Abstract
Objectives:
This study was to explore the effect of protein phosphatase, Mg2+/Mn2+ dependent 1D knockdown on proliferation and apoptosis as well as p38 MAPK/p53 signaling pathway in acute myeloid leukemia.
Methods:
The expression of protein phosphatase, Mg2+/Mn2+ dependent 1D was detected in acute myeloid leukemia cell lines including SKM-1, KG-1, AML-193, and THP-1 cells, and normal bone marrow mononuclear cells isolated from healthy donors. The knockdown of protein phosphatase, Mg2+/Mn2+ dependent 1D was conducted by transfecting small interfering RNA into AML-193 cells and KG-1 cells.
Results:
The relative messenger RNA/protein expressions of protein phosphatase, Mg2+/Mn2+ dependent 1D were higher in SKM-1, KG-1, AML-193, and THP-1 cells compared with control cells (normal bone marrow mononuclear cells). After transfecting protein phosphatase, Mg2+/Mn2+ dependent 1D small interfering RNA into AML-193 cells and KG-1 cells, both messenger RNA and protein expressions of protein phosphatase, Mg2+/Mn2+ dependent 1D were significantly reduced, indicating the successful transfection. Most importantly, knockdown of protein phosphatase, Mg2+/Mn2+ dependent 1D suppressed cell proliferation and promoted cell apoptosis in AML-193 cells and KG-1 cells. In addition, knockdown of protein phosphatase, Mg2+/Mn2+ dependent 1D enhanced the expressions of p-p38 and p53 in AML-193 cells and KG-1 cells. The above observation suggested that protein phosphatase, Mg2+/Mn2+ dependent 1D knockdown suppressed cell proliferation, promoted cell apoptosis, and activated p38 MAPK/p53 signaling pathway in acute myeloid leukemia cells.
Conclusion:
Protein phosphatase, Mg2+/Mn2+ dependent 1D is implicated in acute myeloid leukemia carcinogenesis, which illuminates its potential role as a treatment target for acute myeloid leukemia.
Keywords: PPM1D, AML, p38 MAPK/p53 signaling pathway, proliferation, apoptosis
Introduction
Acute myeloid leukemia (AML), a heterogenous hematological malignancy, is characterized by abnormal myeloid lineage differentiation and accumulation of leukemic blast cells.1 As the most common acute leukemia in adults (accounting for 80% of all cases), the incidence of AML ranges from 1.3/100 000 in population less than 65 years to 12.2/100 000 in those elder than 65 years.2 The currently standard treatments (cytarabine plus anthracycline) or the novel packaging of conventional chemotherapies have achieved certain improvements in treatment response and prognosis, while the treatment outcome is still generally poor.2-4 Recently, the molecular ontogeny of AML has led to the discoveries of genes and pathways that underly the pathogenesis of AML, which stimulates the development of targeted drugs.5 However, the treatment options for AML are currently limited and more molecular studies are still needed for better understanding of the disease and the discovery novel treatment targets.
Protein phosphatase, Mg2+/Mn2+ dependent 1D (PPM1D), also called wild type p53 inducible protein 1 (Wip1) phosphatase, is one of the protein phosphatase 2C family of Ser/Thr protein phosphatases.6 Upregulation or mutation of PPM1D in primary tumors is observed by existing literatures, which indicates the carcinogenic role of PPM1D. For instance, PPM1D protein-truncating variants mutations that possess a gain-of-function effect are associated with increased breast cancer and ovarian cancer risk, and PPM1D facilitates the malignant progression of breast cancer by inactivating wild type p38 MAPK, p53, and p16.7,8 Molecular studies indicate that PPM1D inactivates p38 MAPK and p53 by phosphorylation, and thereby suppressing the UV-induced cell apoptosis in pancreatic cancer and several lung cancer cell lines.9,10 As for hematological malignancies, suppression of PPM1D induces neutrophil differentiation and G1 arrest in human promyelocytic leukemia cells, and inhibition of PPM1D enhances the arsenic trioxide-induced activation of Chk2/p53 and p38 MAPK/p53 signaling pathway.11,12 In addition, mouse model of adult T-cell leukemia/lymphoma discloses that Tax + PPM1D−/− mice present with less tumorigenesis compared with Tax + PPM1D+/+ mice.13
Known that p38 MAPK/p53 signaling pathway is essential for apoptosis regulation in human leukemia including AML, and PPM1D is closely associated with p38 MAPK/p53 signaling,14,15 we hypothesized that PPM1D might mediate carcinogenesis in AML, and regulated p38 MAPK/p53 signaling pathway and serve as a potential treatment target for AML. Therefore, this study aimed to explore the effect of PPM1D knockdown on proliferation and apoptosis as well as p38 MAPK/p53 signaling pathway in AML cells.
Methods
Cell Culture
Human AML cell lines SKM-1, KG-1, AML-193, and THP-1 were all purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen. SKM-1, KG-1, and THP-1 cells were cultured in a medium containing 90% RPMI-1640 medium (Sigma) supplemented with 10% fetal bovine serum (FBS; Sigma). AML-193 cells were cultured in a medium containing 90% Iscove’s Modified Dulbecco’s Medium (Sigma) supplemented with 10% FBS (Sigma). All cells were cultured in a humid incubator at 37°C with 5% CO2.
Expression of PPM1D in Cell Lines
Reverse transcription polymerase chain reaction (RT-qPCR) and Western blot were performed to assess the messenger RNA (mRNA) and protein expressions of PPM1D in SKM-1, KG-1, AML-193, and THP-1 cells. The mRNA and protein expressions of PPM1D in normal bone marrow mononuclear cells (BMMCs), which were isolated from bone marrow sample of a healthy donor after the Ethics Approval of our Hospital and the written informed consent, were also determined and were served as control.
Transfection
Protein phosphatase, Mg2+/Mn2+ dependent 1D was knocked down by using small interfering RNA (siRNA). Protein phosphatase, Mg2+/Mn2+ dependent 1D siRNA (PPM1D siRNA 5′-UAUCCUUAAAGUCAGGGCUUUAGCG-3′) and control siRNA (control siRNA 5′-GAGUGGGUCUGGGUCUUCCCGUAGA-3′) were designed and synthesized by Invitrogen Biotechnology Co, Ltd. Protein phosphatase, Mg2+/Mn2+ dependent 1D siRNA and control siRNA were transfected into AML-193 cells and KG-1 cells using HilyMax (Dojindo) according to the manufacturer’s instruction. Cells transfected with PPM1D siRNA and control siRNA were termed as si-PPM1D cells and control cells accordingly. At 24 hours after transfection, RT-qPCR and Western blot were performed to assess the mRNA and protein expressions of PPM1D; Annexin V/Propidium Iodide assay was carried out to determine cell apoptosis rate by Annexin V-FITC Apoptosis Detection Kit (R&D) according to the manufacturer’s instruction; Cell Counting Kit-8 assay (Dojindo) was carried out to detect the cell proliferation at 0, 24, 48, 72 hours according to the manufacturer’s instruction; cell cycle was detected using Cell Cycle Kit (BD) according to the manufacturer’s instruction. In addition, PPM1D complementary DNA (cDNA) was added back into the PPM1D-knockout to validate the effect of PPM1D silencing. Furthermore, the protein expression of p38, phosphorylation of p38 (p-p38) and p53 were determined by Western blot at 24 hours, and the proteins expression of p38 target gene (p-ATF2) and p53 target gene (Bax) were detected to further validate the effect of PPM1D on p38 MAPK/p53 signaling pathway.
Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted using TRIzol (Invitrogen), and cDNA was reversely transcribed using QuantiNova Reverse Transcription Kit (Qiagen). Polymerase chain reaction was performed using QuantiNova SYBR Green PCR Kit (Qiagen) according to the manufacturer’s instruction and RNA expression was calculated by 2−ΔΔCt method with GAPDH as internal reference. Primers sequence: PPM1D, forward: 5′-CTCAATGTGCCAGGACCAAGA-3′, reverse: 5′-GTCAGTCAGGTTCAGGTATAACTCA-3′; GAPDH, forward: 5′-GACCACAGTCCATGCCATCAC-3′, reverse: 5′-ACGCCTGCTTCACCACCTT-3′.
Western Blot
Cells were firstly lysed by RIPA Buffer (Sigma) and centrifuged. Following that, protein quantification was conducted using Pierce BCA Protein Assay Kit (Thermo), then underwent electrophoresis on NuPAGE 4% to 20% Tris-Acetate Midi Protein Gels (Thermo). Subsequently, proteins were transferred to polyvinylidene fluoride membrane (Millipore) and blocked at room temperature with 5% nonfat dried milk in PBST for 1 hour. Then, the membrane was incubated with primary antibodies, followed by secondary antibodies, which are listed in Table 1. Pierce ECL Plus Western Blotting Substrate (Invitrogen) was used to visualize the protein bands and image using X-ray film (Kodak). The gray value was determined with Image J software (NIH).
Table 1.
Antibodies Applied in Western Blot.
| Antibody | Company | Dilution |
|---|---|---|
| Primary antibody | ||
| PPM1D mouse mAb (sc-376257) | Santa Cruz (USA) | 1:500 |
| ATF2 mouse mAb (sc-242) | Santa Cruz (USA) | 1:500 |
| p-ATF2 mouse mAb (sc-8398) | Santa Cruz (USA) | 1:1000 |
| p38 mouse mAb (#9228) | CST (USA) | 1:1000 |
| p-p38 mouse mAb (#9216) | CST (USA) | 1:1000 |
| p53 mouse mAb (sc-126) | Santa Cruz (USA) | 1:1000 |
| p-p53 mouse mAb (#9284) | CST (USA) | 1:1000 |
| GAPDH mouse mAb (SC-47724) | Santa Cruz (USA) | 1:2000 |
| Secondary antibody | ||
| Goat Antimouse IgG-HRP (SC-2005) | Santa Cruz (USA) | 1:5000 |
Abbreviations: PPM1D, protein phosphatase, Mg2+/Mn2+ dependent 1D; GAPDH, glyceralehyde-3-phosphate dehydrogenase.
Statistical Analysis
Statistical analysis and graph drawing were performed by the GraphPad Prism 7.02 software (GraphPad Software Inc). Data were presented as mean and standard deviation. Comparison between 2 independent samples was determined by the unpaired t test. Comparison among groups was determined by 1-way analysis of variance followed by Dunnett’s multiple comparisons test. Significance was defined as P < .05.
Results
Protein Phosphatase, Mg2+/Mn2+ Dependent 1D Expression in AML Cell Lines
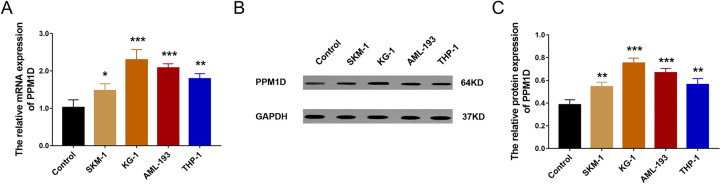
The relative mRNA expression of PPM1D was higher in SKM-1 (P < .05), KG-1 (P < .001), AML-193 (P < .001), and THP-1 cells (P < .01) compared with control cells (normal BMMCs; Figure 1A). Also, the relative protein expression of PPM1D was increased in SKM-1 (P < .01), KG-1 (P < .001), AML-193 (P < .001), and THP-1 (P < .01) cells compared with control cells (Figure 1B and C). Since the aim of this study was to assess the effect of PPM1D silencing on cell activities and signaling pathways in AML cells, we chose the cell lines (KG-1 and AML-193) that overexpressed PPM1D, as the silencing effect would be better in overexpressing cell lines.
Figure 1.
Comparison of PPM1D expression between AML cell lines and control cells. Comparison of PPM1D mRNA expression (A) and protein expression (B and C) between AML cell lines and normal BMMCs. AML indicates acute myeloid leukemia; BMMCs, bone marrow mononuclear cells; mRNA, messenger RNA; PPM1D, protein phosphatase, Mg2+/Mn2+ dependent 1D.
Effect of PPM1D Knockdown on Cell Proliferation
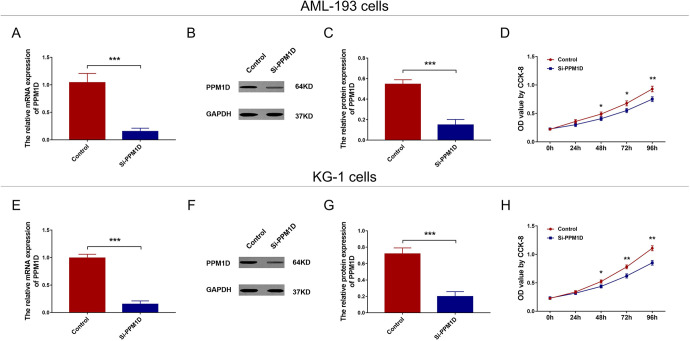
In AML-193 cells, the mRNA (P < .001; Figure 2A) and protein (P < .001; Figure 2B and C) expressions of PPM1D were reduced in si-PPM1D cells compared with control cells. Regarding cell proliferation, the OD value was decreased in si-PPM1D cells compared with control cells at 48 hours (P < .05), 72 hours (P < .05), and 96 hours (P < .01) after transfection (Figure 2D). In KG-1 cells, the mRNA (P < .001; Figure 2E) and protein (P < .001; Figure 2F and G) expressions of PPM1D were suppressed in si-PPM1D cells compared with control cells. And the OD value was lower in si-PPM1D cells compared with control cells at 48 hours (P < .05), 72 hours (P < .01), and 96 hours (P < .01) after transfection (Figure 2H). In addition, to further validate the effect of PPM1D, PPM1D cDNA was added to PPM1D silencing and we observed that adding back PPM1D promoted cell proliferation in both AML-193 cells and KG-1 cells (Supplementary Figure 1A-H).
Figure 2.
PPM1D silencing suppressed cell proliferation in AML cells. The mRNA and protein expression of PPM1D after transfection in AML-193 cells (A-C). Cell proliferation after transfection in AML-193 cells (D). The mRNA and protein expression of PPM1D after transfection in KG-1 cells (E-G). Cell proliferation after transfection in KG-1 cells (H). AML indicates acute myeloid leukemia; mRNA, messenger RNA; PPM1D, protein phosphatase, Mg2+/Mn2+ dependent 1D.
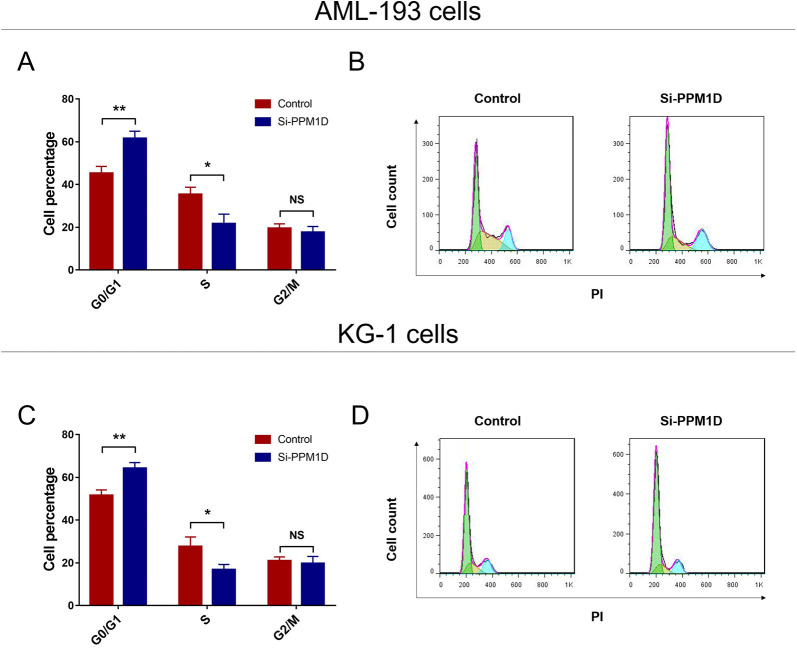
Effect of PPM1D Knockdown on Cell Cycle
Cell cycle was arrested in both AML-193 (Figure 3A and B) and KG-1 cells (Figure 3C and D) by PPM1D silencing, which further supported that PPM1D silencing suppressed cell proliferation in AML. Additionally, adding back PPM1D by cDNA presented a reduction in cell cycle arrest in both AML-193 cells and KG-1 cells (Supplementary Figure 2A-D).
Figure 3.
PPM1D silencing caused cell cycle arrest. Cell cycle after transfection in aml-193 cells (A and B) and KG-1 cells (C and D). PPM1D indicates protein phosphatase, Mg2+/Mn2+ dependent 1D.
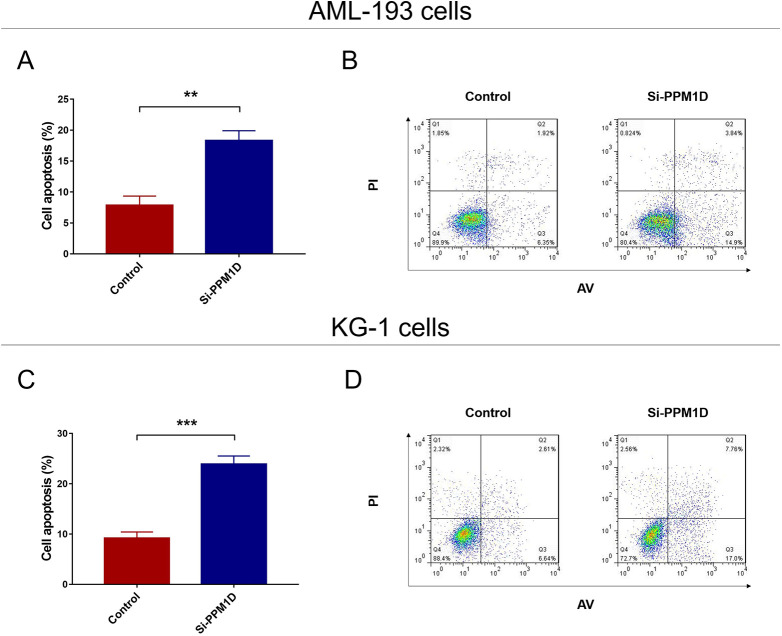
Effect of PPM1D Knockdown on Cell Apoptosis
In AML-193 cells, cell apoptosis was promoted in si-PPM1D cells compared with control cells (P < .01; Figure 4A and B). In KG-1 cells, the cell apoptosis was higher in si-PPM1D cells compared with control cells as well (P < .001; Figure 4C and D). Also, a reduction in cell apoptosis was observed after adding back PPM1D by cDNA in both AML-193 cells and KG-1 cells (Supplementary Figure 3A-D).
Figure 4.
PPM1D silencing promoted cell apoptosis in AML cells. The cell apoptosis rate after transfection in AML-193 cells (A and B) and KG-1 cells (C and D). AML indicates acute myeloid leukemia; PPM1D, protein phosphatase, Mg2+/Mn2+ dependent 1D.
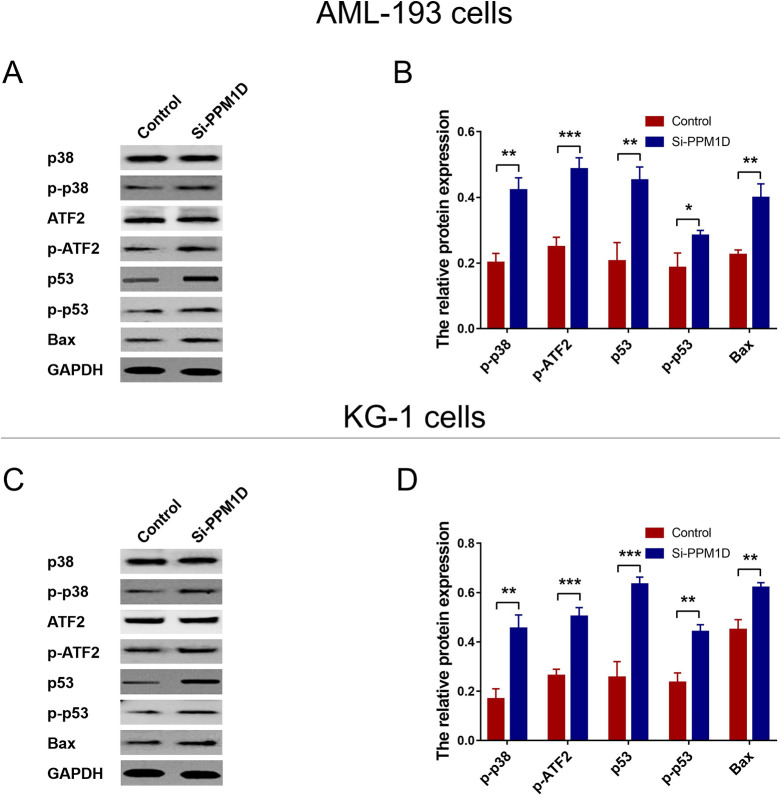
Effect of PPM1D Knockdown on p38 MAPK/p53 Pathway
In AML-193 cells, the relative expressions of p-p38 (P < .01) and p-p53 (P < .05) were enhanced in si-PPM1D cells compared with control; as well as the target gene of p38 (p-ATF2; P < .001) and target gene of p53 (Bax; P < .01; Figure 5A and B). As for in KG-1 cells, the relative expressions of p-p38 (P < .01) and p-p53 (P < .001) were enhanced in si-PPM1D cells compared with control; as well as the target gene of p38 (p-ATF2; P < .001) and target gene of p53 (Bax; P < .01; Figure 5C and D).
Figure 5.
PPM1D silencing activated p38 MAPK/p53 pathway in AML cells. The relative expression of p-p38, p-p53, and the target gene of p38 (p-ATF2), as well as target gene of p53 (Bax) in AML-193 cells (A and B) and KG-1 cells (C and D). AML indicates acute myeloid leukemia; PPM1D, protein phosphatase, Mg2+/Mn2+ dependent 1D.
Discussion
In the present study, we observed that (1) PPM1D was overexpressed in AML cell lines compared with control cells. (2) Silencing of PPM1D suppressed cell proliferation and promoted cell apoptosis in AML cells. (3) Silencing of PPM1D activated p38 MAPK/p53 pathway in AML cells.
Serine/threonine protein phosphatases are essential for homeostasis and cell death, and PPM1D, as one of them, is originally discovered to be a p53 target. The aberrant expression of PPM1D (including overexpression and gain-of-function mutations) is shown to promote carcinogenesis.16 Protein phosphatase, Mg2+/Mn2+ dependent 1D is overexpressed in human pancreatic cancer tissues compared with paired noncancerous tissues, as well as in pancreatic cancer cell lines (MIA PaCa-2 and PANC-1) compared with the human normal pancreatic epithelial cell line HPDE6-C7.10 As for hematological malignancies, PPM1D inhibition facilitates cell differentiation as well as induces G1 arrest in acute promyelocytic leukemia cells.11 In addition, ectopic expression of PPM1D suppresses DNA damage induced Chk and p38 MAPK phosphorylation in acute promyelocytic leukemia.12 Whereas for AML, it still lacks information about the function of PPMID in disease development and progression. In this study, we disclosed that PPM1D was overexpressed in AML cell lines compared with control cells, which supported our hypothesis that PPM1D exerted oncogenic effect in AML. The possible explanations could be that (1) PPM1D might suppress cell differentiation and attenuate cell cycle in myeloid lineage, which promoted the accumulation of leukemic blast cells, thereby increased the risk of AML. (2) PPM1D might suppress cell apoptosis via inhibiting p38 MAPK/p53 signaling pathway and contributed to AML susceptibility. And the regulatory effect of PPM1D on p38 MAPK/p53 signaling pathway was further evaluated in our study, which supported this explanation.
In several solid tumors, the role of PPM1D is insinuated in the regulatory effect of PPM1D on cell activities. For instance, PPM1D overexpression promotes cell migration and invasion, but its suppression inhibits migration and invasion of pancreatic cancer cells.10 In breast cancer cells, PPM1D inhibition poses antiproliferative effect in a p53-dependent manner, and its overexpression promotes sustained cell growth as well as cell survival.16 Protein phosphatase, Mg2+/Mn2+ dependent 1D also promotes cell proliferation, suppresses DNA damage response and cell apoptosis in diffuse intrinsic pontine glioma.17 Whereas for hematological malignancies, although a few studies have revealed the role of PPMID, the effect of PPM1D on cell proliferation and apoptosis in AML is still unclear. In this study, we disclosed that silencing of PPM1D reduced cell proliferation and promoted cell apoptosis in AML-193 and KG-1 cells, which indicated that PPM1D knockdown was antiproliferative in AML cells. This was also supportive to our hypothesis and might be account for that (1) PPM1D might promote stem cell marker expression, which maintained pluripotency and self-renewal of leukemic stem cells, thereby promoted the cell proliferation in AML cells. (2) Cell apoptosis was suppressed by PPM1D via inhibition of p38 MAPK/p53 signaling pathway in AML, which needed further validation.
MAPK is the key part of the 4 signaling cascades (including p38 MAPK, ERK5, extracellular signal-related kinases, and Jun amino-terminal kinases pathways), and p38 MAPK is associated with cell proliferation, apoptosis, differentiation, and migration in various malignancies.18 As a target of p38 MAPK, p53 is activated by p38 MAPK via phosphorylation and it in turn inhibits the activity of PPM1D, forming a negative feedback loop.19 P53 is one of the well-known tumor suppressor genes, whose mutation emerges in over 50% of malignancies, and its role is to trigger cell apoptosis via activation of proapoptotic pathways.13 Inclusively, the regulatory function of p38 MAPK/p53 signaling pathway has been reported in hematological malignancies (including AML, acute promyelocytic leukemia, and acute lymphocytic leukemia).12,18,20,21 Therefore, in order to further explore the regulatory effect of PPM1D in AML, we investigated the impact of PPM1D inhibition on p38 MAPK/p53 signaling pathway in AML cells. Our results showed that the p-p38 and p-p53 expressions, as well as their target genes p-ATF2 and Bax, were promoted by PPM1D inhibition in both AML-193 cells and KG-1 cells, which implied that silencing of PPM1D-activated p38 MAPK/p53 signaling pathway in AML cells. Therefore, gathering the findings of the present study, we deduced that PPM1D knockdown inhibited promoted cell proliferation and promoted cell apoptosis in AML cells, and activates p38 MAPK/p53 signaling pathway.
In conclusion, PPM1D is overexpressed in AML and its knockdown suppresses cell proliferation, promotes cell apoptosis, and activates p38 MAPK/p53 signaling pathway in AML cells.
Supplemental Material
Supplementary_Figure_1-revised for PPM1D Knockdown Suppresses Cell Proliferation, Promotes Cell Apoptosis, and Activates p38 MAPK/p53 Signaling Pathway in Acute Myeloid Leukemia by Bin Li, Jie Hu, Di He, Qi Chen, Suna Liu, Xiaoling Zhu and Meijia Yu in Technology in Cancer Research & Treatment
Supplementary_Figure_2-revised for PPM1D Knockdown Suppresses Cell Proliferation, Promotes Cell Apoptosis, and Activates p38 MAPK/p53 Signaling Pathway in Acute Myeloid Leukemia by Bin Li, Jie Hu, Di He, Qi Chen, Suna Liu, Xiaoling Zhu and Meijia Yu in Technology in Cancer Research & Treatment
Supplementary_Figure_3-revised for PPM1D Knockdown Suppresses Cell Proliferation, Promotes Cell Apoptosis, and Activates p38 MAPK/p53 Signaling Pathway in Acute Myeloid Leukemia by Bin Li, Jie Hu, Di He, Qi Chen, Suna Liu, Xiaoling Zhu and Meijia Yu in Technology in Cancer Research & Treatment
Abbreviations
- AML
acute myeloid leukemia
- BMMC
bone marrow mononuclear cell
- cDNA
complementary DNA
- FBS
fetal bovine serum
- mRNA
messenger RNA
- PPMID
protein phosphatase, Mg2+/Mn2+ dependent 1D
- RT-qPCR
Reverse transcription polymerase chain reaction
- siRNA
small interfering RNA.
Footnotes
Authors’ Note: This study was approved by the The Second People’s Hospital of Yunnan Province Ethical Committee (approval no. 2019177). All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Meijia Yu  https://orcid.org/0000-0001-9370-3773
https://orcid.org/0000-0001-9370-3773
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Assi SA, Imperato MR, Coleman DJL, et al. Subtype-specific regulatory network rewiring in acute myeloid leukemia. Nat Genet. 2019;51(1):151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Kouchkovsky I, Abdul-Hay M. ‘Acute myeloid leukemia: a comprehensive review and 2016 update’. Blood Cancer J. 2016;6(7):e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. [DOI] [PubMed] [Google Scholar]
- 4. Winer ES, Stone RM. Novel therapy in acute myeloid leukemia (AML): moving toward targeted approaches. Ther Adv Hematol. 2019;10:2040620719860645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le Guezennec X, Bulavin DV. WIP1 phosphatase at the crossroads of cancer and aging. Trend Biochem Sci. 2010;35(2):109–114. [DOI] [PubMed] [Google Scholar]
- 7. Ruark E, Snape K, Humburg P, et al. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature. 2013;493(7432):406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu E, Ahn YS, Jang SJ, et al. Overexpression of the wip1 gene abrogates the p38 MAPK/p53/Wip1 pathway and silences p16 expression in human breast cancers. Breast Cancer Res Treat. 2007;101(3):269–278. [DOI] [PubMed] [Google Scholar]
- 9. Takekawa M, Adachi M, Nakahata A, et al. P53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J. 2000;19(23):6517–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu B, Guo BM, Kang J, et al. PPM1D exerts its oncogenic properties in human pancreatic cancer through multiple mechanisms. Apoptosis. 2016;21(3):365–378. [DOI] [PubMed] [Google Scholar]
- 11. Kamada R, Kudoh F, Yoshimura F, Tanino K, Sakaguchi K. Inhibition of Ser/Thr phosphatase PPM1D induces neutrophil differentiation in HL-60 cells. J Biochem. 2017;162(4):303–308. [DOI] [PubMed] [Google Scholar]
- 12. Yoda A, Toyoshima K, Watanabe Y, et al. Arsenic trioxide augments Chk2/p53-mediated apoptosis by inhibiting oncogenic Wip1 phosphatase. J Biol Chem. 2008;283(27):18969–18979. [DOI] [PubMed] [Google Scholar]
- 13. Zane L, Yasunaga J, Mitagami Y, et al. Wip1 and p53 contribute to HTLV-1 Tax-induced tumorigenesis. Retrovirology. 2012;9:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang LJ, Lee YC, Huang CH, et al. Non-mitotic effect of albendazole triggers apoptosis of human leukemia cells via SIRT3/ROS/p38 MAPK/TTP axis-mediated TNF-alpha upregulation. Biochem Pharmacol. 2019;162:154–168. [DOI] [PubMed] [Google Scholar]
- 15. Repicky A, Jantova S, Cipak L. Apoptosis induced by 2-acetyl-3-(6-methoxybenzothiazo)-2-yl-amino-acrylonitrile in human leukemia cells involves ROS-mitochondrial mediated death signaling and activation of p38 MAPK. Cancer Lett. 2009;277(1):55–63. [DOI] [PubMed] [Google Scholar]
- 16. Parssinen J, Alarmo EL, Karhu R, Kallioniemi A. PPM1D silencing by RNA interference inhibits proliferation and induces apoptosis in breast cancer cell lines with wild-type p53. Cancer Genet Cytogenet. 2008;182(1):33–39. [DOI] [PubMed] [Google Scholar]
- 17. Akamandisa MP, Nie K, Nahta R, Hambardzumyan D, Castellino RC. Inhibition of mutant PPM1D enhances DNA damage response and growth suppressive effects of ionizing radiation in diffuse intrinsic pontine glioma. Neuro Oncol. 2019;21(6):786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li H, Dong C, Tian Y, et al. Knockdown of diacylglycerol kinase zeta (DGKZ) induces apoptosis and G2/M phase arrest in human acute myeloid leukemia HL-60 cells through MAPK/survivin/caspase pathway. Pharmazie. 2019;74(7):418–422. [DOI] [PubMed] [Google Scholar]
- 19. Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–417. [DOI] [PubMed] [Google Scholar]
- 20. Ogasawara S, Chuman Y, Michiba T, Kamada R, Imagawa T, Sakaguchi K. Inhibition of protein phosphatase PPM1D enhances retinoic acid-induced differentiation in human embryonic carcinoma cell line. J Biochem. 2019;165(6):471–477. [DOI] [PubMed] [Google Scholar]
- 21. Mahmud H, Ter Elst A, Scherpen FJG, et al. Peptide microarray of pediatric acute myeloid leukemia is related to relapse and reveals involvement of DNA damage response and repair. Oncotarget. 2019;10(45):4679–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Figure_1-revised for PPM1D Knockdown Suppresses Cell Proliferation, Promotes Cell Apoptosis, and Activates p38 MAPK/p53 Signaling Pathway in Acute Myeloid Leukemia by Bin Li, Jie Hu, Di He, Qi Chen, Suna Liu, Xiaoling Zhu and Meijia Yu in Technology in Cancer Research & Treatment
Supplementary_Figure_2-revised for PPM1D Knockdown Suppresses Cell Proliferation, Promotes Cell Apoptosis, and Activates p38 MAPK/p53 Signaling Pathway in Acute Myeloid Leukemia by Bin Li, Jie Hu, Di He, Qi Chen, Suna Liu, Xiaoling Zhu and Meijia Yu in Technology in Cancer Research & Treatment
Supplementary_Figure_3-revised for PPM1D Knockdown Suppresses Cell Proliferation, Promotes Cell Apoptosis, and Activates p38 MAPK/p53 Signaling Pathway in Acute Myeloid Leukemia by Bin Li, Jie Hu, Di He, Qi Chen, Suna Liu, Xiaoling Zhu and Meijia Yu in Technology in Cancer Research & Treatment