Abstract
Breast cancer diagnosis and staging is based on mammography, ultrasound, and magnetic resonance imaging (MRI). Contrast enhanced spectral mammography (CESM) has gained momentum as an innovative and clinically useful method for breast assessment. CESM is based on abnormal enhancement of neoplastic tissue compared to surrounding breast tissue. We performed a systematic review of prospective trial to evaluate its diagnostic performance, following standard PRISMA-DTA. We used a bivariate random-effects regression approach to obtain summary estimates of both sensitivity and specificity of CESM. 8 studies published between 2003 and 2019 were included in the meta-analysis for a total of 945 lesions. The summary area under the curve obtained from all the study was 89% [95% CI 86%–91%], with a sensitivity of 85% [95% CI 73%–93%], and a specificity of 77% [95% CI 60%–88%]. With a pre-test probability of malignancy of 57% a positive finding at CESM gives a post-test probability of 83% while a negative finding a post-test probability of 20%. CESM shows a suboptimal sensitivity and specificity in the diagnosis of breast cancer in a selected population, and at present time, it could be considered only as a possible alternative test for breast lesions assessment when mammography and ultrasound are not conclusive or MRI is contraindicated or not available.
Keywords: Mammography, Breast neoplasms, Meta-analysis, Systematic review, Contrast enhanced spectral mammography
Highlights
-
•
CESM is based on abnormal enhancement of neoplastic tissue compared to surrounding breast tissue.
-
•
The summary AUC for CESM in patients with breast cancer suspicion was 89%, with 85% sensitivity and 77% specificity.
-
•
With a pre-test probability of malignancy of 57% a positive finding at CESM gives a post-test probability of 83%.
-
•
With a pre-test probability of malignancy of 57% a negative finding at CESM gives a post-test probability of 20%.
-
•
CESM shows a suboptimal sensitivity and specificity in the diagnosis of breast cancer in a selected population.
1. Introduction
Contrast-enhanced spectral mammography (CESM) is a recently developed technique based on visualization of iodinated contrast agent uptake that has been proposed as a new breast imaging adjunct [1].
Breast cancer diagnosis and staging are currently based on three main diagnostic techniques, namely full field digital mammography (FFDM), ultrasound (US), and magnetic resonance imaging (MRI).
Specifically, FFDM is now usually integrated with digital breast tomosynthesis (DBT) which is a recent improvement generating quasi–3-dimensional images of the breast, improving mammographic sensitivity and specificity [2] are particularly suitable for analysing fatty breast but, since fibro-glandular tissue can obscure mass lesions [3,4], their sensitivity falls in dense breast, possibly yielding to underdiagnosis [5,6].
On the other hand, while US is particularly suitable for examining dense breasts, its overall diagnostic efficacy is operator dependent [5].
During the last two decades, new methods have been developed and tested to improve the diagnostic performance of breast imaging [7,8]. They are based on the biological principle of neo-angiogenesis that render malignancy associated vessels more permeable to contrast agent than healthy tissue, resulting in tumour enhancement [7]. MRI characterized by high sensitivity, but its specificity is affected by a relatively high false positive (FP) rate (up to 19%) [9]. Furthermore, some studies showed that MRI may not reduce reoperation rates and lead to unnecessary mastectomies, without any improvement in surgical outcomes or prognosis [10,11]. Finally, MRI is associated with high costs, long-time examination and the poor performance in detecting microcalcifications [12].
In this scenario, CESM has recently gained momentum as an innovative and clinically useful method for breast assessment [13,14]. CESM is based on abnormal enhancement of neoplastic tissue compared to surrounding breast tissue [15], hence the principle is similar to MRI, the cost of CESM is far lower (similar to conventional mammography [16]), and the time to image CESM is considerably less than that required for MRI [17]. Finally, implementation of CESM is easier than MRI because it can be implemented with a software upgrade to some existing digital mammography equipment rather than requiring purchase of entirely new machine [18].
Several studies showed high sensitivity of CESM in breast cancer detection, even in women with dense breast, suggesting its possible role in specific scenario like in the breast cancer screening among high risk patients [13,15,19,20]. However, CESM advantages have to be balanced against the radiation dose, especially given the presence of alternative imaging which does not rely on radiation (i.e. MRI or automated breast ultrasound [50]), and the risk of contrast agent reactions, which, although low, is greater than that associated with gadolinium [51].
Although two meta-analyses and one systematic review on CESM have already been published [[21], [22], [23]], over the years the CESM technology kept improving and several new studies on CESM appeared in the literature, reflecting the interest in the new technique. Our purpose is to systematically evaluate the diagnostic accuracy of CESM in women with clinical and/or radiological suspicion of breast cancer. The rationale of our systematic review is to update and further analyse a selection of studies on CESM, also including literature from 2018 that was not considered in previous meta-analyses. Notably, having more studies available, we could perform a meta-analysis based on prospective studies only, which is a distinctive point compared to previous reviews on CESM.
2. Materials and methods
The reporting of this review follows the standard PRISMA-DTA [24]. There is no formal protocol of this review published elsewhere.
2.1. Study identification
We attempted to identify all published studies that reported on the diagnostic accuracy of CESM in patients with suspected breast cancer using the PubMed and EMBASE (till 28th Semptember 2019) electronic databases. The search strategy was developed without any language or other restrictions, and used a combination of the following free text words and MeSH/EMTREE terms: ‘’mammography’ and ‘contrast enhancement’. We supplemented our search by manually reviewing the reference lists of all retrieved articles (“snowballing”).
2.2. Study eligibility
After duplicate records removal two investigators (FP and MS) independently reviewed titles and abstracts from the initial search to determine whether the inclusion criteria were satisfied. After the first screening process, full-texts of all potential included studies were assessed according to pre-specified selection criteria. Any prospective study evaluating the diagnostic performance of CESM in the diagnosis of patients with a clinical or radiological suspicion of breast cancer was eligible if it allowed the calculation of sensitivity and specificity for breast cancer diagnosis and the reconstruction of the original 2x2 table. Case reports and editorials were excluded. Decisions regarding inclusion were made independently, the results were compared, and any disagreement was resolved through discussion or by involving a third reviewer (AS), when necessary.
2.3. Data extraction and risk of bias assessment
Two reviewers (MS and FP) independently extracted data on study and patient’s characteristics, and on the diagnostic accuracy of CESM in this setting, using a standardized form. Information on CESM criteria for breast cancer diagnosis, true positive, true negative, false positive, false negative results were collected. Moreover, a Radiologist (FP) extracted the following technical characteristics of CESM: projection used, type of contrast media, and radiation dose delivered per patient. The Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) checklist was used [25] to assess the risk of bias of the primary studies. The signalling question number 2 on the index test (domain 2 of the QUADAS-2) was adapted for this review as follows: ‘Were CESM criteria pre-specified?‘. An appropriate percentage of patients who underwent follow-up without histological or cytological diagnosis was defined as less or equal to 5%.
Any disagreement concerning the extracted data and risk of bias assessment was resolved by consensus and, if necessary, by involving a third reviewer (AS). No attempts to mask for authorship, journal name, or institution were made here or in any other step of the review process.
3. Statistical analysis
3.1. Weighted mean prevalence of breast cancer
Weighted mean proportion and 95% confidence intervals (CIs) of breast cancer prevalence, ducatal carcinoma in situ (DCIS) prevalence and its percentage of malignant lesion were calculated; these data were pooled using a random-effects model (DerSimonian and Laird method). Statistical heterogeneity was evaluated using the I2 statistic.
3.2. Bivariate analysis for test accuracy
We used a bivariate random-effects regression approach to obtain summary estimates of both sensitivity and specificity of the respective CESM while adjusting for sources of bias and variability. This model assumes that the logit-transformed sensitivities and specificities of the included CESM studies follow a bivariate normal distribution around a common mean of logit-transformed sensitivity and specificity, incorporating any correlation that might exist between logit sensitivity and logit specificity. The number of patients testing positive among the diseased patients in a particular study is assumed to follow a binomial distribution, as is the number of patients testing negative among the non-diseased patients. Sensitivity and specificity were calculated after exclusion of inconclusive CESM results.
3.3. Sensitivity analyses
The study from Brandan et al. [26] used two different sets of prespecified criteria for CESM. As such, the analysis was performed twice, including data with either criteria. We repeated the analyses including only studies with low risk of bias for patient selection according to the QUADAS-2 score in order to highlight potential distortions in our sensitivity and specificity estimates driven by low quality studies. We also performed the following subgroup analyses: number of lesions, number of patients, percentage of DCIS, year of publication, if CESM was the only methods used in evaluating disease, threshold method for assessing malignancy and number of projections.
Statistical analyses were performed using the software STATA (version 14.1, Stata Corp, Austin, Texas) and Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011).
4. Results
4.1. Study characteristics
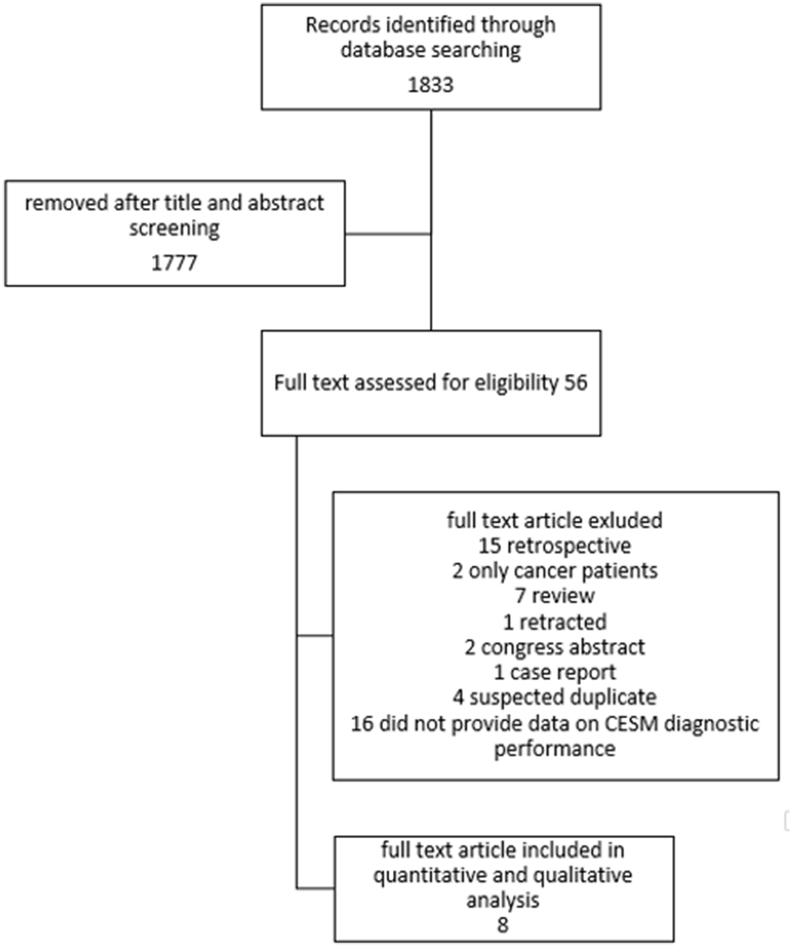
A total of 8 studies published between 2003 and 2019 were included in the meta-analysis analysing a total of 945 lesions, of which 36 were DCIS (range 18–263) [[26], [27], [28], [29], [30], [31], [32], [33]](see Table 1). Total number of included patients were 684 in 7 studies (Tohamey and colleagues did not reported number of included patients and did not reply to our kind request to provide additional data). Full selection process is detailed in the PRISMA flowchart (Fig. 1). Since in the final selection there were multiple publications by the same authors [27,30,[34], [35], [36]] or the same institutions [[37], [38], [39], [40]] with close publication dates, we contacted the corresponding authors for those papers, to enquire if there were any overlap in patients between different publications. As a cautionary measure, given the absence of a response from the corresponding authors, we decided to include only the most recent and numerous paper from each group of suspected duplicates.
Table 1.
Study characteristics.
| Study | Patients | Lesions | BC | DCIS | Median age (range) | Projection used | Criteria evaluated | Inclusion criteria | Exposure (Gy) | Contrast media |
|---|---|---|---|---|---|---|---|---|---|---|
| Brandan 2016 [26] | 18 | 18 | 11 | 1 | 52 | CC | CE | BI-RADS 4–5 on Mx | 6 | Optiray 300 |
| Diekmann 2011]28] | 70 | 80 | 30 | 5 | 55 | CC | CE + morphology | Suspicious lesion on Mx, US, MRI | 1.76 | Ultravist 370 |
| Dromain 2011]27] | 120 | 133 | 80 | NR | 56 (27–86) | CC MLO | CE + morphology | Recall from screening + Suspicious lesion on Mx, US, MRI | 0.7–3.6 | Xenetix 300 |
| Lewin 2003 [29] | 26 | 26 | 14 | 1 | 51 | MLO | CE | Suspicious lesion on Mx | 0.7 | Omnipaque 350 |
| Łuczyńska 2016 [30] | 193 | 225 | 143 | 16 | 55 | CC MLO | CE + morphology | Suspicious lesion on Mx | NR | NR |
| Jong 2003 [31] | 22 | 22 | 10 | 1 | NR (40–74) | CC | CE + morphology | Suspicious lesion on Mx, US | NR | Omnipaque 300 |
| Tohamey 2018 [32] | NR | 178 | 104 | 6 | 46 | Unclear | CE + morphology | Unclear | NR | NR |
| Xing 2019 [33] | 235 | 263 | 177 | 6 | NR | CC MLO | CE + morphology | Suspicious lesion on US or clinical | NR | Iohexol |
Legend: BC breast cancer DCIS ductal carcinoma in situ Gy Grey CC cranio-caudal CE contrast enhancement Mx mammography US ultrasound MRI magnetic resonance imaging MLO mediolateral oblique NR not reported.
Fig. 1.
The figure shows the workflow for study screening and selection.
4.2. Risk of bias of the included studies
The studies we included in the meta-analysis had overall moderate to low risk of bias. Only two studies had a low risk of bias in all the applicability domains (Table 2).
Table 2.
Risk of bias.
| Ref. | Study | Risk of bias |
Applicability concerns |
|||||
|---|---|---|---|---|---|---|---|---|
| Patient selecion | Index Test | Reference Standard | Flow and Timing∗ | Patient selecion | Index Test | Reference Standard | ||
| 26 | Brandan 2016 | H | L | L | L(0%) | H | L | L |
| 28 | Diekmann 2011 | L | L | L | H(17%) | H | L | L |
| 27 | Dromain 2011 | L | L | L | H(10%) | L | L | L |
| 29 | Lewin 2003 | U | L | L | L(1%) | L | L | L |
| 30 | Łuczyńska 2016 | H | L | L | L(0%) | H | L | L |
| 31 | Jong 2003 | L | L | L | L(5%) | H | L | L |
| 32 | Tohamey 2018 | U | H | H | L(8%) | H | H | H |
| 33 | Xing 2019 | U | L | L | L(0%) | U | L | L |
L Low risk U Unclear risk H High risk.
∗ percentage of no histology (our threshold = 5%).
4.3. Weighted mean prevalence of breast cancer
The pooled prevalence of malignancies was 55% (95% CI 48%–63%) with a pooled prevalence of DCIS in all the lesions and only in malignant ones of 5% (95% CI 3%–7%) and 9% (95% CI 6%–12%) respectively.
4.4. CESM accuracy
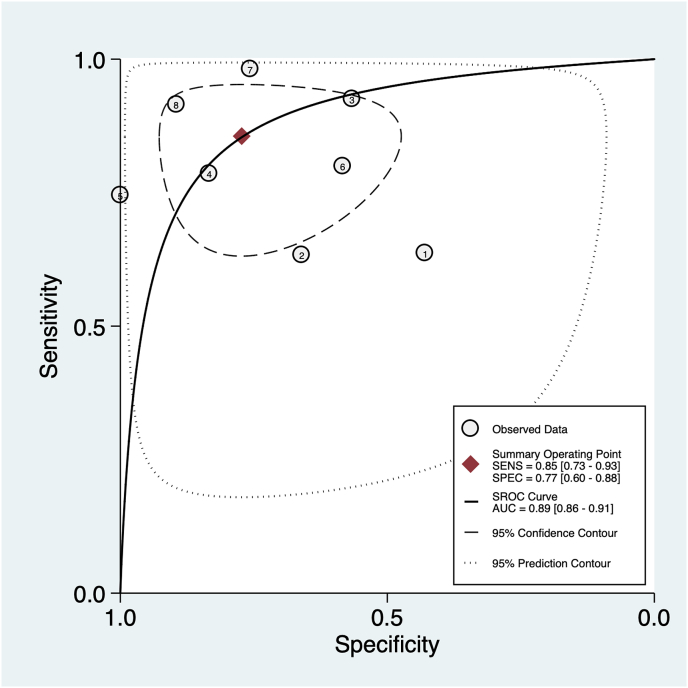
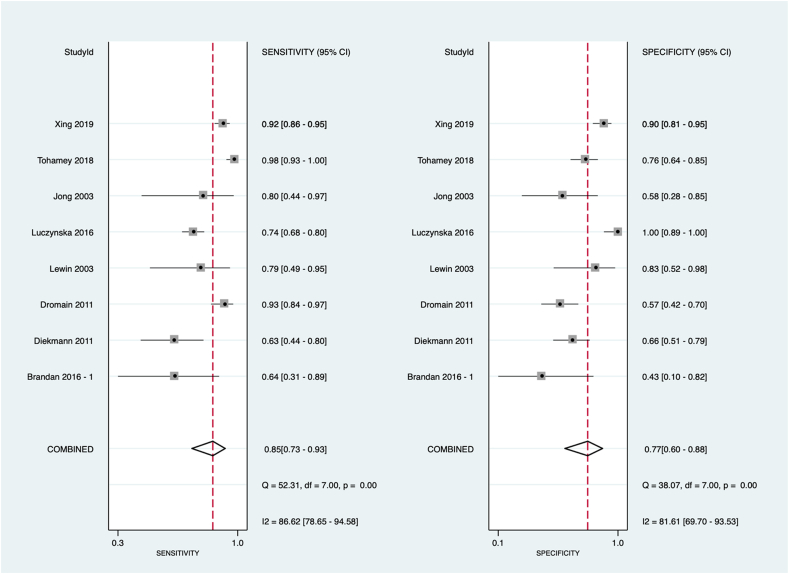
The summary area under the curve obtained from all the study was 89% [95% CI 86%–91%], with a sensitivity of 85% [95% CI 73%–93%], a specificity of 77% [95% CI 60%–88%] and a diagnostic odds ratio of 20 [95% CI 7–60] with a substantial amount of observed heterogeneity (I2 94, 95% CI = [89–99], p < 0.001) (Fig. 2).
Fig. 2.
The plot shows the summary bivariate ROC curve for CESM diagnostic accuracy.
There was no evidence of a threshold effect (Spearman Rho −0.07, p value 0.87) as suggested also by visual inspection of the bivariate box-plot obtained plotting logit sensitivity and specificity and the symmetric aspect of the SROC curve for CESM (Fig. 3).
Fig. 3.
The figure shows the scatterplot obtained using logit sensitivity vs logit specificity suggesting no evidence of a threshold effect.
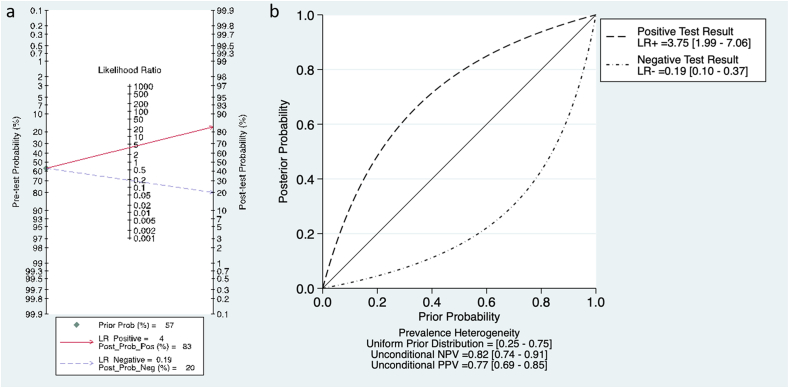
The Fagan plot and the probability modifying plot (Fig. 4) show that with a pre-test probability of malignancy as in our sample of 57% a positive finding at CESM gives a post-test probability of 83% while a negative finding a post-test probability of 20%.
Fig. 4.
The Fagan plot (a) and the probability modifying plot (b) show that with a pre-test probability of malignancy as in our sample of 57% a positive finding at CESM gives a post-test probability of 83% while a negative finding a post-test probability of 20%.
The analysis on the ROC curve were conducted including the results from the study by Brandan according to the first set of types of time–iodine curves used to define malignancy, as it was the better regarding all variables (specificity, sensitivity, positive and negative predictive value). The analysis performed using the second set of types of time–iodine curves used to define malignancy, available on request, did not significantly differ from those carried out with the first set.
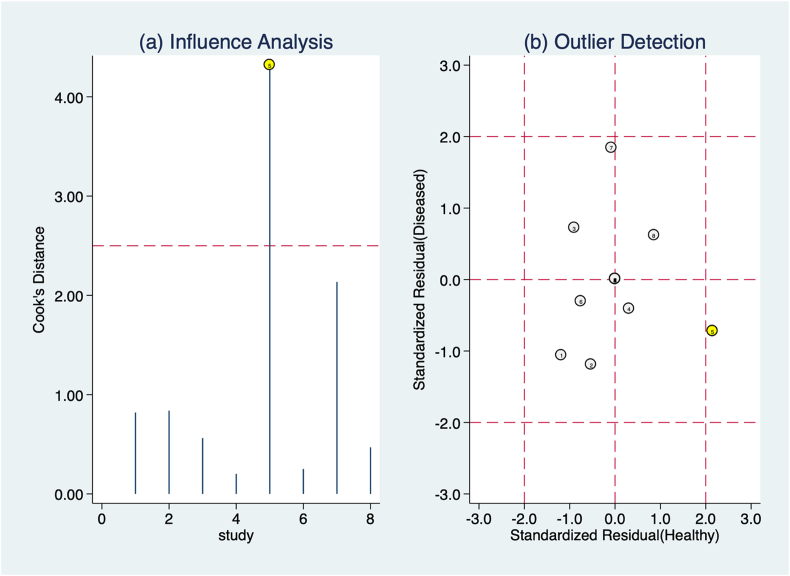
The influence analysis found the study by Luczynska et al. [30] to be the most influential and an outlier compared to the rest of the studies included in the meta-analysis (Fig. 5). This is due to two factor: the study by Luczynska et al. is the only one without false positive, making it the one with the highest specificity by far; this is coupled with the second highest number of lesions analysed, making the result unlikely to be influenced by study size and giving it high weight in any analysis performed.
Fig. 5.
The figure shows influence analysis and outlier analysis based on Cook’s distance and standardized predicted random effects. The study by Luczynska et al. resulted to be both an outlier and the most influential.
4.5. CESM safety
All studies but those by Luczynska et al. and Tohamey et al. [30,32] had exclusion criteria to minimize the risks linked to the administration of iodinated contrast medium. Lewin et al. [29] considered general contraindications to iodinated contrast medium, while Brandan, Diekmann, Dromain, Jong and Xing [[26], [27], [28],31,33]all specify allergy, known or suspected, as a key exclusion criteria. Brandan, Diekmann and Xing [26,28,33]excluded also patient with known renal disease. The only paper to mention a safety event, namely an allergic reaction requiring intensive care treatment, is Diekmann et al. [28].
4.6. Sensitivity analysis and meta-regression
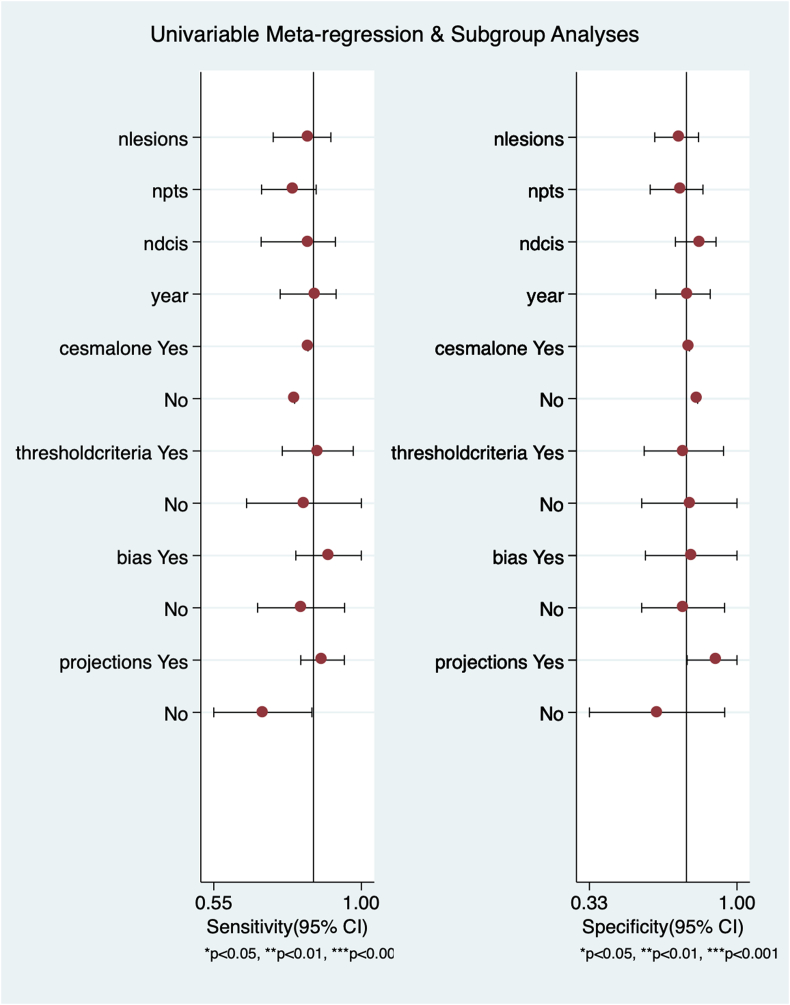
We investigated the possible effect of the following covariates on CESM diagnostic accuracy though with the limit of having a low number of studies included in the meta-analysis (<10): number of lesions, number of patients, number of DCIS, year of publication, if CESM was the only methods and threshold method for assessing malignancy and number of projections. We also did a sensitivity analysis comparing the studies with high quality in all domains of applicability according to QUADAS-2 to the others with higher risk of bias. None of the covariates were associated with an effect on specificity or sensitivity nor study with no evidence of bias in applicability domains did statistically significant differ from the others with higher risk of bias (Fig. 6). We did not perform a meta-analysis including only the studies with low risk of bias in all dominion of QUADAS-2 (Table 2) since these were only two.
Fig. 6.
The figure shows results for univariate metaregression. None of the investigated covariates was associated with statistically significant different sensitivities or specificities.
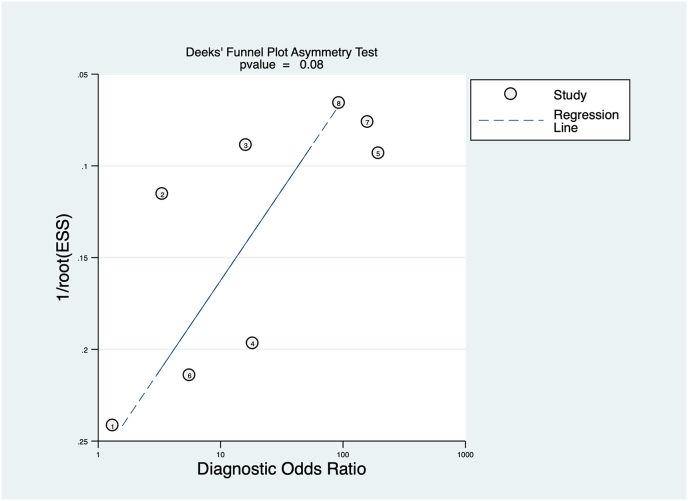
4.7. Publication bias
There was evidence of borderline significance for small study effect (p value 0.08, see Fig. 7).
Fig. 7.
The figure shows the funnel plot with no evidence of small study effect.
5. Discussion
Combining an iodinated contrast agent with FFDM (utilising a dual energy exposure undertaken during a single breast compression), CESM may improve diagnostic accuracy in the diagnosis and staging of primary breast cancer, particularly in women with dense breasts, due to its ability to identify breast cancer that might ordinarily be masked by the dense parenchymal background (DPB) patterns on a FFDM [13,19,20,27,41,42].
Our systematic review showed that CESM may detect a breast cancer with a 85% sensitivity and a 77% specificity in women with clinical or radiological suspicion. Compared with a previous systematic review by Tagliafico et colleagues [43], our review shows a more even picture, with a lower sensitivity but a higher specificity. The slightly lower results in sensitivity is probably due to the stricter paper selection, including prospective studies only, excluding those performed only in breast cancer patients. The higher specificity could be explained with an increased experience with CESM, and better pre-specified criteria evaluating both the standard morphological/architectural features and the contrast enhancement’s features, although we did not find any paper addressing this specific matter. All the studies considered in the present analysis, with the exception of Lewin et al. [29] who considered only the presence of contrast enhancement, assessed both morphological and contrast kinetic features. Brandan et al. [26] clearly showed an increase of specificity in the same data set by 7% using iodine uptake alongside contrast kinetic features.
All the papers included in our systematic review analysed patients with a clinical or radiological suspicion of breast cancer, used CESM as second line diagnostic assessment. This is, at the present time, the field where CESM is gaining more momentum.
Other applications seem burdened with various concerns. Firstly, CESM showed a better performance than conventional mammography in screening breast cancer [39,42], especially in DPB in which the FFDM’s sensitivity is reduced from 75%–85% to 35%–45% [42,44]. CESM may decrease FNs especially for women with dense breasts [42]. Nevertheless, the administration of iodinated contrast agent is fundamental to the CESM and its routinely use as a screening procedure poses problems for allergic reaction and contrast-induced nephropathy, even if the risk of both these adverse reactions were demonstrated to be low [45,46].
Secondly, high-risk patients (e.g. BRCA mutated) may prefer CESM over screening MRI, especially if ongoing trials (NCT02479100; NCT02275871; NCT03517813; EudraCT:2012-002784-10) demonstrate screening CESM to be clinically non-inferior breast MRI [12]. Discomfort, claustrophobia and time constraints are concerns of patients undergo breast MRI possibly leading to declination from patients indeed (up to the 42% reported by the ACRIN 6666 trial in women eligible to receive a screening with MRI after mammography and US) [47]. Thirdly, patients with pacemakers or metallic implants may be excluded from MRI screening. CESM is quicker to perform, the iodinated contrast agent is cheaper than gadolinium ones, and so the procedure is potentially more cost-effective [48]. Fourthly, CESM can detect microcalcification, in contrast with breast, playing an additional help to the contrast enhancement in the detection and staging of breast cancer. Data from studies of women with known breast cancer, CESM approaches the sensitivity of breast MRI and it showed higher specificity, fewer false positives, and a superior positive predictive value (PPV) of biopsy [13,49].
Finally, physiological benign background parenchymal enhancement can be seen with CESM in a similar manner to that observed in breast MRI. Although timing the CESM with the menstrual cycle is unlikely to be such an issue as it is with MR, since no clear pattern in variation of parenchymal background enhancement across the menstrual cycle has yet been demonstrated for CESM [52], we have no information about the timing of examination in the studies we analysed, except from Tohamey et al. [32] who assessed that in their study the menstrual phase was not taken into consideration and therefore its impaction and bias could not be analysed.
The radiation dose of CESM is well within European and UK quality assurance guidelines for mammography [51] as it uses the same X-ray energy spectrum as a standard FFDM. Moreover, when CESM is planned, the FFDM can safely be omitted since no additional information are provided [51]. Considering that the mean average glandular dose for FFDM is in the range 0.5–3.5 mGy [53,54], only one study [26] reported a higher average glandular dose per CESM exposure (6 mGy).
As for safety concern linked to the administration of iodinated contrast medium, only 1.4% of severe allergic reaction was reported [28]. However, one should keep in mind that most of the other papers did not explicitly stated the absence of safety events, and that no study was designed to follow up on renal function to verify any episode of contrast induced renal damage.
The studies we analysed had limited the use of CESM to women presenting with a clinical or radiological suspicion of breast cancer. In such second level setting, we found a pre-test probability of breast cancer that is higher than in the general population, as was in the studies, at 57%. It should be kept in mind that all these studies were designed to perform CESM with any grade of suspicion of breast cancer, thus increasing the pre-test probability of breast cancer beyond that of a usual population undergoing second level imaging. In these patients, positive post-test probability gave 83% chance of malignancy, while negative finding meant a post-test probability of 20% of malignancy. These values, at the present time, fall short of clinical need, especially regarding a 20% of FN.
An accepted reason for a FN is when the lesion is not included on the mammographic field of view [55,56]. However, false negatives include different tumour types. The diffuse infiltrative nature of lobular tumours makes detection more difficult with conventional imaging and can challenge MRI staging, so it is likely that they are more prone to false-negative results with CESM [51]. In addition, mucinous carcinomas can show no enhancement or be misinterpreted as a cyst [57].
Areas of DCIS usually show subtle or no enhancement on the recombined images [57,58], and the mammographic detection of DCIS is essentially based on the depiction of microcalcifications, which are a frequent but not obligatory findings.
We tried to further investigate the FN results we analysed, especially given the possibility that most of them may be low grade DCIS [59]. Unfortunately, most of the papers did not provide sufficient information for this analysis.
All our sub-analysis did not detect any factor that could affect CESM performance, such as bias in patient selection, the use of single or double projection for mammogram, or being blinded to other imaging results for the same patient. This could be due to the small number of studies analysed (<10), possibly not giving enough power to detect differences between the different settings, one of the limitations of our review. Another limitation is the high heterogeneity among studies, which probably reflect the fact that CESM is still an evolving diagnostic technique, and also the low number of included studies (only two) with a low risk of bias in all the applicability domains. Finally, a main limitation in performing sub analysis is due to the fact that this is a study level meta-analysis. A patient level analysis, pooling together data from some of the main groups performing CESM could answer many of the open question presented here.
On the other hand, the strength of our work lies in the rigorous selection of papers including only prospective studies.
Other analyses will be required to assess the value of CESM in other settings, such as screening programmes, especially for groups of women at increased risk of breast cancer who do not currently qualify for MRI screening, and those with dense mammographic background patterns [58]. In addition, the CESM monitoring response to neoadjuvant chemotherapy for patients who are unable to undergo MRI may offer a useful alternative [60,61]. Finally, computer-aided diagnosis systems are being developed using CESM, which may further help the unexperienced reader interpreting images [62].
6. Conclusions
CESM shows a suboptimal sensitivity and specificity in the diagnosis of breast cancer in a selected population with clinical or radiological suspicion of disease, and it could be considered only as a possible alternative test for breast lesions assessment when FFDM and US are not conclusive or when MRI is contraindicated or not available. Moreover, being possibly more accessible, cheaper, and preferred by patients than breast MRI, CESM may be considered as primary imaging test in symptomatic patients, providing improved diagnostic information at the first clinic visit.
Authorship contribution
M.S., F.P. and A.S. designed the study, M.S. and F.P. gathered data, G.A. performed the analysis, M.S. and F.P. drafted the article, A.S., T.G. E.C., O.N., A.B., F.P., S.P., and C.C. revised it critically for important intellectual content. All authors have approved the submitted version of this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Nothing to disclose from all the authors.
Contributor Information
Matteo Basilio Suter, Email: matteosuter@gmail.com.
Filippo Pesapane, Email: filippopesapane@gmail.com.
Giorgio Maria Agazzi, Email: giorgiomaria.agazzi@gmail.com.
Tania Gagliardi, Email: tanja.gagliardi@bluewin.ch.
Olga Nigro, Email: nigro.olga3@gmail.com.
Anna Bozzini, Email: anna.bozzini@ieo.it.
Francesca Priolo, Email: francesca.priolo@ieo.it.
Silvia Penco, Email: silvia.penco@ieo.it.
Enrico Cassano, Email: erinco.cassano@ieo.it.
Claudio Chini, Email: claudio.chini@asst-settelaghi.it.
Alessandro Squizzato, Email: alessandro.squizzato@uninsubria.it.
References
- 1.Bhimani C., Matta D., Roth R.G., Liao L., Tinney E., Brill K. Contrast-enhanced spectral mammography. Acad Radiol. 2017;24:84–88. doi: 10.1016/j.acra.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Zackrisson S., Lång K., Rosso A., Johnson K., Dustler M., Förnvik D. One-view breast tomosynthesis versus two-view mammography in the Malmö Breast Tomosynthesis Screening Trial (MBTST): a prospective, population-based, diagnostic accuracy study. Lancet Oncol. 2018;19:1493–1503. doi: 10.1016/S1470-2045(18)30521-7. [DOI] [PubMed] [Google Scholar]
- 3.Pisano E.D. Digital compared with screen-film mammography: measures of diagnostic accuracy among women screened in the ontario breast screening program—evidence that direct radiography is superior to computed radiography for cancer detection. Radiology. 2016;278:311–312. doi: 10.1148/radiol.2015152344. [DOI] [PubMed] [Google Scholar]
- 4.Berg W.A., Gutierrez L., NessAiver M.S., Carter W.B., Bhargavan M., Lewis R.S. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–849. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 5.Bosch A.M., Kessels A.G.H., Beets G.L., Rupa J.D., Koster D., van Engelshoven J.M.A. Preoperative estimation of the pathological breast tumour size by physical examination, mammography and ultrasound: a prospective study on 105 invasive tumours. Eur J Radiol. 2003;48:285–292. doi: 10.1016/s0720-048x(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 6.Fasching P.A., Heusinger K., Loehberg C.R., Wenkel E., Lux M.P., Schrauder M. Influence of mammographic density on the diagnostic accuracy of tumor size assessment and association with breast cancer tumor characteristics. Eur J Radiol. 2006;60:398–404. doi: 10.1016/j.ejrad.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Kuhl C.K. The changing world of breast cancer: a Radiologistʼs perspective. Plast Surg Nurs. 2016;36:31–49. doi: 10.1097/PSN.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 8.Rotili A., Trimboli R.M., Penco S., Pesapane F., Tantrige P., Cassano E. Double reading of diffusion-weighted magnetic resonance imaging for breast cancer detection. Breast Canc Res Treat. 2020;180:111–120. doi: 10.1007/s10549-019-05519-y. [DOI] [PubMed] [Google Scholar]
- 9.Saslow D., Boetes C., Burke W., Harms S., Leach M.O., Lehman C.D. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. Ca - Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 10.Bleicher R.J., Ciocca R.M., Egleston B.L., Sesa L., Evers K., Sigurdson E.R. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg. 2009;209:180–187. doi: 10.1016/j.jamcollsurg.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houssami N., Turner R., Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg. 2013;257:249–255. doi: 10.1097/SLA.0b013e31827a8d17. [DOI] [PubMed] [Google Scholar]
- 12.Phillips J., Miller M.M., Mehta T.S., Fein-Zachary V., Nathanson A., Hori W. Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: patient preferences and attitudes. Clin Imag. 2017;42:193–197. doi: 10.1016/j.clinimag.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Jochelson M.S., Dershaw D.D., Sung J.S., Heerdt A.S., Thornton C., Moskowitz C.S. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology. 2013;266:743–751. doi: 10.1148/radiol.12121084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallenberg E.M., Schmitzberger F.F., Amer H., Ingold-Heppner B., Balleyguier C., Diekmann F. Contrast-enhanced spectral mammography vs. mammography and MRI – clinical performance in a multi-reader evaluation. Eur Radiol. 2017;27:2752–2764. doi: 10.1007/s00330-016-4650-6. [DOI] [PubMed] [Google Scholar]
- 15.Fallenberg E.M., Dromain C., Diekmann F., Renz D.M., Amer H., Ingold-Heppner B. Contrast-enhanced spectral mammography: does mammography provide additional clinical benefits or can some radiation exposure be avoided? Breast Canc Res Treat. 2014;146:371–381. doi: 10.1007/s10549-014-3023-6. [DOI] [PubMed] [Google Scholar]
- 16.Patel B.K., Gray R.J., Pockaj B.A. Potential cost savings of contrast-enhanced digital mammography. Am J Roentgenol. 2017;208:W231–W237. doi: 10.2214/AJR.16.17239. [DOI] [PubMed] [Google Scholar]
- 17.Kim G., Phillips J., Cole E., Brook A., Mehta T., Slanetz P. Comparison of contrast-enhanced mammography with conventional digital mammography in breast cancer screening: a pilot study. J Am Coll Radiol. 2019 doi: 10.1016/j.jacr.2019.04.007. S1546144019304405. [DOI] [PubMed] [Google Scholar]
- 18.Covington M.F., Pizzitola V.J., Lorans R., Pockaj B.A., Northfelt D.W., Appleton C.M. The future of contrast-enhanced mammography. Am J Roentgenol. 2018;210:292–300. doi: 10.2214/AJR.17.18749. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Roth R., Germaine P., Ren S., Lee M., Hunter K. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): a retrospective comparison in 66 breast lesions. Diagnostic and Interventional Imaging. 2017;98:113–123. doi: 10.1016/j.diii.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Badr S., Laurent N., Régis C., Boulanger L., Lemaille S., Poncelet E. Dual-energy contrast-enhanced digital mammography in routine clinical practice in 2013. Diagnostic and Interventional Imaging. 2014;95:245–258. doi: 10.1016/j.diii.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X., Huang J., Zhang K., Xia L., Feng L., Yang P. Diagnostic value of contrast-enhanced spectral mammography for screening breast cancer: systematic review and meta-analysis. Clin Breast Canc. 2018;18:e985–e995. doi: 10.1016/j.clbc.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Tagliafico A.S., Bignotti B., Rossi F., Signori A., Sormani M.P., Valdora F. Diagnostic performance of contrast-enhanced spectral mammography: systematic review and meta-analysis. Breast. 2016;28:13–19. doi: 10.1016/j.breast.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Zanardo M., Cozzi A., Trimboli R.M., Labaj O., Monti C.B., Schiaffino S. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): a systematic review. Insights Imaging. 2019;10:76. doi: 10.1186/s13244-019-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McInnes M.D.F., Moher D., Thombs B.D., McGrath T.A., Bossuyt P.M., the PRISMA-DTA Group Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. J Am Med Assoc. 2018;319:388. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 25.Whiting P.F., Rutjes A.W.S., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B. Quadas-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 26.Brandan M.-E., Cruz-Bastida J.P., Rosado-Méndez I.M., Villaseñor-Navarro Y., Pérez-Ponce H., Galván H.A. Clinical study of contrast-enhanced digital mammography and the evaluation of blood and lymphatic microvessel density. Br J Radiol. 2016;89:20160232. doi: 10.1259/bjr.20160232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dromain C., Thibault F., Diekmann F., Fallenberg E.M., Jong R.A., Koomen M. Dual-energy contrast-enhanced digital mammography: initial clinical results of a multireader, multicase study. Breast Cancer Res. 2012;14:R94. doi: 10.1186/bcr3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diekmann F., Freyer M., Diekmann S., Fallenberg E.M., Fischer T., Bick U. Evaluation of contrast-enhanced digital mammography. Eur J Radiol. 2011;78:112–121. doi: 10.1016/j.ejrad.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Lewin J.M., Isaacs P.K., Vance V., Larke F.J. Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology. 2003;229:261–268. doi: 10.1148/radiol.2291021276. [DOI] [PubMed] [Google Scholar]
- 30.Luczyńska E., Heinze S., Adamczyk A., Rys J., Mitus J.W., Hendrick E. Comparison of the mammography, contrast-enhanced spectral mammography and ultrasonography in a group of 116 patients. Anticancer Res. 2016;36:4359–4366. [PubMed] [Google Scholar]
- 31.Jong R.A., Yaffe M.J., Skarpathiotakis M., Shumak R.S., Danjoux N.M., Gunesekara A. Contrast-enhanced digital mammography: initial clinical experience. Radiology. 2003;228:842–850. doi: 10.1148/radiol.2283020961. [DOI] [PubMed] [Google Scholar]
- 32.Tohamey Y.M., Youssry S.W., Abd El Aziz A.I. Interpretation of patterns of enhancement on contrast-enhanced spectral mammography: an approach to a standardized scheme. The Egyptian Journal of Radiology and Nuclear Medicine. 2018;49:854–868. doi: 10.1016/j.ejrnm.2018.03.004. [DOI] [Google Scholar]
- 33.Xing D., Lv Y., Sun B., Xie H., Dong J., Hao C. Diagnostic value of contrast-enhanced spectral mammography in comparison to magnetic resonance imaging in breast lesions. J Comput Assist Tomogr. 2019;43:245–251. doi: 10.1097/RCT.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Łuczyńska E., Niemiec J., Hendrick E., Heinze S., Jaszczyński J., Jakubowicz J. Degree of enhancement on contrast enhanced spectral mammography (CESM) and lesion type on mammography (MG): comparison based on histological results. Med Sci Mon Int Med J Exp Clin Res. 2016;22:3886–3893. doi: 10.12659/msm.900371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dromain C., Thibault F., Muller S., Rimareix F., Delaloge S., Tardivon A. Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol. 2011;21:565–574. doi: 10.1007/s00330-010-1944-y. [DOI] [PubMed] [Google Scholar]
- 36.Łuczyńska E., Heinze-Paluchowska S., Hendrick E., Dyczek S., Ryś J., Herman K. Comparison between breast MRI and contrast-enhanced spectral mammography. Med Sci Mon Int Med J Exp Clin Res. 2015;21:1358–1367. doi: 10.12659/MSM.893018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mokhtar O., Mahmoud S. Can contrast enhanced mammography solve the problem of dense breast lesions? The Egyptian Journal of Radiology and Nuclear Medicine. 2014;45:1043–1052. doi: 10.1016/j.ejrnm.2014.04.007. [DOI] [Google Scholar]
- 38.Nada A.M.A.M., Hamdi R., Shokry A. Dual energy contrast enhanced soft tissue digital mammography versus ultrasound elastography in the evaluation of breast masses. The Egyptian Journal of Radiology and Nuclear Medicine. 2017;48:1179–1186. doi: 10.1016/j.ejrnm.2017.05.001. [DOI] [Google Scholar]
- 39.Helal M., Abu Samra M.F., Ibraheem M.A., Salama A., Hassan E.E., Hassan N.E.-H. Accuracy of CESM versus conventional mammography and ultrasound in evaluation of BI-RADS 3 and 4 breast lesions with pathological correlation. The Egyptian Journal of Radiology and Nuclear Medicine. 2017;48:741–750. doi: 10.1016/j.ejrnm.2017.03.004. [DOI] [Google Scholar]
- 40.ElSaid N.A.E., Farouk S., Shetat O.M.M., Khalifa N.M., Nada O.M. Contrast enhanced digital mammography: is it useful in detecting lesions in edematous breast? The Egyptian Journal of Radiology and Nuclear Medicine. 2015;46:811–819. doi: 10.1016/j.ejrnm.2015.04.002. [DOI] [Google Scholar]
- 41.Fallenberg E.M., Dromain C., Diekmann F., Engelken F., Krohn M., Singh J.M. Contrast-enhanced spectral mammography versus MRI: initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol. 2014;24:256–264. doi: 10.1007/s00330-013-3007-7. [DOI] [PubMed] [Google Scholar]
- 42.Mori M., Akashi-Tanaka S., Suzuki S., Daniels M.I., Watanabe C., Hirose M. Diagnostic accuracy of contrast-enhanced spectral mammography in comparison to conventional full-field digital mammography in a population of women with dense breasts. Breast Cancer. 2017;24:104–110. doi: 10.1007/s12282-016-0681-8. [DOI] [PubMed] [Google Scholar]
- 43.Tagliafico A.S., Bignotti B., Rossi F., Signori A., Sormani M.P., Valdora F. Diagnostic performance of contrast-enhanced spectral mammography: systematic review and meta-analysis. Breast. 2016;28:13–19. doi: 10.1016/j.breast.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Boyd N.F., Guo H., Martin L.J., Sun L., Stone J., Fishell E. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 45.Houben I.P.L., Van de Voorde P., Jeukens C.R.L.P.N., Wildberger J.E., Kooreman L.F., Smidt M.L. Contrast-enhanced spectral mammography as work-up tool in patients recalled from breast cancer screening has low risks and might hold clinical benefits. Eur J Radiol. 2017;94:31–37. doi: 10.1016/j.ejrad.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Cope L.H., Drinkwater K.J., Howlett D.C. RCR audit of compliance with UK guidelines for the prevention and detection of acute kidney injury in adult patients undergoing iodinated contrast media injections for CT. Clin Radiol. 2017;72:1047–1052. doi: 10.1016/j.crad.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Berg W.A., Blume J.D., Adams A.M., Jong R.A., Barr R.G., Lehrer D.E. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254:79–87. doi: 10.1148/radiol.2541090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hobbs M.M., Taylor D.B., Buzynski S., Peake R.E. Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): patient preferences and tolerance: CESM and CEMRI preferences and tolerance. Journal of Medical Imaging and Radiation Oncology. 2015;59:300–305. doi: 10.1111/1754-9485.12296. [DOI] [PubMed] [Google Scholar]
- 49.Lee-Felker S.A., Tekchandani L., Thomas M., Gupta E., Andrews-Tang D., Roth A. Newly diagnosed breast cancer: comparison of contrast-enhanced spectral mammography and breast MR imaging in the evaluation of extent of disease. Radiology. 2017;285:389–400. doi: 10.1148/radiol.2017161592. [DOI] [PubMed] [Google Scholar]
- 50.Sung J.S., Dershaw D.D. Breast magnetic resonance imaging for screening high-risk women. Magn Reson Imag Clin N Am. 2013;21:509–517. doi: 10.1016/j.mric.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 51.James J.J., Tennant S.L. Contrast-enhanced spectral mammography (CESM) Clin Radiol. 2018;73:715–723. doi: 10.1016/j.crad.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Sogani J., Morris E.A., Kaplan J.B., D’Alessio D., Goldman D., Moskowitz C.S. Comparison of background parenchymal enhancement at contrast-enhanced spectral mammography and breast MR imaging. Radiology. 2017;282:63–73. doi: 10.1148/radiol.2016160284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischmann A., Siegmann K.C., Wersebe A., Claussen C.D., Müller-Schimpfle M. Comparison of full-field digital mammography and film–screen mammography: image quality and lesion detection. BJR (Br J Radiol) 2005;78:312–315. doi: 10.1259/bjr/33317317. [DOI] [PubMed] [Google Scholar]
- 54.Gennaro G., Baldelli P., Taibi A., Di Maggio C., Gambaccini M. Patient dose in full-field digital mammography: an Italian survey. Eur Radiol. 2004;14:645–652. doi: 10.1007/s00330-003-2010-9. [DOI] [PubMed] [Google Scholar]
- 55.Thibault F., Balleyguier C., Tardivon A., Dromain C. Contrast enhanced spectral mammography: better than MRI? Eur J Radiol. 2012;81:S162–S164. doi: 10.1016/S0720-048X(12)70068-2. [DOI] [PubMed] [Google Scholar]
- 56.Tennant S.L., James J.J., Cornford E.J., Chen Y., Burrell H.C., Hamilton L.J. Contrast-enhanced spectral mammography improves diagnostic accuracy in the symptomatic setting. Clin Radiol. 2016;71:1148–1155. doi: 10.1016/j.crad.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Lalji U.C., Houben I.P.L., Prevos R., Gommers S., van Goethem M., Vanwetswinkel S. Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: validation of results in a large multireader, multicase study. Eur Radiol. 2016;26:4371–4379. doi: 10.1007/s00330-016-4336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jochelson M.S., Pinker K., Dershaw D.D., Hughes M., Gibbons G.F., Rahbar K. Comparison of screening CEDM and MRI for women at increased risk for breast cancer: a pilot study. Eur J Radiol. 2017;97:37–43. doi: 10.1016/j.ejrad.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Guidi A.J., Schnitt S.J., Fischer L., Tognazzi K., Harris J.R., Dvorak H.F. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in patients with ductal carcinoma in situ of the breast. Cancer. 1997;80:1945. doi: 10.1002/(sici)1097-0142(19971115)80:10<1945::aid-cncr11>3.0.co;2-y. –53. [DOI] [PubMed] [Google Scholar]
- 60.Barra F.R., Sobrinho A.B., Barra R.R., Magalhães M.T., Aguiar L.R., Albuquerque GFL de. Contrast-enhanced mammography (cem) for detecting residual disease after neoadjuvant chemotherapy: a comparison with breast magnetic resonance imaging (MRI) BioMed Res Int. 2018;2018:1–9. doi: 10.1155/2018/8531916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park J., Chae E.Y., Cha J.H., Shin H.J., Choi W.J., Choi Y.-W. Comparison of mammography, digital breast tomosynthesis, automated breast ultrasound, magnetic resonance imaging in evaluation of residual tumor after neoadjuvant chemotherapy. Eur J Radiol. 2018;108:261–268. doi: 10.1016/j.ejrad.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 62.Patel B.K., Ranjbar S., Wu T., Pockaj B.A., Li J., Zhang N. Computer-aided diagnosis of contrast-enhanced spectral mammography: a feasibility study. Eur J Radiol. 2018;98:207–213. doi: 10.1016/j.ejrad.2017.11.024. [DOI] [PubMed] [Google Scholar]