Abstract
Objectives
Triple negative breast cancer (TNBC) is a heterogenous disease and associated with unfavorable outcomes. The role of sonographic features and its association with disease outcome in TNBC is uncertain. Our study aimed to determine the prognosis predictive value of sonographic features in TNBC.
Methods
Women with TNBC patients treated between January 2009 and December 2015 were retrospectively included. Patients’ clinic-pathological, sonographic features, recurrence-free survival (RFS), and breast cancer-specific survival (BCSS) events were reviewed and analyzed. Kaplan–Meier analysis and multivariable Cox regression were used to determine the prognostic factors in TNBC.
Results
A total of 433 TNBC patients were included. With a median follow-up of 4.8 years, 58 (13.4%) RFS and 35(8.1%) BCSS events were detected. Besides lymphatic vascular invasion (LVI), nuclear grade III, tumor >2.0 cm, and positive axillary lymph node (ALN), multivariable analysis found that vertical orientation in ultrasound imaging was independently associated with worse RFS (Hazard Ratio (HR) = 3.238; 95% Confidential Interval (CI), 1.661–6.312; P = 0.001) and BCSS (HR = 7.028; 95% CI, 3.199–15.436; P < 0.001). TNBC with vertical orientation in ultrasound imaging had higher ALN metastasis burden than those with sonographic parallel features (2.7 ± 1.0 vs 1.5 ± 0.2, P = 0.003).
Conclusions
TNBC presenting with vertical orientation in ultrasound imaging was associated with worse disease outcome and a greater number of ALN metastasis.
Keywords: Breast neoplasm, Triple negative breast neoplasms, Ultrasonography, Prognosis
Highlights
-
•
In TNBC, vertical orientation in US was associated with inferior RFS.
-
•
Vertical orientation in US independently predicted worse BCSS in TNBC.
-
•
Vertical orientation in US was related with more ALN metastasis in TNBC patients.
All abbreviations
- Terms
Abbreviations
- Triple negative breast cancer
TNBC
- Recurrence-free survival
RFS
- Breast cancer-specific survival
BCSS
- Estrogen receptor
ER
- Progesterone receptor
PR
- Human epidermal growth factor receptor-2
HER2
- Fluorescence in Situ Hybridization
FISH
- Sentinel lymph node biopsy
SLNB
- Axillary lymph node dissection
ALND
- Invasive ductal carcinoma
IDC
- Lymphatic vessel invasion
LVI
- Hazard ratio
HR
- Confidence interval
CI
1. Introduction
Breast cancer, as a global public health issue, has become the second leading cause for cancer death for women worldwide [1,2]. Triple negative breast cancer (TNBC), defined as the type of breast cancer without estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor-2 (HER2) expression, accounts for 15–20% of newly diagnosed cases approximately [[3], [4], [5]]. TNBC is distinguished for its aggressive biological behaviors that patients of this subtype are more likely to have larger tumor size, higher nuclear grade, and more distant recurrence events. What's more, the recurrence pattern of TNBC is strikingly different from other subtypes which had the highest relapse risk in the first three years and then declines rapidly thereafter [[3], [4], [5], [6], [7]].
For years, researchers have been investigating the clinicopathological factors to predict the recurrence and death for TNBC patients [[4], [5], [6]]. Factors including younger age at diagnosis, axillary lymph node (ALN) involvement, and lymphatic vessel invasion (LVI) have been reported to associate with the higher relapse rate of TNBC in long-term follow-up studies [4,6]. However, to further better understand the recurrence pattern of TNBC, more biological and clinical markers need to be brought into clinical practice.
Studies have been conducted to look into the diagnostic and prognostic values of radiological examinations in breast cancer [[8], [9], [10]]. Kinds of preoperative imaging assessments of breasts have been conducted routinely among breast cancer patients, including ultrasound, mammography, and magnetic resonance imaging (MRI). It has been reported that the absence of preoperative MRI and higher breast tissue density indicated higher risk of recurrence in early stage TNBC [8]. Several studies have found that tumors presenting with casting-type calcification and architecture distortion in mammography tended to have worse survival than those without these features [9,10]. Meanwhile, relatively few studies have focused on the sonographic features and disease outcome in TNBC [11]. In one study investigating breast cancers detected at screening ultrasound, tumors with Breast Imaging and Reporting Data System (BI-RADS) 4A category was related with a higher risk of recurrence than tumors with 4B–5 categories [11]. But few studies have looked into the association between the specific sonographic features and prognosis in TNBC patients.
Based on above issues, our study aimed to determine the prognostic value of sonographic features and its association with clinicopathological factors in TNBC patients.
2. Materials and methods
2.1. Study population
We performed a retrospective analysis on consecutive women underwent surgery at the Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiaotong University School of Medicine from January 2009 to December 2015. TNBC patients who had received preoperative ultrasound were considered as eligible. Patients with the history of previous breast surgery, bilateral breast cancer, other malignant cancer, receiving neoadjuvant chemotherapy, or presented as diffuse lesions in ultrasound were excluded. Patients characteristics, imaging data, and follow-up information was recorded and retrieved from Shanghai Jiaotong University Breast Cancer Database (SJTU-BCDB). The protocol was reviewed and approved by the Ethical Committee/Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
2.2. Ultrasound detecting and data record systems
TNBC patients received preoperative sonography testing by ultrasound experts majored in breast imaging, with more than 10 years of experience in assessing breast ultrasound. The MyLab60 (Esaote, Genoa, Italy) was applied for disease detection and equipped with a high frequency (5–12 MHz) linear array transducer and images were stored by Digital Imaging and Communications in Medicine (DICOM). The ultrasound imaging was assessed according to the ACR BI-RADS® Atlas Fourth Edition (before 2013) and ACR BI-RADS® Atlas Fifth Edition (since 2013). The descriptive features both in the 4th and 5th editions were included for further analysis, including orientation, shape, margin, echo pattern, posterior acoustic patterns, architectural distortion, change in Cooper's ligament, vessel morphology, and vessel distortion.
2.3. Pathological and immunohistochemistry evaluation
Pathologic assessment was conducted by Department of Pathology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine. The removed breast tumors were fixed in formalin within 1 h, embedded in paraffin, and stained with hematoxylin-eosin for further examinations. Size of tumor, histopathological types, nuclear grade, LVI, and ALN metastases were recorded for each patient. The ALN metastases were defined as micro-metastasis or macro-metastasis in axillary lymph nodes. Meanwhile, the histopathological types were divided into IDC and other types.
ER, PR, HER2, and Ki67 expression was examined by immunohistochemistry (IHC). ER or PR positivity was defined as at least 1% tumor with nuclear staining [12]. HER2 positivity was determined as IHC HER2 3 + or positive on Fluorescence in Situ Hybridization (FISH), while specimens with IHC HER2 0 or 1 + expression were classified as HER2 negativity. Breast cancer without ER, PR, and HER2 expression was defined as TNBC.
2.4. Data collection and statistical analysis
Following clinicopathological factors were collected: patients’ age, menstrual status, surgery types of breast and axillary, tumor side, tumor size, ALN involvement, TNM stage, tumor grade, LVI, Ki-67 index and adjuvant treatments. Cutoff value for high Ki-67 expression was 30%.
RFS was defined as the time period from the date of surgery to the date of local regional recurrence, distant metastasis, and contralateral breast cancers. BCSS was defined as the interval between the date of diagnosis and breast cancer-related death.
Statistical analyses were conducted by SPSS for Windows version 22.0 (SPSS Inc., Chicago, IL, USA). Tests were two-sided and P value < 0.05 was considered as statistically significant difference. Pearson's Chi-square test (Fisher's exact test when necessary) was used to compare the distribution of clinicopathological features between subgroups. Survival curves were performed by Kaplan–Meier analysis. The Cox regression analysis was performed for univariate and multivariate analyses of RFS and BCSS. Each clinicopathological and sonographic factors with P value < 0.05 by univariate analysis were taken into further multivariate Cox regression analysis with stepwise selection. Hazard ratio (HR) and 95% confidence interval (CI) of each predictive factor were analyzed. Correlation between different ultrasound characteristics were estimated by Spearman correlation test.
3. Results
3.1. Baseline characteristics of the study population
A total of 433 TNBC patients were finally included (Fig. 1). Average age of enrolled patients was 55.5 (27–87) years old and 266 (61.4%) patients were post-menopausal at diagnosis. There were 149 (34.4%) patients underwent breast conserving surgery while 284 (65.6%) received mastectomy. Sentinel lymph node biopsy (SLNB) was performed among 238 (55.0%) patients. There were 219 (50.6%) patients with tumor size > 2.0 cm and 289 (66.7%) with grade III disease. The mean Ki-67 value for enrolled patients was 51.5% (0–95%). ALN metastasis was detected in 120 (27.7%) patients.
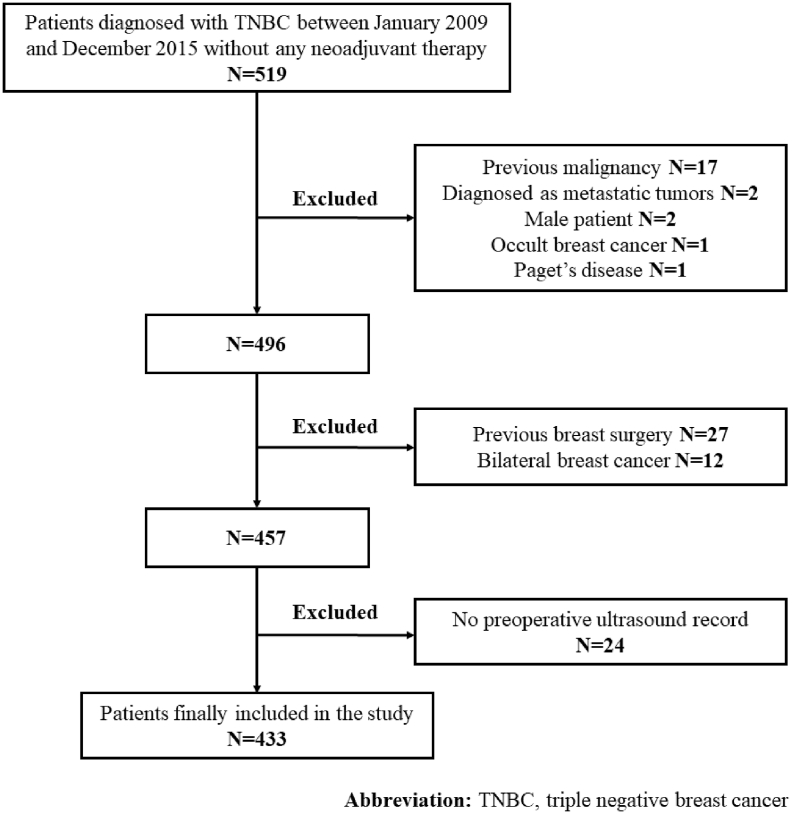
Fig. 1.
Flow chart of inclusion. From January 2009 to December 2015, 519 patients were eligible for this study. Among them, 27 patients were excluded for history of previous breast surgery, 24 for no preoperative ultrasound record in our clinic, 17 for history of previous malignancy, 12 for bilateral breast cancer, 2 for diagnosed as metastatic tumor, 2 for male patients, 1 for Paget's disease and 1 for occult breast cancer. 433 patients were finally included in this study.
3.2. Univariate analysis of clinicopathological features and disease outcomes
With a median follow-up of 4.8 years, a total of 58 and 35 patients had RFS and BCSS events. There were 12 (20.7%) patients with locoregional recurrences, 41 (70.7%) with distant metastasis, and 5 (8.6%) with contralateral breast cancer. A total of 35 patients died due to breast cancer metastasis while other 4 patients due to other causes: 2 cerebrovascular incidents, 1 renal failure, and 1 myocardial infarction. Univariate survival analysis of clinicopathological features and RFS were summarized in Table 1. Histopathological types as IDC (P = 0.038), tumor >2.0 cm (P = 0.001), ALN metastasis (P < 0.001), and higher grade (P = 0.038) was associated with RFS. Additionally, patients with positive LVI had unfavorable RFS than those without (P < 0.001). Ki67 index >30% (P = 0.112) and co-morbidities (P = 0.563) failed to predict RFS in univariate analysis.
Table 1.
Univariable analysis of clinicopathological features as prognostic factors for RFS events.
| Variables | Total N (%) |
RFS events |
P value | |
|---|---|---|---|---|
| Yes | No | |||
| Age (y) | 0.693 | |||
| ≤55 | 221(51.0) | 31(53.4) | 190(50.7) | |
| >55 | 212(49.0) | 27(46.6) | 185(49.3) | |
| Menstrual status | 0.758 | |||
| Pre/peri-menopausal | 167(38.6) | 21(36.2) | 146(38.9) | |
| Post-menopausal | 266(61.4) | 37(63.8) | 229(61.1) | |
| Co-morbidities | 0.563 | |||
| Absent | 279(64.4) | 40(69.0) | 239(63.7) | |
| Present | 154(35.6) | 18(31.0) | 136(36.3) | |
| Surgery type (breast) | 0.435 | |||
| BCS | 149(34.4) | 16(27.6) | 133(35.5) | |
| Mastectomy | 284(65.6) | 42(72.4) | 242(64.5) | |
| Surgery type (axillary) | 0.008 | |||
| SLNB | 238(55.0) | 19(32.8) | 219(57.4) | |
| ALND | 195(45.0) | 39(67.2) | 156(41.6) | |
| Histopathological types | 0.038 | |||
| IDC | 377(87.1) | 56(96.6) | 321(85.6) | |
| Others | 56(12.9) | 2(3.4) | 54(14.4) | |
| Lymphovascular invasion | <0.001 | |||
| Absent | 407(94.0) | 48(82.8) | 359(95.7) | |
| Present | 26(6.0) | 10(17.2) | 16(4.3) | |
| Tumor grade | 0.008 | |||
| I-II | 87(20.1) | 7(12.1) | 80(21.3) | |
| III | 289(66.7) | 50(86.2) | 239(63.7) | |
| NA | 57(13.2) | 1(1.7) | 56(14.9) | |
| Tumor size | 0.001 | |||
| ≤2 cm | 219(50.6) | 16(27.6) | 203(54.1) | |
| >2 cm | 214(49.4) | 42(72.4) | 172(45.9) | |
| Lymph nodes involvement | <0.001 | |||
| Absent | 313(72.3) | 27(46.6) | 286(76.3) | |
| Present | 120(27.7) | 31(53.4) | 79(23.7) | |
| Ki-67 (%) | 0.112 | |||
| ≤30 | 139(32.1) | 14(24.1) | 125(33.3) | |
| >30 | 294(67.9) | 44(75.9) | 250(66.7) | |
| Abbreviations: RFS, recurrence-free survival; BCS, breast conserving surgery; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; IDC, invasive ductal carcinoma; NA, not applicable. | ||||
The words in bold were clinical-pathological and sonographic variables included in our study.
Regarding BCSS, univariate analysis found that patients with tumor >2.0 cm(P = 0.022), ALN metastasis (P < 0.001), and LVI (P < 0.001) had a higher death rate. Furthermore, tumors with Ki67 index >30% were associated with inferior BCSS compared with those with low Ki67 index (P = 0.023) (Supplemental Table 1).
3.3. Sonographic factors and disease outcome
Table 2 illustrated sonographic features of the total population in our study. There were 51(11.8%) lesions presented with vertical orientation. Three hundred and ninety-two (91.2%) mass had an irregular shape while 234 (56.7%) showed angular margin. Posterior acoustic shadow was seen in 168 (39.5%) tumors. Regarding peritumoral vessels, wide and distorted vessels were found in 315 (84.0%) and 321 (85.6%) lesions, respectively.
Table 2.
Univariable analysis of sonographic features as prognostic factors for RFS events.
| Variables | Total |
RFS events |
P value | |
|---|---|---|---|---|
| N (%) | Yes | No | ||
| Orientation | 0.006 | |||
| Parallel | 382(88.2) | 44(75.9) | 338(90.1) | |
| Vertical | 51(11.8) | 14(24.1) | 37(9.9) | |
| Shape | 0.593 | |||
| Regular | 38(8.8) | 4(6.9) | 34(9.1) | |
| Irregular | 395(91.2) | 54(93.1) | 341(90.9) | |
| Margin | 0.623 | |||
| Circumscribed | 38(9.2) | 3(5.6) | 35(9.7) | |
| Indistinct | 42(10.2) | 6(11.1) | 36(10.0) | |
| Micro-lobulated | 80(19.4) | 12(22.2) | 68(18.9) | |
| Angular | 234(56.7) | 29(53.7) | 205(57.1) | |
| Spiculate | 19(4.6) | 4(7.4) | 15(4.2) | |
| Echo pattern | 0.273 | |||
| Hypoechoic | 410(94.7) | 57(98.3) | 353(94.1) | |
| Mixed-echoic | 23(5.3) | 1(1.7) | 22(5.9) | |
| Posterior acoustic pattern | 0.529 | |||
| No change | 188(44.2) | 26(47.3) | 162(43.8) | |
| Enhancement | 58(13.6) | 5(9.1) | 53(14.3) | |
| Shadowing | 168(39.5) | 23(41.8) | 145(39.2) | |
| Mixed change | 11(2.6) | 1(1.8) | 10(2.7) | |
| Architectural distortion | 0.842 | |||
| Absent | 327(76.0) | 46(79.3) | 281(75.5) | |
| Present | 103(24.0) | 12(20.7) | 91(24.5) | |
| Change in Cooper's ligament | 0.640 | |||
| Absent | 345(80.2) | 47(81.0) | 298 (80.1) | |
| Present | 85(19.8) | 11(19.0) | 74(19.9) | |
| Vessel morphology | 0.353 | |||
| Slim | 60(16.0) | 5(10.6) | 55(16.8) | |
| Wide | 315(84.0) | 42(89.4) | 273(83.2) | |
| Vessel distortion | 0.860 | |||
| Absent | 54(14.4) | 6(12.8) | 48(14.6) | |
| Present | 321(85.6) | 41(87.2) | 280(85.4) | |
| Abbreviations: RFS, recurrence-free survival. | ||||
The words in bold were clinical-pathological and sonographic variables included in our study.
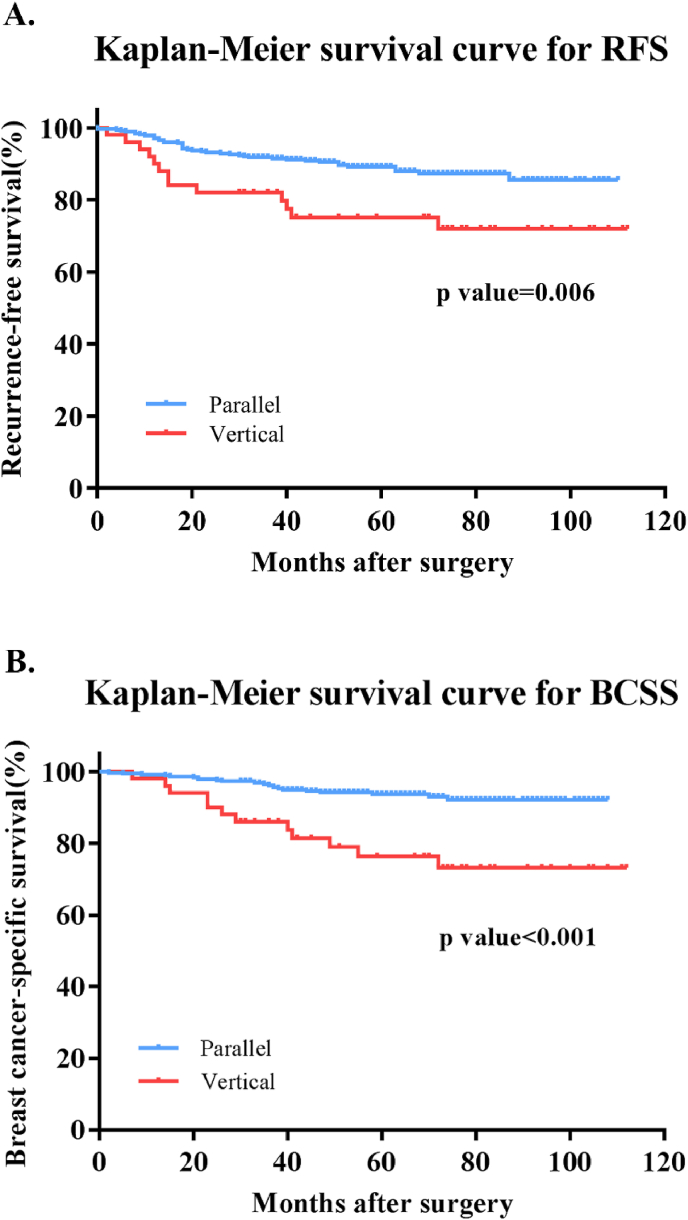
Univariate analysis of sonographic characteristics and disease outcomes was also performed. It turned out that only vertical orientation was predictive for RFS events (P = 0.006). Other sonographic features, including shape (P = 0.593), margin (P = 0.623), posterior acoustic pattern (P = 0.529), architecture distortions (P = 0.842), changes in Cooper's ligament (P = 0.640), and vessel distortions (P = 0.860) were not associated with RFS. The 5-year RFS was 75.1% for TNBC with vertical orientation while 89.2% for those with parallel orientation (Fig. 2A). Regarding BCSS, presence of vertical orientation was also an independent risk factor for breast cancer-related survival (P < 0.001). The 5-year BCSS was 76.3% and 94.1% for vertical-oriented and parallel-oriented TNBCs respectively (Fig. 2B).
Fig. 2.
Kaplan-Meier survival curves for recurrence-free survival and breast cancer-specific survival stratified by orientation in ultrasound in triple negative breast cancer. A) Vertical orientation was strongly associated with recurrence-free survival in TNBC patient (P = 0.006) B) Vertical orientation was highly associated with breast cancer mortality (P < 0.001) in TNBC patients.
3.4. Multivariate analysis of prognostic factors in TNBC
Clinicopathological and sonographic variables with a p-value <0.05 were then inputted into the multivariate predict model. As a result, tumor size, ALN metastasis, nuclear grade, LVI, Ki67 index, histopathological type and vertical sonographic features were included for RFS and BCSS multivariate analysis. Regarding RFS associated factors, tumors >2.0 cm (HR = 2.38; 95% CI, 1.30–4.37; P = 0.005), ALN positive (HR = 2.00; 95% CI, 1.11–3.59; P = 0.021), higher tumor grade (HR = 2.37; 95% CI, 1.06–5.29; P = 0.035), LVI (HR = 2.70; 95% CI, 1.25–5.78; P = 0.011), and vertical orientation in ultrasound (HR = 3.24; 95% CI, 1.66–6.31; P = 0.001) were independently associated with worse RFS (Table 3). Meanwhile, it was indicated that tumors >2.0 cm (HR = 2.59; 95% CI, 1.17–5.73; P = 0.019), positive ALN (HR = 3.43; 95% CI, 1.60–7.32; P = 0.001), LVI (HR = 2.69; 95% CI, 1.13–6.40; P = 0.025), and Ki67 index >30% (HR = 3.03; 95% CI, 1.13–8.13; P = 0.028) were independent prognostic factors for BCSS events. More importantly, patients with vertical sonographic features had significantly higher likelihood to die of breast cancer (HR = 7.03; 95% CI, 3.20–15.44; P < 0.001) (Table 4).
Table 3.
Multivariable analysis of clinicopathological and sonographic features as prognostic factors for RFS events.
| Variables | HR | 95% CI | P value | |
|---|---|---|---|---|
| LVI | Yes vs no | 2.69 | 1.25–5.78 | 0.011 |
| Tumor grade | III vs I-II | 2.37 | 1.06–5.29 | 0.035 |
| Tumor size | >2 cm vs ≤ 2 cm | 2.38 | 1.30–4.37 | 0.005 |
| Lymph nodes involvement | Yes vs no | 2.00 | 1.11–3.59 | 0.021 |
| Vertical orientation | Yes vs no | 3.24 | 1.66–6.31 | 0.001 |
| Abbreviations: RFS, recurrence-free survival; LVI, lymphovascular invasion; HR, hazard ratio; CI, confidential interval. | ||||
Table 4.
Multivariable analysis of clinicopathological and sonographic features as prognostic factors for BCSS events.
| Variables | HR | 95% CI | P value | |
|---|---|---|---|---|
| LVI | Yes vs no | 2.69 | 1.13–6.40 | 0.025 |
| Tumor size | >2 cm vs ≤ 2 cm | 2.59 | 1.17–5.73 | 0.019 |
| Lymph nodes involvement | Yes vs no | 3.43 | 1.60–7.32 | 0.001 |
| Ki67 index | >30% vs ≤ 30% | 3.03 | 1.13–8.13 | 0.028 |
| Vertical orientation | Yes vs no | 7.03 | 3.20–15.44 | <0.001 |
| Abbreviations: BCSS, breast cancer-specific survival; LVI, lymphovascular invasion; HR, hazard ratio; CI, confidential interval. | ||||
3.5. Association between ultrasound vertical-oriented feature and clinicopathological factors
Differences of clinicopathological factors between vertical-oriented and parallel-oriented groups were illustrated in Table 5. Two groups had similar distribution in histopathological types, tumor size, nuclear grade, and LVI (all P > 0.05). Notably, the average ALN metastasis number in the vertical group was 2.7 ± 1.0, which was significantly higher than that in the parallel group (1.5 ± 0.2, P = 0.003).
Table 5.
Differences of clinicopathological features between parallel subgroup and vertical subgroup.
| Variables | Vertical Group (N = 51) | Parallel Group (N = 382) | P value |
|---|---|---|---|
| Age (y) | 0.138 | ||
| ≤55 | 31 (60.8) | 190 (49.7) | |
| >55 | 20 (39.2) | 192 (50.3) | |
| Menstrual status | 0.261 | ||
| Pre/peri-menopausal | 16 (31.4) | 151 (39.5) | |
| Postmenopausal | 35 (68.6) | 231 (60.5) | |
| Histopathological types | 0.791 | ||
| IDC | 45 (88.2) | 332 (86.9) | |
| Others | 6 (11.8) | 50 (13.1) | |
| LVI | 0.505 | ||
| Absent | 24 (94.7) | 358 (93.7) | |
| Present | 2 (5.3) | 36 (6.3) | |
| Tumor grade | 0.087 | ||
| I-II | 16 (31.4) | 71 (18.6) | |
| III | 28 (54.9) | 261 (68.3) | |
| NA | 7 (13.7) | 50 (13.1) | |
| Tumor size | 0.210 | ||
| ≤2 cm | 30 (58.8) | 189 (49.5) | |
| >2 cm | 21 (41.2) | 193 (50.5) | |
| Lymph node metastasis (mean ± SD) | 2.7 ± 1.0 | 1.5 ± 0.2 | 0.003 |
| Ki-67 (%, mean ± SD) | 42.7 ± 4.2 | 52.2 ± 1.4 | 0.067 |
| Abbreviations: IDC, invasive ductal carcinoma; LVI, lymphovascular invasion; NA, not applicable; SD, standard deviation. | |||
The words in bold were clinical-pathological and sonographic variables included in our study.
3.6. Correlation between vertical orientation and other sonographic features
Relationship between vertical orientation and other ultrasound characteristics was shown in Supplemental Table 3. The presence of vertical orientation was statistically correlated with angular margin (r = 0.149, P = 0.002), posterior acoustic shadow (r = 0.152, P = 0.002), wide (r = 0.115, P = 0.026), and distorted peritumoral vessels (r = 0.132, P = 0.011) in ultrasound.
4. Discussion
Triple negative breast cancer is a highly heterogeneous subtype of breast cancer and has distinct recurrence pattern. Our present study aimed to take a deep insight into the prognosis predictive value of sonographic features in TNBC. To the best of our knowledge, vertical orientation in ultrasound was revealed to be predictive for poor prognosis in TNBC for the first time. Vertical orientation was also found to be associated with higher ALN metastasis burden in TNBC patients.
Clinicopathological factors including larger tumor size, ALN metastasis and higher nuclear grade have been commonly reported to be related to more recurrence events and more breast cancer-related deaths [[13], [14], [15]], which was in concordance with our findings. LVI was defined as the presence of tumor emboli in the lymphatics or vessels in peritumoral environment. Although elucidation of the mechanism of LVI remained unclear, invasive breast cancers with LVI have been confirmed to develop distant metastasis and overall recurrence more likely [16,17]. A large population-based study from Denmark of over 15000 patients found LVI as an independent risk factor in patients of high risk, including TNBC subtype [18]. Likewise, LVI was found to be an independent prognostic factor of inferior RFS and BCSS in our study. In the other hand, the role of proliferation factor Ki67 in predicting prognosis of TNBC was still conflicting. Several articles found higher Ki-67 index an independent prognosis factor for poor DFS, RFS and OS in TNBC [19,20], while other studies came to the inverse conclusion [21,22]. A population-based study of 841 TNBCs concluded that the Ki67 index was predictive for mortality but not for recurrence, which was comparable with our findings [21]. The discordances between these articles may be owing to different cut-off values of Ki-67 stratification, ranging from 10% to 40%. Furthermore, novel biomarkers and gene signatures were found to exhibit predictive value for TNBC outcomes. According to a pooled study from C. Denkert et al., higher concentration of tumor-infiltrating leukocytes was associated with superior DFS and OS in TNBC [23]. However, information on these biomarkers were not included in our study.
Apart from the conventional tumor attributes, the association between preoperative imaging features and prognosis of TNBC has been taken into consideration in a few literatures. However, regarding the predictive value for preoperative breast imaging features, studies mainly focused on MRI and mammography [[8], [9], [10],24,25]. A Swedish cohort study of 498 breast cancer patients discovered that casting type calcification (HR = 3.47; 95% CI, 2.21–5.53) and architecture distortion (HR = 4.43; 95% CI, 2.02–9.50) in mammography indicated poorer survival in TNBC population [9]. Additionally, M. S. Bae et al. found an increased recurrence risk in TNBC patients presenting with dense breast tissue in mammography [8]. Considering the preoperative MRI, its role in predicting outcomes in TNBCs were still under debate. Some articles reported that the absence of preoperative MRI was related with unfavorable outcomes in TNBC patients while the others came to the opposite conclusion [8,24]. Nevertheless, for those underwent preoperative MRI, a research from Netherland once reported that the presence of rim enhancement was predictive for worse long-term outcomes in TNBC [25]. Literatures concerning the prognosis and sonographic traits were limited [11,26]. Soo-Yeon Kim et al. investigated the association between ultrasound factors and breast cancer recurrences and it concluded that tumors with BI-RADS 4A category at screening had the worst outcome compared to other categories [11]. However, only 40 (8%) TNBC patients were included and no specific descriptive US factors were explored in this study. Furthermore, in another research to study sonographic features and prognosis in breast cancer, Sae Rom Chung et al. divided the type of ultrasound lesion into mass or non-mass categories and found there was no correlation with prognosis [26]. In our study, we included 433 TNBC patients and analyzed the association between detailed sonographic characteristics and disease outcome. It was first reported that the presence of vertical orientation in ultrasound may indicate inferior RFS (P = 0.001) and BCSS (P < 0.001) in TNBC in our study population. For TNBC with vertical orientation, the 5-year RFS and BCSS was 75.1% and 76.1% respectively, significantly poorer than 89.2% and 94.1% for those with parallel orientation. Furthermore, sonographic vertical orientation was found to be associated with higher burden of ALN metastasis than parallel orientation (2.7 ± 1.0 vs 1.5 ± 0.2, P = 0.003).
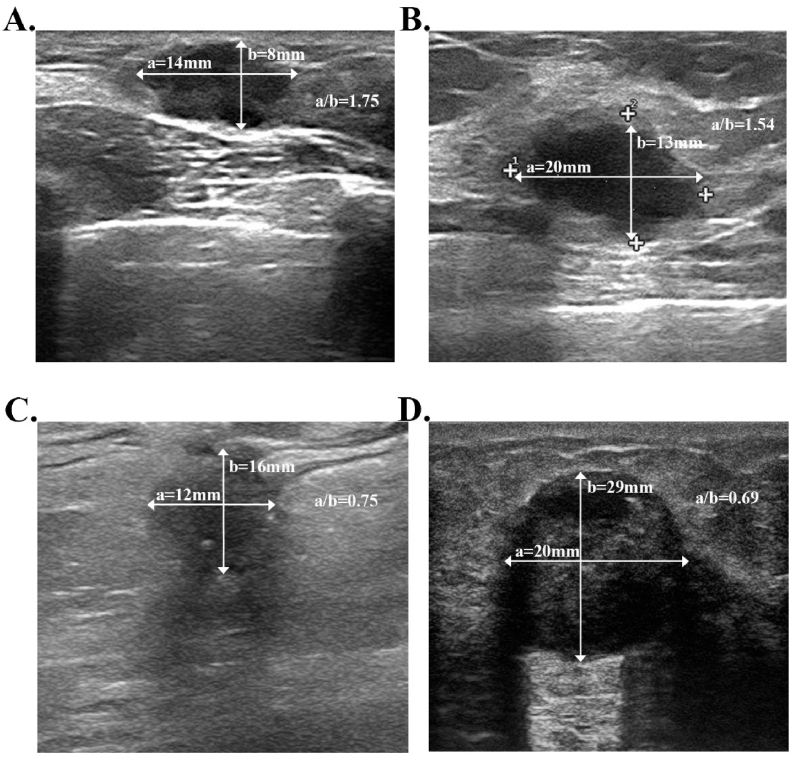
A vertical orientation was commonly interpreted as a horizonal-to-anteroposterior ratio less than 1.0 (Fig. 3). Otherwise, the mass would be defined as parallelly oriented. It reflected a growth process that went through normal tissue-plane boundaries in breast and was reported to indicate more aggressive biological behavior in previous studies [27,28]. SH Kim et al. investigated sonogram features and histological markers in 458 breast cancers and concluded that invasive breast cancers tend to display as vertical orientation compared with in situ breast cancers (p < 0.05), indicating that vertical orientation may be the interpretation of higher proliferative activity [29]. Additionally, it was reported by Q Guo et al. that vertical growth orientation was more likely to present in images of high-risk group (p < 0.001), which was defined by 2007 St. Gallen Criteria: ≥4 positive ALN or 1–3 positive ALN with either ER−/PR− or HER2+ [30], which was in concordance with our finding that tumors with more ALN burden tend to display as vertical orientation in sonograms. Additionally, in our study, the presence of vertical orientation was positively correlated with angular margin and posterior acoustic shadow in ultrasound. An angular margin may reflect higher growing speed and inconsistent growing direction of a mass [31]. Likewise, the posterior acoustic shadow was related with higher mitotic rate which caused more attenuation of US waves than surrounding tissue [30]. These sonographic patterns correlated with vertical orientation also indicated its value in reflecting aggressive behaviors and predicting worse outcomes in TNBC. Controversially, some articles came to the conclusion that tumors with higher recurrence risk may share the sonographic traits of a benign neoplasm, especially in TNBC [[31], [32], [33]]. Eun Y. Chae tried to identify the relationship between ultrasound characteristics and Recurrence Score (RS) of 21 genes in 267 HR+/HER2-breast cancers and reported that parallel orientation was related to higher RS score (OR = 5.53) [31]. However, the study did not take ALN metastasis as a risk criterion and the biological behaviors of ER+/HER2-breast cancer may be distinct from that of TNBC. Further research was warranted to study the relationship between tumor orientation and pathological features in different molecular subtypes.
Fig. 3.
Sonogram imaging for different tumor growth orientations in triple negative breast cancer patients. The lines with arrow illustrated different diameters of the lesions. Line “a” showed the horizonal diameter and line “b” showed the anteroposterior diameter. A vertical orientation was commonly interpreted as a horizonal-to-anteroposterior ratio less than 1.0. Otherwise, the mass would be defined as parallelly oriented. A) 30-year-old woman with triple negative breast cancer and with no recurrence after surgery. B) 56-year-old woman with triple negative breast cancer and with no recurrence. C) 67-year-old women with triple negative breast cancer and no recurrence. D) 51-year-old women with triple negative breast cancer and died of distant metastasis 23 months after surgery. A) and B) both showed breast tumors of parallel orientation. C) and D) were two lesions both displayed vertical orientation.
Compared with mammography and MRI, breast ultrasound has the advantages of no radiation, well tolerance, and wide availability. The strength of our study was that we comprehensively analyzed association between detailed ultrasound characteristics and disease prognosis in TNBC. To the best of our knowledge, this is the first report that tumors presenting vertical orientation in ultrasound may have worse outcome in TNBC patients. It can provide a clue that certain features of sonography could help us better evaluate biological behaviors and outcomes of TNBC in clinical practice. According to the long-term follow-up results of the ACOSOG Z0011 trial, further ALND could be omitted for patients who meet the eligibility criteria of the ACOSOG Z0011 trial [34,35]. In our study, TNBC presenting with vertical orientation was associated with higher burden of ALN metastases compared with parallel orientation (2.7 ± 1.0 vs 1.5 ± 0.2, P = 0.003). Thus, we need to pay more attention to apply the ACOSOG Z0011 trial procedure for TNBC patients with vertical orientation. Furthermore, vertical orientation in ultrasound was associated with inferior disease outcomes in TNBC patient in out cohort. As shown in the CREATE-X trial, additional capecitabine adjuvant treatment could significantly improve tumor prognosis among TNBC patients with residual invasive tumor after neoadjuvant therapy [36]. As a result, for TNBC patients with vertical orientation in the ultrasound, neoadjuvant treatment may be firstly recommended and capecitabine could be assigned in the adjuvant settings for those with residual tumor, thus to improve the long-term outcomes for TNBC patients. Further studies are also needed to validate the relationship between specific sonographic findings and prognosis in a larger scale of TNBC populations as well as other molecular subtypes.
There were certainly several limitations in this study. First of all, the study was retrospective and single-centered, which may lead to unavoidable bias in basic characteristics of study population. Secondly, regarding the heterogeneous nature of TNBC, different subtypes of TNBC such as basal-like and normal-like TNBCs may present with distinct ultrasound features [37]. However, more detailed information on molecular biomarkers such as CK5/6, BRCA, and EGFR were not available in this study.
5. Conclusion
In conclusion, vertical orientation in preoperative ultrasound was independently associated with worse prognosis and a higher burden of ALN metastasis in TNBC patients. Besides traditional clinicopathological factors, tumor orientation in ultrasound could be considered as a complementary risk factor for TNBC, which deserves further clinical evaluation.
Declaration of competing interest
None.
Acknowledgement
The authors would like to thank Ms. Yidong Du for her assistance in the operation and management of SJTU-BCDB. We also appreciate the radiologists, study coordinators, nurses, and physicians for their great assistance in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2019.10.006.
Contributor Information
Xiaosong Chen, Email: kwshen@medmail.com.cn.
Kunwei Shen, Email: chenxiaosong0156@hotmail.com.
Funding
The authors appreciated the financial support from the National Natural Science Foundation of China (Grant Number: 81772797), Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20172007); Ruijin Hospital, Shanghai Jiaotong University School of Medicine-"Guangci Excellent Youth Training Program” (GCQN-2017-A18). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Siegel Rebecca L., Miller Kimberly D., Jemal A. Cancer statistics, 2018. Ca - Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C.E., Ma J., Goding Sauer A., Newman L.A., Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. Ca - Cancer J Clin. 2017;67(6):439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 3.Goldhirsch A., Winer E.P., Coates A.S., Gelber R.D., Piccart-Gebhart M., Thürlimann B., Bergh J. Personalizing the treatment of women with early breast cancer: highlights of the St gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent R., Trudeau M., Pritchard K.I., Hanna W.M., Kahn H.K., Sawka C.A., Narod S.A. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 6.Penault-Llorca F., Viale G. Pathological and molecular diagnosis of triple-negative breast cancer: a clinical perspective. Ann Oncol. 2012;23(suppl_6):vi19–vi22. doi: 10.1093/annonc/mds190. [DOI] [PubMed] [Google Scholar]
- 7.Bianchini G., Balko J.M., Mayer I.A., Sanders M.E., Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae M.S., Moon H.G., Han W., Noh D.Y., Ryu H.S., Park I.A., Moon W.K. Early stage triple-negative breast cancer: imaging and clinical-pathologic factors associated with recurrence. Radiology. 2015;278(2):356–364. doi: 10.1148/radiol.2015150089. [DOI] [PubMed] [Google Scholar]
- 9.Tsau H.S., Yen A.M.F., Fann J.C.Y., Wu W.Y.Y., Yu C.P., Chen S.L.S., Chang K.J. Mammographic tumour appearance and triple-negative breast cancer associated with long-term prognosis of breast cancer death: a Swedish Cohort Study. Canc epidemiol. 2015;39(2):200–208. doi: 10.1016/j.canep.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Tabár L., Chen H.H., Duffy S.W., Yen M.F., Chiang C.F., Dean P.B., Smith R.A. A novel method for prediction of long-term outcome of women with T1a, T1b, and 10–14 mm invasive breast cancers: a prospective study. The Lancet. 2000;355(9202):429–433. doi: 10.1016/s0140-6736(00)82008-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim S.Y., Han B.K., Kim E.K., Choi W.J., Choi Y., Kim H.H., Moon W.K. Breast cancer detected at screening US: survival rates and clinical-pathologic and imaging factors associated with recurrence. Radiology. 2017;284(2):354–364. doi: 10.1148/radiol.2017162348. [DOI] [PubMed] [Google Scholar]
- 12.Hammond M.E.H., Hayes D.F., Dowsett M., Allred D.C., Hagerty K.L., Badve S., Hicks D.G. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134(7):e48–e72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 13.Rakha E.A., El-Sayed M.E., Green A.R., Lee A.H., Robertson J.F., Ellis I.O. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109(1):25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 14.Costa R.L., Gradishar W.J. Triple-negative breast cancer: current practice and future directions. J oncol pract. 2017;13(5):301–303. doi: 10.1200/JOP.2017.023333. [DOI] [PubMed] [Google Scholar]
- 15.Dent R., Hanna W.M., Trudeau M., Rawlinson E., Sun P., Narod S.A. Pattern of metastatic spread in triple-negative breast cancer. Breast Canc Res Treat. 2009;115(2):423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 16.Lee A.H.S., Pinder S.E., Macmillan R.D., Mitchell M., Ellis I.O., Elston C.W., Blamey R.W. Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer. 2006;42(3):357–362. doi: 10.1016/j.ejca.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Song Y.J., Shin S.H., Cho J.S., Park M.H., Yoon J.H., Jegal Y.J. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J breast canc. 2011;14(3):198–203. doi: 10.4048/jbc.2011.14.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ejlertsen B., Jensen M.B., Rank F., Rasmussen B.B., Christiansen P., Kroman N., Danish Breast Cancer Cooperative Group Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. JNCI: J Natl Cancer Inst. 2009;101(10):729–735. doi: 10.1093/jnci/djp090. [DOI] [PubMed] [Google Scholar]
- 19.Keam B., Im S.A., Lee K.H., Han S.W., Oh D.Y., Kim J.H., Park I.A. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Canc Res. 2011;13(2):R22. doi: 10.1186/bcr2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Y., Yuan Y., Liu G., Wei Y. P53 and Ki-67 as prognostic markers in triple-negative breast cancer patients. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0172324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urru S.A.M., Gallus S., Bosetti C., Moi T., Medda R., Sollai E., Palmas D. Clinical and pathological factors influencing survival in a large cohort of triple-negative breast cancer patients. BMC Canc. 2018;18(1):56. doi: 10.1186/s12885-017-3969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aleskandarany M.A., Green A.R., Benhasouna A.A., Barros F.F., Neal K., Reis-Filho J.S., Rakha E.A. Prognostic value of proliferation assay in the luminal, HER2-positive, and triple-negative biologic classes of breast cancer. Breast Canc Res. 2012;14(1):R3. doi: 10.1186/bcr3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denkert C., von Minckwitz G., Darb-Esfahani S., Lederer B., Heppner B.I., Weber K.E., Schmitt W.D. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 24.Houssami N., Turner R., Macaskill P., Turnbull L.W., McCready D.R., Tuttle T.M., Solin L.J. An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol. 2014;32(5):392–401. doi: 10.1200/JCO.2013.52.7515. [DOI] [PubMed] [Google Scholar]
- 25.Alexander M.T., Loo C.E., Wesseling J., Pijnappel R.M., Gilhuijs K.G. Association between rim enhancement of breast cancer on dynamic contrast-enhanced MRI and patient outcome: impact of subtype. Breast Canc Res Treat. 2014;148(3):541–551. doi: 10.1007/s10549-014-3170-9. [DOI] [PubMed] [Google Scholar]
- 26.Chung S.R., Choi W.J., Cha J.H. Prognostic factors predicting recurrence in invasive breast cancer: an analysis of radiological and clinicopathological factors[J] Asian J Surg. 2019;42(5):613–620. doi: 10.1016/j.asjsur.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Hong A.S., Rosen E.L., Soo M.S., Baker J.A. BI-RADS for sonography: positive and negative predictive values of sonographic features. Am J Roentgenol. 2005;184(4):1260–1265. doi: 10.2214/ajr.184.4.01841260. [DOI] [PubMed] [Google Scholar]
- 28.Paulinelli R.R., Freitas-Junior R., de Lucena C.Ê.M., Moreira M.A.R., de Moraes V.A., Bernardes-Júnior J.R.M., Teixeira D.A. Sonobreast: predicting individualized probabilities of malignancy in solid breast masses with echographic expression. Breast J. 2011;17(2):152–159. doi: 10.1111/j.1524-4741.2010.01046.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.H., Seo B.K., Lee J., Kim S.J., Cho K.R., Lee K.Y., Lee J.H. Correlation of ultrasound findings with histology, tumor grade, and biological markers in breast cancer. Acta Oncol. 2008;47(8):1531–1538. doi: 10.1080/02841860801971413. [DOI] [PubMed] [Google Scholar]
- 30.Guo Q., Zhang L., Di Z., Ning C., Dong Z., Li Z., Tian J. Assessing risk category of breast cancer by ultrasound imaging characteristics. Ultrasound Med Biol. 2018;44(4):815–824. doi: 10.1016/j.ultrasmedbio.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Chae E.Y., Moon W.K., Kim H.H., Kim W.H., Cha J.H., Shin H.J., Ahn S.H. Association between ultrasound features and the 21-gene recurrence score assays in patients with oestrogen receptor-positive, HER2-negative, invasive breast cancer. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0158461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dialani V., Gaur S., Mehta T.S., Venkataraman S., Fein-Zachary V., Phillips J., Slanetz P.J. Prediction of low versus high recurrence scores in estrogen receptor–positive, lymph node–negative invasive breast cancer on the basis of radiologic-pathologic features: comparison with oncotype DX test recurrence scores. Radiology. 2016;280(2):370–378. doi: 10.1148/radiol.2016151149. [DOI] [PubMed] [Google Scholar]
- 33.Yepes M.M., Romilly A.P., Collado-Mesa F., Net J.M., Kiszonas R., Arheart K.L., Glück S. Can mammographic and sonographic imaging features predict the Oncotype DX™ recurrence score in T1 and T2, hormone receptor positive, HER2 negative and axillary lymph node negative breast cancers? Breast Canc Res Treat. 2014;148(1):117–123. doi: 10.1007/s10549-014-3143-z. [DOI] [PubMed] [Google Scholar]
- 34.Giuliano A.E., Hunt K.K., Ballman K.V., Beitsch P.D., Whitworth P.W., Blumencranz P.W., Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. Jama. 2011;305(6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giuliano A.E., Ballman K.V., McCall L., Beitsch P.D., Brennan M.B., Kelemen P.R., Leitch A.M. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. Jama. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masuda N., Lee S.J., Ohtani S., Im Y.H., Lee E.S., Yokota I., Yanagita Y. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 37.Li Z., Ren M., Tian J., Jiang S., Liu Y., Zhang L., Wu T. The differences in ultrasound and clinicopathological features between basal-like and normal-like subtypes of triple negative breast cancer. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0114820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.