the compound in real-world use is comparable to the result obtained in the registration trial (CLEOPATRA). This study also highlighted some of the differences between results from randomized trials and the real-world setting. With society’s demand regarding value for money after the introduction of expensive treatments, the need for real-world data will increase. Therefore, better handling and registration of the new treatment strategies and their effects are highly desired.
6. Study limitations
The DBCG data may be limited in various aspects.
The DBCG data are collected as electronic healthcare records, meaning the data are not collected for research purpose but for clinical practice reasons. This may impede the standardization of the data entry among the sites.
The completeness of data on patients was not sufficient. Patients could only be followed if reported to the DBCG database and we did not have the essential tools to identify patients not reported to DBCG.
All patients were seen every 12 weeks in the different centers, according to our national guidelines, thus reducing the risk of follow-up bias.
Declaration of Competing Interest
This study was funded by F. Hoffmann–La Roche and Genentech.
References
- 1.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Slamon D.J., Leyland-Jones B., Shak S. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Jones R.L., Swanton C., Ewer M.S. Anthracycline cardiotoxicity. Expet Opin Drug Saf. 2006;5(6):791–809. doi: 10.1517/14740338.5.6.791. [DOI] [PubMed] [Google Scholar]
- 4.Marty M., Cognetti F., Maraninchi D. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(19):4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 5.Andersson M., Lidbrink E., Bjerre K. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(3):264–271. doi: 10.1200/JCO.2010.30.8213. [DOI] [PubMed] [Google Scholar]
- 6.Valero V., Forbes J., Pegram M.D. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(2):149–156. doi: 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J., Manikhas A., Cortés J. Phase III trial of nonpegylated liposomal doxorubicin in combination with trastuzumab and paclitaxel in HER2-positive metastatic breast cancer. Ann Oncol Off J Eur Soc Med Oncol. 2014;25(3):592–598. doi: 10.1093/annonc/mdt543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baselga J., Cortés J., Kim S.B. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swain S.M., Kim S.-B., Cortés J. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolff A.C., Hammond M.E.H., Hicks D.G. Recommendations for human epidermal growth factor receptor 2 testing in breast. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 11.Perez E.A., Lopez-Vega J.M., Petit T. Safety and efficacy of vinorelbine in combination with pertuzumab for first-line treatment of patients with HER2-positive locally advanced or metastatic breast cancer: VELVET cohort 1 final results. Breast Canc Res. 2016;18:126. doi: 10.1186/s13058-016-0773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson M., López-Vega J.M., Petit T. Efficacy and safety of pertuzumab and trastuzumab administered in a single infusion bag, followed by vinorelbine: VELVET cohort 2 final results. Oncol. 2017;22(10):1160–1168. doi: 10.1634/theoncologist.2017-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert N.J., Goertz H.P., Chopra P. HER2-Positive metastatic breast cancer patients receiving pertuzumab in a community Oncology practice setting: treatment patterns and outcomes. Drugs - Real World Outcomes. 2017;4(1):1–7. doi: 10.1007/s40801-016-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Placido S., Giuliano M., Schettini F. Human epidermal growth factor receptor 2 dual blockade with trastuzumab and pertuzumab in real life: Italian clinical practice versus the CLEOPATRA trial results. Breast Edinb Scotl. 2018;38:86–91. doi: 10.1016/j.breast.2017.12.012. [DOI] [PubMed] [Google Scholar]