Abstract
Acute interstitial nephritis is an uncommon but classic complication of minocycline therapy for acne. A 14-year-old African American girl was started on oral minocycline for the treatment of acne 6 weeks before presentation. After 4 weeks on minocycline, she developed a generalized rash, anasarca, fever, myalgia, nausea, vomiting, sore throat, and generalized body weakness. The evaluation showed increased levels of serum creatinine, urea nitrogen, and serum alanine and aspartate aminotransferases. Renal ultrasonography showed bilateral enlarged, echogenic kidneys, and percutaneous renal biopsy showed features of acute allergic interstitial nephritis. Treatment included methylprednisolone and intravenous fluids and discontinuation of minocycline. The elevated serum creatinine level (12.9 mg/dL (reference, 0.40–0.70 mg/dL)) suggests marked renal impairment corresponding with Kidney Disease Improving Global Outcomes acute kidney injury classification stage 3. The kidney injury improved from stage 3 to stage 1 within 3 days, and early treatment with steroids might have prevented chronic renal failure. The creatinine level promptly decreased to normal, and liver enzyme results also improved. In summary, the diagnosis of acute interstitial nephritis should be considered in patients who present with renal failure associated with recent use of minocycline, and treatment with corticosteroids should be considered early during the hospitalization.
Keywords: Renal failure, antibiotic, adverse event, acne
Introduction
Acute interstitial nephritis (AIN) is a decline in renal function secondary to inflammation and the accumulation of inflammatory debris in the renal interstitium. AIN commonly is caused by drugs, most commonly antibiotics (penicillin, cephalosporins, and minocycline), nonsteroidal anti-inflammatory drugs, and proton pump inhibitors.1,2 Drug-induced–acute interstitial nephritis (DI-AIN) is a delayed T-cell-mediated hypersensitivity reaction that manifests 7–10 days after drug exposure as a rise in serum creatinine or urea nitrogen level.3–5
Minocycline is a bacteriostatic antibiotic that may have protective effects on the kidneys including antiapoptotic, antioxidant, cytoprotective, and anti-inflammatory properties.6 Although minocycline is not a first-line drug for treatment of acne, it still is used in practice and may have substantial adverse events. Minocycline treatment for acne may be associated with the development of systemic lupus erythematosus, hyperpigmentation, liver dysfunction, or drug reaction with eosinophilia and systemic symptoms (DRESS).7,8 In patients who have renal failure, minocycline does not accumulate but may aggravate uremia.9,10 Literature search showed only rare reports of isolated AIN or chronic interstitial nephritis associated with minocycline2,4,11–13 and very few cases with concomitant liver or systemic involvement (Table 1).14,15 However, physicians should be aware of the potential adverse effects of minocycline because these may include life-threatening complications and chronic comorbidities such as the need for hemodialysis or liver transplant (Table 1).12,15
Table 1.
Published reports of acute interstitial nephritis associated with minocycline.
| Age (years) | Sex | Indication for treatment | Serum creatinine (mg/dL) | Recovery time | Reference |
|---|---|---|---|---|---|
| 43 | F | Upper respiratory infection | 7.9 | 2 weeks | Walker et al.11 |
| 16 | M | Sycosis barbae and acne vulgaris | 14.2 | 6 weeksa | Wilkinson et al.12 |
| 47 | F | Acne | 5.8 | 6 monthsb | Fletcher and Sellars4 |
| 15 | M | Acne | 11.7 | DRESSc | Kiessling et al.14 |
| 13 | F | Acne | 1.5 | DRESSd | Lan et al.15 |
Literature search performed with PubMed and review of references in published studies. DRESS: drug reaction with eosinophilia and systemic symptoms; F: female; M: male.
Required dialysis, and had protracted illness for 6 weeks.
Patient developed chronic renal failure and was treated with steroids for 6 weeks.
Patient needed hemodialysis for 15 days, BUN and creatinine was normalized in 1 month, steroid was given for 3 months.
Patient had fulminant liver failure requiring a liver transplant and developed autoimmune type 1 diabetes mellitus. Chronic illness, recovery time not applicable.
We treated an adolescent girl who had minocycline-induced AIN that improved with corticosteroid therapy.
Case report
A 14-year-old African American girl (body mass index, 21.5 kg/m2) was started on oral minocycline (100 mg capsule once daily) for treatment of acne at 6 weeks prior to presentation. After 4 weeks on minocycline, she developed a generalized rash and anasarca that persisted. She also developed fever, myalgia, nausea, vomiting, sore throat, and generalized body weakness. There was no history of flank or lower abdominal pain. She took diphenhydramine for the rash and pruritus, but she had only partial relief and presented to the emergency department.
Physical examination showed that she was alert and nontoxic. Vital signs were normal. She had a generalized, palpable, erythematous rash that did not blanch with external pressure, mild anasarca, erythematous tonsils with exudate, and bilateral posterior cervical lymphadenopathy. The examination otherwise was normal, including the lungs, heart, and abdomen. There was no costovertebral angle or suprapubic tenderness.
Laboratory tests showed serum leukocyte count 26 × 109/L (reference, 4.5–11 × 109/L), hemoglobin level 11.9 gm/dL (reference, 12–16 gm/dL), and platelet count 156 × 109/L (reference, 150–250 × 109/L). The differential showed the absence of eosinophilia (1% eosinophils), with an eosinophil count of 0.26 × 109/L (reference, 0–0.5 × 109/L) and presence of atypical lymphocytes (1.29 × 109/L). Coagulation profile showed mildly prolonged prothrombin time (16.3 s (reference, 10–13 s)). Comprehensive metabolic panel showed elevated serum creatinine (12.9 mg/dL (reference, 0.40–0.70 mg/dL)), urea nitrogen (75 mg/dL (reference, 7–18 mg/dL)), and liver enzyme levels (alanine aminotransferase, 378 U/L (reference, 7–45 U/L); aspartate aminotransferase, 146 U/L (reference, 8–48 U/L). Complement C3 level was 0.89 g/L (reference, 0.8–1.6 g/L) and C4 level was 0.08 g/L (reference, 0.16–0.48 g/L). Urinalysis showed that the urine was leukocyte esterase positive, nitrite negative, had red blood cells (0–2 per high-power field (HPF)), and leukocytes (6–15 per HPF). In addition, the urine peripheral smear showed few renal epithelial cells, leukocyte casts (0–2 per HPF), granular casts (0–2 per HPF), and few eosinophils. There was mild proteinuria (protein-to-creatinine ratio, 44.1 mg/mmol). Urine, blood, and throat culture results were negative. Epstein-Barr virus (EBV) immunoglobulin M (IgM) titer was positive, but serum EBV polymerase chain reaction and Monospot tests were negative. Renal ultrasonography showed bilateral enlarged, echogenic kidneys, and percutaneous renal biopsy showed features consistent with allergic AIN (Figure 1). Skin biopsy was not performed. HIV test was deferred because there was no history of sexual activity or intravenous drug abuse.
Figure 1.
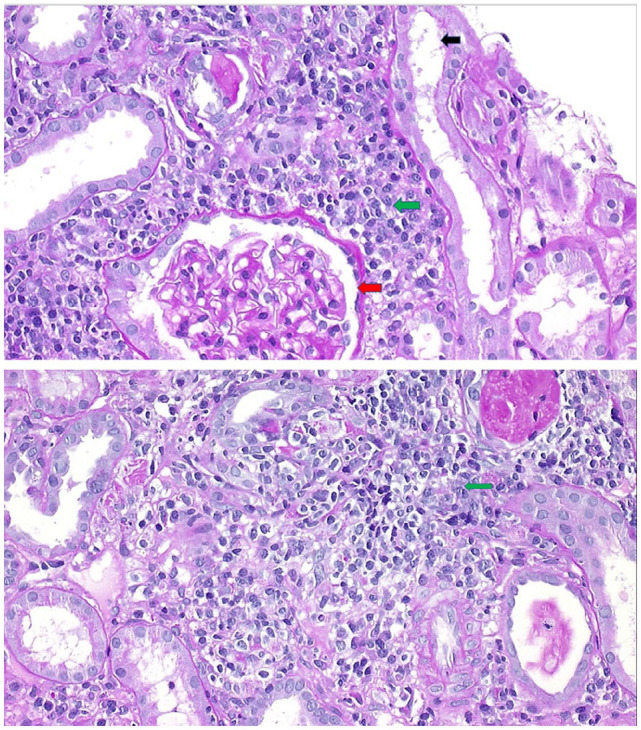
A 14-year-old girl who presented with renal failure after starting minocycline treatment for acne. Renal biopsy (periodic acid–Schiff stain; magnification ×400) showed normal glomerulus (red arrow) but marked interstitial edema with patchy interstitial lymphoplasmacytic infiltrates (green arrows). The tubules had dilated lumens and decreased positive periodic acid–Schiff stain positivity of the brush border (black arrow).
The minocycline was discontinued. The patient was admitted to the hospital and treated with methylprednisolone (120 mg intravenous loading dose followed by 60 mg intravenous twice daily for 5 days), and steroid therapy was tapered with oral prednisone over 3 weeks and stopped (20 mg once daily at 6–12 days; 10 mg once daily at 13–24 days). The patient received normal saline bolus intravenously followed by twice maintenance intravenous fluid for × 2 days (0.9% saline with 5% dextrose and 20 meq kcal/L). Serum creatinine level decreased to 9 mg/dL at 1 day, 4.3 mg/dL at 2 days, 2.17 mg/dL at 3 days, 1.61 mg/dL at 4 days, 1.1 mg/dL at 6 days, and 0.89 mg/dL at 9 days. The liver aminotransferase levels were decreased at 9 days (alanine aminotransferase, 271 U/L; aspartate aminotransferase, 48 U/L). The cervical lymphadenopathy resolved when the patient was discharged home after 9 days of hospitalization. The renal function returned to normal on follow-up laboratory assessment at 1 month, and there was no further recurrence of renal or hepatic dysfunction.
Discussion
The present patient had minocycline-induced AIN that responded rapidly to discontinuation of minocycline and treatment with systemic methylprednisolone and intravenous fluids. Previous cases of minocycline- or tetracycline-induced AIN in adolescents and adults had been treated successfully with steroids, but some patients may require hemodialysis.4,11,12,14–16
A high index of suspicion for minocycline-induced AIN is important if there is an abnormal renal function test with recent use of minocycline. Patients with AIN may be asymptomatic or present with nonspecific symptoms, and only one-third of patients who have AIN may present with the classic triad of fever, rash, and eosinophilia.17 Diagnosis of AIN can also be suspected when the fever, rash, and arthralgias are associated with an elevation in serum immunoglobulin E level and the presence of eosinophils or other inflammatory infiltrate in the renal interstitium.3 Although some patients may have an abnormal lymphocyte stimulation test, renal biopsy is the most reliable diagnostic procedure.1,2,18 Characteristic renal biopsy findings of AIN include tubulitis, tubular epithelial degeneration, interstitial edema, and interstitial mononuclear cells or T lymphocytes.2
Due to the patient’s constellation of symptoms and signs including fever, anasarca, rash, lymphadenopathy, visceral organ involvement in form of nephritis and hepatitis and presence of atypical lymphocyte on peripheral smear, DRESS syndrome was considered.19 While minocycline has been reported to cause DRESS syndrome, it is usually associated with pulmonary involvement which was not the case here. Furthermore, DRESS often have eosinophilia which was absent in this case. RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions) score was 5 which classified this case as probable DRESS, hence it cannot be excluded with certainty.19 It is important to note that treatment of DRESS also includes steroids, particularly when visceral organs are involved which is the same treatment we deployed in this case of likely AIN.
Prior case studies have shown findings on renal biopsies following tetracycline use consistent with severe AIN.17 Common findings at the onset of renal failure secondary to DI-AIN include smooth swollen kidneys on ultrasonography and eosinophiluria, as observed in our patient.12 Patients may be asymptomatic or have nonspecific symptoms leading to a delay in diagnosis.1,17 The temporal association of use of the drug and onset of symptoms is helpful for diagnosis of AIN.11,12,14,16 Our patient did not have urinary symptoms, and the diagnosis of acute renal failure was not established until a basic metabolic profile showed elevated serum urea nitrogen and creatinine levels. This confirms that AIN should be considered even in the absence of oliguria or other urinary symptoms.
Treatment of drug-induced AIN includes stopping the causative drug. The use of corticosteroids in the treatment of DI-AIN is controversial and has not demonstrated consistent benefit.5,20 Some studies have shown improvement of kidney function recovery with early initiation of corticosteroid therapy.17,20 DI-AIN is a T-cell-mediated hypersensitivity reaction, and the suppressor effect of steroids on T cells and eosinophils may control inflammation.3 The lack of high-quality randomized controlled trials regarding the use of corticosteroids in the treatment of DI-AIN suggests that more research should be performed.1,3 The present patient was treated with corticosteroids early during the hospitalization and had rapid clinical improvement, with a return of normal kidney function within 1 week. It is important to be cautious when giving high-dose steroids in a patient with active infection. The low positive titer of EBV IgM suggests reactivation of an EBV infection. The positive Epstein-Barr Virus Nuclear Antigen (EBNA) excludes an acute infection and negative blood EBV PCR suggests there is no evidence of viremia. The creatinine level in our patient was high, suggesting marked renal impairment corresponding to Kidney Disease Improving Global Outcomes acute kidney injury stage 3 which improved to stage 1 within 3 days of treatment and steroids in part may have prevented chronic renal failure. The transaminitis was possibly due to drug-induced liver injury which improved after the minocycline was stopped.
A previous case series of 133 biopsy-proven cases of AIN showed that 70% of cases were drug-induced in which more than 25 different types of drugs were implicated.21,22 The present case illustrates the importance of being vigilant for DI-AIN and DRESS associated with drugs that may not be commonly associated with these syndromes.
Conclusion
In summary, the present case suggests that the diagnosis of DI-AIN should be considered in patients who present with renal failure associated with recent use of minocycline. Early steroid therapy was associated with rapid recovery in this patient. A high index of suspicion and routine laboratory assessment for renal function are vital for early detection.
Acknowledgments
The authors thank John V. Marymont, Mary I. Townsley, and Elly Trepman for writing and editorial support.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: Kamal Sharma  https://orcid.org/0000-0002-9860-2047
https://orcid.org/0000-0002-9860-2047
References
- 1. Moledina DG, Perazella MA. Drug-induced acute interstitial nephritis. Clin J Am Soc Nephrol 2017; 12: 2046–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joh K, Aizawa S, Yamaguchi Y, et al. Drug-induced hypersensitivity nephritis: lymphocyte stimulation testing and renal biopsy in 10 cases. Am J Nephrol 1990; 10(3): 222–230. [DOI] [PubMed] [Google Scholar]
- 3. Raghavan R, Shawar S. Mechanisms of drug-induced interstitial nephritis. Adv Chronic Kidney Dis 2017; 24: 64–71. [DOI] [PubMed] [Google Scholar]
- 4. Fletcher S, Sellars L. Minocycline-induced chronic interstitial nephritis. Nephrol Dial Transplant 1996; 11(3): 540–541. [PubMed] [Google Scholar]
- 5. Kodner CM, Kudrimoti A. Diagnosis and management of acute interstitial nephritis. Am Fam Physician 2003; 67: 2527–2534. [PubMed] [Google Scholar]
- 6. Haghi-Aminjan H, Asghari MH, Goharbari MH, et al. A systematic review on potential mechanisms of minocycline in kidney diseases. Pharmacol Rep 2017; 69(4): 602–609. [DOI] [PubMed] [Google Scholar]
- 7. Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev 2012; 2012: Cd002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walsh TR, Efthimiou J, Dreno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis 2016; 16(3): e23–e33. [DOI] [PubMed] [Google Scholar]
- 9. Welling PG, Shaw WR, Uman SJ, et al. Pharmacokinetics of minocycline in renal failure. Antimicrob Agents Chemother 1975; 8(5): 532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. George CR, Guinness MD, Lark DJ, et al. Minocycline toxicity in renal failure. Med J Aust 1973; 1: 640–641. [DOI] [PubMed] [Google Scholar]
- 11. Walker RG, Thomson NM, Dowling JP, et al. Minocycline-induced acute interstitial nephritis. Br Med J 1979; 1: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilkinson SP, Stewart WK, Spiers EM, et al. Protracted systemic illness and interstitial nephritis due to minocycline. Postgrad Med J 1989; 65(759): 53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hill GW, Roach M. Minocycline-induced interstitial nephritis. Br Med J 1979; 1: 820–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kiessling S, Forrest K, Moscow J, et al. Interstitial nephritis, hepatic failure, and systemic eosinophilia after minocycline treatment. Am J Kidney Dis 2001; 38(6): E36. [DOI] [PubMed] [Google Scholar]
- 15. Lan J, Lahoti A, Lew DB. A severe case of minocycline-induced DRESS resulting in liver transplantation and autoimmune sequelae. Ann Allergy Asthma Immunol 2016; 116(4): 367–368. [DOI] [PubMed] [Google Scholar]
- 16. Bihorac A, Ozener C, Akoglu E, et al. Tetracycline-induced acute interstitial nephritis as a cause of acute renal failure. Nephron 1999; 81(1): 72–75. [DOI] [PubMed] [Google Scholar]
- 17. Fernandez-Juarez G, Perez JV, Caravaca-Fontan F, et al. Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol 2018; 13: 1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shibasaki T, Ishimoto F, Sakai O, et al. Clinical characterization of drug-induced allergic nephritis. Am J Nephrol 1991; 11(3): 174–180. [DOI] [PubMed] [Google Scholar]
- 19. Taweesedt PT, Nordstrom CW, Stoeckel J, et al. Pulmonary manifestations of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a systematic review. Biomed Res Int 2019; 2019: 7863815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quinto LR, Sukkar L, Gallagher M. Effectiveness of corticosteroid compared with non-corticosteroid therapy for the treatment of drug-induced acute interstitial nephritis: a systematic review. Intern Med J 2019; 49(5): 562–569. [DOI] [PubMed] [Google Scholar]
- 21. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis 2014; 64(4): 558–566. [DOI] [PubMed] [Google Scholar]
- 22. Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol 2010; 6: 461–470. [DOI] [PubMed] [Google Scholar]


