Abstract
Background
Randomized controlled trials (RCT) of scalp cooling (SC) to prevent chemotherapy induced alopecia (CIA) did not evaluate its effect on hair regrowth (HR) and was conducted in a predominantly taxane (T) treated population. We conducted an RCT of SC in a setting of anthracycline (A) and taxane chemotherapy (CT) and assessed its effect on CIA and HR.
Methods
Non-metastatic breast cancer women undergoing (neo) adjuvant CT were randomized to receive SC using the Paxman scalp cooling system during every cycle of CT, or no SC. The primary end point (PEP) was successful hair preservation (HP) assessed clinically and by review of photographs after CT. HR was assessed at 6 and 12 weeks.
Results
51 patients were randomized to SC (34) or control arm (17) in a 2:1 ratio. Twenty-five (49%) patients received A followed by T and the two arms were balanced with respect to this factor. HP rate was significantly higher in SC arm compared to control arm (56.3% vs 0%, P = 0.000004). HR was higher in SC arm compared to control at 6 weeks (89% vs 12%; P < 0.001) and 12 weeks (100% vs 59%, P = 0.0003). Loss of hair at PEP evaluation, which was a quality of life measure, was significantly lower in SC versus control arm (45% vs 82%, P = 0.016). There were no grade 3–4 cold related adverse effects.
Conclusions
Women with breast cancer receiving A or T chemotherapy receiving SC were significantly more likely to have less than 50% hair loss after CT, superior hair regrowth and improvement in patient reported outcomes, with acceptable tolerance. It merits wider usage.
Keywords: Scalp cooling, Chemotherapy, Alopecia, Breast cancer, Quality of life
1. Introduction
Chemotherapy-induced alopecia (CIA) is not life threatening; however, it is one of the most visibly distressing adverse effects (AE) of chemotherapy (CT). Clinicians often neglect alopecia, owing to the fact that it is completely reversible, and focus more towards treating the disease [1,2].
CT attacks rapidly dividing cells, including cancer cells, as well as normal cells lining mucosal surfaces and hair follicles. At any time point, around 85–90% of hair follicles are in a rapidly dividing phase and are hence, prone to damage by chemotherapeutic agents [3]. Scalp cooling (SC) causes scalp vasoconstriction, reduces follicular metabolic rate, with resultant diminished cellular drug uptake [3,4]. This diminished CT delivery leading to potential increase in scalp metastasis was a concern for a long period. However, long-term safety data allayed this fear [3,[5], [6], [7]]. An increase in the risk of systemic recurrences, after the use of scalp cooling in patients with advanced breast cancer, still remains an unanswered question [8]. Over the years, there have been a wide variety of cooling techniques used, from basic cryo gel caps, to modern scalp cooling systems. Even the success rate has had a wide variation (0%–90%) [[9], [10], [11]]. This could be attributed to multiple cancer types being enrolled in trials, varied CT regimens, intrinsic patient/hair characteristics, technical reasons like optimal cap fit, and use of non-validated outcome measures (use of wigs, subjective hair loss assessment) [3,6,11]. A meta-analysis as well as other randomized control trials (RCTs) showed positive results with SC with acceptable tolerance [9,[12], [13], [14]]. Various other non-randomized studies have also reported beneficial outcomes with SC [[15], [16], [17], [18], [19]]. However, in most studies, taxanes (T) were preferentially used, and only CIA was measured, not hair regrowth (HR), which is also an important landmark towards attaining normalcy [12]. It was imperative to test SC for CIA and HR with a regimen containing both anthracyclines (A) and T, as it is the commonest CT regimen in breast cancer and many other cancers. Patient characteristics, climatic variations and cultural practices vary in different parts of the world, and hence efficacy of SC merited testing in an Asian population.
2. Methods
2.1. Study design and patients
This was an investigator initiated, open label, single centre, RCT that recruited consecutive women with non-metastatic breast cancer planned for curative intent (neo) adjuvant CT containing both A and T in sequential fashion between December 16, 2016 to July 24, 2018. The clinical trial registration number was CTRI/2017/02/007896.
Women were not eligible if they had Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v 4.0) alopecia grade higher than 0, history of prior CT, personal history of migraines, cluster or tension headaches, cold agglutinin disease or cold urticaria and lichen planus or lupus.
The institutional review board approved the protocol and written informed consent was obtained from all participants prior to randomization.
Access devices (sole authorized distributor of Paxman Coolers Limited), Bengaluru provided the Paxman scalp cooling system and funding for the research coordinator and study nurse. However, they had no involvement in the design or conduct of the study, the collection, management, analysis, or interpretation of the data, the preparation, review, or approval of the manuscript.
2.2. Randomization and intervention
Randomization was performed centrally using the method of minimization, that made assignments based on a computer-generated random number. Subjects were stratified by the sequence of CT (A or T first), and subsequently randomized to the SC or to no SC (control) arm in a 2:1 ratio.
2.3. Scalp cooling procedure
SC was done using Paxman scalp cooling system which is an FDA approved refrigerated scalp cooling system. SC was initiated 30 min prior to each CT cycle, with scalp temperature maintained throughout CT, and for 90 min post CT completion. Prior to starting the first cycle, the study nurse determined the appropriate sized cap for each woman and at the start of each cycle, the cap was optimally fitted on participant’s head in accordance with the product information recommendations.
2.4. Assessment of estimated hair loss
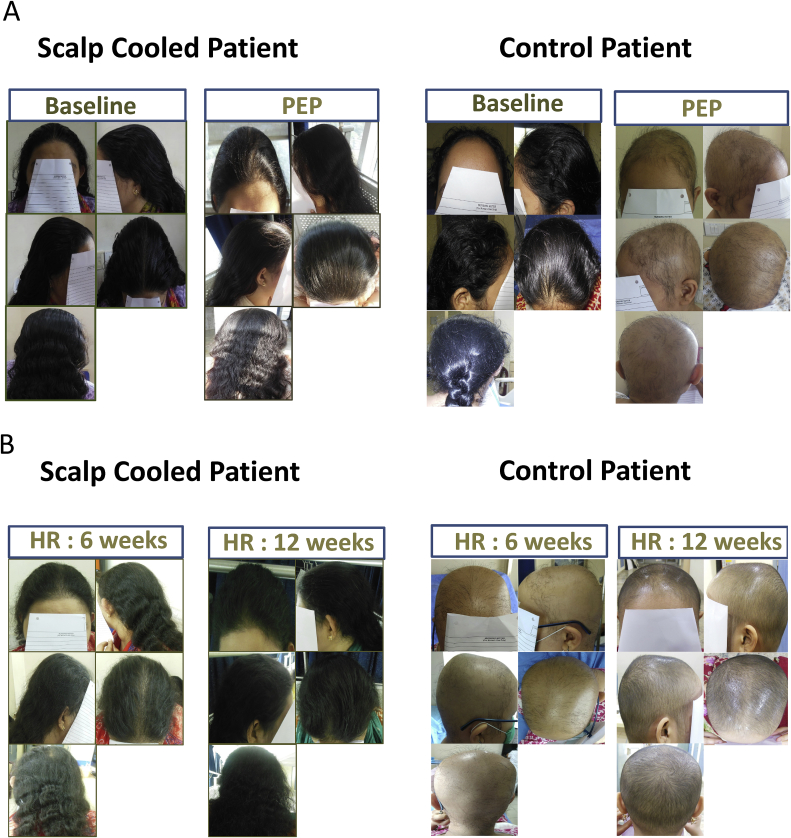
Alopecia grading was carried out clinically (visual inspection) by the primary investigator (PI) and by photographing the participant’s scalps in both the SC and control groups.
The clinical assessment was carried out at baseline, before the start of each CT cycle and at the primary and secondary efficacy endpoints (PEP and SEP). Photographs of the first 25 subjects who completed at least 1 cycle of CT were obtained at each alopecia assessment (3 weekly) while for subjects enrolled afterwards, photographs were taken at baseline, at PEP and at SEP. Photographs captured hair from the front, back, both sides, and from above.
For further validation, independent observers (IO) outside the study, and the subject themselves, also assessed the hair fall and hair regrowth.
2.5. Study endpoints
The PEP was successful hair preservation (HP) assessed clinically and by review of 5 photographs, using the CTCAE version 4.0 scale for alopecia (grade 0 = no hair loss, grade1 = < 50% hair loss, not requiring a wig) after 4 cycles or 12 weeks of CT. Success was defined as grade 0 and 1 alopecia. Failure was defined as grade 2 alopecia (>50% hair loss, requiring the use of a wig). Participant withdrawal after completion of one cycle of CT were deemed treatment failures.
SEP comprised of HR assessment at 6 and 12 weeks (defined as attainment of grade 0/1 alopecia post completion of all CT). Other SEP were device related AEs, determined by patient reported symptoms and by scalp examination and tolerance to SC, defined as the percentage of patients who could complete all planned CT cycles using SC. Patients reported AEs (feeling cold, headache, heaviness etc.) were recorded using the case record form (CRF) (eFig. 1 in Supplement) along with compulsion to use head coverings/wigs. In addition, CRF had provision to record cap device malfunctions. None of the standard CT related AEs were captured.
Participant reported scores on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Breast cancer module (EORTC-QOL-questionnaire–BR23) were assessed at baseline and at PEP as planned. Later on, a post hoc QOL analysis was carried out at the SEP of HR assessment at 6 weeks post completion of all CT.
The other exploratory efficacy SEPs were success in HP, assessed by the IO and by the subject at PEP, as well as HR assessment at 6 weeks post completion of all CT cycles, to further validate investigator’s assessment.
2.6. Trial amendment
The clinical trial was amended after inception to include other commonly practiced CT regimens and schedules for breast cancer (still A or T based). Stratification was introduced to test the success rate of sequence of administration of A and T. SEP of HR at 6 and 12 weeks after completion of all CT was also included. The protocol amendments are available in the online supplement.
2.7. Statistical methods
We had set the HP rate to 40% in the SC group and 5.8% in the control group based on prior study results [[20], [21], [22]]. A sample size of 51 with calculations using chi-square test and patient allocation 2:1 ratio in SC and control arms, respectively, (with assumption of 25% drop out rates) provided 80% power at 5% level of significance (one-sided) for this trial.
Analyses for efficacy of SC was done on the modified intent-to-treat population (M-ITT), defined as eligible and randomized participants who received at least one cycle of CT (including precooling, cooling during chemotherapy and post cooling). The primary efficacy analysis compared the success of HP (as assessed by the investigator) between SC and controls, using Fisher exact test. The 95% confidence interval (CI) of the success proportion for patients using SC was estimated using Wilson method without continuity correction. Concordance with respect to PEP (success of HP) and SEP (HR) between PI, IO and the subject was assessed using Cohen’s Kappa Agreement statistics and the agreement results are presented along with 95% CI. Data were analysed using a logistic regression model with penalized maximum likelihood estimation, as the control group did not show any success rate. Independent variables included treatment group, and CT regimen.
Changes in QOL from baseline to the PEP were assessed by the EORTC BR-23 questionnaires [23]. For questions related to QOL, response categories were collapsed to (1) not at all or a little bit and (2) quite a bit or very much. Category (2) of the QOL was compared between SC and control groups using chi-square test and the 95% (CI) of the differences were estimated using an exact method on binomial distribution. Planned exploratory secondary analyses summarized perceived HP, based on the use of wigs and/or head wraps by descriptive statistics. Safety analyses were planned to report frequencies of device-associated AEs. P ≤ 0.05 was considered statistically significant. Median follow-up time was calculated from the date of randomization until last follow-up. All analyses were performed in SPSS version 24 and STATA 14 statistical software.
3. Results
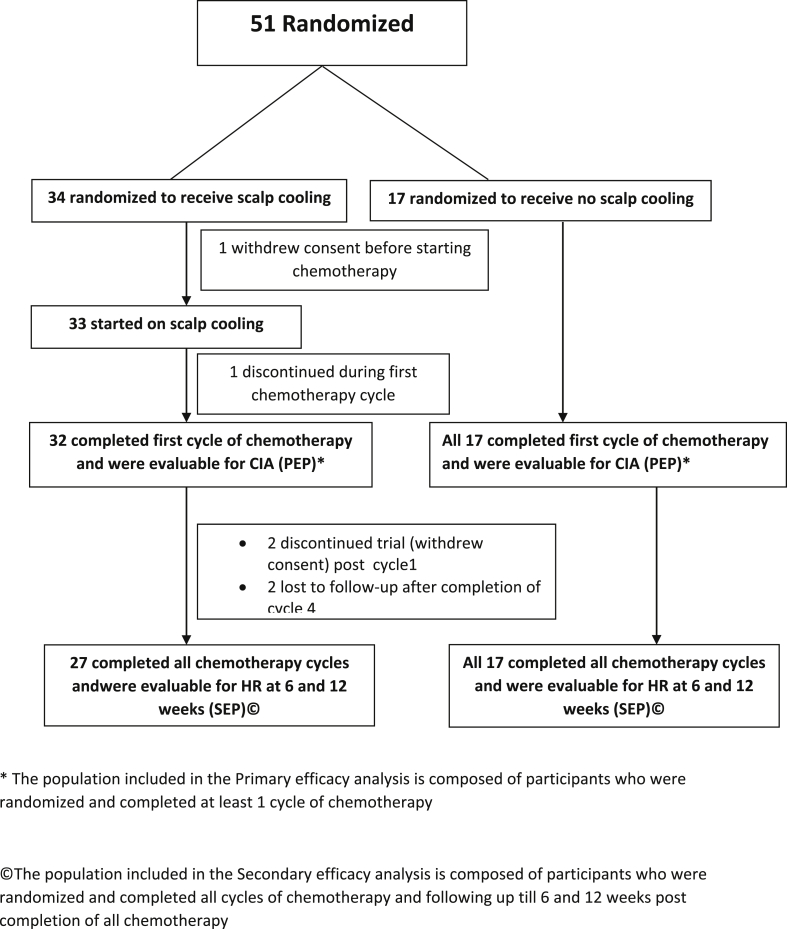
Between December 2016 to July 2018, 51 participants were enrolled. Afterobtaining written informed consent, they were then randomized to SC (34) or to control arm (17) in the ratio of 2:1. Among these 51 randomized patients, 49 participants could complete at least 1 cycle of CT, were evaluable for the PEP, and constituted the M-ITT population (Fig. 1). The median age was 38 (21–58) years and 86% of women were pre or perimenopausal (Table 1). Of these, 25 (49%) received A followed by T, while 51% received the reverse sequence. The two arms were balanced with respect to this factor eTable 1(Table 2).
Fig. 1.
Flow Diagram of the Trial, December 16, 2016, Through July 24, 2018, at analysis.
Table 1.
Baseline Characteristics (n = 49, modified intention to treat population).
| Parameter |
No. (%) of Patientsa |
|
|---|---|---|
| Cooling(n = 32) | Non-Cooling (n = 17) | |
| Age [Median (range)] | 37 (21–49) | 44 (30–58) |
| Menopausal status | ||
| Premenopausal Postmenopausal |
29 (91) | 13 (77) |
| 03 (9) | 04 (23) | |
| Body Mass Index [Median (range)]b | 24.19 (17.03–31.88) | 27.3 (21.77–43.14) |
|
Thyroid Status Euthyroid Hypothyroid |
– | – |
| 31 (97) | 15 (88) | |
| 01 (3) | 02 (12) | |
|
Education Qualification Primary/>Primary Secondary Higher |
||
| 07 (22) | 03 (18) | |
| 08 (25) | 05 (29) | |
| 17 (53) | 09 (53) | |
|
Chemotherapy Regimen sequence Anthracycline f/b Taxane Taxane f/b anthracycline |
15 (47) | 09 (53) |
| 17 (53) | 08 (47) | |
|
Breast Cancer Stage Early LABCc |
||
| 19 (59) | 11 (65) | |
| 13 (41) | 06 (35) | |
| Family history | ||
| Yes | 09 (28) | 05 (29) |
| No | 23 (72) | 12 (71) |
Unless otherwise indicated.
Calculated as weight in kilograms divided by height in meters square.
LABC: Locally advanced breast cancer.
Table 2.
Chemotherapy regimen in treatment and control group.
| Sr. No. | Chemotherapy Regimen assigned |
No. of Patients |
|
|---|---|---|---|
| Scalp cooling group (n = 34) | Control group (n = 17) | ||
| 1 | Adriamycin (60 mg/m2)/Epirubicin (90 mg/m2) and Cyclophosphamide (600 mg/m2) for 4 cycles every 2 or 3 weekly followed by Paclitaxel (80 mg/m2) weekly for 12 weeks/Paclitaxel (175 mg/m2) for 4 cycles every 2 or 3 weekly/Docetaxel (90 mg/m2)a for 4 cycles every 3 weekly | 16 | 09 |
| 2 | Paclitaxel (80 mg/m2) weekly for 12 weeks/Paclitaxel (175 mg/m2) for 4 cycles every 2 or 3 weekly/Docetaxel (90 mg/m2)a for 4 cycles every 3 weekly followed by Adriamycin (60 mg/m2)/Epirubicin (90 mg/m2) and Cyclophosphamide (600 mg/m2) for 4 cycles every 2 or 3 weekly | 18 | 08 |
2 patients in control arm were assigned for paclitaxel, however due to significant side effects (peripheral neuropathy), they were given 3 weekly Docetaxel.
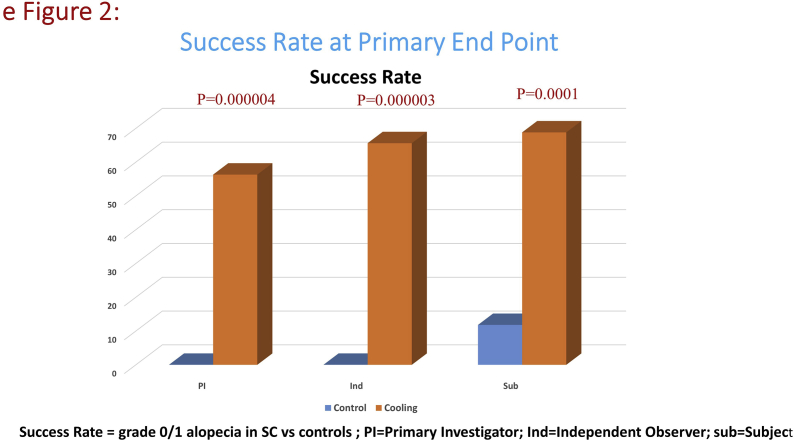
At the time of analysis, 18 of 32 evaluable participants in the SC arm (56.3%; 95%CI 37.9%–73.1%) and 0 of 17 (0%) participants in the control group had successful HP (difference 56.3%; 95% CI 31%–73%, P = 0.00004) (Fig. 2 A, eFig. 2). There were significant differences in the success of HP depending on drug sequence. In patients who received T first, 13 of 17 (77%) patients had successful HP. In those who received A first, only 5 of 15 (33%) had successful HP. The difference was statistically significant (P = 0.0307).
Fig. 2.
A: Scalp cooling with the Paxman Cooler System versus control (comparison of CIA at Primary End Point). B: Scalp cooling with the Paxman Cooler System versus control (Hair Regrowth Rate at Primary End Point of 6 and 12 weeks post chemotherapy).
The HR rate was also significantly better in SC group. At 6 weeks, 89% in SC arm while 12% in control arm (difference 77%; 95%CI 49%–88%, P < 0.0001) and at 12 weeks, 100% in SC versus 59% in control arm (difference 41%; 95%CI18%-64%, P = 0.0003) had grade 0/1 alopecia indicative of attainment of adequate HR (Fig. 2 B).
The concordance rate with PI and IO with regard to successful HP was 0.914 (0.798–1.000) at PEP and 0.858 (0.703–1.000) at 6 weeks. The PI and subject concordance rate was 0.754 (0.575–0.933) at PEP and 0.858 (0.703–1.000) at 6 weeks.
There was compulsion to use headcovers/wigs by 15/32 (46.9%; 95%CI 30.9–63.6) of the patients who received SC and 17/17 (100%; 95% CI 81.6–100) among controls (P = 0.0001).
On logistic regression analysis, chemotherapy regimen (T first or A first) and treatment group (scalp cooling or no scalp cooling) were independently associated with success rates as assessed by PI, with T first performing better.
3.1. Quality of life
One of five QOL measures were significantly better in SC group at the time of PEP as well as at SEP (after 6 weeks of CT completion) assessment. Loss of hair was reported by 45% and 21% in SC arm, versus 82% and 53% in the control arm at PEP and SEP respectively (P = 0.016 and 0.0297 respectively).
3.2. Adverse Events
SC was well tolerated with no grade 3/4 AEs; majority of the women had grade 1 (57%) and 12% had grade 2 AEs. There were a total of 98 episodes of AEs among 325 cycles administered (30%); of which 92 (28%) were grade 1 and 6 (2%) were grade 2. The most common AEs were Grade1/2 headache and grade1/2 feeling of coldness constituting 30% and 42% of all AE episodes, respectively (eTable 3). Of 34 randomized to SC, 27 (80%) participants completed all the CT cycles; however, only 2 discontinued SC due to AEs, 2 discontinued due to logistic reasons, 2 were lost to follow-up and 1 withdrew even before starting SC due to apprehension. On verbal questioning based on the comfort scale, most patients were reasonably comfortable and none were very uncomfortable wearing the device (eTable 2). No patient had developed scalp metastases with a median follow-up of 17.1 [inter quartile range (IQR) 13.3–21.8] months.
4. Discussion
CIA is an outward sign of cancer and can even be detrimental to oncologic outcome, as stress and depression can affect the immune system and prognosis [24]. Though SC has been used to prevent CIA since 1970 [5], to date, this has not been integrated into routine clinical practice [25]. Our study suggests that women with breast cancer who received SC with both A and T chemotherapy were significantly more likely (57% versus 0%) to have successful HP than those who did not receive SC. This is consistent with results from recent studies which show success of scalp cooling in ∼30–80% patients [[11], [12], [13], [14], [15], [16], [17], [18], [19]]. Also, though SC has mostly been studied in females undergoing treatment for breast cancer, many of the studies are in patients with early breast cancer [13,14,16,[26], [27], [28], [29]]. Our study included patients with locally advanced breast cancer as well. Our results also indicate that SC is more effective in women who received T followed by A compared with the reverse sequence. Similar trends have been observed in other studies, with rate of HP with T reported to be 45–94% and with A (with/without T) 0–40% [11,12,16,19,30]. It was noted that patients who were started on anthracycline first, had a high chance of losing hair. Interestingly, these patients then regrow hairs during taxane therapy and majority resume normal hair density at the end of chemotherapy or within 6 weeks, thus facilitating the resumption of their normal lifestyle.
Differential HP potential of different chemotherapeutics with use of SC may have clinical relevance in decision-making. For example, with Her2-positive breast cancer, the choice of regimen may bank towards TCH regimen (docetaxel, carboplatin, and trastuzumab) rather than doxorubicin and cyclophosphamide followed by taxanes and trastuzumab (AC→TH) owing to its superior HP potential. Another important factor would be cost; currently, SC devices in India cost about INR 1500 to 5000 (USD 50 to 75) per CT cycle per patient and are not yet reimbursed by health insurance. The reimbursement issue is prevailing the world over.
The results of the QOL analysis suggest better QOL in the SC arm versus the controls with respect to only one question: retention of hair. However, this is clinically meaningful, as this was the very aim of the study and was analogous to the findings reported by Rugo [11]. Interestingly, the other QOL measures show no difference, which was in concurrence with other investigators [12,16]. This demonstrates that other elements, including receipt of treatment and their toxicities are important determinants of QOL in a woman with cancer [16,31,32]. Furthermore, we lack precise QOL tools to measure the effects of CIA on a woman’s perception of body image, sexuality and mental wellbeing [11,12]. Clinical observation as well as literature suggest that CIA is extremely distressing and sometimes more tormenting than losing a breast [16,32]. The use of SC devices may help alleviate some of this agony, though we need to develop explicit QOL tools.
The fear of scalp metastasis has largely been allayed by various studies, including a meta-analysis by Rugo [7], which found no increase in incidence of scalp metastases. However, there may be the concern of increased systemic recurrence, rather than scalp metastases. SC may protect circulating tumor cells due to vasoconstriction and reduced metabolic activity in the scalp [33]. These cells may then lead to systemic spread to other metastatic sites in breast cancer, especially in advanced tumors. We need large long term studies in patients with advanced breast cancer to answer the question of whether SC will lead to increased systemic metastasis in the long run [8].
The most rate limiting step in the use of SC in patients in our center and in most low and middle income countries, would be the lack of space and time. Day care beds are limited and with a large volume of patients, it would be logistically difficult to provide SC to all female patients on chemotherapy. The treatment time would also be considerably longer consisting of precooling for 30 min, followed by administration of chemo and 90 min post chemotherapy cooling period. Longer time would result in lesser patients being administered treatment in a day, and this could lead to delays in starting treatment in subsequent patients.
This study has its own limitations, including a small sample size. Furthermore, the PEP was successful HP after 4 cycles of CT, which could be higher than the efficacy at the end of all the cycles. However, majority of the women attained grade 2 alopecia after 1–2 cycles of A containing CT. Notably, the RCT by Nangia, also assessed the alopecia at this time point, which again proves its clinical relevance [12]. Chemotherapy type, sequence and technicality related with optimal cap fit are potential confounders for real world outcomes.
Overall, the SC device was well tolerated with no serious AE, and most participants perceived it as reasonably comfortable. At median follow up, there were no scalp metastasis noted. The participants will be followed up, according to routine institution practice, after the study completion for safety (time and site of first recurrence, including scalp metastasis) and overall survival.
5. Conclusions
Among women with non-metastatic breast cancer receiving chemotherapy with a taxane, anthracycline, or both, those who underwent SC were significantly more likely to have less than 50% hair loss after the fourth chemotherapy cycle compared with those who received no scalp cooling SC and this merits wider application. More large long term studies are needed in patients with advanced breast cancer to comment on safety in these patients.
Acknowledgements
We would like to thank Access devices, Bengaluru for providing the scalp cooling device and the Breast disease management group and department of medical oncology for their support and input.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2019.12.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Zdenkowski N., Tesson S., Lombard J., Lovell M., Hayes S., Francis P.A. Supportive care of women with breast cancer: key concerns and practical solutions. Med J Aust. 2016;205:471–475. doi: 10.5694/mja16.00947. [DOI] [PubMed] [Google Scholar]
- 2.Trusson D., Pilnick A. The role of hair loss in cancer identity: perceptions of chemotherapy-induced alopecia among women treated for early-stage breast cancer or ductal carcinoma in situ. Cancer Nurs. 2017;40 doi: 10.1097/NCC.0000000000000373. E9–16. [DOI] [PubMed] [Google Scholar]
- 3.Massey C.S. A multicentre study to determine the efficacy and patient acceptability of the Paxman Scalp Cooler to prevent hair loss in patients receiving chemotherapy. Eur J Oncol Nurs. 2004;8:121–130. doi: 10.1016/j.ejon.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Dean J.C., Salmon S.E., Griffith K.S. Prevention of doxorubicin-induced hair loss with scalp hypothermia. N Engl J Med. 1979;301:1427–1429. doi: 10.1056/NEJM197912273012605. [DOI] [PubMed] [Google Scholar]
- 5.Lemieux J., Desbiens C., Hogue J.-C. Breast cancer scalp metastasis as first metastatic site after scalp cooling: two cases of occurrence after 7- and 9-year follow-up. Breast Canc Res Treat. 2011;128:563–566. doi: 10.1007/s10549-011-1453-y. [DOI] [PubMed] [Google Scholar]
- 6.van den Hurk C.J.G., van de Poll-Franse L.V., Breed W.P.M., Coebergh J.W.W., Nortier J.W.R. Scalp cooling to prevent alopecia after chemotherapy can be considered safe in patients with breast cancer. Breast. 2013;22:1001–1004. doi: 10.1016/j.breast.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 7.Rugo H.S., Melin S.A., Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Canc Res Treat. 2017;163:199–205. doi: 10.1007/s10549-017-4185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altundag K. Do systemic recurrences increase after the use of scalp cooling in patients treated for breast cancer with chemotherapy? J BUON. 2017;22:1362–1363. [PubMed] [Google Scholar]
- 9.Shin H., Jo S.J., Kim D.H., Kwon O., Myung S.-K. Efficacy of interventions for prevention of chemotherapy-induced alopecia: a systematic review and meta-analysis. Int J Cancer. 2015;136:E442–E454. doi: 10.1002/ijc.29115. [DOI] [PubMed] [Google Scholar]
- 10.Breed W.P.M., van den Hurk C.J.G., Peerbooms M. Presentation, impact and prevention of chemotherapy-induced hair loss: scalp cooling potentials and limitations. Expert Rev Dermatol. 2011;6:109–125. [Google Scholar]
- 11.Rugo H.S., Klein P., Melin S.A., Hurvitz S.A., Melisko M.E., Moore A. Association between use of a scalp cooling device and alopecia after chemotherapy for breast cancer. J Am Med Assoc. 2017;317:606–614. doi: 10.1001/jama.2016.21038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nangia J., Wang T., Osborne C., Niravath P., Otte K., Papish S. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. J Am Med Assoc. 2017;317:596–605. doi: 10.1001/jama.2016.20939. [DOI] [PubMed] [Google Scholar]
- 13.Smetanay K., Junio P., Feißt M., Seitz J., Hassel J.C., Mayer L. COOLHAIR: a prospective randomized trial to investigate the efficacy and tolerability of scalp cooling in patients undergoing (neo)adjuvant chemotherapy for early breast cancer. Breast Canc Res Treat. 2019;173:135–143. doi: 10.1007/s10549-018-4983-8. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita T., Nakayama T., Fukuma E., Inokuchi M., Ishiguro H., Ogo E. Efficacy of scalp cooling in preventing and recovering from chemotherapy-induced alopecia in breast cancer patients: the HOPE study. Front Oncol. 2019;9:733. doi: 10.3389/fonc.2019.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betticher D.C., Delmore G., Breitenstein U., Anchisi S., Zimmerli-Schwab B., Müller A. Efficacy and tolerability of two scalp cooling systems for the prevention of alopecia associated with docetaxel treatment. Support Care Cancer. 2013;21:2565–2573. doi: 10.1007/s00520-013-1804-9. [DOI] [PubMed] [Google Scholar]
- 16.Chan A., Bauwens A., Pontre S., Jackson S., McGlone F., Ernenwein T. Efficacy of scalp cooling in reducing alopecia in early breast cancer patients receiving contemporary chemotherapy regimens. Breast. 2018;41:127–132. doi: 10.1016/j.breast.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Vasconcelos I., Wiesske A., Schoenegg W. Scalp cooling successfully prevents alopecia in breast cancer patients undergoing anthracycline/taxane-based chemotherapy. Breast. 2018;40:1–3. doi: 10.1016/j.breast.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Rice B.A., Ver Hoeve E.S., DeLuca A.N., Esserman L.J., Rugo H.S., Melisko M.E. Registry study to assess hair loss prevention with the Penguin Cold Cap in breast cancer patients receiving chemotherapy. Breast Canc Res Treat. 2018;167:117–122. doi: 10.1007/s10549-017-4506-z. [DOI] [PubMed] [Google Scholar]
- 19.van den Hurk C.J., Peerbooms M., van de Poll-Franse L.V., Nortier J.W., Coebergh J.W.W., Breed W.P. Scalp cooling for hair preservation and associated characteristics in 1411 chemotherapy patients - results of the Dutch Scalp Cooling Registry. Acta Oncol. 2012;51:497–504. doi: 10.3109/0284186X.2012.658966. [DOI] [PubMed] [Google Scholar]
- 20.van den Hurk C.J.G., van den Akker-van Marle M.E., Breed W.P.M., van de Poll-Franse L.V., Nortier J.W.R., Coebergh J.W.W. Impact of scalp cooling on chemotherapy-induced alopecia, wig use and hair growth of patients with cancer. Eur J Oncol Nurs. 2013;17:536–540. doi: 10.1016/j.ejon.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa T., Shimizu S., Katayama K., Chishima T., Hamaguchi Y., Doi T. Feasibility of AC/EC followed by weekly paclitaxel in node-positive breast cancer in Japan. Anticancer Res. 2009;29:1515–1520. [PubMed] [Google Scholar]
- 22.Takabatake D., Taira N., Hara F., Sien T., Kiyoto S., Takashima S. Feasibility study of docetaxel with cyclophosphamide as adjuvant chemotherapy for Japanese breast cancer patients. Jpn J Clin Oncol. 2009;39:478–483. doi: 10.1093/jjco/hyp050. [DOI] [PubMed] [Google Scholar]
- 23.Fayers P., Aaronson N.K., Bjordal K., Groenvold M., Curran D., Bottomley A. European Organisation for Research and Treatment of Cancer; 2001. EORTC QLQ-C30 scoring manual. [Google Scholar]
- 24.Choi E.K., Kim I.-R., Chang O., Kang D., Nam S.-J., Lee J.E. Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psycho Oncol. 2014;23:1103–1110. doi: 10.1002/pon.3531. [DOI] [PubMed] [Google Scholar]
- 25.Nangia J. Quality of life matters: it is time to integrate scalp cooling in routine clinical practice. J Oncol Pract. 2018;14:157–158. doi: 10.1200/JOP.18.00033. [DOI] [PubMed] [Google Scholar]
- 26.Gianotti E., Razzini G., Bini M., Crivellaro C., Righi A., Darecchio S. Scalp cooling in daily clinical practice for breast cancer patients undergoing curative chemotherapy: a multicenter interventional study. Asia Pac J Oncol Nurs. 2019;6:277–282. doi: 10.4103/apjon.apjon_4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prochilo T., Huscher A., Andreis F., Mirandola M., Zaina E., Pomentale B. Hair loss prevention by a scalp cooling device in early breast cancer patients: the poliambulanza preliminary experience. Rev Recent Clin Trials. 2019;14:66–71. doi: 10.2174/1574887113666181120111104. [DOI] [PubMed] [Google Scholar]
- 28.Komen M.M.C., van den Hurk C.J.G., Nortier J.W.R., van der Ploeg T., Nieboer P., van der Hoeven J.J.M. Prolonging the duration of post-infusion scalp cooling in the prevention of anthracycline-induced alopecia: a randomised trial in patients with breast cancer treated with adjuvant chemotherapy. Support Care Cancer. 2019;27:1919–1925. doi: 10.1007/s00520-018-4432-6. [DOI] [PubMed] [Google Scholar]
- 29.Martín M., de la Torre-Montero J.C., López-Tarruella S., Pinilla K., Casado A., Fernandez S. Persistent major alopecia following adjuvant docetaxel for breast cancer: incidence, characteristics, and prevention with scalp cooling. Breast Canc Res Treat. 2018;171:627–634. doi: 10.1007/s10549-018-4855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mols F., van den Hurk C.J., Vingerhoets A.J.J.M., Breed W.P.M. Scalp cooling to prevent chemotherapy-induced hair loss: practical and clinical considerations. Support Care Cancer. 2009;17:181–189. doi: 10.1007/s00520-008-0475-4. [DOI] [PubMed] [Google Scholar]
- 31.Komen M.M.C., Smorenburg C.H., van den Hurk C.J.G., Nortier J.W.R. Factors influencing the effectiveness of scalp cooling in the prevention of chemotherapy-induced alopecia. The Oncologist. 2013;18:885–891. doi: 10.1634/theoncologist.2012-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemieux J., Maunsell E., Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psycho Oncol. 2008;17:317–328. doi: 10.1002/pon.1245. [DOI] [PubMed] [Google Scholar]
- 33.Lee J.S., Magbanua M.J.M., Park J.W. Circulating tumor cells in breast cancer: applications in personalized medicine. Breast Canc Res Treat. 2016;160:411–424. doi: 10.1007/s10549-016-4014-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.