Abstract
Objective
The impact of mammography screening recall on quality-of-life (QOL) has been studied in women at average risk for breast cancer, but it is unknown whether these effects differ by breast cancer risk level. We used a vignette-based survey to evaluate how women across the spectrum of breast cancer risk perceive the experience of screening recall.
Methods
Women participating in mammography or breast MRI screening were recruited to complete a vignette-based survey. Using a numerical rating scale (0–100), women rated QOL for hypothetical scenarios of screening recall, both before and after benign results were known. Lifetime breast cancer risk was calculated using Gail and BRCAPRO risk models. Risk perception, trait anxiety, and breast cancer worry were assessed using validated instruments.
Results
The final study cohort included 162 women at low (n = 43, 26%), intermediate (n = 66, 41%), and high-risk (n = 53, 33%). Actual breast cancer risk was not a predictor of QOL for any of the presented scenarios. Across all risk levels, QOL ratings were significantly lower for the period during diagnostic uncertainty compared to after benign results were known (p < 0.05). In multivariable regression analyses, breast cancer worry was a significant predictor of decreased QoL for all screening scenarios while awaiting results, including scenarios with non-invasive imaging alone or with biopsy. High trait anxiety and family history predicted lower QOL scores after receipt of benign test results (p < 0.05).
Conclusions
Women with high trait anxiety and family history may particularly benefit from discussions about the risk of recall when choosing a screening regimen.
Highlights
-
•
Impact of screening recall on quality-of-life does not vary by breast cancer risk.
-
•
Breast cancer worry predicts lower quality-of-life ratings while awaiting results.
-
•
Quality-of-life ratings improve after receipt of benign results.
-
•
High trait anxiety predicts lower quality-of-life after benign results are known.
1. Introduction
Mammography is the current standard for breast cancer screening as it has been shown to decrease breast cancer mortality in the general population [1,2]. However, in women at high risk for breast cancer such as BRCA mutation carriers, mammography detects less than half of all breast cancers, possibly due to young patient age and increased breast density, or tumor pathologic factors [3,4]. As such, women at high risk for breast cancer are offered more intensive screening at younger ages and with supplemental modalities such as magnetic resonance imaging (MRI) [5,6] which has been shown to improve cancer detection [7,8] and reduce the incidence of advanced stage breast cancers [9] when used for high-risk screening.
Although there are benefits to more intensive screening regimens in high-risk women, an inherent drawback to screening at younger ages and with multiple modalities is the increased frequency of recall for additional imaging and interventions in women who do not have cancer [10]. Studies that have examined the impact of false positive screening mammograms in average risk women have suggested that the consequences of screening recalls include increased worry about breast cancer, psychological distress, and anxiety [[11], [12], [13], [14], [15], [16]]. The majority of these studies have suggested that these effects are transient, although some studies suggest that even these short-term effects may affect health behaviors such as future screening decisions [17]. Despite these findings, a survey of US women’s attitudes toward false-positive mammography results found that women largely view false-positive test results as an acceptable consequence of screening [18].
While the impact of false positive examinations has been previously studied in average risk women, it is not known how these perceptions compare to those of women who are at high risk for breast cancer. As additional genetic and other risk factors for breast cancer are identified that qualify women for high-risk screening, it is important to understand the experience of false-positive screening examinations in this patient population to inform clinical guidelines and facilitate shared decision-making. In addition, this information is needed to inform cost-effectiveness analyses of high-risk screening strategies, as the cost-effectiveness of screening mammography has been shown to be highly sensitive to small, short-term effects on quality-of-life (QOL) related to the screening test [19].
The purpose of this study was to better understand how women across a spectrum of breast cancer risk perceive the impact of false-positive screening examinations and biopsies on QOL. We hypothesized that perceived QOL during and after the experience of screening recall would vary by underlying breast cancer risk.
2. Methods
This study was HIPAA-compliant and approved by the Institutional Review Board of Massachusetts General Hospital.
2.1. Subject recruitment
We recruited women ages ≥25 scheduled for routine mammographic screening or high-risk breast MRI screening at Massachusetts General Hospital from July 2011 through December 2012. Women were eligible to participate regardless of history of prior screening recall and/or biopsy, or history of prior breast cancer. Eligible women received a written invitation by mail to participate in the study four weeks prior to their scheduled exam and were subsequently contacted by telephone after 10 days if no response was received. Participants were given the option to complete the survey online, by mail, or with assistance over the telephone with a study coordinator. Women could complete the survey at any time during the four weeks leading up to their screening exam appointment. On the day of the screening exam, the survey was closed.
2.2. Survey instrument
We developed a vignette-based survey to assess women’s beliefs about breast cancer risk and the experience and consequences of false positive screening examinations leading to additional imaging and procedures. The survey instrument comprised of a series of vignettes describing temporary health state scenarios related to recall from screening mammography or MRI which participants were asked to rate using a numerical QOL rating scale[20], as detailed below. Demographic information was also collected for each participant, including age, sex, and race/ethnicity, personal breast cancer screening history (including prior screening recalls or biopsies), prior breast cancer history, and breast cancer risk factors. Previously validated measures of breast cancer worry, trait anxiety, and numeracy were also administered (described below). The survey was available in paper format and online, using the online REDCap survey design tool (version 5.0.15, Vanderbilt University). The complete version of the survey is available in the online Supplement.
2.3. Vignettes for false positive screening recall
Participants were presented with a series of vignettes describing the temporary health states related to recall after a) abnormal screening mammography; or b) abnormal screening breast MRI. Descriptions of temporary health states such as vignettes can be used to elicit preferences and quality-of-life perceptions from the general population[21,22], and have been previously used to quantify utilities for early and advanced breast cancer stages [23,24] and breast cancer treatment effects [24]. For this study, vignettes were developed by a team of investigators with expertise in breast imaging and image-guided procedures and survey science. For each vignette, participants were asked to rate the experience after recall for a scenario requiring additional non-invasive imaging alone, and a scenario in which a biopsy was performed. All vignettes included communication of benign results after non-invasive diagnostic imaging, or after a percutaneous biopsy was performed. For each scenario, we asked participants to rate QOL as defined by health utility theory using a 0 to 100 numerical scale (anchored by 0 = death, 100 = optimal health)[20], both during the period of diagnostic uncertainty and after benign results were known. The survey was pilot tested with 29 participants meeting study eligibility criteria, and vignette text was revised for clarity based on pilot participant feedback prior to the launch of the full study.
2.4. Actual and perceived breast cancer risk
Demographic information and breast cancer risk factors were used to calculate lifetime breast cancer risk using the Gail model [25] and the BRCAPRO [26] risk model in women without and with a family history of breast cancer, respectively. Each individual’s breast cancer risk was then stratified into one of the following risk groups: low/average risk (lifetime risk <15%); intermediate risk (lifetime risk 15–20%); and high risk (lifetime risk >20%) [5].
To assess perceived breast cancer risk, participants were asked to estimate their risk of developing breast cancer in their lifetime (expressed as a percentage, from 0 to 100%). Women who endorsed a personal history of breast cancer were asked to estimate their risk of developing a new or recurrent breast cancer in their lifetime (expressed as a percentage, from 0 to 100%).
2.5. Trait anxiety
The Spielberger State Trait Anxiety Index (STAI) is an established instrument used to assess general and trait anxiety [27]. For the current study, participants completed the trait-anxiety component of the instrument, which assigns a percentile for each participant based on her index score and age. Anxiety percentiles in the highest quartile (≥75%) were considered high anxiety.
2.6. Numeracy
Numeracy was assessed using the following multiple choice items taken from a previously validated instrument[28]: 1) Which of the following numbers represents the biggest risk of getting a disease? (Options: 1 in 100, 1 in 1000, 1 in 10, or Don’t Know/Not Sure); and 2) Which of the following represents the biggest risk of getting a disease (Options: 1%, 10%, 5%, or Don’t Know/Not Sure). Women who answered both items correctly were considered to have high numeracy.
2.7. Breast cancer worry
The Lerman Breast Cancer Worry Scale [29] is a 3-item scale which assesses the impact of concern about breast cancer on daily function and activities. We assessed breast cancer worry by adapting two of the items from the scale: “How often do you worry about developing a new or recurrent breast cancer?” and “How much does worrying about a new or recurrent breast cancer interfere with your daily life?” Women rated both questions on a scale of 1 (not at all) to 7 (all the time). Responses of 6 or 7 were considered to indicate “high” breast cancer worry.
2.8. Regression and statistical analysis
We performed t-tests, chi-square tests, univariate and multivariable regression to examine whether short-term quality of life ratings for each vignette were associated with the following variables: age (continuous), race, numeracy, personal and family history of breast cancer, breast cancer risk category (low/intermediate versus high), breast cancer worry and trait anxiety. Because breast cancer risk perception has been previously shown to be inaccurate among women[30,31], we performed univariate regression to examine the relationship between perceived risk and QOL ratings in the vignette. Spearman’s correlation was used to compare perceived risk by actual risk category. All statistical analyses were performed in SAS (version 9.4, Cary, NC), using stepwise linear regression.
3. Results
3.1. Participant recruitment and characteristics
Participant recruitment is summarized in Fig. 1. Of the 404 women who were initially identified, 16 women were excluded due to: incorrect contact information (n = 5), language barrier (n = 8), or cancelled screening exam (n = 3). An additional 47 were unable to be reached via mail or phone. Of 341 women who were successfully contacted, 194 completed the survey (cooperation rate: 194 of 341 women who were contacted, 57%; response rate: 194 of 388 women who were eligible, 50%). Of the 147 women who did not complete the survey, 63 (43%) refused the survey, 56 (38%) did not log on to the online survey, and 28 (19%) logged on but did not complete any survey items. Finally, we excluded women with incomplete responses for screening scenario questions or incomplete information for risk calculation (32 women).
Fig. 1.
Survey recruitment and participation. Contact rate was 341/404 (84%), cooperation rate was 194/341 contacted women (57%), and response rate was 194/388 eligible women (50%).
The cohort included for final analysis included 162 women at low (n = 43, 26%), intermediate (n = 66, 41%) and high risk (n = 53, 33%). Characteristics of the final cohort are summarized in Table 1. Of note, 95% of the women surveyed were non-Hispanic white, and more than two-thirds had a college degree or higher. Approximately 43% of the women had a personal history of breast cancer, and approximately half had a family history of breast cancer. Almost all women (99%) had previously undergone mammography for breast cancer screening. Approximately 74% of women had undergone a biopsy in the past, and 59% had experienced a prior benign biopsy due to a false-positive screen. STAI trait anxiety scores were evenly distributed across all four quartiles. Ten percent of participants indicated “high worry” about breast cancer.
Table 1.
Characteristics of women completing vignettes based on survey responses (n = 162).
| Completed vignettes, n (%) | |
|---|---|
| Age, mean (range) | 53 (26–85) |
| Race/Ethnicity (missing: n = 2) | |
| White, non-Hispanic | 154 (95) |
| White, Hispanic | 1 (1) |
| Nonwhite, non-Hispanic | 4 (3) |
| Nonwhite, Hispanic | 1 (1) |
| Education level (missing: n = 2) | |
| College graduate or higher | 109 (67) |
| Numeracy Questions (missing: n = 3) | |
| Both correct | 114 (70) |
| One correct | 19 (12) |
| None correct | 26 (16) |
| Personal history of breast cancer | 70 (43) |
| Invasive carcinoma | 41 (59) |
| Ductal carcinoma in situ | 19 (27) |
| Unknown type | 10 (14) |
| First degree family history of breast cancer | 80 (49) |
| Screening History | |
| Prior screening mammogram (missing: n = 1) | 160 (99) |
| Prior breast MRI (missing: n = 1) | 122 (75) |
| Prior screening recall (missing: n = 4) | 117 (72) |
| Prior biopsy (missing = 2) | 119 (74) |
| Lifetime breast cancer risk∗ | |
| Low/Average (<15%) | 43 (27) |
| Intermediate (15–20%) | 66 (41) |
| Personal history of breast cancer | 56 (35) |
| Personal history of lobular carcinoma in situ (LCIS) | 10 (6) |
| High (>20%) | 53 (33) |
| Known germline mutation | 41 (25) |
| History of chest radiation | 9 (6) |
| Untested 1PstP degree relative of germline mutation carrier | 2 (1) |
| Lifetime risk >20% based on risk models | 1 (1) |
| Perceived lifetime breast cancer risk (%), mean (range) (missing: n = 15) |
51% (1–100%) |
| Trait Anxiety (STAI) | |
| Top Quartile | 45 (28) |
| 51-75PthP percentile scores | 45 (28) |
| 26-50PthP percentile scores | 35 (22) |
| Bottom Quartile | 37 (23) |
aEstimated using the Gail and BRCAPRO models in women without and with family history of breast cancer, respectively.
3.2. Perceived versus actual breast cancer risk
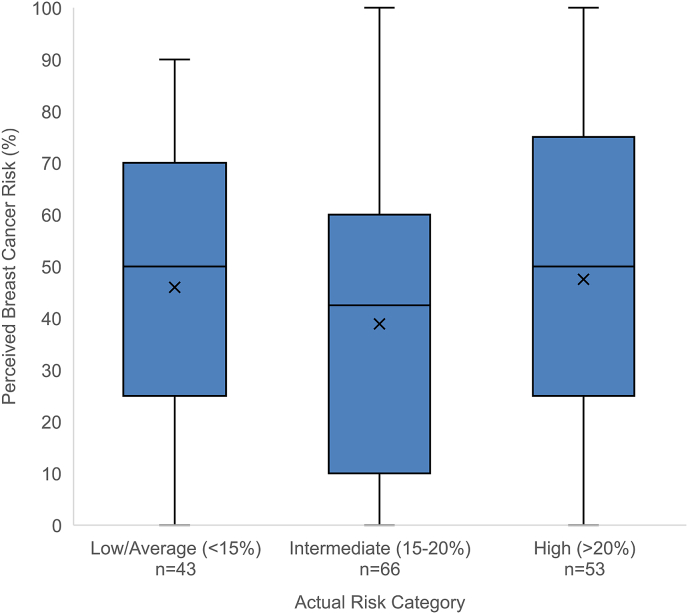
The majority of women in the low/average and intermediate breast cancer risk groups overestimated their breast cancer risk (81% of low/average risk women and 68% of intermediate risk women). Perceived risk of breast cancer did not vary by actual risk category (Spearman’s r = 0.048, p = 0.54); average perceived lifetime risk of breast cancer was 46%, 39%, and 48% in low/average, intermediate, and high-risk women, respectively (Fig. 2). Among low/average risk women, 75% incorrectly reported that they were at high risk with >20% lifetime risk of breast cancer.
Fig. 2.
Perceived versus actual lifetime risk of breast cancer. Perceived risk of breast cancer was estimated as a percentage by study participants; actual risk was calculated using the Gail and BRCAPRO models in women without and with family history of breast cancer, respectively. Perceived risk did not vary by actual breast cancer risk category (low/average < 15%, intermediate 15–20%, versus high >20%). Means for each category are denoted by ‘X’.
3.3. QOL ratings during and after diagnostic uncertainty
Univariate and multivariable regression results for QOL ratings during screening mammography recall are shown in Table 2 (QOL scores during recall for additional non-invasive imaging) and Table 3 (QOL scores during recall with biopsy performed). Additional tables are included in the online appendix with QOL scores during recall from screening MRI for additional non-invasive imaging (Table E1) and with MR-guided biopsy performed (Table E2). Results from regression models using stepwise selection are shown; results using forward and backward selection produced similar results (not shown).
Table 2.
Quality-of-life (QOL) scores during and after recall from screening mammography for non-invasive additional imaging.
| Characteristic | During Diagnostic Uncertainty after Mammography Recall |
After Benign Results Received after Mammography Recall |
||||
|---|---|---|---|---|---|---|
| Mean QOL Score (SD) | Univariate p value | Multivariable p value | Mean QOL Score (SD) | Univariate p value | Multivariable p value | |
| Age | 54.5 (26.2) | 0.51 | 67.2 (25.8) | 0.70 | 0.08 | |
| Race | 0.21 | 0.40 | ||||
| White | 54.3 (26.2) | 67.2 (25.8) | ||||
| Nonwhite | 60.1 (27.0) | 77.0 (24.9) | ||||
| Numeracy Questions | 0.02 | 0.11 | 0.39 | |||
| Both correct | 57.8 (25.7) | 66.0 (26.1) | ||||
| 1+ incorrect | 46.9 (26.3) | 69.9 (25.2) | ||||
| Family History of BC | 0.42 | 0.51 | 0.04 | |||
| Yes | 56.2 (26.6) | 65.8 (26.3) | ||||
| No | 52.9 (25.9) | 68.5 (25.4) | ||||
| Personal History of BC | 0.73 | 0.80 | ||||
| Yes | 52.3 (30.6) | 66.8 (28.0) | ||||
| No | 54.1 (23.4) | 68.1 (23.3) | ||||
| Actual BC Risk | 0.10 | 0.43 | ||||
| Low/Intermediate | 52.2 (26.9) | 66.0 (24.8) | ||||
| High | 59.4 (24.4) | 69.5 (27.9) | ||||
| Perceived BC Risk (%) | 54.9 (26.7) | 0.29 | 66.3 (26.2) | 0.46 | ||
| Anxiety Level | 0.27 | 0.03 | 0.04 | |||
| Top quartile | 50.8 (27.9) | 60.1 (27.5) | ||||
| Bottom 3 quartiles | 56.0 (25.6) | 69.9 (24.7) | ||||
| BC worrya | <0.001 | <0.001 | 0.35 | |||
| High worry | 32.8 (28.5) | 61.6 (31.9) | ||||
| Low-moderate worry | 57.1 (24.8) | 67.8 (25.0) | ||||
BC=Breast Cancer.
High worry was defined as a score ≥6 on the Breast Cancer Worry scale.
Table 3.
Quality-of-life (QOL) scores during and after recall from screening mammography with biopsy performed.
| Characteristic | During Diagnostic Uncertainty After Biopsy |
After Benign Results Received After Biopsy |
||||
|---|---|---|---|---|---|---|
| Mean QOL Score (SD) | Univariate p value | Multivariable p value | Mean QOL Score (SD) | Univariate p value | Multivariable p value | |
| Age | 45.4 (26.9) | 0.45 | 67.2 (27.0) | 0.48 | 0.12 | |
| Race | 0.13 | 0.52 | ||||
| White | 44.9 (26.8) | 67.0 (27.2) | ||||
| Nonwhite | 63.8 (31.7) | 75.0 (25.0) | ||||
| Numeracy Questions | 0.06 | 0.11 | 0.53 | |||
| Both correct | 48.0 (26.7) | 66.3 (27.2) | ||||
| 1+ incorrect | 39.3 (26.7) | 69.2 (26.6) | ||||
| Family History of BC | 0.81 | 0.05 | 0.04 | |||
| Yes | 45.1 (27.1) | 63.1 (28.9) | ||||
| No | 45.7 (26.9) | 71.2 (24.5) | ||||
| Personal History of BC | 0.70 | 0.92 | ||||
| Yes | 44.0 (30.0) | 67.5 (28.6) | ||||
| No | 46.0 (25.0) | 66.9 (25.9) | ||||
| Risk | 0.30 | 0.50 | ||||
| Low/Intermediate | 43.9 (26.8) | 66.2 (26.6) | ||||
| High | 48.6 (27.1) | 69.2 (27.9) | ||||
| Perceived BC Risk (%) | 45.3 (27.0) | 0.37 | 66.1 (26.9) | 0.18 | ||
| Anxiety Level | 0.35 | 0.04 | 0.02 | |||
| Top quartile | 42.2 (25.8) | 60.0 (27.6) | ||||
| Bottom 3 quartiles | 46.7 (27.3) | 69.9 (26.3) | ||||
| BC worrya | 0.001 | 0.002 | 0.18 | |||
| High worry | 25.8 (25.6) | 58.9 (31.8) | ||||
| Low-moderate worry | 47.7 (26.2) | 68.1 (26.3) | ||||
BC=Breast Cancer.
High worry was defined as a score ≥6 on the Breast Cancer Worry scale.
QOL ratings did not vary by risk group for any of the presented scenarios across breast cancer risk groups (Fig. 3). Across all risk levels and all screening scenarios, QOL scores were significantly lower during the period of diagnostic uncertainty versus after benign results were received; on average, QOL scores increased by 16 after receipt of benign results compared to the period of uncertainty (p < 0.05 for all scenarios). In the mammography recall scenario requiring non-invasive additional imaging, mean QOL increased from 55 during the period of diagnostic uncertainty to 68 after results were received. Similarly, mean QOL increased from 46 for a scenario in which biopsy was performed but results were not yet known, to 68 after benign results were delivered. In the MRI recall scenario requiring additional evaluation (Table E1), QOL increased from 47 during diagnostic uncertainty to 58 after results are received. In the MRI recall scenario requiring biopsy (Table E2), QOL increased from 44 prior to results being known to 62 after results are received. Of note, there was substantial within-woman variability in changes in QOL ratings before and after results were known, ranging from a decrease in QOL score by 52 to an improvement in QOL score of 95 across scenarios. Perceived risk was not a predictor of QOL scores in any of the scenarios.
Fig. 3.
A–3B. QOL ratings by participants across risk categories (low/intermediate versus high) for scenarios of screening mammography recall with additional imaging (panel A) and biopsy (panel B). In both scenarios, QOL ratings significantly increased after receipt of benign results compared to before results were known (p < 0.05 for all scenarios). QOL ratings for each scenario did not differ by breast cancer risk category. Blue bars = low/intermediate risk women; orange bars = high-risk women. Means for each category are denoted by ‘X’.
Multivariable regression analysis indicated that across all screening recall scenarios, breast cancer worry was a significant predictor of decreased QOL during the period of diagnostic uncertainty while awaiting results from non-invasive additional imaging or biopsy (mean QOL scores ranged from 25 to 33 in women with high worry versus 45–57 in women without high worry). High trait anxiety (top quartile STAI percentile score) was a predictor of persistently lower QOL scores in one scenario of diagnostic uncertainty after MRI-guided biopsy (mean QOL scores 37 versus 45 in women with high versus low anxiety, respectively) and in all scenarios after receipt of benign results (mean QOL scores ranged from 50 to 60 in women with high anxiety versus 58–68 in women without high anxiety). Family history of breast cancer was also a predictor of persistently lower QOL scores after receipt of benign results in most (3 of 4) scenarios. Personal history of breast cancer and prior history of screening recall were not predictors of QOL ratings for any scenarios. Women who incorrectly answered one or more items on the numeracy measure had lower QOL ratings for screening mammography recall for additional imaging, but not for other scenarios. Age was a predictor of lower QOL score after MR-guided biopsy, but not in other scenarios. No other variables were predictors of QOL scores in multivariate analyses in any of the presented scenarios.
4. Discussion
Our vignette-based survey of women across a spectrum of risk undergoing breast cancer screening suggests that perceived QOL during recall after screening mammography or MRI does not appear to vary by breast cancer risk level. Across a spectrum of risk, QOL was lower during the period of diagnostic uncertainty, and then improved after benign results were received. Breast cancer worry was a predictor of lower QOL during the period of diagnostic uncertainty, and trait anxiety and family history were significant predictors of persistently low QOL after receipt of benign results.
Our finding that the perceived impact of screening recall does not differ between women at average and high risk of breast cancer may be explained by inaccurate breast cancer risk perceptions among the participants in our study. Interestingly, perceived breast cancer risk did not vary by actual risk category, with average and high-risk women estimating their lifetime risk of breast cancer to be 46% and 48%, respectively, and most (75%) average risk women incorrectly reporting >20% lifetime risk of breast cancer. These findings corroborate prior studies which have shown that women’s perceived risk of breast cancer is often inaccurate [[30], [31], [32], [33], [34]], although these studies have produced mixed results regarding whether women tend to overestimate their risk or underestimate their risk [32]. This discrepancy may in part be due to the different instruments used to assess perceived risk, as studies using numerical risk estimates (such as ours) suggest that women tend to overestimate their breast cancer risk [30,31,33], while studies using verbal scales suggest women tend to underestimate their risk [[33], [34], [35]].
Our findings are in keeping with the results of prior studies of women at average risk which have suggested that the period of diagnostic uncertainty is most distressing for women who have been recalled from screening, and that quality of life ratings improve on average after receipt of benign results [14]. However, the duration of psychosocial consequences in average risk women recalled from screening is unclear, as studies have demonstrated mixed results. While some studies have suggested that these effects are short-term[16,36], other studies have demonstrated evidence of persistent psychological distress for at least 12 months [11] to 36 months [14]. Interestingly, studies of high-risk women who have experienced screening recall suggest that breast cancer worry significantly decreases by 6 months after recall [36,37]. As breast cancer risk assessment improves, understanding the impact of screening recall by breast cancer risk level is important to informing risk-tailored breast cancer screening strategies. Our study is unique in that it included women at average, intermediate, and high risk for breast cancer, and our results suggest that across breast cancer risk levels, QOL scores improve on average after receipt of benign results.
In our study, trait anxiety was a predictor of persistently low QOL scores after receipt of benign results. In a prior study of Danish women recalled from screening mammography[38], trait anxiety predicted lower QOL ratings across all time points up to 12 months after screening. In particular, women with high trait anxiety who had a false positive result endorsed persistently lower QOL ratings than women with low trait anxiety and a breast cancer diagnosis. These findings suggest that trait anxiety may be an important characteristic to consider during individualized decision-making regarding breast cancer screening.
Our study has a few limitations worth noting. The participants in our study provided QOL ratings for hypothetical vignettes of the experience of screening recall for additional imaging and biopsy, and their perceptions may differ from women experiencing actual screening recall episodes. In addition, because of the nature of the scenario we could not assess short versus long-term consequences of screening recall. Finally, the women in our study received screening at a single institution and were predominantly non-Hispanic white women and highly educated, limiting generalizability.
In conclusion, our vignette-based survey study suggests breast cancer risk affects QOL during breast cancer screening recall less than other factors such as breast cancer worry and trait anxiety. Across the spectrum of risk, breast cancer worry predicted lower QOL ratings for recall during the period of diagnostic uncertainty while awaiting test results. While quality of life generally improved after receipt of benign results, women with high trait anxiety levels reported persistently decreased quality of life even after results were known. Our results suggest that these women may particularly benefit from discussions regarding the potential for false-positive test results when choosing a breast cancer screening regimen.
Ethical approval
This study was HIPAA-compliant and approved by the Institutional Review Board of Massachusetts General Hospital.
Declaration of competing interest
JML and KPL receive research support from GE Healthcare. GSG was previously a consultant for GE Healthcare until 2018.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant NIH K07-CA128816 (JML), American Cancer Society (ACS) grant RSGHP-07-266-01-CPHS (KD and JSS), a National Cancer Institute grant P01 CA154292 (ANAT) and by the Eleanor and Miles Shore Scholars in Medicine Fellowship (JML, TM).
Appendix Tables.
Table E1.
Quality-of-life (QOL) scores during and after recall from screening magnetic resonance imaging (MRI) for additional ultrasound evaluation.
| Characteristic | During Diagnostic Uncertainty After MRI Recall |
After Benign Results Received After MRI Recall |
||||
|---|---|---|---|---|---|---|
| Mean QOL Score (SD) | Univariate p value | Multivariable p value | Mean QOL Score (SD) | Univariate p value | Multivariable p value | |
| Age | 46.4 (27.0) | 0.26 | 57.1 (27.2) | 0.80 | ||
| Race | 0.50 | |||||
| White | 46.1 (26.7) | 57.1 (25.1) | ||||
| Nonwhite | 60.2 (39.1) | 64.8 (33.3) | ||||
| Numeracy Questions | 0.08 | 0.22 | ||||
| Both correct | 48.8 (27.0) | 58.7 (25.0) | ||||
| 1+ incorrect | 40.8 (26.4) | 53.3 (25.8) | ||||
| Family History of BC^ | 0.91 | 0.13 | ||||
| Yes | 47.0 (27.5) | 54.4 (26.1) | ||||
| No | 45.9 (26.6) | 59.7 (24.4) | ||||
| Personal History of BC^ | 0.91 | 0.90 | ||||
| Yes | 45.3 (29.5) | 57.8 (25.9) | ||||
| No | 45.9 (26.5) | 57.2 (25.4) | ||||
| Risk | 0.088 | 0.94 | ||||
| Low/Intermediate | 43.9 (27.1) | 57.2 (24.4) | ||||
| High | 51.6 (26.4) | 56.9 (27.2) | ||||
| Perceived BC Risk (%) | 46.9 (27.1) | 0.52 | 57.2 (24.9) | 0.69 | ||
| Anxiety Level | 0.10 | 0.04 | 0.0382 | |||
| Top quartile | 40.9 (26.1) | 50.5 (24.8) | ||||
| Bottom 3 quartiles | 48.6 (27.1) | 59.6 (25.1) | ||||
| BC worry∗ | 0.002 | 0.004 | 0.20 | |||
| High worry | 27.6 (25.37) | 49.6 (23.8) | ||||
| Low-moderate worry | 48.6 (26.40) | 58.0 (25.4) | ||||
BC=Breast Cancer.
∗High worry was defined as a score ≥6 on the Breast Cancer Worry scale.
Table E2.
Quality-of-life (QOL) scores during and after recall from screening MRI with MR-guided biopsy performed.
| Characteristic | During Diagnostic Uncertainty After Biopsy |
After Benign Results Received After Biopsy |
||||
|---|---|---|---|---|---|---|
| Mean QOL Score (SD) | Univariate p value | Multivariable p value | Mean QOL Score (SD) | Univariate p value | Multivariable p value | |
| Age | 43.2 (27.5) | 0.28 | 60.8 (26.7) | 0.20 | 0.008 | |
| Race | 0.09 | 0.27 | ||||
| White | 42.5 (27.3) | 60.7 (26.7) | ||||
| Nonwhite | 64.0 (32.3) | 74.0 (23.8) | ||||
| Numeracy Questions | 0.30 | 0.46 | ||||
| Both correct | 44.6 (27.2) | 59.8 (26.1) | ||||
| 1+ incorrect | 39.7 (28.1) | 63.2 (28.0) | ||||
| Family History of BC | 0.50 | 0.02 | 0.02 | |||
| Yes | 41.9 (28.1) | 55.8 (28.1) | ||||
| No | 44.4 (26.9) | 65.8 (24.3) | ||||
| Personal History of BC | 0.48 | 0.41 | ||||
| Yes | 41.1 (29.4) | 59.5 (27.5) | ||||
| No | 44.9 (26.6) | 63.7 (27.1) | ||||
| Risk | 0.17 | 0.94 | ||||
| Low/Intermediate | 41.1 (27.0) | 61.0 (25.5) | ||||
| High | 47.4 (28.3) | 60.6 (29.2) | ||||
| Perceived BC Risk (%) | 42.8 (27.5) | 0.75 | 59.4 (26.5) | 0.06 | ||
| Anxiety Level | 0.10 | 0.04 | 0.04 | 0.04 | ||
| Top quartile | 37.4 (25.4) | 54.0 (25.0) | ||||
| Bottom 3 quartiles | 45.4 (28.0) | 64.0 (27.0) | ||||
| BC worry∗ | 0.003 | 0.009 | 0.34 | |||
| High worry | 24.8 (23.4) | 54.9 (24.3) | ||||
| Low-moderate worry | 45.3 (27.2) | 61.5 (26.9) | ||||
BC=Breast Cancer.
∗High worry was defined as a score ≥6 on the Breast Cancer Worry scale.
References
- 1.Nelson H.D., Fu R., Cantor A., Pappas M., Daeges M., Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive services task force recommendation. Ann Intern Med. 2016;164(4):244–255. doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- 2.Marmot M.G., Altman D.G., Cameron D.A., Dewar J.A., Thompson S.G., Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Canc. 2013;108(11):2205–2240. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komenaka I.K., Ditkoff B.A., Joseph K.A. The development of interval breast malignancies in patients with BRCA mutations. Cancer. 2004;100(10):2079–2083. doi: 10.1002/cncr.20221. [DOI] [PubMed] [Google Scholar]
- 4.van Zelst J.C.M., Mus R.D.M., Woldringh G. Surveillance of women with the BRCA1 or BRCA2 mutation by using biannual automated breast US, MR imaging, and mammography. Radiology. 2017;285(2):376–388. doi: 10.1148/radiol.2017161218. [DOI] [PubMed] [Google Scholar]
- 5.Saslow D., Boetes C., Burke W. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA A Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 6.Mainiero M.B., Moy L., Baron P. ACR appropriateness criteria((R)) breast cancer screening. J Am Coll Radiol : JACR. 2017;14(11s):383–390. doi: 10.1016/j.jacr.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 7.Leach M.O., Boggis C.R., Dixon A.K. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005;365(9473):1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 8.Chiarelli A.M., Blackmore K.M., Muradali D. Performance measures of magnetic resonance imaging plus mammography in the high risk ontario breast screening program. J Natl Canc Inst. 2019;112(2):136–144. doi: 10.1093/jnci/djz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warner E., Hill K., Causer P. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669. doi: 10.1200/JCO.2009.27.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson H.D., Pappas M., Cantor A., Griffin J., Daeges M., Humphrey L. Harms of breast cancer screening: systematic review to update the 2009 U.S. Preventive services task force recommendation. Ann Intern Med. 2016;164(4):256–267. doi: 10.7326/M15-0970. [DOI] [PubMed] [Google Scholar]
- 11.Bolejko A., Hagell P., Wann-Hansson C., Zackrisson S. Prevalence, long-term development, and predictors of psychosocial consequences of false-positive mammography among women attending population-based screening. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1388–1397. doi: 10.1158/1055-9965.EPI-15-0060. [DOI] [PubMed] [Google Scholar]
- 12.Brett J., Bankhead C., Henderson B., Watson E., Austoker J. The psychological impact of mammographic screening. A systematic review. Psycho Oncol. 2005;14(11):917–938. doi: 10.1002/pon.904. [DOI] [PubMed] [Google Scholar]
- 13.Brewer N.T., Salz T., Lillie S.E. Systematic review: the long-term effects of false-positive mammograms. Ann Intern Med. 2007;146(7):502–510. doi: 10.7326/0003-4819-146-7-200704030-00006. [DOI] [PubMed] [Google Scholar]
- 14.Brodersen J., Siersma V.D. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11(2):106–115. doi: 10.1370/afm.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hafslund B., Espehaug B., Nortvedt M.W. Effects of false-positive results in a breast screening program on anxiety, depression and health-related quality of life. Canc Nurs. 2012;35(5):E26–E34. doi: 10.1097/NCC.0b013e3182341ddb. [DOI] [PubMed] [Google Scholar]
- 16.Tosteson A.N., Fryback D.G., Hammond C.S. Consequences of false-positive screening mammograms. JAMA Intern Med. 2014;174(6):954–961. doi: 10.1001/jamainternmed.2014.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullen J., Schwartz M.D., Lawrence W.F., Selby J.V., Mandelblatt J.S. Short-term impact of cancer prevention and screening activities on quality of life. J Clin Oncol. 2004;22(5):943–952. doi: 10.1200/JCO.2004.05.191. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz L.M., Woloshin S., Sox H.C., Fischhoff B., Welch H.G. US women’s attitudes to false positive mammography results and detection of ductal carcinoma in situ: cross sectional survey. BMJ. 2000;320(7250):1635–1640. doi: 10.1136/bmj.320.7250.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stout N.K., Rosenberg M.A., Trentham-Dietz A., Smith M.A., Robinson S.M., Fryback D.G. Retrospective cost-effectiveness analysis of screening mammography. J Natl Canc Inst. 2006;98(11):774–782. doi: 10.1093/jnci/djj210. [DOI] [PubMed] [Google Scholar]
- 20.Drummond M. Methods for the economic evaluation of health care programmes. fourth ed. Oxford University Press; Oxford, United Kingdom ; New York, NY, USA: 2015. [Google Scholar]
- 21.Neumann P.J., Sanders G.D., Russell L.B., Siegel J.E., Ganiats T.G. second ed. Oxford University Press; Oxford ; New York: 2017. Cost effectiveness in health and medicine. [Google Scholar]
- 22.Wolowacz S.E., Briggs A., Belozeroff V. Estimating health-state utility for economic models in clinical studies: an ISPOR good research practices task force report. Value Health. 2016;19(6):704–719. doi: 10.1016/j.jval.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd A., Nafees B., Narewska J., Dewilde S., Watkins J. Health state utilities for metastatic breast cancer. Br J Canc. 2006;95(6):683–690. doi: 10.1038/sj.bjc.6603326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frederix G.W., Quadri N., Hovels A.M. Utility and work productivity data for economic evaluation of breast cancer therapies in The Netherlands and Sweden. Clin Therapeut. 2013;35(4):1–7. doi: 10.1016/j.clinthera.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Gail M.H., Brinton L.A., Byar D.P. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Canc Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 26.Berry D.A., Iversen E.S., Jr., Gudbjartsson D.F. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol. 2002;20(11):2701–2712. doi: 10.1200/JCO.2002.05.121. [DOI] [PubMed] [Google Scholar]
- 27.Spielberger C.D.G.R., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the state-trait anxiety inventory. [Google Scholar]
- 28.Lipkus I.M., Samsa G., Rimer B.K. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 29.Lerman C., Trock B., Rimer B.K., Jepson C., Brody D., Boyce A. Psychological side effects of breast cancer screening. Health Psychol. 1991;10(4):259–267. doi: 10.1037//0278-6133.10.4.259. [DOI] [PubMed] [Google Scholar]
- 30.Buxton J.A., Bottorff J.L., Balneaves L.G. Women’s perceptions of breast cancer risk: are they accurate? Can J Public Health. 2003;94(6):422–426. doi: 10.1007/BF03405078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivell S., Elwyn G., Gaff C.L. How risk is perceived, constructed and interpreted by clients in clinical genetics, and the effects on decision making: systematic review. J Genet Counsel. 2008;17(1):30–63. doi: 10.1007/s10897-007-9132-1. [DOI] [PubMed] [Google Scholar]
- 32.Katapodi M.C., Lee K.A., Facione N.C., Dodd M.J. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev Med. 2004;38(4):388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Lipkus I.M., Kuchibhatla M., McBride C.M. Relationships among breast cancer perceived absolute risk, comparative risk, and worries. Cancer Epidemiol Biomarkers Prev. 2000;9(9):973–975. [PubMed] [Google Scholar]
- 34.Facione N.C. Perceived risk of breast cancer: influence of heuristic thinking. Canc Pract. 2002;10(5):256–262. doi: 10.1046/j.1523-5394.2002.105005.x. [DOI] [PubMed] [Google Scholar]
- 35.Katapodi M.C., Dodd M.J., Facione N.C., Humphreys J.C., Lee K.A. Why some women have an optimistic or a pessimistic bias about their breast cancer risk: experiences, heuristics, and knowledge of risk factors. Canc Nurs. 2010;33(1):64–73. doi: 10.1097/NCC.0b013e3181b430f9. [DOI] [PubMed] [Google Scholar]
- 36.Tyndel S., Austoker J., Henderson B.J. What is the psychological impact of mammographic screening on younger women with a family history of breast cancer? Findings from a prospective cohort study by the PIMMS Management Group. J Clin Oncol. 2007;25(25):3823–3830. doi: 10.1200/JCO.2007.11.0437. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel T.N., Esplen M.J., Hill K.A., Wong J., Causer P.A., Warner E. Psychological impact of recall on women with BRCA mutations undergoing MRI surveillance. Breast. 2011;20(5):424–430. doi: 10.1016/j.breast.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 38.van der Steeg A.F., Keyzer-Dekker C.M., De Vries J., Roukema J.A. Effect of abnormal screening mammogram on quality of life. Br J Surg. 2011;98(4):537–542. doi: 10.1002/bjs.7371. [DOI] [PubMed] [Google Scholar]