Abstract
Objective
To investigate the association of body mass index (BMI) with multivessel coronary artery disease in patients with myocardial infarction.
Methods
This study was performed in 1566 patients with myocardial infarction in the Department of Cardiology, Affiliated Hospital of Jining Medical University, China. Independent and dependent variables were BMI measured at baseline and multivessel coronary artery disease, respectively. The covariates examined in this study were age, systolic blood pressure, diastolic blood pressure, heart rate, creatinine, uric acid, bilirubin, cholesterol, triacylglycerol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, left ventricular ejection fraction, sex, heart failure, atrial fibrillation, chronic obstructive pulmonary disease, stroke, hypertension, diabetes mellitus, and smoking.
Results
A nonlinear relationship was detected between BMI and multivessel coronary artery disease, and this was an inverted U-shaped curve and the cutoff point was 26.3 kg/m2. The effect sizes and confidence intervals on the left and right sides of the inflection point were 1.10 (1.01–1.20) and 0.85 (0.74–0.97), respectively.
Conclusions
There is an obesity paradox for BMI > 26.3 kg/m2. Future studies should examine the relationship between BMI and prognosis in patients with myocardial infarction, which may be important for improving the prognosis through control of BMI.
Keywords: Body mass index, coronary artery disease, obesity paradox, myocardial infarction, multivessel lesion, inverted U-shaped curve
Introduction
Cardiovascular disease is a global burden and remains the leading cause of death in Europe, with more than 4 million people dying from cardiovascular disease each year and 37% of patients dying prematurely from cardiovascular disease.1,2 The situation of cardiovascular disease in China is also not optimistic. The incidence of myocardial infarction in China is nearly 81.3/10 million.3 The relative risk of all-cause death and cardiovascular outcomes (recurrent myocardial infarction and cardiovascular death) in 1 to 3 years and 3 to 5 years after myocardial infarction is at least 30% higher than that of the general reference population.4
Approximately 50% of patients with myocardial infarction have multivessel lesions5 and 60% of patients with non-ST-segment elevation have multivessel lesions.6 Studies have shown that the clinical outcome of patients with multivessel coronary artery disease is poor, including high mortality, readmission, and target vessel revascularization rates. In patients with multivessel disease, the risk of major cardiovascular events within 12 months is doubled and the risk of myocardial infarction is nearly tripled.7 In patients with multivessel coronary artery disease, the incidence of postinfarction cardiosclerosis is 36.2% and the incidence of cardiogenic edema is 38.4%.8
Extreme obesity is associated with a higher risk of mortality,9–11 but an obesity paradox exists where obesity is a protective factor for long-term outcome in patients with myocardial infarction.12–14 Faggioni et al.15 showed that underweight patients (body mass index [BMI] < 18.5 kg/m2) and patients with severe obesity (BMI ≥ 35 kg/m2) had an increased risk of all-cause death, but overweight (BMI between 25 and 29.9 kg/m2) and obesity (BMI between 30 and 34.9 kg/m2) had a lower risk of death.
Although obesity is a risk factor for coronary atherosclerosis and myocardial infarction,16–19 the quantitative relationship between obesity and multivessel coronary artery disease is unclear. Evidence regarding the association of obesity with multivessel coronary disease in Chinese patients is lacking. Therefore, this study aimed to determine the effect of BMI on multivessel lesions in patients with myocardial infarction.
Patients & Methods
Study design
This cross-sectional study was performed to address the relationship between BMI and multivessel coronary artery disease. The target-independent variable was BMI obtained at baseline. The dependent variable was multivessel coronary artery disease (dichotomous variable: 1 = patients with multivessel coronary artery disease; 0 = patients without multivessel coronary artery disease).
Study population
Data of Chinese patients with newly diagnosed myocardial infarction were nonselectively and consecutively collected from the Department of Cardiology, Affiliated Hospital of Jining Medical University, Shandong Province, China. The data did not include those of identifiable participants to protect patients’ privacy. Data were compiled from the hospital electronic medical record system. The ethics committee of the Affiliated Hospital of Jining Medical University approved this study (2019-B003). The patients participating in the study provided written informed consent.
The study initially involved 1566 participants. The entry time and deadline for inclusion were 1 January 2016 and 1 December 2018, respectively. The clinical practice for every participant was performed according to the European Society of Cardiology guidelines on myocardial infarction with ST-segment elevation, 2017.20 The inclusion criteria were as follows: patients who underwent coronary angiography, patients who were diagnosed with myocardial infarction, and patients who signed an informed consent form. The exclusion criteria were as follows: patients in a serious condition who could not accurately measure their height and weight, and patients who died before or during the operation and did not complete coronary angiography.
Variables
BMI at baseline was obtained and recorded as a continuous variable. The detailed process is described as follows. BMI was calculated as the weight (kg) divided by the square of height (m).21 BMI was measured by a nurse when the patient was admitted to hospital. The nurses were trained by the research group, and the measurement methods of height and weight were consistent. When measuring height and weight, the participants wore lightweight indoor clothing and no shoes, and used calibrated electronic scales and height gauges. The height and weight were accurate to 0.1 m and 0.1 kg, respectively.
Multivessel coronary artery disease was used as the final outcome variable (dichotomous variable). The detailed process of defining multivessel coronary artery disease was as follows: more than two coronary arteries (anterior descending artery, circumflex artery, and right coronary artery or its branches) had > 50% stenosis and/or left main artery lesions in coronary angiography.22,23 The degree of stenosis of the coronary artery was diagnosed by angiography.24,25
In this study, the following covariates were included: (1) demographic data, (2) variables affecting BMI or multivessel coronary artery disease reported by previous studies,16,26,27 and (3) clinical experience-based data. Therefore, the following variables were used to construct the fully adjusted model: (1) continuous variables, including age, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate, creatinine (Cr), uric acid (UA), bilirubin (BIL), total cholesterol (TC), triacylglycerol (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and the ejection fraction (EF) (obtained at baseline); and (2) categorical variables, including sex, heart failure, atrial fibrillation, chronic obstructive pulmonary disease (COPD), stroke, hypertension, diabetes mellitus, and smoking (obtained at baseline). Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg and patients with a history of taking antihypertensive drugs in the last 2 weeks.28 Diabetes mellitus was defined as having previously diagnosed diabetes and using insulin or hypoglycemic agents or fasting blood glucose levels ≥7.0 mmol/L. Smoking was defined as a person smoking more than one cigarette a day or more than 10 packs a month.
Statistical analysis
Continuous variables are presented in two forms. The K-S method was used to conduct a normality test. In the first form, continuous variables with a normal distribution are expressed as mean ± standard deviation. In the second form, continuous variables with a skewed distribution are presented as median (minimum, maximum). Categorical variables are expressed as frequency or percentage. The chi-square test (categorical variables), one-way analysis of variance (normal distribution), or the Kruskal–Wallis H test (skewed distribution) was used to compare differences among different BMI groups (quartiles based on BMI data). Data analysis was based on the following three criteria: (1) the relationship between X and Y (linear or nonlinear), (2) the factors modifying or interfering with the relationship between X and Y, and (3) the interference factors or the true relationship between X and Y after stratified analysis. Therefore, data analysis was performed in three steps. In step 1, univariate and multivariate binary logistic regression analyses were performed.
Three models were constructed as follows: model 1, a crude model with no covariates adjusted; model 2, adjusted only for sociodemographic data; and model 3, model 2 + other covariates shown in Table 1. In step 2, a generalized additive model and smooth curve fitting (penalized spline method) were used to address the nonlinearity of BMI and multivessel coronary artery disease. If nonlinearity was detected, the inflection point was determined using the recursive algorithm and then binary logistic regression was constructed on both sides of the inflection point. Determining which model was more suitable for fitting the correlation between the target independent and dependent variables was based mainly on the P value of the log likelihood ratio test. The continuous variable was first converted into a quartile categorical variable and then an interaction test was performed. The tests for effect modification for subgroup indicators were followed by the likelihood ratio test. A sensitivity analysis was conducted to ensure the robustness of data analysis. BMI was converted into a categorical variable and the P value was calculated for the trend. The purpose of this analysis was to verify the results of BMI as a continuous variable and observe the possibility of nonlinearity.
Table 1.
Baseline characteristics of the patients.
| BMI | Q1 | Q2 | Q3 | Q4 | P value |
|---|---|---|---|---|---|
| n | 357 | 358 | 357 | 358 | |
| Age (years) | 57.63 ± 11.07 | 59.33 ± 10.83 | 61.15 ± 11.27 | 60.75 ± 11.08 | <0.001 |
| Sex, n (%) | <0.001 | ||||
| Female | 41 (11.48) | 83 (23.18) | 138 (38.66) | 192 (53.63) | |
| Male | 316 (88.52) | 275 (76.82) | 219 (61.34) | 166 (46.37) | |
| SBP (mm Hg) | 78.27 ± 16.79 | 88.46 ± 22.34 | 99.31 ± 26.10 | 102.69 ± 24.61 | <0.001 |
| DBP (mm Hg) | 73.52 ± 12.44 | 74.89 ± 10.81 | 76.11 ± 11.65 | 77.59 ± 11.55 | <0.001 |
| Heart rate | 73.81 ± 11.06 | 73.28 ± 10.36 | 71.03 ± 12.16 | 69.54 ± 12.01 | <0.001 |
| CR (mmol/L) | 73.30 ± 23.61 | 73.19 ± 26.95 | 72.39 ± 32.51 | 69.67 ± 33.32 | <0.001 |
| UA (mmol/L) | 307.74 ± 90.60 | 316.34 ± 109.36 | 296.53 ± 89.05 | 297.70 ± 96.59 | 0.019 |
| BIL (mmol/L) | 9.57 ± 5.07 | 9.19 ± 5.02 | 9.87 ± 4.70 | 9.43 ± 5.19 | 0.064 |
| TC (mmol/L) | 4.28 ± 1.12 | 4.23 ± 0.95 | 4.32 ± 1.06 | 4.42 ± 1.15 | 0.216 |
| TG (mmol/L) | 1.95 ± 1.75 | 1.90 ± 1.45 | 1.90 ± 1.20 | 2.12 ± 1.38 | 0.042 |
| HDL-C (mmol/L) | 1.10 ± 0.36 | 1.05 ± 0.30 | 1.09 ± 0.32 | 1.09 ± 0.36 | 0.333 |
| LDL-C (mmol/L) | 2.75 ± 0.94 | 2.70 ± 0.91 | 2.75 ± 0.95 | 2.78 ± 1.02 | 0.680 |
| LVEF (%) | 60.42 ± 7.12 | 61.46 ± 6.21 | 60.60 ± 8.48 | 62.64 ± 5.71 | 0.027 |
| Smoking, n (%) | <0.001 | ||||
| No | 192 (53.78) | 225 (62.85) | 249 (69.75) | 265 (74.02) | |
| Yes | 165 (46.22) | 133 (37.15) | 108 (30.25) | 93 (25.98) | |
| COPD, n (%) | 0.219 | ||||
| No | 353 (98.88) | 354 (98.88) | 357 (100.00) | 356 (99.44) | |
| Yes | 4 (1.12) | 4 (1.12) | 0 (0.00) | 2 (0.56) | |
| DM, n (%) | 0.054 | ||||
| No | 288 (80.67) | 285 (79.61) | 273 (76.47) | 261 (72.91) | |
| Yes | 69 (19.33) | 73 (20.39) | 84 (23.53) | 97 (27.09) | |
| Heart failure, n (%) | 0.034 | ||||
| No | 316 (88.52) | 332 (92.70) | 316 (88.76) | 309 (86.31) | |
| Yes | 41 (11.48) | 26 (7.30) | 40 (11.24) | 49 (13.69) | |
| Atrial fibrillation, n (%) | 0.513 | ||||
| No | 351 (98.32) | 352 (98.32) | 352 (98.60) | 356 (99.44) | |
| Yes | 6 (1.68) | 6 (1.68) | 5 (1.40) | 2 (0.56) | |
| Stroke, n (%) | 0.776 | ||||
| No | 341 (95.52) | 339 (94.69) | 343 (96.08) | 344 (96.09) | |
| Yes | 16 (4.48) | 19 (5.31) | 14 (3.92) | 14 (3.91) | |
| Hypertension, n (%) | <0.001 | ||||
| No | 236 (66.11) | 173 (48.32) | 176 (49.30) | 119 (33.24) | |
| Yes | 121 (33.89) | 185 (51.68) | 181 (50.70) | 239 (66.76) |
Values are mean ± standard deviation or n (%). Abbreviations: BMI, body mass index; BIL, bilirubin; COPD, chronic obstructive pulmonary disease; Cr, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; Q, quartile; SBP, systolic blood pressure; TC, total cholesterol; TG, triacylglycerol; UA, uric acid.
All of the analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA). P values < 0.05 (two-sided) were considered statistically significant.
Results
Baseline characteristics of selected participants
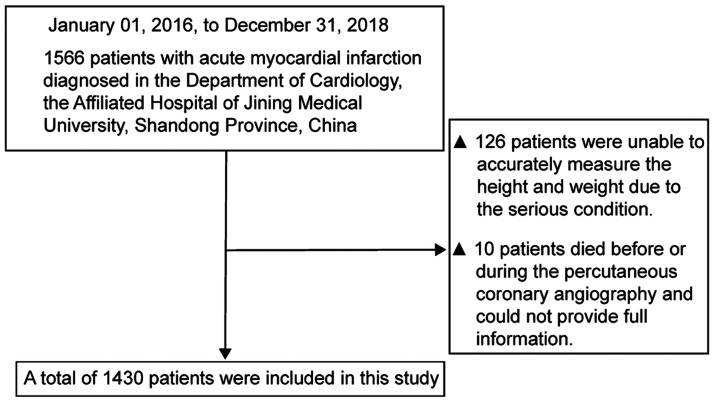
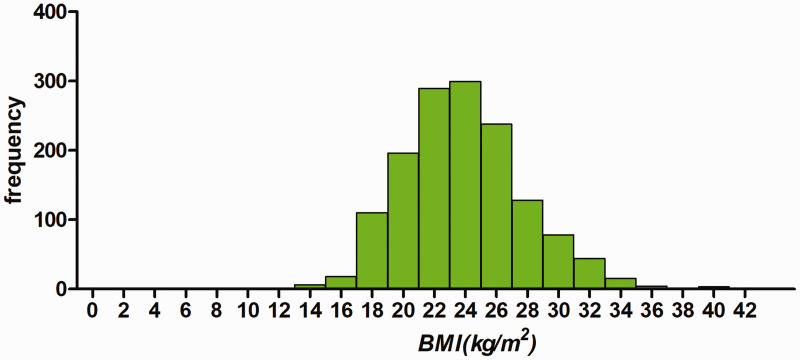
A total of 1430 participants were selected for the final data analysis using strict screening criteria (see inclusion and exclusion criteria for detail above and Figure 1). The baseline characteristics of these selected participants are shown in Table 1 according to quartiles of BMI. The distribution of BMI in the research participants is shown in Figure 2. The mean age of the 1430 selected participants was 59.71 ± 11.14 years and 68.25% of them were men. The average BMI was 23.85 kg/m2. There were no significant differences in levels of TC, HDL-C, and LDL-C, atrial fibrillation, COPD, diabetes mellitus, and stroke among the different BMI groups. Participants with the highest BMI (quartile 4) had higher values of SBP and DBP (both P < 0.001), and they were mostly nonsmoking female individuals. Opposite patterns were observed in heart rate, Cr, male sex, and smoking (all P < 0.001).
Figure 1.
Inclusion/exclusion criteria of the patients.
Figure 2.
Distribution of BMI in 1430 patients with myocardial infarction. BMI, body mass index.
Univariate analysis
The results of univariate analyses are shown in Table 2. Univariate binary logistic regression analysis showed that sex, COPD, stroke, smoking, DBP, heart rate, Cr, BIL, TC, HDL-C, LDL-C, and the EF were not associated with multivessel coronary artery disease. In contrast, univariate analysis showed that SBP (odds ratio [OR] = 1.01, 95% confidence interval [CI] 1.00–1.01), UA (OR = 1.00, 95% CI 1.00–1.00), TG (OR = 1.09, 95% CI 1.00–1.18), diabetes mellitus (OR = 1.75, 95% CI 1.33–2.29), and hypertension (OR = 1.45, 95% CI 1.14–1.86) were positively associated with multivessel coronary artery disease.
Table 2.
Univariate analysis for multivessel coronary artery lesions in acute myocardial infarction.
| Covariate | Value | OR (95% CI) | P value |
|---|---|---|---|
| Age (years) | 59.71 ± 11.14 | 1.03 (1.02–1.04) | <0.001 |
| Sex, n (%) | |||
| Male | 976 (68.25) | Reference | |
| Female | 454 (31.75) | 0.86 (0.67–1.11) | 0.252 |
| BMI (kg/m2) | 23.85 ± 3.89 | 1.02 (0.99–1.05) | 0.270 |
| SBP (mm Hg) | 92.17 ± 24.66 | 1.01 (1.00–1.01) | 0.005 |
| DBP (mm Hg) | 75.52 ± 11.71 | 1.01 (1.00–1.02) | 0.069 |
| Heart rate | 71.86 ± 11.55 | 1.01 (0.99–1.02) | 0.326 |
| Creatinine (mmol/L) | 72.13 ± 29.35 | 1.00 (1.00–1.01) | 0.164 |
| UA (mmol/L) | 304.55 ± 96.96 | 1.00 (1.00–1.00) | 0.012 |
| BIL (mmol/L) | 9.95 ± 8.74 | 1.00 (0.99–1.02) | 0.716 |
| TC (mmol/L) | 4.31 ± 1.07 | 0.98 (0.88–1.11) | 0.797 |
| TG (mmol/L) | 1.97 ± 1.46 | 1.09 (1.00–1.18) | 0.038 |
| HDL-C (mmol/L) | 1.08 ± 0.34 | 0.99 (0.68–1.43) | 0.944 |
| LDL-C (mmol/L) | 2.74 ± 0.96 | 0.93 (0.81–1.06) | 0.278 |
| LVEF (%) | 61.28 ± 7.01 | 0.98 (0.96–1.00) | 0.116 |
| Smoking, n (%) | |||
| No | 931 (65.10) | Reference | |
| Yes | 499 (34.90) | 0.88 (0.68–1.14) | 0.326 |
| COPD, n (%) | |||
| No | 1420 (99.30) | Reference | |
| Yes | 10 (0.70) | 1.33 (0.34–5.17) | 0.680 |
| Diabetes mellitus, n (%) | |||
| No | 1107 (77.41) | Reference | |
| Yes | 323 (22.59) | 1.75 (1.33–2.29) | <0.001 |
| Heart failure, n (%) | |||
| No | 1274 (89.10) | Reference | |
| Yes | 156 (10.90) | 1.21 (0.83–1.76) | 0.321 |
| Atrial fibrillation, n (%) | |||
| No | 1411 (98.67) | Reference | |
| Yes | 19 (1.33) | 1.11 (0.40–3.10) | 0.845 |
| History of stroke, n (%) | |||
| No | 1367 (95.59) | Reference | |
| Yes | 63 (4.41) | 1.31 (0.75–2.29) | 0.340 |
| Hypertension, n (%) | |||
| No | 704 (49.23) | Reference | |
| Yes | 726 (50.77) | 1.45 (1.14–1.86) | 0.003 |
Values are mean ± standard deviation or n (%). Abbreviations: BMI, body mass index; BIL, bilirubin; CI, confidence interval; COPD, chronic obstructive pulmonary disease; Cr, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; OR, odds ratio; SBP, systolic blood pressure; TC, total cholesterol; TG, triacylglycerol; UA, uric acid.
Results of unadjusted and adjusted binary logistic regression analyses
In this study, three models were constructed to analyze the independent effects of BMI on multivessel coronary artery disease (univariate and multivariate binary logistic regression analyses). The effect sizes (OR) and 95% CIs are shown in Table 3. In the unadjusted model (model 1), the model-based effect size was explained as the difference in 1 unit of BMI associated with the risk of multivessel coronary artery disease. An example of this model is that an effect size of 1.02 for multivessel coronary artery disease in an unadjusted model meant that a difference in 1 unit of BMI was associated with an increased 2% difference in the risk of multivessel coronary artery disease (1.02, 95% CI 0.99–1.05). In the minimum-adjusted model (model 2), the BMI increased by 1 unit, and the risk of multivessel coronary artery disease increased by 1% (1.01, 95% CI 0.98–1.05). In the fully adjusted model (model 3) (all adjusted covariates are shown in Table 1), for each additional 1-unit increase in BMI, the risk of multivessel coronary artery disease increased by 1% (1.01, 95% CI 0.95–1.06). For sensitivity analysis, BMI was converted from a continuous variable into a categorical variable (quartiles of BMI), and the P value for the trend of BMI with categorical variables in a fully adjusted model was consistent with the result when BMI was a continuous variable. Additionally, the trend of the effect size in different BMI groups was non-equidistant.
Table 3.
Relationship between BMI and multivessel coronary artery lesions in different models.
|
Crude model (model 1) |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| BMI (kg/m2) | 1.02 (0.99–1.05) | 0.204 | 1.01 (0.98–1.05) | 0.506 | 1.01 (0.95–1.06) | 0.848 |
| BMI | ||||||
| Q1 | Reference | Reference | Reference | |||
| Q2 | 1.40 (0.99–1.99) | 0.059 | 1.35 (0.95–1.93) | 0.097 | 1.28 (0.76–2.15) | 0.357 |
| Q3 | 1.46 (1.03–2.07) | 0.034 | 1.35 (0.94–1.93) | 0.108 | 1.40 (0.82–2.38) | 0.217 |
| Q4 | 1.42 (1.00–2.02) | 0.051 | 1.33 (0.91–1.93) | 0.136 | 1.20 (0.67–2.14) | 0.549 |
| P for trend | 0.059 | 0.172 | 0.546 | |||
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; Q, quartile.
Model 2: adjusted for age and sex.
Model 3: adjusted for sex, age, chronic obstructive pulmonary disease, stroke, diabetes mellitus, smoking, systolic blood pressure, diastolic blood pressure, heart rate, creatinine, uric acid, bilirubin, total cholesterol, triacylglycerol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and the ejection fraction.
Results of nonlinearity of BMI and multivessel coronary artery disease
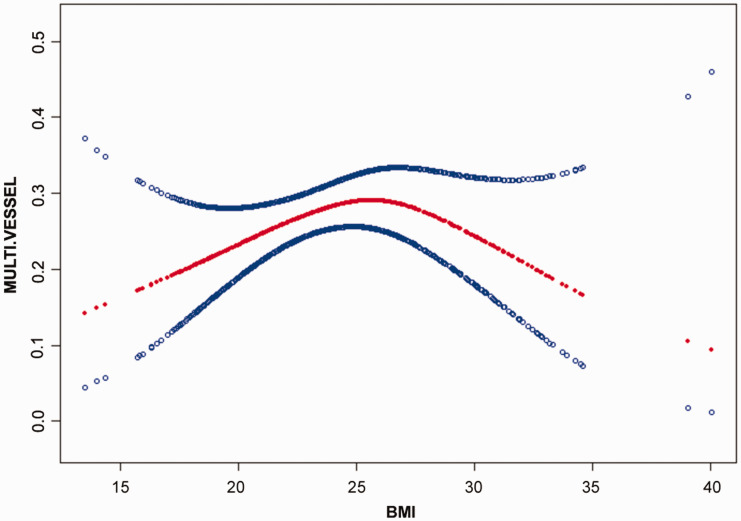
The nonlinear relationship between BMI and multivessel coronary artery disease was analyzed (Figure 3). The smooth curve and the result of the generalized additive model showed that the relationship between BMI and multivessel coronary artery disease was nonlinear after adjusting for age, SBP, DBP, heart rate, Cr, UA, BIL, TC, TG, HDL-C, LDL, the EF, sex, heart failure, atrial fibrillation, COPD, stroke, hypertension, diabetes mellitus, and smoking. Binary logistic regression was used to fit the association and select the best-fit model on the basis of the P value for the log likelihood ratio test.
Figure 3.
Association between BMI and multivessel coronary artery lesions of myocardial infarction. A threshold, nonlinear relationship between BMI and multivessel coronary artery lesion was found (P = 0.038) in a generalized additive model. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. Models were adjusted for sex, age, chronic obstructive pulmonary disease, stroke, diabetes mellitus, smoking, systolic blood pressure, diastolic blood pressure, heart rate, creatinine, uric acid, bilirubin, total cholesterol, triacylglycerol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and the ejection fraction. BMI, body mass index.
Because the P value for the log likelihood ratio test was < 0.05, binary logistic regression was chosen for fitting the association between BMI and multivessel coronary artery disease because it accurately represented the relationship. Using binary logistic regression and a recursive algorithm, the inflection point was calculated as 26.3 (Table 4). On the left side of the inflection point, the effect size and 95% CI were 1.1 and 1.01–1.20, respectively. On the right side of the inflection point, the effect size and 95% CI were 0.85 and 0.74–0.97, respectively.
Table 4.
Results of BMI and multivessel coronary artery lesions using binary linear regression.
| Inflection point of BMI (kg/m2) | Effect size | 95% CI | P value |
|---|---|---|---|
| <26.3 | 1.10 | 1.01–1.20 | 0.023 |
| ≥26.3 | 0.85 | 0.74–0.97 | 0.018 |
Effect: multivessel coronary artery lesions; cause: BMI. BMI, body mass index; CI, confidence interval.
The regression model was adjusted for sex, age, chronic obstructive pulmonary disease, stroke, diabetes mellitus, smoking, systolic blood pressure, diastolic blood pressure, heart rate, creatinine, uric acid, bilirubin, total cholesterol, triacylglycerol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and the ejection fraction.
Discussion
The findings of this study indicated a nonlinear relationship between BMI and multivessel coronary artery disease after adjusting for other covariates. This relationship was an inverted U-shaped curve and the cutoff point was 26.3 kg/m2. The effect sizes on the left and right sides of the inflection point were not consistent (left: 1.1; right: 0.85). This result suggested a threshold effect on the independent association between BMI and multivessel coronary artery disease.
The results of our study are similar to those of Choi et al.16 who showed that changes in BMI were related to coronary heart disease or acute myocardial infarction. The present study further showed an association between BMI and multivessel coronary artery disease. However, an interesting finding was an inverted U-shaped relationship between BMI and the occurrence of coronary artery disease, with a cutoff threshold of BMI of 26.3 kg/m2. This finding has guiding significance for prevention of coronary artery disease in Chinese patients with coronary heart disease. Whitlock et al.29 studied the European population and showed that in the BMI range of 22.5 to 25 kg/m2, the vessel-induced mortality rate increased by 40% for each increase in 5 kg/m2 of the BMI. This previous finding is similar to the results of the present study. However, Whitlock et al.’s29 study did not show vascular hazards in patients with a BMI > 26 kg/m2, which might be related to ethnographic differences. Kovacic et al.13 studied the relationship between BMI and the degree of coronary artery calcification. The average value of BMI was 28.2 kg/m2, and BMI was negatively correlated with the degree of coronary artery calcification. This finding is consistent with the trend of a BMI > 26.3 kg/m2 in the present study. Adipose tissue was previously regarded as a reservoir, and is now considered an endocrine organ. Obesity is associated with neurohormonal activation and metabolic abnormalities, including renin–angiotensin–aldosterone system activation, sympathetic system activation, hyperleptinemia, and growth factor regulation disorders. Fat cells synthesize many hormones or active molecules, called adipokines, which may have a protective effect on heart muscle.30 These facts may explain the obesity paradox in the present study. Additionally, previous studies have shown that patients with obesity are more likely to have cardiovascular disease diagnosed early, which may also be one reason for the existence of the obesity paradox.31,32
There is controversy regarding the relationship between obesity and the prognosis of patients with coronary heart disease. Faggioni et al. showed that patients who were underweight (BMI <18.5 kg/m2) and severely obese (BMI ≥ 35 kg/m2) had an increased risk of all-cause death. However, in our study, overweight patients tended to have a lower incidence of multivessel coronary artery disease than patients with a normal body mass index. A meta-analysis of 40 cohort studies showed that patients who were overweight/obese with coronary heart disease had a lower risk of total and cardiovascular death than those who were underweight or normal weight with coronary heart disease.33 This previous finding is consistent with the results of our study.
The clinical value of the current study is as follows: (1) our study was novel in examining the independent association between BMI and multivessel coronary artery disease in Chinese patients with myocardial infarction; and (2) our findings might be helpful in future studies for establishment of diagnostic or predictive models of multivessel coronary artery disease.
The present study has some strengths as follows. (1) Our study addressed and further explored nonlinearity of the relationship between BMI and multivessel coronary artery disease. (2) We performed an observational study, and therefore, it was susceptible to potential confounding. Consequently, strict statistical adjustment was used to minimize residual confounders. (3) Target independent variables were used as both continuous and categorical variables. Such an approach could reduce contingency in the data analysis and enhance robustness of the results. However, the present study also has some limitations. (1) Our study was performed in Chinese patients with myocardial infarction. Therefore, universality and extrapolation of findings were not possible. Some patients were in a serious condition and could not accurately measure their height and weight. (2) Some patients died before or during the surgery and did not complete coronary angiography. Therefore, the findings of this study cannot be used for such cases.
In conclusion, there is an inverted U-shaped relationship between BMI and multivessel coronary artery disease in Chinese patients with myocardial infarction, and the threshold is 26.3 kg/m2. The obesity paradox is present when BMI is >26.3 kg/m2. Future studies should investigate the relationship between BMI and prognosis of Chinese patients with myocardial infarction. Additionally, research needs to be performed to determine the standard of weight control after myocardial infarction, which may be important for improving the prognosis of patients with myocardial infarction through control of BMI.
Acknowledgements
The authors thank all of the staff members of the institution. We also thank Chen Xinglin and Chen Chi for their guidance in the design and analysis of this study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by MiaoPu Project of the Affiliated Hospital of Jining Medical University (MP-2018-016).
ORCID iD
Qingyun Zhang https://orcid.org/0000-0002-0110-8609
References
- 1.Nichols M, Townsend N, Scarborough P, et al. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 2014; 35: 2929. [DOI] [PubMed] [Google Scholar]
- 2.Moran AE, Forouzanfar MH, Roth GA, et al. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation 2014; 129: 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng W, Chen G, Cai D, et al. Inflammatory bowel disease and risk of ischemic heart disease: an updated meta-analysis of cohort studies. J Am Heart Assoc 2017; 6: e005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson S, Rosengren A, Young K, et al. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: a systematic review. BMC Cardiovasc Disord 2017; 17: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel B, Mehta SR, Mehran R. Reperfusion strategies in acute myocardial infarction and multivessel disease. Nat Rev Cardiol 2017; 14: 665–678. [DOI] [PubMed] [Google Scholar]
- 6.Pineda AM, Carvalho N, Gowani SA, et al. Managing multivessel coronary artery disease in patients with ST-elevation myocardial infarction: a comprehensive review. Cardiol Rev 2017; 25: 179–188. [DOI] [PubMed] [Google Scholar]
- 7.Chang M, Lee CW, Ahn JM, et al. Impact of multivessel coronary artery disease with versus without left main coronary artery disease on long-term mortality after coronary bypass grafting versus drug-eluting stent implantation. Am J Cardiol 2017; 119: 225–230. [DOI] [PubMed] [Google Scholar]
- 8.Rezende PC, Hueb W, Rahmi RM, et al. Myocardial injury in diabetic patients with multivessel coronary artery disease after revascularization interventions. Diabetol Metab Syndr 2017; 9: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das SR, Alexander KP, Chen AY, et al. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol 2011; 58: 2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing Z, Pei J, Huang J, et al. Relationship of obesity to adverse events in myocardial infarction patients without primary percutaneous coronary intervention: results from the Occluded Artery Trial (OAT). Curr Med Res Opin 2019; 35: 1563–1569. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Jeong MH, Kim JH, et al. Influence of obesity and metabolic syndrome on clinical outcomes of ST-segment elevation myocardial infarction in men undergoing primary percutaneous coronary intervention. J Cardiol 2018; 72: 328–334. [DOI] [PubMed] [Google Scholar]
- 12.Ueshima D, Yoshikawa S, Sasaoka T, et al. Obesity paradox in the era of percutaneous coronary intervention with 2nd-generation drug-eluting stents: an analysis of a multicenter PCI registry. Heart Vessels 2019; 34: 218–226. [DOI] [PubMed] [Google Scholar]
- 13.Kovacic JC, Lee P, Baber U, et al. Inverse relationship between body mass index and coronary artery calcification in patients with clinically significant coronary lesions. Atherosclerosis 2012; 221: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Wu C, Sun Y, et al. Obesity paradox: clinical benefits not observed in obese patients with ST-segment elevation myocardial infarction: a multicenter, prospective, cohort study of the northern region of China. Int J Cardiol Heart Vessel 2013; 168: 2949–2950. [DOI] [PubMed] [Google Scholar]
- 15.Faggioni M, Baber U, Afshar AE, et al. Effects of body mass index on clinical outcomes in female patients undergoing percutaneous coronary intervention with drug-eluting stents: results from a patient-level pooled analysis of randomized controlled trials. JACC Cardiovasc Interv 2018; 11: 68–76. [DOI] [PubMed] [Google Scholar]
- 16.Choi S, Kim K, Kim SM, et al. Association of obesity or weight change with coronary heart disease among young adults in South Korea. JAMA Intern Med 2018; 178: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renninger M, Lochen ML, Ekelund U, et al. The independent and joint associations of physical activity and body mass index with myocardial infarction: the Tromso Study. Prev Med 2018; 116: 94–98. [DOI] [PubMed] [Google Scholar]
- 18.Kachur S, Lavie CJ, De Schutter A. Obesity and cardiovascular diseases. Minerva Med 2017; 108: 212–228. [DOI] [PubMed] [Google Scholar]
- 19.Desai R, Singh S, Baikpour M, et al. Does obesity affect the outcomes in takotsubo cardiomyopathy? Analysis of the Nationwide Inpatient Sample database, 2010-2014. Clin Cardiol 2018; 41: 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. [DOI] [PubMed] [Google Scholar]
- 21.Calabro P, Moscarella E, Gragnano F, et al. Effect of body mass index on ischemic and bleeding events in patients presenting with acute coronary syndromes (from the START-ANTIPLATELET Registry). Am J Cardiol 2019; 124: 1662–1668. [DOI] [PubMed] [Google Scholar]
- 22.Kerkmeijer LS, Farhan S, Mehran R. Diabetes mellitus and multivessel coronary artery disease: an ongoing battle for an ideal treatment strategy. Ann Transl Med 2017; 5: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kayatta MO, Halkos ME, Puskas JD. Hybrid coronary revascularization for the treatment of multivessel coronary artery disease. Ann Cardiothorac Surg 2018; 7: 500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sardar P, Kundu A, Bischoff M, et al. Hybrid coronary revascularization versus coronary artery bypass grafting in patients with multivessel coronary artery disease: a meta-analysis. Catheter Cardiovasc Interv 2018; 91: 203–212. [DOI] [PubMed] [Google Scholar]
- 25.Bajraktari G, Jashari H, Ibrahimi P, et al. Complete revascularization for patients with ST-segment elevation myocardial infarction and multivessel coronary artery disease: a meta-analysis of randomized trials. Coron Artery Dis 2018; 29: 204–215. [DOI] [PubMed] [Google Scholar]
- 26.Collet C, Onuma Y, Andreini D, et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J 2018; 39: 3689–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holroyd EW, Sirker A, Kwok CS, et al. The relationship of body mass index to percutaneous coronary intervention outcomes: does the obesity paradox exist in contemporary percutaneous coronary intervention cohorts? Insights from the British Cardiovascular Intervention Society Registry. JACC Cardiovasc Interv 2017; 10: 1283–1292. [DOI] [PubMed] [Google Scholar]
- 28.McCormack T, Boffa RJ, Jones NR, et al. The 2018 ESC/ESH hypertension guideline and the 2019 NICE hypertension guideline, how and why they differ. Eur Heart J 2019; 40: 3456–3458. [DOI] [PubMed] [Google Scholar]
- 29.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parto P, Lavie CJ. Obesity and cardiovascular diseases. Curr Probl Cardiol 2017; 42: 376–394. [DOI] [PubMed] [Google Scholar]
- 31.Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol 2018; 3: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elagizi A, Kachur S, Lavie CJ, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis 2018; 61: 142–150. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet 2006; 368: 666–678. [DOI] [PubMed] [Google Scholar]