Abstract
Purpose
To investigate the effect of 21-gene recurrence score (RS) on chemotherapy-decision making and prognosis in breast cancer patients aged <40 years.
Methods
Using the Surveillance, Epidemiology, and End Results program, we included patients aged <40 years with tumor size ≤5 cm, node negative, and estrogen receptor-positive breast cancer between 2004 and 2015. Correlations among the 21-gene RS, chemotherapy decision-making and prognosis were analyzed.
Results
We included 2721 patients in this study. According to TAILORx cutoffs, 352 (12.9%), 1814 (66.7%), and 555 (20.4%) patients were classified as low-, intermediate-, and high-risk cohorts, respectively. The 21-gene RS categories were associated with the probability of receiving chemotherapy, with 7.1%, 33.4%, and 77.1% of patients in low-, intermediate-, and high-risk cohorts treated with chemotherapy, respectively (P < 0.001). Those in the intermediate-risk cohort were significantly less likely to receive chemotherapy over time (P = 0.008), and the trends of chemotherapy receipt were stable in the low-risk and high-risk cohorts over time. Multivariate analysis showed that the 21-gene RS was an independent prognostic indicator for breast cancer specific survival. In the stratified analysis, the receipt of chemotherapy was associated with better breast cancer specific survival in the high-risk cohort (P = 0.028), but not in the intermediate-risk cohort (P = 0.223).
Conclusions
21-gene RS has clinical implications for young breast cancer patients with respect to optimizing chemotherapy-decisions. Despite increasing rates of chemotherapy receipt in young patients, more studies are needed to determine the definitive effect of chemotherapy in young patients with three RS categories.
Keywords: Breast cancer, Oncotype, Age, Prognosis, Chemotherapy, Recurrence score
Highlights
-
•
Intermediate-risk patients were less likely to receive chemotherapy over time.
-
•
The 21-gene RS was an independent prognostic indicator for BCSS.
-
•
Survival benefit of chemotherapy was observed in the high-risk cohort.
1. Introduction
Breast cancer remains a major public health problem in women worldwide, and studies have been attracted by breast cancer arising in young women [[1], [2], [3], [4]]. Young breast cancer, usually defined as women diagnosed with breast cancer under the age of 40, accounting for approximately 5–7% of new breast cancer cases [1,2]. Although this represents a small proportion of the cancer burden, young breast cancer patients tend to experience more aggressive behavior, which contributes to poor prognosis [3,4]. Young women have several adverse biological factors contributing to poorer outcomes including lower hormone receptor expression, overexpression of human epidermal growth factor receptor 2 (HER-2), higher tumor grade, higher risk of lymphovascular invasion and nodal involvement [[4], [5], [6], [7]]. In hormone receptor-positive breast cancer, patients with younger age were associated with lower survival compared to those with older age [[8], [9], [10]]. Genomic analysis suggests there is a distinct biological entity between young and older breast cancer patients, which may contribute to lower hormone sensitivity in young patients [[11], [12], [13], [14]]. Therefore, age <40 years has itself been demonstrated to be an independently unfavorable prognostic factor.
The 21-gene recurrence score (RS) (Oncotype Dx™, Redwood, CA) is one of the most widely used gene expression profiles to guide the personalized management of breast cancer. The 21-gene RS model was constructed from 16 breast cancer related genes and 5 reference genes to both predict prognosis and estimate the benefits of adjuvant chemotherapy in patients with hormone receptor-positive, HER-2 negative, nodal negative (N0) breast cancer [15,16]. However, most patients in previous studies have been older and data regarding the decision-making process of chemotherapy and prognostic assessment of the 21-gene RS in young breast cancers are limited [[17], [18], [19], [20], [21], [22], [23]]. Due to the longer life expectancy of young women, genomic testing may be more valuable in determining the chemotherapy-decision making and assessing prognosis. Using data from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 study, patients aged <40 years had a significantly higher proportion of high-risk RS score (55.9%) compared to patients aged 40–50 years (29.6%), 50–60 years (25.4%), and >60 years (21.3%) (traditional RS cutoffs) [24]. However, only 668 patients were included in this study, which may affect the representativeness of these findings.
The recent trials including Trial Assigning Individualized Options for Treatment (TAILORx) and West German Study Group PlanB (WSG-PlanB) have confirmed both prognosis and the predictive value of chemotherapy by the 21-gene RS assay in N0 and hormone receptor-positive breast cancer [25,26]. In addition, the results of 21-gene RS have incorporated into the 8th American Joint Committee on Cancer staging system of breast cancer [27]. In the current National Comprehensive Cancer Network breast cancer clinical practice guidelines, the 21-gene RS assay also have been incorporated into the decision of systemic chemotherapy and adjuvant endocrine therapy in early stage breast cancer [28]. However, only 4.6% of patients were aged ≤40 years in TAILORx trial and the age distribution was unavailable in WSG-PlanB trial [25,26]. Therefore, more studies are needed to validate the genomic signatures in clinical practice of young patients aged <40 years. In light of this, the aims of the current study were to assess the effect of 21-gene RS on chemotherapy-decision making and survival outcome in breast cancer patients aged <40 years.
2. Materials and methods
Patients We included female patients diagnosed with breast cancer between 2004 and 2015 using the data from the Surveillance, Epidemiology, and End Results (SEER) program. The National Cancer Institute’s SEER database includes de-identified patient information including demographic, clinicopathological factors, and survival outcomes for approximately 28% of the United States population [29]. Patients aged <40 years with tumor size ≤5 cm (T1-2), N0, and estrogen receptor (ER)-positive invasive breast carcinoma who received surgical treatment and had available 21-gene RS results were included. Patients with no pathological diagnosis and metastatic stage were excluded. Our study was exempted from the Institutional Review Board approval process because of the de-identified patient information in the SEER dataset.
2.1. Variables
We included the following variables of interest: age at diagnosis, race/ethnicity, grade, tumor size, progesterone receptor (PR) status, and histology. The management including surgical procedure, chemotherapy, and radiotherapy were also identified. In addition, two 21-gene RS cutoffs including traditional RS cutoffs and TAILORx RS cutoffs were identified. Traditional RS cutoffs were classified into the following three categories: low-risk (RS < 18), intermediate-risk (RS 18–30), and high-risk (RS > 30) cohorts [15,16]. The optimal cutoffs have changed from traditional cutoffs to TAILORx cutoffs in recent clinical trials, which have been optimized to minimize under-treatment of potential high-risk patients. The TAILORx RS cutoffs were also stratified into three following risk categories: low-risk (RS < 11), intermediate-risk (RS 11–25), and high-risk (RS > 25) cohorts [25].
2.2. Statistical analysis
The categorical variables were compared using the chi-squared test. Binomial logistic regression and receiver operating characteristics (ROC) were used to assess the predictive factors related to chemotherapy administration. Breast cancer specific survival (BCSS) curves were evaluated using the Kaplan-Meier method and compared using the log-rank test. Multivariate Cox proportional analysis was used to determine the independent prognostic indicators related to BCSS. Statistical analysis was conducted using SPSS, version 22.0 (IBM Corp., Armonk, NY, USA), and a P-value < 0.05 was determined to be statistically significant.
3. Results
We included 2721 patients in this study. The majority of patients had invasive ductal carcinoma subtype (89.1%) and PR-positive disease (94.3%). In 1776 patients with available HER-2 status, 97.8% (n = 1737) of them had HER-2 negative disease. In the entire cohort, 53.8% of them received mastectomy, and approximately 40% of patients received radiotherapy and chemotherapy. The median RS was 18 with a range of 0–74. According to traditional RS cutoffs, 1293 (47.5%), 1122 (41.2%), and 306 (11.2%) patients were classified as low-, intermediate-, and high-risk cohorts, respectively. Using TAILORx RS cutoffs, 352 (12.9%), 1814 (66.7%), and 555 (20.4%) patients were classified as low-, intermediate-, and high-risk cohorts, respectively. The patient baseline characteristics are listed in Table 1.
Table 1.
The patient baseline characteristic.
| Variables | n (%) |
|---|---|
| Age (mean ± SD) (years) | 35.5 ± 3.5 |
| Race/ethnicity | |
| Non-Hispanic White | 1664 (61.2) |
| Non-Hispanic Black | 268 (9.8) |
| Hispanic (All Races) | 373 (13.7) |
| Other | 416 (15.3) |
| Grade | |
| Well differentiated | 640 (23.5) |
| Moderately differentiated | 1443 (53.0) |
| Poorly/undifferentiated | 578 (21.2) |
| Unknown | 60 (2.2) |
| Histology | |
| IDC or mixed with other types of carcinoma | 2425 (89.1) |
| ILC or mixed with other types of carcinoma | 112 (4.1) |
| Other | 184 (6.8) |
| Tumor stage | |
| T1 | 2083 (76.6) |
| T2 | 638 (23.4) |
| PR status | |
| Negative | 145 (5.3) |
| Positive | 2567 (94.3) |
| Unknown | 9 (0.3) |
| Surgical procedure | |
| BCS | 1257 (46.2) |
| Mastectomy | 1464 (53.8) |
| Radiotherapy | |
| No | 1611 (59.2) |
| Yes | 1110 (40.8) |
| Chemotherapy | |
| No | 1662 (61.1) |
| Yes | 1059 (38.9) |
| Traditional RS cutoffs | |
| Low-risk | 1293 (47.5) |
| Intermediate-risk | 1122 (41.2) |
| High-risk | 306 (11.2) |
| TAILORx RS cutoffs | |
| Low-risk | 352 (12.9) |
| Intermediate-risk | 1814 (66.7) |
| High-risk | 555 (20.4) |
Abbreviations: BCS, breast conserving surgery; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; PR, progesterone receptor; RS, recurrence score; SD, standard deviation; T, tumor; TAILORx, Trial Assigning Individualized Options for Treatment.
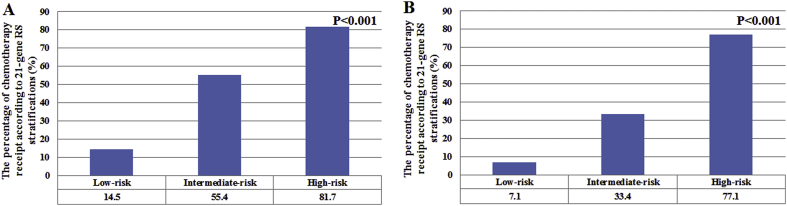
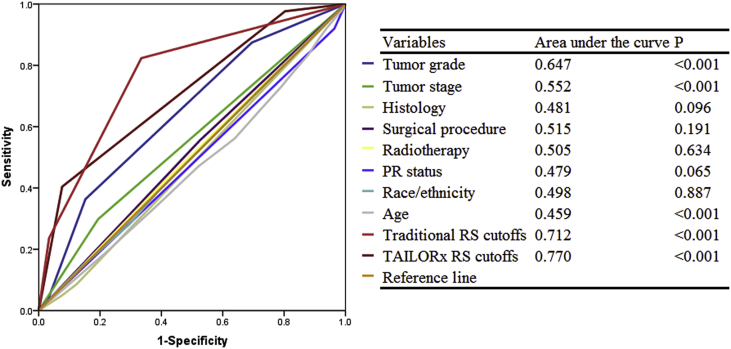
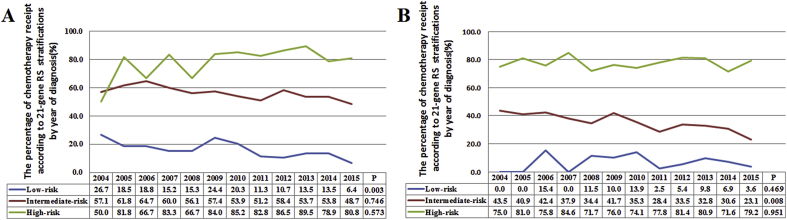
Patients with a higher RS were more likely to receive chemotherapy. In the traditional RS cutoffs, 14.5%, 55.4%, and 81.7% of patients in the low-, intermediate-, and high-risk cohorts were treated with chemotherapy, respectively (P < 0.001). According to the TAILORx RS cutoffs, 7.1%, 33.4%, and 77.1% of patients in low-, intermediate-, and high-risk cohorts were treated with chemotherapy, respectively (P < 0.001) (Fig. 1A-B). In the traditional RS cutoffs, 21-gene RS was an independent predictive factor for receipt of chemotherapy. Patients at intermediate-risk (odds ratio [OR] 6.802, 95% confidence interval [CI] 5.570–8.306, P < 0.001) and high-risk (OR 18.930, 95%CI 13.391–26.759, P < 0.001) cohorts were more likely to receive chemotherapy compared to low-risk cohort. Similar trends were found using the TAILORx cutoffs. In addition, age, tumor size, tumor grade, surgical procedure, and radiotherapy were also independent predictors associated with chemotherapy receipt (Table 2). Moreover, the 21-gene RS was also an effective predictor of chemotherapy receipt using ROC analysis (Fig. 2). Using traditional RS cutoffs, there was a significantly decreased percentage of chemotherapy receipt in the low-risk cohort over time (P = 0.003), and the trends of chemotherapy receipt were not significantly changed in the intermediate- (P = 0.746) and high-risk (P = 0.573) cohorts over time (Fig. 3A). However, there was a significantly decreased percentage of chemotherapy receipt in the intermediate-risk cohort over time (P = 0.008), and the trends of chemotherapy receipt were stable in low- (P = 0.469) and high-risk (P = 0.951) cohorts over time using TAILORx RS cutoffs (Fig. 3B).
Fig. 1.
The percentage of chemotherapy receipt according to 21-gene RS categories (traditional RS cutoffs, A; TAILORx cutoffs, B).
Table 2.
Multivariable logistic regression model for chemotherapy receipt.
| Variables | Traditional RS cutoffs |
TAILORx RS cutoffs |
||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |
| Age (continuous variable) (years) | 0.972 | 0.947–0.997 | 0.030 | 0.972 | 0.948–0.996 | 0.023 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 1 | 1 | ||||
| Non-Hispanic Black | 0.797 | 0.583–1.088 | 0.153 | 0.857 | 0.632–1.163 | 0.323 |
| Hispanic (All Races) | 0.856 | 0.647–1.133 | 0.278 | 0.859 | 0.659–1.119 | 0.259 |
| Other | 1.049 | 0.807–1.361 | 0.719 | 1.106 | 0.860–1.423 | 0.432 |
| Grade | ||||||
| Well differentiated | 1 | 1 | ||||
| Moderately differentiated | 1.816 | 1.424–2.316 | <0.001 | 1.821 | 1.443–2.297 | <0.001 |
| Poorly/undifferentiated | 2.885 | 2.152–3.868 | <0.001 | 2.942 | 2.211–3.915 | <0.001 |
| Unknown | 1.456 | 0.749–2.831 | 0.268 | 1.392 | 0.741–2.616 | 0.304 |
| Histology | ||||||
| IDC or mixed with other types of carcinoma | 1 | 1 | ||||
| ILC or mixed with other types of carcinoma | 1.091 | 0.685–1.736 | 0.714 | 1.054 | 0.681–1.631 | 0.814 |
| Other | 0.725 | 0.491–1.070 | 0.105 | 0.758 | 0.523–1.099 | 0.144 |
| Tumor stage | ||||||
| T1 | 1 | 1 | ||||
| T2 | 1.458 | 1.179–1.803 | 0.001 | 1.471 | 1.202–1.801 | <0.001 |
| PR status | 0 | |||||
| Negative | 1 | 1 | ||||
| Positive | 0.984 | 0.657–1.474 | 0.938 | 0.87 | 0.584–1.297 | 0.495 |
| Unknown | 2.827 | 0.535–14.950 | 0.221 | 2.065 | 0.468–9.124 | 0.339 |
| Surgical procedure | ||||||
| BCS | 1 | 1 | ||||
| Mastectomy | 1.718 | 1.287–2.292 | <0.001 | 1.683 | 1.269–2.232 | <0.001 |
| Radiotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 2.022 | 1.508–2.713 | <0.001 | 1.803 | 1.354–2.400 | <0.001 |
| 21-gene recurrence score | ||||||
| Low-risk | 1 | 1 | ||||
| Intermediate-risk | 6.802 | 5.570–8.306 | <0.001 | 5.995 | 3.933–9.138 | <0.001 |
| High-risk | 18.93 | 13.391–26.759 | <0.001 | 31.231 | 19.650–49.637 | <0.001 |
Abbreviations: BCS, breast conserving surgery; CI, confidence interval; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; OR, odds ratio; PR, progesterone receptor; RS, recurrence score; T, tumor; TAILORx, Trial Assigning Individualized Options for Treatment.
Fig. 2.
Receiver operating characteristics analyses for prediction of chemotherapy receipt.
Fig. 3.
The trends of chemotherapy receipt according to 21-gene RS categories over time (traditional RS cutoffs, A; TAILORx cutoffs, B).
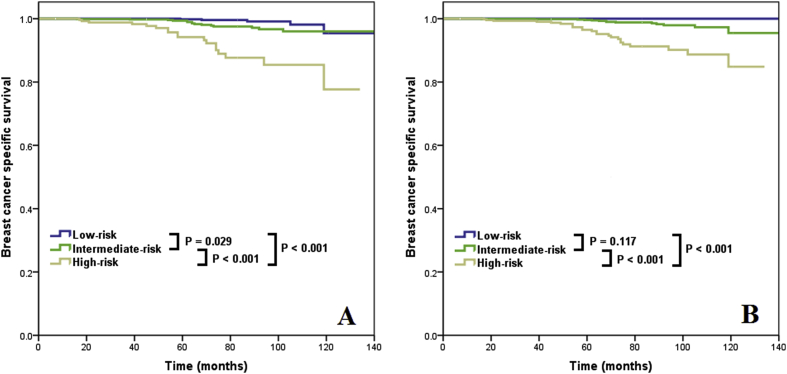
With a median follow-up of 50 months, 38 patients died with breast cancer-related disease. The 5-year BCSS was 99.0%. Using the traditional RS cutoffs, the 5-year BCSS was 99.8%, 99.4%, and 94.2% in low-, intermediate-, and high-risk cohorts, respectively (log-rank test, P < 0.001) (Fig. 4A). Using the TAILORx cutoffs, the 5-year BCSS was 100%, 99.6%, and 96.5% in low-, intermediate-, and high-risk cohorts, respectively, respectively (log-rank test, P < 0.001) (Fig. 4B). The multivariate prognostic analysis showed that the 21-gene RS was an independent prognostic factor related to BCSS. In the traditional RS cutoffs, the low-risk cohort had comparable BCSS compared to the intermediate-risk cohort (hazard ratio [HR] 0.363, 95% CI 0.131–1.000, P = 0.050), and the high-risk cohort had significantly lower BCSS compared to the intermediate-risk cohort (HR 4.664, 95%CI 2.342–9.288, P < 0.001) (Table 3). Similar trends were also observed for the TAILORx RS cutoffs.
Fig. 4.
Breast cancer specific survival curves by 21-gene RS categories (traditional RS cutoffs, A; TAILORx cutoffs, B).
Table 3.
Multivariate prognostic analyses in the entire cohort.
| Variables | Traditional RS cutoffs |
TAILORx RS cutoffs |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Age (continuous variable) (years) | 0.978 | 0.905–1.057 | 0.579 | 0.984 | 0.909–1.065 | 0.686 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 1 | 1 | ||||
| Non-Hispanic Black | 1.275 | 0.543–2.996 | 0.577 | 1.474 | 0.631–3.446 | 0.370 |
| Hispanic (All Races) | 0.242 | 0.033–1.789 | 0.164 | 0.264 | 0.036–1.952 | 0.192 |
| Other | 1.017 | 0.348–2.975 | 0.975 | 1.096 | 0.375–3.201 | 0.867 |
| Grade | ||||||
| Well differentiated | 1 | 1 | ||||
| Moderately differentiated | 1.279 | 0.418–3.915 | 0.666 | 1.078 | 0.350–3.318 | 0.895 |
| Poorly/undifferentiated | 1.171 | 0.343–3.994 | 0.801 | 1.037 | 0.303–3.548 | 0.953 |
| Unknown | – | – | 0.978 | – | – | 0.981 |
| Histology | ||||||
| IDC or mixed with other types of carcinoma | 1 | 1 | ||||
| ILC or mixed with other types of carcinoma | 1.030 | 0.137–7.730 | 0.977 | 1.177 | 0.156–8.900 | 0.875 |
| Other | 0.409 | 0.054–3.104 | 0.387 | 0.424 | 0.056–3.195 | 0.405 |
| Tumor stage | ||||||
| T1 | 1 | 1 | ||||
| T2 | 2.627 | 1.376–5.017 | 0.003 | 2.786 | 1.448–5.361 | 0.002 |
| PR status | ||||||
| Negative | 1 | 1 | ||||
| Positive | 0.781 | 0.320–1.907 | 0.588 | 0.654 | 0.275–1.555 | 0.336 |
| Unknown | – | – | 0.990 | – | – | 0.992 |
| Surgical procedure | ||||||
| BCS | 1 | 1 | ||||
| Mastectomy | 1.430 | 0.525–3.890 | 0.484 | 1.374 | 0.511–3.692 | 0.529 |
| Radiotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 0.899 | 0.317–2.551 | 0.842 | 0.807 | 0.289–2.256 | 0.683 |
| Chemotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 0.844 | 0.414–1.717 | 0.639 | 0.790 | 0.387–1.610 | 0.516 |
| 21-gene recurrence score | ||||||
| Intermediate-risk | 1 | 1 | ||||
| Low-risk | 0.363 | 0.131–1.000 | 0.050 | – | – | 0.968 |
| High-risk | 2.764 | 1.048–7.295 | 0.040 | 4.68 | 2.102–10.419 | <0.001 |
Abbreviations: BCS, breast conserving surgery; CI, confidence interval; HR, hazard ratio; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; PR, progesterone receptor; RS, recurrence score; T, tumor; TAILORx, Trial Assigning Individualized Options for Treatment.
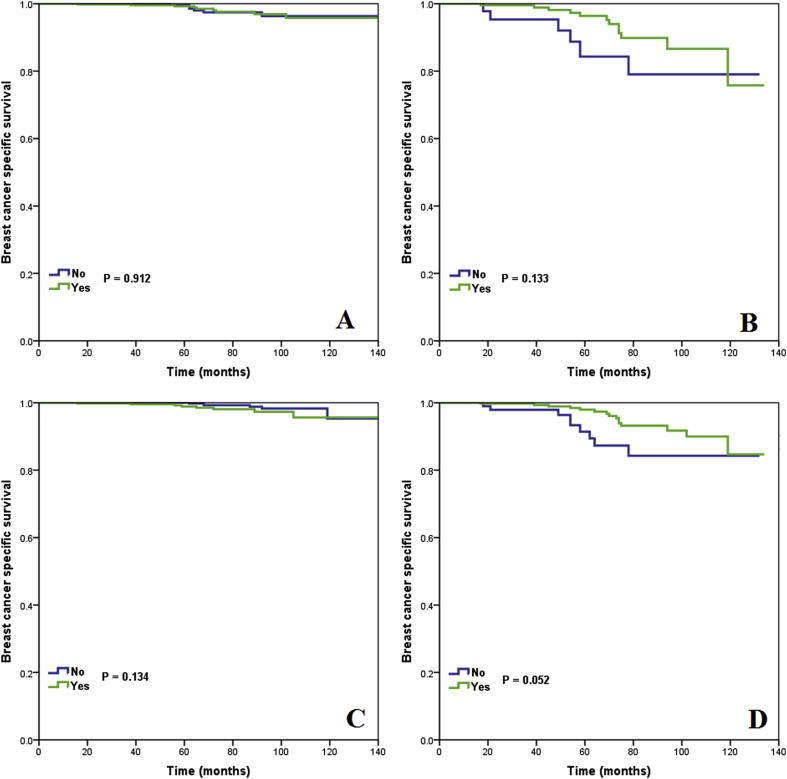
Finally, we analyzed the effect of chemotherapy on survival stratified by 21-gene RS categories. In the TAILORx RS cutoffs, after adjustment of age, race/ethnicity, histology, tumor stage, PR status, and treatment variables, the receipt of chemotherapy was correlated with better BCSS in the high-risk cohort (HR 0.369, 95%CI 0.151–0.897, P = 0.028), but was not associated with better BCSS in the intermediate-risk cohort (HR 1.975, 95%CI 0.660–5.906, P = 0.223). We could not perform multivariate analysis in the low-risk cohort because no patients died with breast cancer in this patient subset. The survival curves between patients treated with and without chemotherapy in the intermediate- and high-risk cohorts are shown in Fig. 5. The 5-year BCSS was comparable between chemotherapy and non-chemotherapy cohorts in the intermediate-risk cohort (log-rank test, P = 0.134) (Fig. 5A), and was 98.0% and 91.4% in the high-risk cohort, respectively (log-rank test, P = 0.052) (Fig. 5B). However, in the traditional RS cutoffs, the receipt of chemotherapy was not associated with improved BCSS according to the three risk categories in univariate and multivariate prognostic analysis (Fig. 5C-D) (Table 4).
Fig. 5.
Breast cancer specific survival curves in patients treated with and without chemotherapy by 21-gene RS categories (TAILORx cutoffs: intermediate-risk, A, high-risk, B; traditional RS cutoffs: intermediate-risk, C, high-risk, D).
Table 4.
Multivariate prognostic analyses by 21-gene RS stratifications.
| Variables | Low-risk |
Intermediate-risk |
High-risk |
|||
|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Traditional RS cutoffs | ||||||
| No chemotherapy | 1 | 1 | 1 | |||
| Chemotherapy | 2.155 (0.324–14.326) | 0.427 | 0.877 (0.297–2.592) | 0.812 | 0.337 (0.113–1.003) | 0.051 |
| TAILORx RS cutoffs | ||||||
| No chemotherapy | – | 1 | ||||
| Chemotherapy | – | – | 1.907 (0.641–5.673) | 0.246 | 0.368 (0.151–0.897) | 0.025 |
Abbreviations: CI, confidence interval; HR, hazard ratio; RS, recurrence score; TAILORx, Trial Assigning Individualized Options for Treatment.
4. Discussion
In this study, we used a population-based cohort to assess the effect of 21-gene RS in chemotherapy-decision making and prognostic assessment in breast cancer patients aged <40 years. Our results showed that a higher RS was independently associated with a higher percentage of chemotherapy administration and lower BCSS.
Although young patients aged <40 years were more likely to experience large tumors, nodal involvement, and hormone receptor negative, approximately 50% of them had T1-2N0 and ER-positive disease [30]. Therefore, RS testing should also be considered for half of young patients. In our previous large cohort study including all patient ages, the distribution of 21-gene RS was 56.7%, 35.7%, and 7.6% in low-, intermediate-, and high-risk cohorts using traditional RS categories, respectively [31]. However, in this study, we found that 11.2% of patients <40 years were in the high-risk RS cohort, which was similar to the results from the Genomic Health central lab (14.1% vs. 8.8% in women aged <40 vs. ≥70 years) [23]. The results from NSABP B-14 study also showed significantly higher probability of high-risk RS in patients aged <40 years compared to those aged ≥40 years (55.9% vs. 24.3%) [24]. Our results showed that young patients were also presented with aggressive behavior in the era of genetic testing. Given that younger women tend to experience less medical comorbidities, it is possible that providers can be more ‘aggressive’ in their treatment recommendations for younger patients, thus precluding the need for genetic testing.
The optimal cutoffs of RS have changed from traditional cutoffs to TAILORx cutoffs in recent clinical trials, which have been optimized to minimize under-treatment of potential high-risk patients [25]. In the latest American Society of Clinical Oncology (ASCO) guideline, chemoendocrine therapy may be offered for intermediate-risk patients (RS of 16–25) aged ≤50 years [32]. Using the TAILORx cutoffs, more patients were reclassified into intermediate-risk (25.5%) and high-risk (8.9%) cohorts. With the ASCO clinical practice guideline, approximately half of the low-risk cohort in the traditional RS cutoffs would have been candidates for chemotherapy after being reclassified into the intermediate-risk cohort using the TAILORx cutoffs. As listed the study characteristics of our study and previous studies in Supplementary Table 1, there were significantly differences in the percentage of chemotherapy receipt between traditional RS cutoffs and TAILORx RS cutoffs.
In this study, we observed excellent prognosis of young patients in the low- and intermediate-risk cohorts. Recently, a large cohort study also indicated the 5-year disease recurrence rate was 1.5%, 2.9%, and 11.1% of patients aged <50 years with low-, intermediate-, and high-risk cohort, respectively (Supplementary Table 1) [33]. Our results showed that the 21-gene RS was a robust prognostic indicator in young patients, while comparable BCSS was found between low- and intermediate-risk cohorts. Therefore, our findings raise questions about the benefit of adjuvant chemotherapy in the intermediate-risk cohort.
21-gene RS is extensively used worldwide to guide chemotherapy decisions for breast cancer with T1-2N0, hormone receptor-positive, and HER-2 negative disease. However, studies in young patients are limited [17–23]. A study by Mutonga et al. showed that 0%, 39%, and 100% of patients aged <50 years received chemotherapy in low-, intermediate-, and high-risk cohorts, respectively, which was not significantly different from older patients [34]. However, a recent study from the National Cancer Database showed that the recommendation or receipt of chemotherapy was significantly more likely in patients aged <50 years with low- (16.8% vs. 10.8%, P < 0.001), intermediate- (64.4% vs. 46.3%, P < 0.001), and high-risk (93.1% vs. 88.6%, P < 0.001) cohorts, compared to patients aged ≥50 years [35]. Another study including intermediate-risk RS patients (RS of 16–25) from the National Cancer Database also showed that 47.8% of patients aged <40 years were treated with chemotherapy, while only 25.3% of patients aged ≥40 years received chemotherapy [36]. Here, we also showed that 21-gene RS was associated with receipt of chemotherapy, there was 14.5%, 55.4%, and 81.7% of traditional RS cutoffs patients in low-, intermediate-, and high-risk cohorts treated with chemotherapy, respectively, while it was 7.1%, 33.4%, and 77.1% in TAILORx RS cutoffs. The percentage of chemotherapy receipt in the three risk categories were higher than in previous studies in all ages of patients using both traditional RS cutoffs (low-risk RS, 4.5%–7%; intermediate-risk RS, 32.3%–34%; high-risk RS, 72%) and TAILORx RS cutoffs (low-risk RS, 1.7%–5%; intermediated-risk RS, 15%–18%; high-risk RS, 63%–73.4%) [31,37,38]. Therefore, although the 21-gene RS assay affects the decision-making of chemotherapy in young patients, the overall rate of chemotherapy receipt in young patients is higher than for other ages. However, our study showed that the percentage of chemotherapy receipt in young patients with lower RS gradually decreased over time.
The survival benefit of adjuvant chemotherapy for intermediate-risk patients remains unclear. Our results showed that the TAILORx RS cutoffs may be more optimal to select young patients for chemotherapy. The recent results of the TAILORx trial showed that endocrine therapy was non-inferior to chemoendocrine therapy in patients with intermediate-risk (RS of 16–25) (HR 1.08, 95% CI 0.94–1.24, P = 0.262) [25]. However, in an exploratory subgroup analysis among patients aged ≤50 years, a lower rate of distant recurrence was observed in patients who received chemotherapy. Notably, only one-third of patients in the TAILORx study were aged ≤50 years, which may be under-representative of those affected. In addition, several studies also failed to observe survival benefit in the intermediate-risk cohort (TAILORx RS cutoffs). However, only 2.6%–6.8% of patients were aged <40 years [33,36,37]. Owing to potential long-term chemotherapy toxicities including cardiovascular toxicity, cognitive change, secondary cancers, and irreversible ovarian failure, which may occur in young women with longer life expectancy. Moreover, approximately two-thirds of patients were diagnosed as intermediate-risk RS. Therefore, more studies are needed to investigate the potential effect of chemotherapy in this patient subset.
The results from moxifen and Exemestane Trial/Suppression of Ovarian Function Trial trials have indicated that the addition of ovarian function suppression to endocrine therapy could improve survival outcomes in high risk premenopausal patients with hormone receptor-positive/HER2-negative breast cancer, including patients aged <40 years and node negative disease [39]. In the TAILORx trial, there were also 13% of premenopausal women treated with ovarian function suppression [25]. Younger patients have a lower incidence of chemotherapy-induced amenorrhea (52%, 70.8%, and 95.1% in aged <40, 40–45, and >45 years, P < 0.0001), which may contribute to worse survival outcomes in hormone receptor-positive breast cancer [40]. However, there were currently no studies assessing the role of ovarian function suppression in intermediate- and high-risk RS cohorts, and it is not known if an escalated adjuvant endocrine treatment will reduce the impact of adjuvant chemotherapy or not. We should underline that the data of ovarian function suppression and endocrine therapy were not include in the SEER database. More studies are needed to assess the effect of ovarian function suppression on prognosis in intermediate- and high-risk RS cohorts.
Several inherent limitations should be considered in our study. First, there was potential selective bias using a retrospective database. However, our study reflects the real-world outcomes for young breast cancer patients using a population-based national registry. Secondly, the functional status, socioeconomic status, and comorbidities of patients were not recorded in the SEER database, which may likely influence the treatment decisions for patients. However, similar to our results, younger patients might be treated more aggressively because of fewer comorbidities compared to older patients. Third, HER-2 status was not included in the analysis of our study. However, only 2.2% of patients had HER-2 positive disease in patients with available HER-2 status. We believe that this would have limited impact on our results. In addition, the treatment information including types of chemotherapy regimen, completion of chemotherapy, and endocrine therapy were also not recorded in the SEER program. Moreover, the median follow-up time was only 50 months and only 38 events have been recorded in our study, therefore long-term follow-up with additional events are necessary to draw definitive conclusions regarding 21-gene RS testing in decision-making of chemotherapy and prognostic assessment of young women.
In conclusion, our study suggests that 21-gene RS has clinical implications for young breast cancer patients with respect to optimizing chemotherapy-decisions. Despite increasing rates of chemotherapy receipt in young patients, future studies are needed to obtain more evidence-based management strategies in young patients with three RS categories.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Acknowledgments
This work was partly supported by the National Natural Science Foundation of China (No. 81872459), the Commission Young and Middle-aged Talents Training Project of Fujian Health Commission (No. 2019-ZQNB-25), the Science and Technology Planning Projects of Xiamen Science & Technology Bureau (No. 3502Z20174070), and the Baise City Scientific Research and Technology Development Plan (No. 20183008).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2019.12.013.
Contributor Information
Zhen-Yu He, Email: hezhy@sysucc.org.cn.
San-Gang Wu, Email: unowu12345@hotmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Reyna C., Lee M.C. Breast cancer in young women: special considerations in multidisciplinary care. J Multidiscip Healthc. 2014;7:419–429. doi: 10.2147/JMDH.S49994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu P., Li X., Mittendorf E.A., Li J., Du X.L., He J. Comparison of clinicopathologic features and survival in young American women aged 18-39 years in different ethnic groups with breast cancer. Br J Canc. 2013;109:1302–1309. doi: 10.1038/bjc.2013.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H.B., Han W. Unique features of young age breast cancer and its management. J Breast Cancer. 2014;17:301–307. doi: 10.4048/jbc.2014.17.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anastasiadi Z., Lianos G.D., Ignatiadou E., Harissis H.V., Mitsis M. Breast cancer in young women: an overview. Updates Surg. 2017;69:313–337. doi: 10.1007/s13304-017-0424-1. [DOI] [PubMed] [Google Scholar]
- 5.Gnerlich J.L., Deshpande A.D., Jeffe D.B., Sweet A., White N., Margenthaler J.A. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–347. doi: 10.1016/j.jamcollsurg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kheirelseid E.H., Boggs J.M., Curran C., Glynn R.W., Dooley C., Sweeney K.J. Younger age as a prognostic indicator in breast cancer: a cohort study. BMC Canc. 2011;11:383. doi: 10.1186/1471-2407-11-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin N.U., Vanderplas A., Hughes M.E., Theriault R.L., Edge S.B., Wong Y.N. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheridan W., Scott T., Caroline S., Yvonne Z., Vanessa B., David V. Breast cancer in young women: have the prognostic implications of breast cancer subtypes changed over time? Breast Canc Res Treat. 2014;147:617–629. doi: 10.1007/s10549-014-3125-1. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z., Sahli Z., Wang Y., Wolff A.C., Cope L.M., Umbricht C.B. Young age at diagnosis is associated with worse prognosis in the Luminal A breast cancersubtype: a retrospective institutional cohort study. Breast Canc Res Treat. 2018;172:689–702. doi: 10.1007/s10549-018-4950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lian W., Fu F., Lin Y., Lu M., Chen B., Yang P. The impact of young age for prognosis by subtype in women with early breast cancer. Sci Rep. 2017;7:11625. doi: 10.1038/s41598-017-10414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anders C.K., Hsu D.S., Broadwater G., Acharya C.R., Foekens J.A., Zhang Y. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 12.Azim H.A., Jr., Michiels S., Bedard P.L., Singhal S.K., Criscitiello C., Ignatiadis M. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012;18:1341–1351. doi: 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 13.Anders C.K., Acharya C.R., Hsu D.S., Broadwater G., Garman K., Foekens J.A. Age-specific differences in oncogenic pathway deregulation seen in human breast tumors. PLoS One. 2008;3:e1373. doi: 10.1371/journal.pone.0001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobi Á., Kelemen G., Kaizer L., Weiczner R., Thurzó L., Kahán Z. Breast cancer under 40 years of age: increasing number and worse prognosis. Pathol Oncol Res. 2011;17:425–428. doi: 10.1007/s12253-010-9305-3. [DOI] [PubMed] [Google Scholar]
- 15.Paik S., Shak S., Tang G., Kim C., Baker J., Cronin M. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 16.Paik S. Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. Oncologist. 2007;12:631–635. doi: 10.1634/theoncologist.12-6-631. [DOI] [PubMed] [Google Scholar]
- 17.Gage M.M., Rosman M., Mylander W.C., Giblin E., Kim H.S., Cope L. A validated model for identifying patients unlikely to benefit from the 21-gene recurrence score assay. Clin Breast Canc. 2015;15:467–472. doi: 10.1016/j.clbc.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H.S., Umbricht C.B., Illei P.B., Cimino-Mathews A., Cho S., Chowdhury N. Optimizing the use of gene expression profiling in early-stage breast cancer. J Clin Oncol. 2016;34:4390–4397. doi: 10.1200/JCO.2016.67.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanna M.G., Bleiweiss I.J., Nayak A., Jaffer S. Correlation of oncotype DX recurrence score with histomorphology and immunohistochemistry in over 500 patients. Int J Breast Cancer. 2017;2017:1257078. doi: 10.1155/2017/1257078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bomeisl P.E., Thompson C.L., Harris L.N., Gilmore H.L. Comparison of oncotype DX recurrence score by histologic types of breast carcinoma. Arch Pathol Lab Med. 2015;139:1546–1549. doi: 10.5858/arpa.2014-0557-OA. [DOI] [PubMed] [Google Scholar]
- 21.Clark B.Z., Dabbs D.J., Cooper K.L., Bhargava R. Impact of progesterone receptor semiquantitative immunohistochemical result on Oncotype DXrecurrence score: a quality assurance study of 1074 cases. Appl Immunohistochem Mol Morphol. 2013;21:287–291. doi: 10.1097/PAI.0b013e31826f80c9. [DOI] [PubMed] [Google Scholar]
- 22.Auerbach J., Kim M., Fineberg S. Can features evaluated in the routine pathologic assessment of lymph node-negative estrogen receptor-positive stage I or II invasive breast cancer be used to predict the Oncotype DX recurrence score? Arch Pathol Lab Med. 2010;134:1697–1701. doi: 10.5858/2009-0439-OAR.1. [DOI] [PubMed] [Google Scholar]
- 23.Swain S.M., Nunes R., Yoshizawa C., Rothney M., Sing A.P. Quantitative gene expression by recurrence score in ER-positive breast cancer, by age. Adv Ther. 2015;32:1222–1236. doi: 10.1007/s12325-015-0268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik S., Shak S., Tang G., Kim C., Baker J., Cronin M. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 25.Sparano J.A., Gray R.J., Makower D.F., Pritchard K.I., Albain K.S., Hayes D.F. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitz U., Gluz O., Christgen M., Kates R.E., Clemens M., Malter W. Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 earlybreast cancer patients: five-year data from the prospective, randomised phase 3 West GermanStudy Group (WSG) PlanB trial. Breast Canc Res Treat. 2017;165(3):573–583. doi: 10.1007/s10549-017-4358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giuliano A.E., Edge S.B., Hortobagyi G.N. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25(7):1783–1785. doi: 10.1245/s10434-018-6486-6. [DOI] [PubMed] [Google Scholar]
- 28.NCCN NCCN clinical Practice guidelines in oncology V.2.2019. Breast Cancer. 2019. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Available online at:
- 29.Surveillance, Epidemiology, and End results (SEER) program. National Cancer Institute, DCCPS, Surveillance Research Program; Nov 2017. www.seer.cancer.gov SEER*Stat Database: Incidence - SEER 18 Regs (Excl AK) Custom Data Malignant Breast (with Oncotype DX and Additional Treatment Fields) Sub (2004-2015) - Linked To County Attributes - Total U.S., 1969-2016 Counties. released April 2018, based on the November 2017 submission. [Google Scholar]
- 30.Fu J., Wu L., Fu W., Tan Y., Xu T., Hong Z. How young is too young in breast cancer?-young breast cancer is not a unique biological subtype. Clin Breast Canc. 2018;18:e25–e39. doi: 10.1016/j.clbc.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Wang J., He Z.Y., Dong Y., Sun J.Y., Zhang W.W., Wu S.G. The distribution and outcomes of the 21-gene recurrence score in T1-T2N0 estrogen receptor-positive breast cancer with different histologic subtypes. Front Genet. 2018;9:638. doi: 10.3389/fgene.2018.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andre F., Ismaila N., Henry N.L., Somerfield M.R., Bast R.C., Barlow W. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update-integration of results from TAILORx. J Clin Oncol. 2019;37:1956–1964. doi: 10.1200/JCO.19.00945. [DOI] [PubMed] [Google Scholar]
- 33.Stemmer S.M., Steiner M., Rizel S., Soussan-Gutman L., Ben-Baruch N., Bareket-Samish A. Clinical outcomes in patients with node-negative breast cancer treated based on the recurrence score results: evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017;3:33. doi: 10.1038/s41523-017-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutonga M., Speedy S., Rademaker A., Liu D., Uthe R., Jain S. Relationship of pathological features and a 21 gene expression assay in younger versus older women with node-negative endocrine receptor-positive breast cancer. Breast Canc Res Treat. 2019;176:95–100. doi: 10.1007/s10549-018-05088-6. [DOI] [PubMed] [Google Scholar]
- 35.Williams A.D., Reyes S.A., Arlow R.L., Tchou J., De La Cruz L.M. Is age trumping genetic profiling in clinical practice? Relationship of chemotherapy recommendation and oncotype DX recurrence score in patients aged < 50 Years versus ≥ 50 Years, and trends over time. Ann Surg Oncol. 2018;25:2875–2883. doi: 10.1245/s10434-018-6600-9. [DOI] [PubMed] [Google Scholar]
- 36.Chen J., Wu X., Christos P.J., Formenti S., Nagar H. Practice patterns and outcomes for patients with node-negative hormone receptor-positive breast cancer and intermediate 21-gene Recurrence Scores. Breast Cancer Res. 2018;20:26. doi: 10.1186/s13058-018-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barcenas C.H., Raghavendra A., Sinha A.K., Syed M.P., Hsu L., Patangan M.G., Jr. Outcomes in patients with early-stage breast cancer who underwent a 21-gene expression assay. Cancer. 2017;123:2422–2431. doi: 10.1002/cncr.30618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kizy S., Huang J.L., Marmor S., Blaes A., Yuan J., Beckwith H. Distribution of 21-gene recurrence scores among breast cancer histologic subtypes. Arch Pathol Lab Med. 2018;142:735–741. doi: 10.5858/arpa.2017-0169-OA. [DOI] [PubMed] [Google Scholar]
- 39.Francis P.A., Fleming G.F., Walley B.A., Viale G., Colleoni M.6, Láng I. Absolute improvements in freedom from distant recurrence to tailor adjuvant EndocrineTherapies for premenopausal women: results from TEXT and SOFT. J Clin Oncol. 2019 doi: 10.1200/JCO.18.01967. Oct 16:JCO1801967. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-Fidalgo J.A., Rosello S., Garcia-Garre E., Jorda E., MartinMartorell P., Bermejo B. Incidence of chemotherapy-induced amenorrhea in hormonesensitive breast cancer patients: the impact of addition of taxanes to anthracycline-based regimens. Breast Canc Res Treat. 2010;120:245–251. doi: 10.1007/s10549-009-0426-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.