Abstract
Branchial cleft abnormality is a common congenital neck malformation in children, which is caused by the abnormal development of the gill sac or gill groove. It is mainly manifested as a cyst in the sinus tract and fistula in the neck, as well as branchio-oto-renal syndrome (BORS). As a rare autosomal dominant genetic disease, the typical manifestations of BORS are hearing loss, abnormal branchial cleft development and renal dysplasia. In this paper, a patient was admitted to the hospital for bilateral branchial cleft fistulas combined with bilateral anterior auricular fistulas, auricular appendix, auricle dysplasia, external auditory canal stenosis, and hearing loss. The patient was diagnosed with BORS, and underwent fistulectomy of the neck and anterior ear, external auditory canal formation, and tympanoplasty. The aim of this report is to strengthen clinicians’ understanding of BORS and reduce the rate of clinical missed diagnosis through our case report and literature review.
Keywords: Branchial cleft abnormality, branchio-oto-renal syndrome, hearing loss, case report, fistulectomy, tympanoplasty, congenital neck malformation, pediatric
Introduction
Branchio-oto-renal syndrome (BORS) is an autosomal dominant disorder that is characterized by branchiogenic anomalies (branchial fistula or cyst), hearing loss, and renal disorders including congenital anomalies of the kidney and urinary tract.1 The incidence of BORS is approximately one in 40,000 in the general population and 2% among profoundly deaf children.2 If there is no kidney malformation, it can also be called branchiootic syndrome (BOS). High clinical heterogeneity makes it difficult for clinicians to diagnose BORS. In clinical practice, patients are more likely to see the doctor with one of these manifestations, so it is difficult to suspect that a patient may have BORS. There are a few reports on BORS around the world, and most of them are individual cases. Through this case report and review of relevant literature, clinicians’ awareness of, and attention to, BORS could be strengthened to reduce missed diagnosis and achieve an accurate diagnosis and treatment.
Case report
The patient was a 7-year-old girl and she was admitted to the otolaryngology department for “bilateral neck fistula with repeated discharge since birth”. On physical examination, her clinical features showed normal intelligence, hearing loss, cup-shaped auricle in both ears, a bulge on each anterior auricle, a fistula on the front of each crus helicis, a small amount of secretions that were found by extrusion, and left external auditory canal stenosis (Figure 1). In addition, a fistula was found on the lower one-third leading edge of both sternocleidomastoid muscles, with viscous secretions leaking out (Figure 1). Objective audiometry results showed mixed bilateral hearing loss with a hearing threshold of 56.25 and 62.5 dB for the right and left ears, respectively, for the hearing level in air conduction (Figure 2a). A computed tomography (CT) scan on temporal bone showed bilateral otitis media papillae (Figure 3). The Jahrsdoerfer score3 in both ears was 9. The rest of the systemic examination was unremarkable. Because BORS was suspected, an abdominal ultrasonography was requested, and it revealed left kidney dysplasia (Figure 4) and a normal right kidney. The serum creatinine level was normal with 31 μmol/L. The patient was born from a non-consanguineous marriage, and there was no history of hearing deficits or similar discharge from sinuses in the patient’s parents and her only sibling.
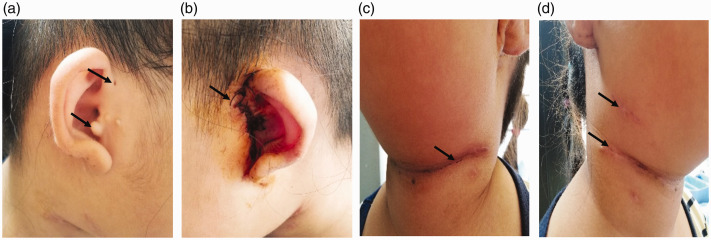
Figure 1.
Auricles in both ears are cup-shaped. (a) Right anterior auricular fistula and accessory ear. (b) After left auricular fistula and auricular appendage resection and external auditory meatus and tympanoplasty. (c) The second branchial fistula 10 days after surgery by transverse incision on the left neck. (d) The second branchial fistula 10 days after surgery by two incisions on the right neck.
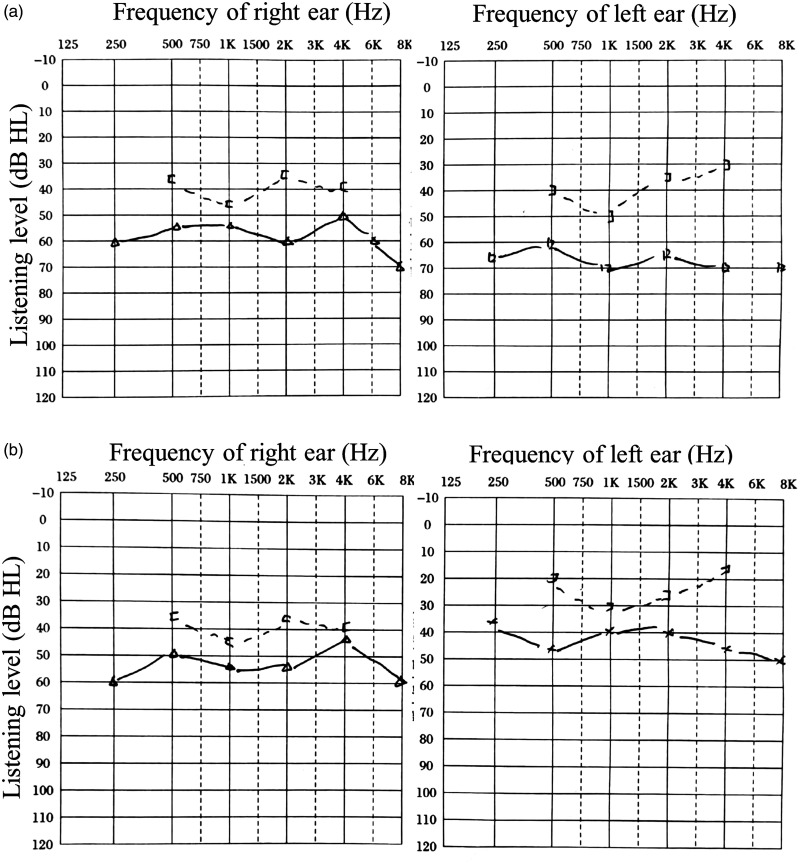
Figure 2.
The objective audiometry before and after surgery. (a) The objective audiometry showed mixed hearing loss bilaterally with hearing threshold of 56.25 and 62.5 dB hearing level in air conduction for the right and left ears preoperatively, respectively. (b) Objective audiometry results showed mixed hearing loss bilaterally with a hearing threshold of 52.5 and 25 dB hearing level in air conduction for the right and left ears 1 year after surgery, respectively.
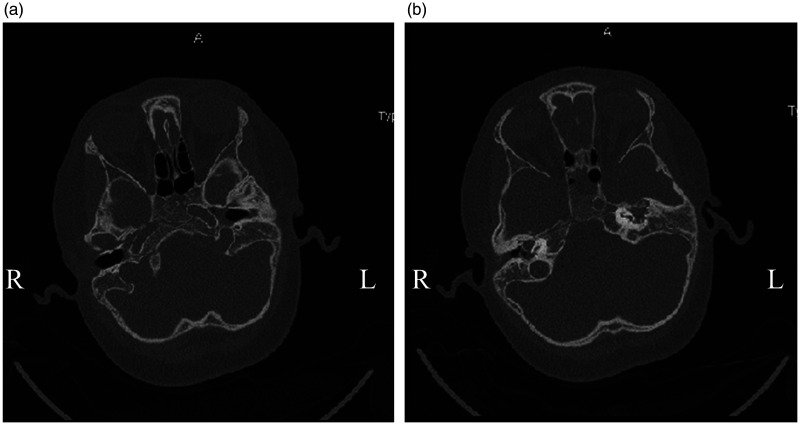
Figure 3.
CT scan before surgery. (a) Preoperative CT scan on the temporal bone showed left otitis media. (b) Preoperative CT scan on temporal bone showed left external auditory canal stenosis.
CT, computed tomography.
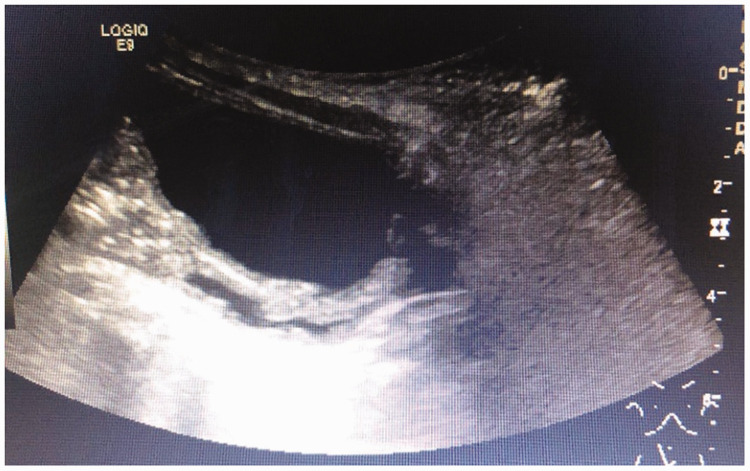
Figure 4.
Abdominal ultrasonography indicating dysplasia of the left kidney.
The patient was prepared for and underwent surgery. Methylene blue was injected into the cervical fistula, the fistula tissues were carefully dissected from the surrounding structures. The fistula then continued to move upward along the anterior margin of the sternocleidomastoid muscle to the superficial carotid sheath, and it finally reached the ipsilateral tonsil fossa. The root of the fistula was not connected to the pharyngeal side wall. A single-incision method was used on the left side, the stepladder method was used on the right side, and the bilateral fistulas were resected completely.
Using the same staining method, the preauricular fistula tissue was observed under a microscope, and it was shown to extend deep into the upper middle ear tympanum and the tympanum sinus. After methylene blue staining of the fistula tissue, a large number of granulation tissues were observed inside. The auditory bone chain was continuous and complete with good activity. Finally, the anterior auricular fistula was completely removed, external auditory canal surgery and tympanoplasty were performed, and accessory ear tissue was resected.
Postoperative pathological results showed that all the tested tissues were consistent with the tissue changes caused by the fistula. The patient was followed-up for 1 year, and the patient’s hearing in the left ear was significantly improved after surgery (Figure 2b). There was no recurrence of infection in the neck or ear.
Discussion
Branchial cleft is a common congenital cervical anomaly in children, accounting for 20% of cervical anomalies in children.4 It is usually characterized by a cyst, sinus, and fistula in the neck.5 Branchial cleft malformation can also be accompanied by congenital developmental abnormalities of local tissues and organs, such as the first and second branchial arch syndrome, preauricular fistula, accessory auricles, auricle dysplasia, and atresia of the external auditory canal. Occasionally, a branchial anomaly may be part of BORS, which was first described by Melnick et al.6 and Fraser et al.7
BORS is a rare genetic disorder that affects multiple organ systems. Although it is very uncommon, several cases of BORS have been reported since it was first described. Abdelhak et al.8 proposed the widely accepted diagnostic criteria for BORS using the major and minor criteria in 2004. The major criteria included second branchial anomaly, hearing loss, preauricular pits, and renal anomaly. The minor criteria included external ear anomaly, middle ear anomaly, inner ear anomaly, preauricular tags, facial asymmetry, and palate abnormality. The syndrome is clinically heterogeneous with highly variable penetrance even within the same family with the disease. Patients without a family history are diagnosed with BORS if they meet three or more of the above major criteria, or two major and at least two minor criteria. Stinckens et al.9 reported the frequency of the main features of BOR syndrome as follows: hearing impairment, 95.4%; malformed auricles, 86.8%; second branchial arch fistula/cyst, 86.5%; preauricular sinus, 87.0%; and renal anomalies, 58.3%. In the early stages, most patients have no signs of kidney disease. In addition, a case reported that hypospadias may be part of the defining features that manifest as the congenital symptom complex in a child.10 Two cases of BORS with dysplastic cochleae were also reported by Williams et al.11 The manifestations of this case included branchial cleft fistula, anterior auricular fistula, external ear malformation, external auditory canal stenosis, hearing loss, and a small kidney.
Hearing loss is an important feature of BORS and it may present as conductive, sensorineural, or mixed severe hearing loss. Kemperman et al.12 analyzed long-term serial audiometry data from patients with BORS, and showed that patients with BORS with enlarged endolymphatic duct and/or sac on MRI seemed to be predisposed to developing more severe hearing impairment. Depending on the degree of middle and outer ear deformity, surgical treatment is generally not recommended for patients with the Jahrsdoerfer score of less than 6.3 Because the Jahrsdoerfer scores in both ears were 9 in our patient, we operated on the patient, and the patient’s hearing improved after surgery. A hearing-aid or cochlear implant may be necessary based on the patient’s results. It is also important for postoperative speech rehabilitation training. Additionally, ototoxic drugs should also be avoided to improve hearing and make better use of residual hearing. Some patients with preauricular or branchial fistulas had no infection or conscious symptoms throughout their life, and the patients who had repeated infection and abscess formation require treatment. The treatment strategy is complete surgical excision of pathological tissue to reduce recurrence.
In this case, otitis media may be partly related to preauricular fistulas because the preauricular fistula tissue was observed under a microscope to extend deep into the upper middle ear tympanum and the tympanum sinus. Clinically, it is necessary to pay attention to variants of anterior auricular fistulas. When all organs are involved in the syndrome, comprehensive treatment should be used, such as preventing fistula infection, resecting the fistula when a fistula infection or abscess forms, improving hearing by surgical treatment, a hearing aid, or cochlear implant, and protecting renal function, dialysis, or renal transplantation for end-stage renal disease patients.
BORS also has a high genotype heterogeneity. Wen et al.13 described the clinical manifestations and pathogenic genes using copy number variations in detail. The known pathogenic genes of this disease include EYA1, SIX1, and SIX5.14 EYA1, which was first reported by Abdelhak in 1997,8 is the most frequent causative gene of BORS.15 However, a study in a Japanese population showed that there was no obvious genotype–phenotype correlation based on genetic analysis in patients with BORS. Therefore, it is proposed that multiplex ligation-dependent probe amplification (MLPA) analysis for EYA1 and comprehensive next generation sequencing (NGS) analysis would be useful for detection of BORS-related genes.16 However, because of the high cost, most patients do not receive genetic testing clinically.
In conclusion, BORS is a rare disease that causes abnormalities in multiple organ systems. Management of this syndrome requires multidisciplinary collaboration. Otolaryngologists and pediatricians should increase their awareness of BORS and diagnose and treat it as early as possible. Wen et al.13 emphasized the criteria clinically and genetically to provide a basis for clinical diagnosis of BORS and genetic counseling.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics and consent
Ethics permission was not sought because our paper is a case report. The patient’s parents provided consent to publish this paper.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Yan Zheng https://orcid.org/0000-0002-5515-3667
References
- 1.Morisada N, Nozu K, Iijima K. Branchio-oto-renal syndrome: comprehensive review based on nationwide surveillance in Japan. Pediatr Int 2014; 56: 309–314. [DOI] [PubMed] [Google Scholar]
- 2.Fraser FC, Sproule JR, Halal F. Frequency of the branchio-oto-renal (BOR) syndrome in children with profound hearing loss. Am J Med Genet 1980; 7: 341–349. [DOI] [PubMed] [Google Scholar]
- 3.Jahrsdoerfer RA, Yeakley JW, Aguilar EA, et al. Grading system for the selection of patients with congenital aural atresia. Am J Otol 1992; 13: 6–12. [PubMed] [Google Scholar]
- 4.Teo NWY, Ibrahim SI, Tan KKH. Distribution of branchial anomalies in a paediatric Asian population. Singapore Med J 2015; 56: 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magdy EA, Ashram YA. First branchial cleft anomalies: presentation, variability and safe surgical management. Eur Arch Otorhinolaryngol 2013; 270: 1917–1925. [DOI] [PubMed] [Google Scholar]
- 6.Melnick M, Bixler D, Silk K, et al. Autosomal dominant branchiootorenal dysplasia. Birth Defects Orig Artic Ser 1975; 11: 121–128. [PubMed] [Google Scholar]
- 7.Fraser FC, Ling D, Clogg D, et al. Genetic aspects of the BOR syndrome–branchial fistulas, ear pits, hearing loss, and renal anomalies. Am J Med Genet 1978; 2: 241–252. [DOI] [PubMed] [Google Scholar]
- 8.Abdelhak S, Kalatzis V, Heilig R, et al. Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYA1. Human Molec Genet 1997; 6: 2247–2255. DOI: 10.1093/hmg/6.13.2247. [DOI] [PubMed] [Google Scholar]
- 9.Stinckens C, Standaert L, Casselman JW, et al. The presence of a widened vestibular aqueduct and progressive sensorineural hearing loss in the branchio-oto-renal syndrome. A family study. Int J Pediatr Otorhinolaryngol 2001; 59: 163–172. [DOI] [PubMed] [Google Scholar]
- 10.Dutta M, Chatterjee I. Hypospadias as a new entity to define the branchio-oto-renal spectrum disorders. Ear Nose Throat J 2019; 98: 20–22. [DOI] [PubMed] [Google Scholar]
- 11.Williams LE, Sohner MT, Sibley RC, 3rd, et al. Multimodality depiction of findings in branchio-oto-renal syndrome: Two case reports. Acta Radiol Open 2019; 8: 2058460119861606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemperman MH, Koch SMP, Kumar S, et al. Evidence of progression and fluctuation of hearing impairment in branchio-oto-renal syndrome. Int J Audiol 2004; 43: 523–532. [DOI] [PubMed] [Google Scholar]
- 13.Wen YY, Sun Y, Kong WJ. [ Emphasizing the application of genetic diagnosis in branchio-oto-renal syndrome]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2018; 32: 1226–1231. [DOI] [PubMed] [Google Scholar]
- 14.Brophy PD, Alasti F, Darbro BW, et al. Genome-wide copy number variation analysis of a Branchio-oto-renal syndrome cohort identifies a recombination hotspot and implicates new candidate genes. Hum Genet 2013; 132: 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith RJH. Branchiootorenal spectrum disorder In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews(®). Seattle (WA): University of Washington, 1993. [PubMed] [Google Scholar]
- 16.Unzaki A, Morisada N, Nozu K, et al. Clinically diverse phenotypes and genotypes of patients with branchio-oto-renal syndrome. J Hum Genet 2018; 63: 647–656. [DOI] [PubMed] [Google Scholar]