Abstract
Signaling pathways of the vitamin D receptor (VDR) and the triggering receptor expressed on myeloid cells (TREM) have been independently implicated in the biology of numerous of cutaneous pathologies. There is substantial evidence for possible crosstalk between these pathways, though the relationship between VDR and TREMs remains unclear. In this study, we characterize the effects of vitamin D-deficiency and sufficiency on the cutaneous expression of TREM-1, TREM-2, VDR, HMGB1, and RAGE. Cutaneous tissue isolated from Yucatan microswine were immunohistochemically evaluated for epidermal expression of TREM-1, TREM-2, VDR, HMGB1, and RAGE. The swine were fed a vitamin D-deficient or vitamin D-sufficient diet to examine the role of vitamin D state on levels of these markers. In vitamin D-sufficient animals, keratinocytes exhibited elevated levels of TREM-1, TREM-2. Additionally, TREM-1 expression predominated in basal cells, whereas TREM-2 levels were higher in keratinocytes, regardless of vitamin D state. Levels of HMGB1 and RAGE did not differ by vitamin D state. VDR expression was consistently higher in the cytoplasm and nuclei of basal cells, when compared to keratinocytes. Our findings suggest a role of vitamin D in signaling of TREM pathways. Additionally, the TREM ratio may play a role in keratinocyte differentiation and should be explored further. Possible signaling crosstalk between these pathways has a potential role in progression of cutaneous malignancies and other inflammatory pathologies.
Keywords: Triggering receptor expressed on myeloid cells, vitamin D receptor (VDR), high mobility group box 1 (HMGB1), receptor for advanced glycation endproducts (RAGE), vitamin D deficiency
Introduction
Expanding knowledge of the immunomodulatory functions of vitamin D and its receptor, VDR, create a role for these molecules in a broad spectrum of pathophysiologic cutaneous processes, including wound healing, [1] psoriasis, [2] skin aging, [3] and malignancies [4]. In basal cell carcinoma, immunostaining of VDR and mRNA levels were consistently stronger in tumor cells than in unaffected skin [5]. In melanocytic lesions, immunohistochemical analysis of VDR expression demonstrated higher VDR expression to be associated with less advanced malignancies and longer overall survival [6].
The triggering receptors expressed on myeloid cells (TREMs) receptor family plays a considerable role in innate immunity and inflammatory signaling. TREM-1 has been implicated as a pro-inflammatory molecule expressed by neutrophils and monocytes [7]. Conversely, TREM-2 is suggested to have anti-inflammatory actions and promote phagocytosis [8]. These receptors have demonstrated roles in numerous chronic inflammatory conditions and malignancies [9]. Early study of TREMs in melanoma suggests levels to be altered in the malignant state [10]. Furthermore, vitamin D has been shown to induce TREM-1 expression in airway epithelium, [11]. suggesting a complex interplay between these signaling pathways.
High-mobility group box-1 (HMGB1) is a ubiquitously expressed protein with dual function as a nonhistone DNA binding protein and as a cytokine mediator of inflammation secreted from immune cells such as monocytes/macrophages and dendritic cells [12]. It is a putative ligand for TREM-1, with demonstrated direct interaction and binding capability to this receptor [13]. Increased expression of HMGB1 is associated with several malignancies, [14], including epidermal tumors [15, 16]. Involvement in other cutaneous pathologies has been reported, including keloids [17] and inherited blistering skin disease [18]. One of the many receptors for HGMB1 is the receptor for advanced glycation endproducts (RAGE). This multi-ligand cell-surface receptor is an immunoglobulin superfamily member and is thought to be a pattern recognition receptor [19]. RAGE has implications in numerous malignancies, playing possible roles in tumor cell proliferation, survival, migration, and invasion [20]. HMGB1 and RAGE likely play a role in cutaneous malignancies, and may have clinical value in cutaneous cancer prognosis [21].
In this investigation, we immunohistochemically examined the cutaneous expression of VDR, TREM-1, TREM-2, HMGB1, and RAGE. Using a porcine model of vitamin D-deficiency and sufficiency, we investigated the effects of vitamin D levels on these molecules.
Methods
Porcine model
Female Yucatan microswine (Lonestar Laboratories, Sioux Center, IA, USA) were fed vitamin D-deficient(0 IU 25-hydroxycholecalciferol/day) or sufficient (2000 IU 25-hydroxycholecalciferol/day) high-cholesterol diets (Harlan, USA). Vitamin D-deficient diets consisted of 23.9% corn starch, 23.5%sucrose, 19% “vitamin free” casein, 13% maltodextrin, 10% cellulose, 4% soybean oil, and 4% cholesterol. The vitamin D-sufficient high cholesterol diet (Harlan, USA) consisted of 37.2% corn (8.5% protein), 23.5% soybean meal (44% protein), 20% chocolate mix, 5% alfalfa, 4% cholesterol, 4% peanut oil, 1.5% sodium cholate, and 1% lard. The swine were housed under controlled 12:12 hour light-dark cycles at 20-24°C, without sunlight to avoid variations in serum 25-hydroxyvitamin D levels due to UV exposure. Vitamin D levels and lipid profiles were routinely monitored every two weeks using peripheral blood obtained via ear venipuncture. The study protocol was approved by the Creighton University Institutional Animal Care and Use Committee and adhered to NIH standards and USDA guidelines.
Immunostaining
Skin was obtained from female Yucatan microswine postmortem. Tissue available for assay belonged to three vitamin D-sufficient animals and three vitamin D-deficient animals. Tissue sections of 4 μm in thickness were deparaffinized in xylene and rehydrated. Heat-induced antigen retrieval was performed in citrate buffer solution (S1699, Dako, Glostrup, Denmark). Endogenous peroxidases were blocked using Bloxall (Vector Laboratories, Burlingame, CA, USA). Immunostaining was performed with the standard streptavidin-biotin peroxidase technique using Vectastain ABC elite kits (Vector Laboratories, Burlingame, CA, USA) and 3, 30-diaminobenzidine chromogen solution (SK4100, Vector Laboratories, Burlingame, CA, USA) as an immunoperoxidase substrate. Primary antibodies were raised against TREM-1, TREM-2, VDR, HMGB1, and RAGE (TREM-1, 1:100, sc-19309; TREM-2, 1:250, sc-48764; VDR, 1:100, sc-13133; HMGB1, 1:250, sc-26-351; RAGE, 1:100, sc-8230; Santa Cruz Biotechnology, Dallas, TX, USA). Tissue was counter-stained with hematoxylin.
Quantification and statistical analysis
Immunoreactivity was quantified using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA) as previously described [22]. Five randomly selected cells in representative areas of the epidermal keratinocytes and basal cells were used for quantification of staining intensity at 40x magnification. Nuclear staining intensity was quantified in positively expressingcells. Cases were stained and quantified in duplicate. Data were collected in Microsoft Excel 14.4 statistical software package (Redmond, WA, USA). Statistical analysis was performed using R version 3.2.3 “Wooden Christmas-Tree” statistical software (R Foundation for Statistical Computing, Vienna, Austria). Comparison of mean staining intensities was performed using one-way ANOVA with Tukey tests for post-hoc analysis for each antibody and subcellular location (nuclear and cytoplasmic); given probability values are multiplicity-adjusted. An a equal to 0.05 was selected for all statistical analyses.
Results
Serum 25-hydroxycholecalciferol levels in vitamin D-deficient pigs ranged from 7.8 to 19.7 ng/mL, whereas levels in vitamin D-sufficient pigs ranged from 39.3 to 41.3 ng/mL. Immunostain intensities of TREM-1, TREM-2, VDR, HMGB1, and RAGE in the skin of vitamin D-sufficient and deficient swine are displayed in Figure 1 with representative micrographs exhibited in Figure 2.
Figure 1.
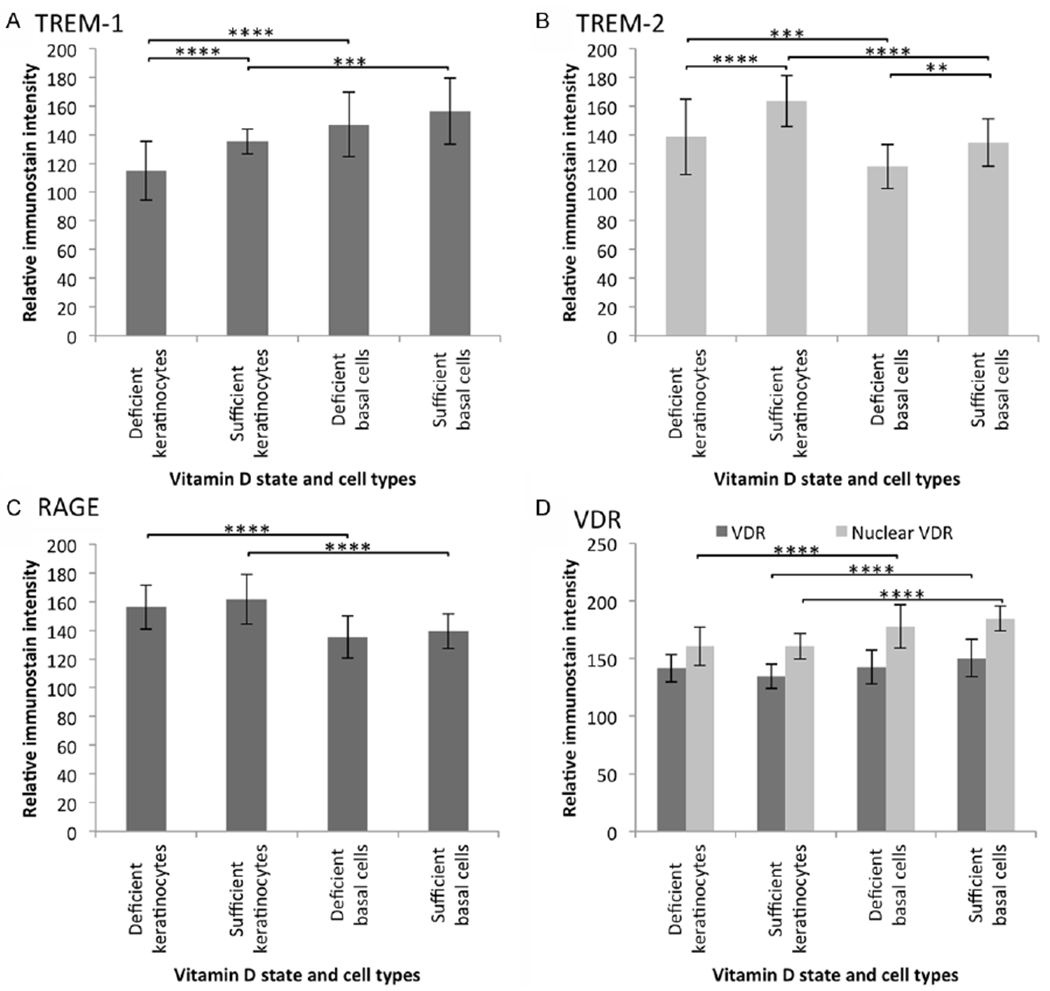
Mean relative immunostain intensity of (A) TREM-1, (B) TREM-2, (C) RAGE, and (D) nuclear and cytoplasmic VDR. One-way ANOVA analysis demonstrated a P < 0.0001 for immunoreactivity grouped by antibody (VDR: cytoplasmic P < 0.001, nuclear P < 0.0001). Expression of TREM-1 was increased in keratinocytes and TREM-2 was increased in both keratinocytes and basal cells with vitamin D sufficient diet. Additionally, TREM-1 was higher in basal cells, whereas TREM-2 levels predominated in keratinocytes. RAGE and nuclear VDR was increased in expression in keratinocytes in both vitamin D states. Cytoplasmic VDR expression was increased in keratinocytes of sufficient animals, compared to basal cells. Standard deviations are shown and statistical significance (post-hoc Tukeytest) is indicated (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Figure 2.
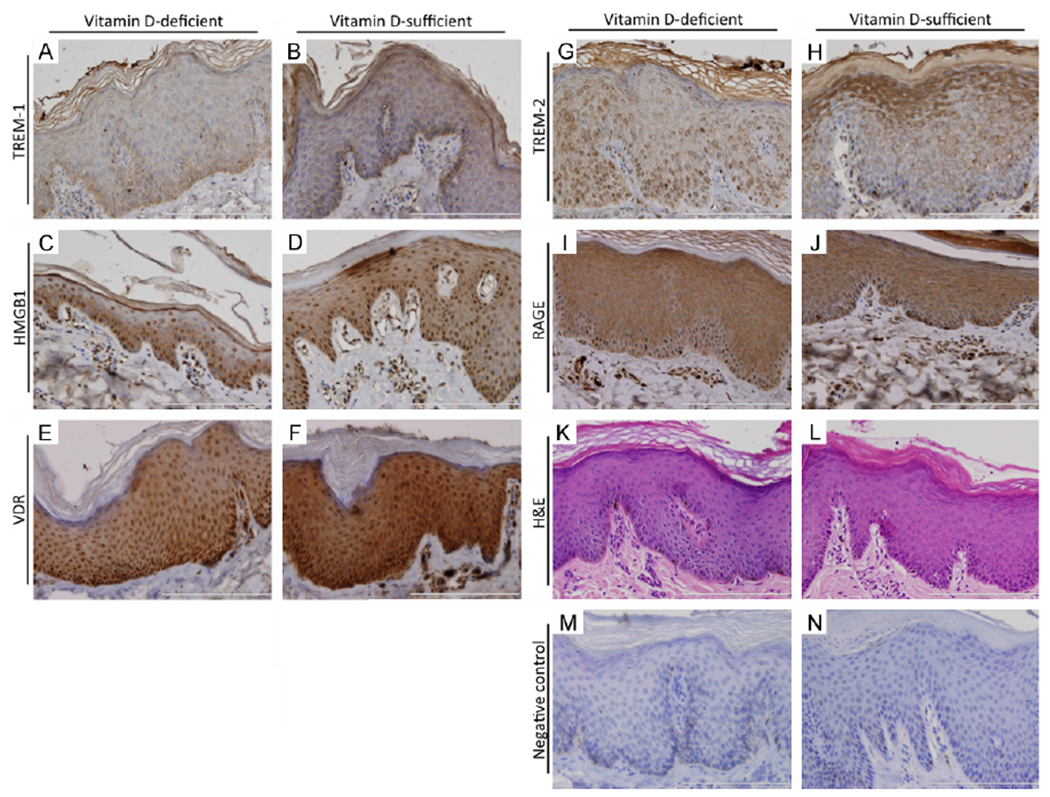
Representative micrographs of immunostained cutaneous tissue from vitamin D-deficient and vitamin D-sufficient swine. TREM-1 (A, B), TREM-2 (G, H), and RAGE (I, J) were localized to the cytoplasm, though TREM-2 demonstrated occasional nuclear staining. HMGB1 (C, D) and VDR (E, F) demonstrated both cytoplasmic and nuclear immunoreactivity. Sections stained with hematoxylin and eosin (K, L) are also shown along with negative controls (M, N) subjected to staining with exclusion of primary antibodies.
Staining of TREM-1 exhibited cytoplasmic expression with a full-thickness epidermal blush. Stronger expression tended to be in the basal layers, when compared to upper keratinocytes in both vitamin D-deficient and sufficient animals (both: ANOVA, P < 0.0001; Tukey, deficient: P < 0.0001, sufficient: P = 0.0003). Vascular bundles demonstrated endothelial positivity, whereas glands were negative in expression. Additionally, TREM-1 expression was increased in keratinocytes of vitamin D-sufficient cases, when compared to deficient cases (ANOVA, P < 0.0001; Tukey, P = 0.0005).
TREM-2 expression was focal and granular in quality in the cytoplasm of keratinocytes and basal cells. Of note, occasional nuclear staining was observed (not quantified). Endothelial cells in vascular structures and sweat glands demonstrated positive expression. TREM-2 immunostaining intensity was markedly higher in both keratinocytes and basal cells of vitamin D-sufficient cases, when compared to those of deficient cases (ANOVA, P < 0.0001; Tukey, keratinocytes: P < 0.0001, basal cells: P = 0.0072). Additionally, in both vitamin D states, keratinocyte expression of TREM-2 was significantly higher than that of basal cells (ANOVA, P < 0.0001; Tukey, sufficient: P < 0.0001, deficient: P = 0.0005).
HMGB1 was expressed in the cytoplasm and nuclei of epidermal cells. Cytoplasmic expression was not markedly different by cell type or vitamin D state (ANOVA, P = 0.769). Nuclear stippling was observed in HMGB1 expression, with observable nucleolar predominance in positivity. No differences in nuclear staining intensity were detected (ANOVA, P = 0.843).
RAGE immunoreactivity demonstrated cytoplasmic localization. In both vitamin D states, expression was elevated in keratinocytes, when compared to basal cells (ANOVA, P < 0.0001; Tukey, deficient: P < 0.0001, sufficient: P < 0.0001). No significant differences were detected when comparing immunostain intensity by vitamin D state (Tukey, keratinocytes: P = 0.4900, basal cells: P = 0.7450).
VDR was expressed in the skin of both vitamin D-deficient and sufficient swine. Expression was of a granular quality, with positivity in both the cytoplasm and nucleus of cells (ANOVA, cytoplasm: P = 0.0002, nuclei: P < 0.0001). A full-thickness blush was observed in both states, though in vitamin D-sufficient cases a marked increase in expression was observed in the cytoplasm of the basal cells of when compared to keratinocytes (Tukey, P < 0.0001). Comparison by cell type in deficient cases, on the other hand, demonstrated VDR cytoplasmic immunoreactivity to be equally expressed in the keratinocytes and basal cells (Tukey, P = 0.9940). Comparison by vitamin D state demonstrated a tendency of immunoreactivity in sufficient cases to be elevated in basal cells and decreased in keratinocytes (Tukey, P = 0.1093 and 0.1840, respectively). Analysis of nuclear staining intensity showed clear differences in cell type, with higher expression in the basal cells in either vitamin D-state (Tukey, both P < 0.0001).
Discussion
Expression of both TREM-1 and TREM-2 in keratinocytes was increased in the sufficient vitamin D state. Previous in vitro investigation has demonstrated vitamin D to be an inducer of TREM-1 in airway epithelium [23]. However, the interaction of cutaneous TREM expression with vitamin D has not been thoroughly investigated. In particular, the role of vitamin D in TREM-2 signaling has had little exploration. TREM-1 has been shown to promote inflammation, while TREM-2 is thought to have anti-inflammatory function [9]. Considering the suggested anti-inflammatory role of vitamin D, [24] the increase in expression of both TREM-1 and TREM-2 in vitamin D-sufficient swine was unexpected. In the light of inflammatory signaling, TREM-2 could be expected to decrease with a higher vitamin D state. This increase in both TREMs could, therefore, be reflective of more complex signaling crosstalk, as opposed to a simply dichotomy of anti- and pro-inflammatory signaling. Additionally, the TREM isoforms demonstrated a reciprocal pattern of expression, when considering cell type in either vitamin D state. TREM-1 immunoreactivity was higher in basal cells, compared to keratinocytes. TREM-2, on the other hand, was elevated in the keratinocytes. This could be indicative of the TREM-1 to TREM-2 ratio playing a role in epidermal cell differentiation. The possible importance of this TREM ratio has been previously suggested [10].
Expression of HMGB1 demonstrated no significant changes with vitamin D state and cell type. RAGE, while increased in keratinocytes when compared to basal cells, demonstrated no changes in immunoreactivity with vitamin D state. These data are suggestive of vitamin D to not have a direct impact on levels of HMGB1 and its RAGE receptor. Increased HMGB1 expression is associated with a number of cutaneous pathologies, including cutaneous malignancies, [15, 16] recessive dystrophic epidermolysis bullosa [18], and keloids [17]. Notably, ultraviolet irradiation of primary cutaneous melanomas in a mouse model caused enhanced expansion of tumour cells along abluminal blood vessel surfaces and increased the number of lungmetastases [25]. This effect depended on neutrophil recruitment and activation that was in turn initiated by release of HMGB1 from damaged keratinocytes. HMGB1 has been suggested to have a coregulatory role in steroid receptor-mediated gene transcription, though no direct interaction was found with VDR [26]. This does not preclude the possibility of interplay between HMGB1 and the vitamin D signaling pathway. Such crosstalk should be investigated, considering the opposing effects on inflammatory signaling of HMGB1 and vitamin D [24, 27].
The currently available literature reporting immunohistochemical evaluation of VDR demonstrates a widely variable localization of this receptor, ranging from nuclear, cytoplasmic and membranous in vulvar carcinomas [28] to solely nuclear reactivity in basal cell carcinomas [5]. Additionally, VDR subcellular localization will vary with vitamin D state, exhibiting a nuclear and cytoplasmic distribution in vitamin D deficiency versus a nucleus-dominated state in the presence of its ligand [29, 30]. The VDR expression observed presently is consistent with previously reported [31] cutaneous VDR staining patterns in vitamin D-sufficient and deficient swine.
Crosstalk of TREM and VDR signaling may be permissible through a number of molecular pathways. In hepatocellular carcinoma, AKT has been suggested as a down-stream effector of TREM-1 signal transduction, along with p65, STAT3, and ERK [32]. Furthermore, ablation of the TREM-1 murine analogue diminished activation of the p38, ERK 1/2, JNK, MAPK, and NF-κΒ signaling pathways in Kupffer cells [33]. In skeletal muscle cells, MAPK activation has been shown to be dependent on VDR expression [34]. Further evidence suggests that VDR inhibits pro-inflammatory cytokines in monocytes and macrophages via MAPK phosphatase-1 [35]. Further exploration of these interlinked pathways is warranted in cutaneous models, as they may have wide implications in epidermal pathologies. Altered expression of TREMs has been previously demonstrated in cutaneous melanoma [10] and could play a role in cutaneous cancer progression. In psoriasis, TREM-1 has even been suggested as a potential therapeutic target [36]. VDR has a similarly wide involvement in cutaneous pathology, including melanoma in which it may serve as a marker for tumor progression [6]. Exploitation of these interlinked pathways has broad potential for clinical application. Thus further investigation aimed at elucidating the nature of these interactions is fully merited.
Our results indicate cutaneous TREM and VDR expression to be influenced by vitamin D status and cell type. Additionally, the TREM-1 to TREM-2 ratio may play a role in keratinocyte differentiation. Furthermore, a complex interplay involving vitamin D, VDR, HMGB1 and the TREM signaling pathway may exist, requiring further exploration. These molecules have been individually implicated in various high impact cutaneous malignancies and other pathologies, though their interplay has had minimal investigation. Future studies are warranted to further understanding of the complex relationship of these molecules in cutaneous pathology.
Acknowledgements
This work was supported by research grants R01 HL116042, R01 HL104516, and R01 FIL120659to DK Agrawal from the Office of the Director, National Institutes of Health, and National Fleart, Lung and Blood Institute, NlH USA.
Footnotes
Disclosure of conflict of interest
None.
References
- [1].Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol 2015; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stefanic M, Rucevic I, Barisic-Drusko V. Meta-analysis of vitamin D receptor polymorphisms and psoriasis risk. Int J Dermatol 2013; 52: 705–10. [DOI] [PubMed] [Google Scholar]
- [3].Reichrath J Unravelling of hidden secrets: The role of vitamin D in skin aging. Dermatoendocrinol 2012; 4: 241–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu X, Zhou T, Cao N, Ni J, Wang X. Role of Vitamin D Metabolism and Activity on Carcinogenesis. Oncol Res 2015; 22: 129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reichrath J, Kamradt J, Zhu XH, Kong XF, Tilgen W, Holick MF. Analysis of 1,25-dihydroxyvitamin D(3) receptors (VDR) in basal cell carcinomas. Am J Pathol 1999; 155: 583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brożyna AA, Jóźwicki W, Slominski AT. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: new data and analyses. Anticancer Res 2014; 34: 2735–43. [PMC free article] [PubMed] [Google Scholar]
- [7].Bouchon A, Dietrich J, Colonna M. Cutting Edge: Inflammatory Responses Can Be Triggered by TREM-1, a Novel Receptor Expressed on Neutrophils and Monocytes. J Immunol 2000; 164: 4991–5. [DOI] [PubMed] [Google Scholar]
- [8].Sharif O, Knapp S. From expression to signaling: roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology 2008; 213: 701–13. [DOI] [PubMed] [Google Scholar]
- [9].Nguyen AH, Berim IG, Agrawal DK. Chronic inflammation and cancer: emerging roles of triggering receptors expressed on myeloid cells. Expert Rev Clin Immunol 2015; 11: 849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nguyen AH, Koenck C, Quirk SK, Lim VM, Mitkov Μ V, Trowbridge RM, Hunter WJ 3rd, Agrawal DK. Triggering Receptor Expressed on Myeloid Cells in Cutaneous Melanoma. Clin Transl Sci 2015; 8: 441–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rigo I, McMahon L, Dhawan P, Christakos S, Yim S, Ryan LK, Diamond G. Induction of triggering receptor expressed on myeloid cells (TREM-1) in airway epithelial cells by 1,25(OH)2 vitamin D3. Innate Immun 2012; 18: 250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Czura CJ, Wang H, Tracey KJ. Dual roles for HMGB1: DNA bindingand cytokine. J Endotoxin Res 2001; 7: 315–21. [DOI] [PubMed] [Google Scholar]
- [13].Wu J, Li J, Salcedo R, Mivechi NF, Trinchieri G, Horuzsko A. The Proinflammatory Myeloid Cell Receptor TREM-1 Controls Kupffer Cell Activation and Development of Hepatocellular Carcinoma. Cancer Res 2012; 72: 3977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nguyen A, Bhavsr S, Riley E, Caponetti G, Agrawal D. Clinical value of high mobility group box 1 and the receptor for advanced glycation endproducs in head and neck cancer: A systematic review. Int Arch Otorhinolaryngol 2016; In press, accepted Feb 28, 2016. [DOI] [PMC free article] [PubMed]
- [15].Weng H, Deng Y, Xie Y, Liu H, Gong F. Expression and significance of HMGB1, TLR4 and NF-κB p65 in human epidermal tumors. BMC Cancer 2013; 13: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sharma A, Ray R, Rajeswari MR. Overexpression of high mobility group (HMG) B1 and B2 proteins directly correlates with the progression of squamous cell carcinoma in skin. Cancer Invest 2008; 26: 843–51. [DOI] [PubMed] [Google Scholar]
- [17].Lee DE, Trowbridge RM, Ayoub NT, Agrawal DK. High-mobility Group Box Protein-1, Matrix Metalloproteinases, and Vitamin D in Keloids and Hypertrophic Scars. Plast Reconstr Surg - Glob Open 2015; 3: e425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Petrof G, Abdul-Wahab A, Proudfoot L, Pramanik R, Mellerio JE, McGrath J a. Serum levels of high mobility group box 1 correlate with disease severity in recessive dystrophic epidermolysis bullosa. Exp Dermatol 2013; 22: 433–5. [DOI] [PubMed] [Google Scholar]
- [19].Malik P, Chaudhry N, Mittal R, Mukherjee TK. Role of receptor for advanced glycation end products in the complication and progression of various types of cancers. Biochim Biophys Acta 2015; 1850: 1898–904. [DOI] [PubMed] [Google Scholar]
- [20].Logsdon CD, Fuentes MK, Huang EH, Arumugam T. RAGE and RAGE ligands in cancer. Curr Mol Med 2007; 7: 777–89. [DOI] [PubMed] [Google Scholar]
- [21].Nguyen AH, Quirk S, Agrawal D. Clinical implications of high mobility group box-1 (HMGB1) and the receptor for advanced glycation endproducts in cutaneous malignancy: A systematic review. 2016. [DOI] [PubMed] [Google Scholar]
- [22].Nguyen D Quantifying chromogen intensity in immunohistochemistry via reciprocal intensity. Nature Publishing Group 2013. [Google Scholar]
- [23].Rigo I, McMahon L, Dhawan P, Christakos S, Yim S, Ryan LK, Diamond G. Induction of triggering receptor expressed on myeloid cells (TREM-1) in airway epithelial cells by 1,25(0H)2 vitamin D3. Innate Immunol 2012; 18: 250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Toniato E, Spinas E, Saggini A, Kritas SK, Caraffa A, Antinolfi P, Saggini R, Pandolfi F, Conti P. Immunomodulatory Effects Of Vita-min D On Skin Inflammation. J Biol Regul Homeost Agents 2015; 29: 563–7. [PubMed] [Google Scholar]
- [25].Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Hömig-Hölzel C, Reuten R, Schadow B, Weighardt H, Wenzel D, Helfrich I, Schadendorf D, Bloch W, Bianchi ME, Lugassy C, Barnhill RL, Koch M, Fleischmann BK, Förster I, Kastenmüller W, Kolanus W, Hölzel M, Gaffal E, Tüting T. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014; 507: 109–13. [DOI] [PubMed] [Google Scholar]
- [26].Boonyaratanakornkit V, Melvin V, Prendergast P, Altmann M, Ronfani L, Bianchi ME, Taraseviciene L, Nordeen SK, Allegretto EA, Edwards DP. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol Cell Biol 1998; 18: 4471–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yamada S, Maruyama I, Takemoto T, Akahoshi T. Recent advances in inflammatory markers. HMGB1 and TREM-1. Inflamm Regen 2007; 23: 88–95. [Google Scholar]
- [28].Salehin D, Haugk C, Thill M, Cordes T, William M, Hemmerlein B, Friedrich M. Vitamin D receptor expression in patients with vulvar cancer. Anticancer Res 2012; 32: 283–9. [PubMed] [Google Scholar]
- [29].Prufer K, Barsony J. Retinoid X receptor dominates the nuclear import and export of the unliganded vitamin D receptor. Mol Endocrinol 2002; 16: 1738–51. [DOI] [PubMed] [Google Scholar]
- [30].Prufer K, Racz A, Lin GC, Barsony J. Dimerization with retinoid X receptors promotes nuclear localization and subnuclear targeting of vitamin D receptors. J Biol Chem 2000; 275: 41114–23. [DOI] [PubMed] [Google Scholar]
- [31].Trowbridge RM, Mitkov MV, Hunter WJ, Agrawal DK. Vitamin D receptor and CD86 expression in the skin of vitamin D-deficient swine. Exp Mol Pathol 2014; 96: 42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Duan M, Wang ZC, Wang XY, Shi JY, Yang LX, Ding ZB, Gao Q, Zhou J, Fan J. TREM-1, an Inflammatory Modulator, is Expressed in Hepatocellular Carcinoma Cells and Significantly Promotes Tumor Progression. Ann Surg Oncol 2015; 22: 3121–9. [DOI] [PubMed] [Google Scholar]
- [33].Wu J, Li J, Salcedo R, Mivechi NF, Trinchieri G, Horuzsko A. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res 2012; 72: 3977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Buitrago C, Pardo VG, Boland R. Role of VDR in 1α,25-dihydroxyvitamin D3-dependent nongenomic activation of MAPKs, Src and Akt in skeletal muscle cells. J Steroid Biochem Mol Biol 2013; 136: 125–30. [DOI] [PubMed] [Google Scholar]
- [35].Zhang Y, Leung DYM, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 2012; 188: 2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hyder L a, Gonzalez J, Harden JL, Johnson-Huang LM, Zaba LC, Pierson KC, Eungdamrong NJ, Lentini T, Gulati N, Fuentes-Duculan J, Suárez-Fariñas M, Lowes MA. TREM-1 as a potential therapeutic target in psoriasis. J Invest Dermatol 2013; 133: 1742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


