Abstract
Interactions with the microenvironment modulate the fate of stem cells in perivascular niches in tissues (e.g., bone) and organs (e.g., liver). However, the functional relevance of the molecular crosstalk between endothelial cells and stem cells within the perivascular niche in dental pulps is unclear. Here, we tested the hypothesis that endothelial cell–initiated signaling is necessary to maintain self-renewal of dental pulp stem cells. Confocal microscopy showed that ALDH1high and Bmi-1high stem cells are preferentially localized in close proximity to blood vessels in physiological human dental pulps. Secondary orosphere assays revealed that endothelial cell–derived factors (e.g., interleukin-6 [IL-6]) promote self-renewal of dental pulp stem cells cultured in low-attachment conditions. Mechanistic studies demonstrated that endothelial cell–derived IL-6 activates IL-6R (IL-6 Receptor) and signal transducer and activator of transcription 3 (STAT3) signaling and induces expression of Bmi-1 (master regulator of stem cell self-renewal) in dental pulp stem cells. Transplantation of dental pulp stem cells stably transduced with small hairpin RNA (shRNA)–STAT3 into immunodeficient mice revealed a decrease in the number of blood vessels surrounded by ALDH1high or Bmi-1high cells (perivascular niches) compared to tissues formed upon transplantation of vector control stem cells. And finally, in vitro capillary sprouting assays revealed that inhibition of IL-6 or STAT3 signaling decreases the vasculogenic potential of dental pulp stem cells. Collectively, these data demonstrate that endothelial cell–derived IL-6 enhances the self-renewal of dental pulp stem cells via STAT3 signaling and induction of Bmi-1. These data suggest that a crosstalk between endothelial cells and stem cells within the perivascular niche is required for the maintenance of stem cell pools in dental pulps.
Keywords: cell differentiation, endothelium, pulp biology, regeneration, stem cells, vascular biology
Introduction
Dental tissue engineering is a biologically inspired approach for replacement of tooth tissues lost to caries, infection, trauma, or developmental defects (Nakashima and Reddi 2003). The discovery and characterization of dental pulp stem cells (DPSCs) in permanent teeth and stem cells from human exfoliated deciduous teeth (SHED) inspired the development of cell-based therapies for dental pulp tissue regeneration (Gronthos et al. 2000; Miura et al. 2003; Rosa et al. 2013). These discoveries enabled clinical studies that provided initial evidence of the efficacy and safety of dental pulp stem cell transplantation for treatment of necrotic teeth (Nakashima et al. 2017; Xuan et al. 2018). Emerging evidence suggests that the long-term viability of an engineered dental pulp requires the regeneration of perivascular niches and maintenance of a stem cell pool (Oh and Nör 2015). Indeed, to enable tissue repair of a tissue engineered with stem cells, it is necessary that some of these cells remain undifferentiated, while others differentiate into tissue-forming cells. As such, understanding mechanisms underlying the maintenance of a dental pulp stem cell pool is critical for the translation and long-term outcome of cell-based regenerative endodontics.
Emerging evidence has suggested the importance of stem cell niches that maintain the self-renewing capacity and multipotency of stem cell populations postnatally (Sugiyama et al. 2006; Sacchetti et al. 2007; Zhao et al. 2018). Studies of perivascular niches in bone suggest that endothelial factors are necessary to promote self-renewal of hematopoietic stem cells (Ding et al. 2012), but their role in the maintenance of dental pulp stem cells is not understood. Thus, the objective of this study was to define the functional impact and regulatory mechanisms that mediate the crosstalk between endothelial cells and dental pulp stem cells. Here, we showed that endothelial cell–derived interleukin (IL)–6 signals through IL-6 receptor (IL-6R) to activate signal transducer and activator of transcription 3 (STAT3) signaling and Bmi-1 activity in DPSCs or SHED (hereby referred to collectively as dental pulp stem cells, unless specified). We also demonstrated that these signaling events enhance the self-renewal of dental pulp stem cells. These data suggest that the endothelial–stem cell crosstalk might enable the maintenance of stem cell pools throughout the life of a tooth.
Materials and Methods
Cell Culture
Human DPSCs were purchased from Lonza, and SHED were obtained from Dr. Songtao Shi (University of Pennsylvania). DPSCs and SHED were cultured in α–minimum essential medium (α-MEM; Invitrogen) supplemented with 20% fetal bovine serum (FBS; Invitrogen), 1% antibiotic-antimycotic (Invitrogen), and 20 µg/mL Plasmocin (InVivogen). Human dermal microvascular endothelial cells (HDMECs; Lonza) were cultured in endothelial cell growth medium (EGM-2MV; Lonza). HDMEC-conditioned medium was obtained by culturing HDMECs (80%–90% confluency) in serum-free endothelial basal medium (EBM-2; Lonza) for 24 h. The resulting solution underwent centrifugation at 3,000 rpm (10 min), and the supernatant was collected. A more detailed list of compounds used in this work can be found in the Appendix.
Lentiviral-Mediated Gene Silencing
DPSCs or SHED underwent lentiviral-mediated gene silencing via small hairpin RNA (shRNA). Briefly, HEK-293T cells were cotransfected transiently with packaging vectors psPAX2, pMD2.G, and the following constructs: pGIPZ-shRNA-Control (Vector Core, University of Michigan) and pGIPZ-shRNA-STAT3 (Vector Core, University of Michigan) using the calcium phosphate method. The resulting supernatants were used to infect DPSCs/SHED overnight. Cells were selected with 1 µg/mL puromycin (InVivogen) in 15% FBS-supplemented α-MEM for at least 1 wk. The resulting silenced cells were cultured thereon with puromycin-supplemented media to maintain selection pressure.
Secondary Orosphere Assay for Self-renewal
DPSCs or SHED (750,000–1,000,000 cells) were cultured on ultralow-attachment 75-cm2 flasks (ULA; cat. 3814; Corning) for 7 d with serum-free Dulbecco’s modified Eagle medium/Nutrient Mixture F-12 (DMEM/F12; Life Technologies) supplemented with 10 ng/mL basic fibroblast growth factor (bFGF; cat. GF003AP-MG; Millipore), 10 ng/mL epidermal growth factor (EGF; cat. 236-EG-200; R&D Systems), and 2% FBS. Media were added to flasks every 2 d. After 7 d, cells were treated with 0.05% Trypsin-EDTA (Life Technologies) and gently agitated mechanically for a single cell suspension. Cells were then plated and treated onto 6-well ULA plates (cat. 3471; Corning) at a cell density of 10,000 cells/well. Orospheres were defined as nonadherent spheres greater than 25 cells in diameter (Krishnamurthy and Nör 2013), counted over the span of 7 to 10 d, and photographed under phase-contrast light microscopy.
Functional Sprouting Assay for Endothelial Differentiation
DPSCs or SHED (5 × 104 cells/well) were seeded on standard 12-well plates coated with growth factor reduced Matrigel (BD Biosciences) and cultured with EGM2-MV medium (Lonza). The number of capillary sprouts was counted in triplicate wells per condition. Representative phase contrast microscopy images were taken at 100× magnification.
In Vivo Model of Vasculogenic Differentiation of DPSCs
Highly porous, biodegradable poly-L-lactic acid (PLLA) (Boehringer Ingelheim) scaffolds were prepared, as described (Nör et al. 2001). DPSCs or SHED stably transduced with shRNA-control (n = 6) or shRNA-STAT3 (n = 6) (106 cells/scaffold) were mixed in a 1:1 ratio with Matrigel (BD Biosciences) and seeded onto PLLA scaffolds (6 × 6 × 1 mm). Resulting scaffolds were implanted into female 2- to 3-mo-old CB.17 severe combined immunodeficient mice (SCID; Charles River) housed under specific pathogen-free (SPF) conditions. Specimens were harvested after 28 d and fixed in 10% buffered formalin for 24 h at 4°C and then processed by the University of Michigan School of Dentistry Histology Core. All animal experiments followed ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines under a protocol that was approved by the Institutional Animal Use and Care Committee of University of Michigan (approval number: PRO00007065).
Imaging and Quantification
For immunofluorescence, tissue sections were deparaffinized and rehydrated, and antigen retrieval was performed in citrate buffer (cat. AP-9003-500; Thermo Scientific) with a decloaking chamber (Biocare Medical). Tissue sections were incubated overnight at 4°C with primary antibodies and then exposed to secondary antibodies (list of antibodies in the Appendix). Confocal images were obtained using an inverted Leica SP5 Confocal Microscope (Leica). For excitation, the 405-nm laser and the tunable white light laser were used. The detectors were set to 415 to 478 nm (blue), 498 to 550 nm (green), and 571 to 727 nm (red) for the spectral ranges. Channels were acquired sequentially between lines. Scanning speed was set to 50 Hz in bidirectional mode with line average set to 1 or 2 depending on the experiment. Pinhole size was set to 67 µm, and images were recorded in 1,024 × 1,024-pixel format. Zoom factor varied according to the area analyzed.
The distance between dental pulp stem cells and blood vessels was determined by measuring the distances between ALDH1high or Bmi-1high cells and nearest CD31+ blood vessel. The area of CD31+ or ALDH1highBmi-1high (pixels/field) was calculated using standardized confocal microscopy images of identical zoom factor, pinhole size, excitation/detector range/intensity, and image resolution. Five random images were obtained per sample, and identical threshold parameters were set to determine positivity between shRNA-control versus shRNA-STAT3 samples in FIJI ImageJ (National Institutes of Health) software. The surface area of positive pixels was then measured among images. The upper/lower outlier values (3 each) were omitted prior to statistical analyses via Prism 8 software (GraphPad Software).
Statistical Analysis
Data were evaluated by t test, 1-way analysis of variance (ANOVA), or 2-way ANOVA followed by appropriate post hoc tests using Prism v8.1.2 software (GraphPad Software). Statistical significance was determined at P < 0.05.
Results
Dental Pulp Stem Cells Are Located in Perivascular Niches and Exhibit Self-renewal
Immunofluorescence analyses of healthy human pulps demonstrated the presence of perivascular stem cell niches, with ALDH1high and Bmi-1high cells preferentially located in close proximity to blood vessels (Fig. 1A). Frequency distribution analyses revealed that 52.9% of all ALDH1high cells are located within 15 µm of blood vessels (Fig. 1B, C). Indeed, ALDH1high cells are located on average 14.0 ± 0.6 µm and Bmi-1high cells 16.3 ± 1.1 µm from blood vessels. Interestingly, we observed a heterogeneous pattern of expression of markers of stemness in the dental pulp—that is, while some cells expressed both ALDH1 and Bmi-1, other cells expressed only 1 of these stemness markers (Appendix Fig. 1).
Figure 1.
Dental pulp stem cells reside near blood vessels and are endowed with self-renewal. (A) Confocal microscopy of human dental pulp. Green color depicts dental pulp stem cells as indicated by aldehyde dehydrogenase 1 (ALDH1) staining, and red color depicts endothelial cells as indicated by CD31 staining. (B) Frequency distribution of the distance of between ALDH1high (stem) cells and CD31+ blood vessels (n = 173 perivascular niches). (C) Frequency distribution of the distance between Bmi-1high (self-renewing) cells and CD31+ blood vessels (n = 92 perivascular niches). (D) Phase contrast microscopy of secondary orospheres (dental pulp stem cells [DPSCs] or stem cells from human exfoliated deciduous teeth [SHED]) cultured in ultralow-attachment conditions with either Dulbecco’s modified Eagle medium/Nutrient Mixture F-12 (DMEM/F12) or Sphere Media (SM; DMEM/F12 supplemented with 10 ng/mL epidermal growth factor [EGF], 10 ng/mL basic fibroblast growth factor [bFGF], 2% fetal bovine serum [FBS]) for 7 d. (E) Graph depicting the number of secondary orospheres generated by DPSCs or SHED cultured with DMEM/F12 or SM in ultralow-attachment plates (105 cells/well) after 7 d. (F) Western blots for Bmi-1 expression in DPSCs or SHED cultured in DMEM/F12 (standard culture conditions), SM (standard culture conditions), or SM (ultralow-attachment conditions) for 7 d. Bmi-1 band density was normalized to respective GAPDH lanes. (G) Phase contrast microscopy of DPSC secondary orospheres cultured in SM or SM containing 10 µM PTC-209 (Bmi-1 inhibitor). (H) Graph depicting the number of secondary orospheres generated by DPSCs or SHED cultured in SM containing 0, 0.1, 1, or 10 µM PTC-209 for 7 d. *P < 0.05.
Self-renewing stem cells are able to form secondary spheres when cultured in suspension (Krishnamurthy and Nör 2013). We observed that stem cells from human dental pulps (DPSCs or SHED) are capable of forming secondary orospheres when cultured in sphere culture media in ultralow-attachment conditions, demonstrating that these cells are endowed with self-renewing capacity (Fig. 1D, E). Western blot analyses show increased expression of Bmi-1 (a marker of self-renewing stem cells) in dental pulp stem cells cultured in low-attachment conditions compared to cells cultured in standard conditions (Fig. 1F). Treatment of dental pulp stem cells with nonlethal doses of the Bmi-1 inhibitor PTC-209 (Appendix Fig. 2A) results in a decrease in the number of secondary orospheres in a dose-dependent manner (Fig. 1G, H). These data confirmed the functional role of Bmi-1 in dental pulp stem cells as an inducer of self-renewal.
Endothelial Cell-Derived IL-6 Promotes Dental Pulp Stem Cell Self-renewal
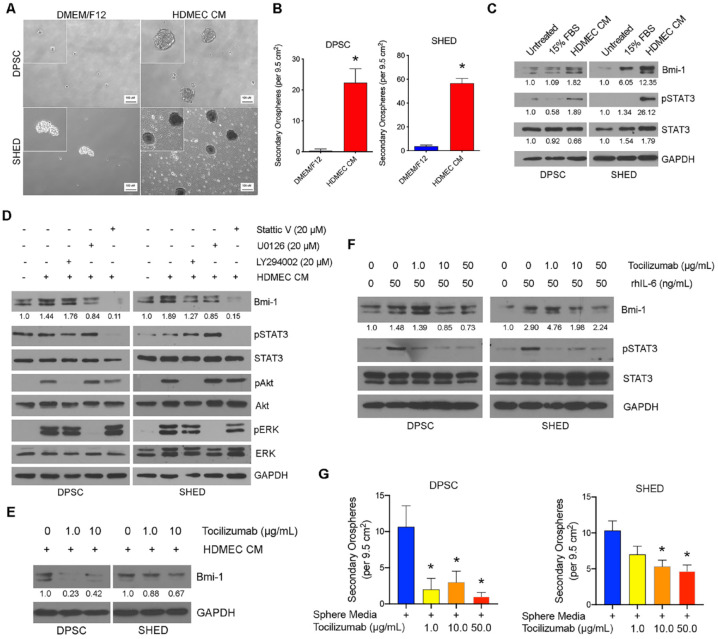
To understand the impact of endothelial cell–secreted factors on self-renewal of dental pulp stem cells, we exposed these cells to conditioned medium from primary human endothelial cells. We observed that the endothelial growth factor milieu causes a major increase in the number of secondary orospheres compared to unconditioned medium (Fig. 2A, B). Western blots demonstrated an upregulation of Bmi-1 expression and induction of STAT3 phosphorylation in dental pulp stem cells (DPSCs or SHED) cultured in endothelial conditioned medium, showing that endothelial cell–derived factors promote activation of pathways regulating self-renewal (Fig. 2C).
Figure 2.
Endothelial cell–derived interleukin (IL)–6 promotes self-renewal of dental pulp stem cells. (A) Phase contrast microscopy of secondary orospheres generated by dental pulp stem cells (DPSCs) or stem cells from human exfoliated deciduous teeth (SHED) cultured in Dulbecco’s modified Eagle medium/Nutrient Mixture F-12 (DMEM/F12) or conditioned media from primary human dermal microvascular endothelial cells (HDMEC CM). (B) Graph depicting the number of secondary orospheres in 6-well ultralow-attachment plates (105 cells/well) after treatment with endothelial conditioned media (HDMEC CM) or control media for 7 d. (C) Western blot analysis of DPSCs or SHED treated with basal medium containing 0% or 15% fetal bovine serum (FBS) or endothelial conditioned media (HDMEC CM). (D) Western blot analysis of DPSCs or SHED after 15-min exposure to HDMEC CM or HDMEC CM containing 20 µM Stattic V (STAT3 inhibitor), 20 µM U0126 (ERK/MAPK inhibitor), or 20 µM LY294002 (Akt/PI3K inhibitor). (E) Western blots of DPSCs or SHED after 15-min exposure to HDMEC CM containing 0, 1, or 10 µg/mL tocilizumab (IL-6R inhibitor). (F) Western blots of DPSCs or SHED exposed to 0 or 50 ng/mL recombinant human interleukin-6 (rhIL-6) in the presence of 0, 1, 10, or 50 µg/mL tocilizumab for 15 min. (G) Graph depicting the number of secondary orospheres generated by DPSCs or SHED cultured in Sphere Media (SM) containing 0, 1, 10, or 50 µg/mL tocilizumab for 7 d. *P < 0.05. Bmi-1 and STAT3 band density was normalized to respective GAPDH lanes in Western blots.
To begin to understand mechanisms responsible for endothelial cell–regulated self-renewal of dental pulp stem cells, we used chemical inhibitors of major signaling pathways (i.e., STAT3, ERK, PI3K-Akt) (Appendix Fig. 3A). We observed a marked decrease in Bmi-1 expression when dental pulp stem cells exposed to endothelial conditioned medium were treated with Stattic V (STAT3 inhibitor) but not when ERK or PI3K-Akt was inhibited (Fig. 2D). These data suggested a role for STAT3 signaling in the regulation of self-renewal of dental pulp stem cells. Knowing that endothelial cells secrete high levels of IL-6 and that IL-6 is a potent inducer of STAT3 (Krishnamurthy et al. 2014), we anticipated that Bmi-1 expression would be decreased in dental pulp stem cells exposed to endothelial cell conditioned medium containing sublethal doses of tocilizumab (anti-IL-6R antibody) (Fig. 2E; Appendix Fig. 3B). To confirm these data, we exposed dental pulp stem cells to recombinant human IL-6 (rhIL-6) in the presence of increasing concentrations of tocilizumab and observed that tocilizumab caused a dose-dependent inhibition of IL-6-induced pSTAT3 and Bmi-1 expression in dental pulp stem cells (Fig. 2F). To determine the functional impact of endothelial cell–derived IL-6 on orospheres generated by dental pulp stem cells, we cultured secondary orospheres in the presence of increasing concentrations of tocilizumab. We observed a decrease (P < 0.05) in secondary orosphere formation in the tocilizumab-treated group when compared to controls, suggesting that endothelial-derived IL-6 mediates self-renewal of dental pulp stem cells (Fig. 2G).
STAT3 Is a Critical Regulator of Dental Pulp Stem Cell Self-renewal
Western blots showed a marked increase in STAT3 phosphorylation and Bmi-1 expression when dental pulp stem cells are exposed to endothelial conditioned medium or rhIL-6 (Fig. 2C–F), and blockade of STAT3 signaling with sublethal Stattic V caused a dose-dependent inhibition of Bmi-1 (Fig. 3A; Appendix Fig. 3C). To confirm these data, we stably silenced STAT3 in both DPSCs and SHED using shRNA. Western blots confirmed the silencing of STAT3 expression and demonstrated a significant decrease in Bmi-1 expression when compared to cells stably transduced with scramble vector control shRNAs without affecting cell proliferation (Fig. 3B, C).
Figure 3.
STAT3 regulates self-renewal of dental pulp stem cells. (A) Western blot analysis of dental pulp stem cells (DPSCs) or stem cells from human exfoliated deciduous teeth (SHED) after treatment with 10 ng/mL recombinant human interleukin-6 (rhIL-6) in the presence of 0, 1, 2.5, or 5 µM Stattic V for 15 min. (B) Western blot analysis of DPSCs or SHED stably transduced with shRNA-control (scrambled sequence) or shRNA-STAT3 with or without exposure to endothelial conditioned media from primary human dermal microvascular endothelial cells (HDMEC CM) for 15 min. (C) Sulforhodamine B (SRB) assay to measure the impact of DPSCs or SHED stably transduced with shRNA-STAT3 up to 72 h (P < 0.05). (D) Graph depicting the number of secondary orospheres of DPSCs or SHED cultured in Sphere Media (SM) containing 0, 0.1, 1, or 5 µM Stattic V for 7 d. (E) Phase contrast microscopy depicting secondary orospheres generated by SHED stably transduced with shRNA-control or shRNA-STAT3 in SM at 7 d. (F) Graph depicting the number of secondary orospheres generated by SHED stably transduced with shRNA-control or shRNA-STAT3 cultured in SM for 7 d. *P < 0.05. Bmi-1 band density was normalized to respective GAPDH lanes in Western blots.
To determine the role of STAT3 signaling on self-renewal, dental pulp stem cells were cultured in low-attachment conditions and treated with sublethal doses of Stattic V (Fig. 3D; Appendix Fig. 3C). Inhibition of STAT3 signaling decreased the number of secondary orospheres in a dose-dependent manner (Fig. 3D). To verify these data, we cultured stem cells in low-attachment conditions and observed that dental pulp stem cells silenced for STAT3 generated fewer orospheres than cells transduced with the control vector (Fig. 3E, F). Together, these results illustrate a functional role for STAT3 signaling in the regulation of dental pulp stem cell self-renewal, as demonstrated by impacts on Bmi-1 expression and number of orospheres.
STAT3 Pathway Is Critical for the Establishment of Perivascular Niches
To determine the role of the STAT3 pathway in the generation and maintenance of perivascular niches in vivo, dental pulp stem cells stably transduced with shRNA-STAT3 (or control vector) were seeded in poly-L-lactic acid (PLLA) scaffolds and transplanted in the subcutaneous space of immunodeficient mice (Fig. 4A). After 28 d, scaffolds were retrieved and processed for histology. While the overall cellular density of the tissues generated in the 2 experimental conditions was similar (Fig. 4A), we observed a decrease (P < 0.05) in the number and area of blood vessels in the tissues generated with STAT3-silenced cells (n = 6) when compared to tissues generated with control cells (n = 6) (Fig. 4B–D). Concomitantly, we observed a significant decrease in the number of ALDH1high and Bmi-1high cells in tissues generated by STAT3-silenced cells when compared to tissues generated by control cells (Fig. 4E, F). These results demonstrate the important role of the STAT3 pathway in the establishment of the perivascular niche in vivo.
Figure 4.
STAT3 pathway is critical for the establishment of perivascular niches in vivo. (A) Graphical representation of the experimental model used here. Dental pulp stem cells (DPSCs) or stem cells from human exfoliated deciduous teeth (SHED) stably transduced with either small hairpin RNA (shRNA)–control or shRNA-STAT3 (106 cells) were mixed with growth factor reduced Matrigel and seeded into biodegradable scaffolds that were transplanted subcutaneously in immunodeficient mice. Representative hematoxylin and eosin (H&E) images of resulting tissues 28 d after transplantation. (B) Representative confocal microscopy images of scaffolds containing DPSCs or SHED stably transduced with either shRNA-control or shRNA-STAT3. CD31 immunofluorescence depicts blood vessels (green) and DAPI staining depicts nuclei (blue). (C) Graph depicting the quantification of CD31+ blood vessels generated by DPSCs or SHED stably transduced with shRNA-STAT3 (vs. shRNA-control) 28 d after transplantation. (D) Graph depicting the quantification of CD31+ surface area (pixels/field) in tissues generated by SHED-shRNA-STAT3 (vs. SHED-shRNA-control) in normalized confocal microscopy images. (E) Graph depicting the quantification of ALDH1high surface area (pixels/field) in tissues generated by SHED-shRNA-STAT3 (vs. SHED-shRNA-control) in normalized confocal microscopy images. (F) Graph depicting the quantification of Bmi-1high surface area (pixels/field) in tissues generated by SHED-shRNA-STAT3 (vs. SHED-shRNA-control) in normalized confocal microscopy images. *P < 0.05. (G) Graphical representation of the proposed mechanism for regulation of self-renewal of dental pulp stem cells.
As tissues generated with STAT3-silenced dental pulp stem cells showed a decrease in vascularity, we decided to study the role of STAT3 in vasculogenic potential differentiation of these cells in vitro using the capillary sprouting assay. We observed a decrease in the number of capillary sprouts (P < 0.05) when dental pulp stem cells were treated with sublethal concentrations of tocilizumab or Stattic V (Fig. 5A–C). Similarly, a decrease in number of sprouts was observed when SHED stably transduced with shRNA-STAT3 were tested in this assay (Fig. 5D, E).
Figure 5.
STAT3 pathway regulates the vasculogenic differentiation potential in dental pulp stem cells. (A) Representative phase contrast microscopy images of stem cells from human exfoliated deciduous teeth (SHED) cultured in growth factor reduced Matrigel with endothelial growth medium 2 (EGM2-MV) supplemented with 0 or 1 µg/mL tocilizumab for 7 d. (B) Graph depicting the number of capillary sprouts generated by SHED cultured in growth factor reduced Matrigel with endothelial growth medium 2 (EGM2-MV) supplemented with 0, 0.1, or 1 µg/mL tocilizumab for 7 d. (C) Representative phase contrast microscopy images of dental pulp stem cells (DPSCs) or SHED cultured in growth factor reduced Matrigel with EGM2-MV supplemented with 0 or 2.5 µM Stattic V for 7 d. (D) Representative phase contrast microscopy images of SHED stably transduced small hairpin RNA (shRNA)–control or shRNA-STAT3 cultured in growth factor reduced Matrigel with EGM2-MV medium for 7 d. (E) Graph depicting the number of capillary sprouts generated by SHED-shRNA-control or SHED-shRNA-STAT3 cultured in growth factor reduced Matrigel with EGM2-MV for 7 d. *P < 0.05.
Discussion
Perivascular niches, where stem cells reside near blood vessels, have been initially described in bone (Kollet et al. 2006; Sugiyama et al. 2006; Sacchetti et al. 2007). A seminal study from the Shi laboratory demonstrated colocalization of blood vessels and stem cells in bone marrow and the dental pulp (Shi and Gronthos 2003). More recently, the role of endothelial cell–stem cell crosstalk in the maintenance of stem cell pools has been unveiled in physiology (Ding et al. 2012) and in cancer (Krishnamurthy et al. 2010). However, the nature of the molecular crosstalk between endothelial cells and stem cells within the perivascular niche in the dental pulp remains unclear.
Aldehyde dehydrogenase (ALDH) has been extensively investigated as a marker of self-renewing stem cells (Corti et al. 2006; Ginestier et al. 2007). Investigation of dental pulp tissues suggested that ALDH1high cells colocalize with cells expressing mesenchymal stem cell surface markers, such as STRO1 and CD90 (Machado et al. 2016). Furthermore, Bmi-1 was shown to be a major regulator of self-renewal in various stem cell populations, including dental stem cells (Molofsky et al. 2003; Liu et al. 2006; Biehs et al. 2013). Here, we demonstrated that ALDH1high and Bmi-1high cells are preferentially found in close proximity to blood vessels in human dental pulps, suggesting the existence of perivascular niches. We observed that endothelial cell–secreted factors induce self-renewal of dental pulp stem cells, as demonstrated by the increase in the efficiency of secondary orosphere formation using the orosphere assay with cells cultured in low-attachment conditions (Reynolds and Weiss 1992; Dontu et al. 2003; Krishnamurthy and Nör 2013). These data provided the first evidence for the functional impact of having stem cells in close proximity to blood vessels in the dental pulp. In other words, endothelial cell–derived factors can potentially enhance asymmetric stem cell division and self-renewal of stem cells, which would enable the maintenance of stem cell pools while other stem cells undergo differentiation.
Bmi-1 was shown to play a critical role in tooth development using the continuously growing murine incisor model (Biehs et al. 2013). Here, we studied the function of Bmi-1 in the self-renewal of dental pulp stem cells by allowing them to form primary orospheres in low-attachment conditions and then serially passaging these cells into secondary orospheres. The ability of endothelial cell–derived factors to influence the formation of secondary orospheres upon dissociation and passaging of primary orospheres indicates a role for these factors on the self-renewal of dental pulp stem cells. Furthermore, the dose-dependent decrease in the number of secondary orospheres observed upon direct inhibition of Bmi-1 activity with PTC-209 provides evidence for a causal link between Bmi-1 activity and self-renewal of dental pulp stem cells.
Knowing that endothelial cell–derived IL-6 promotes the self-renewal of oral cancer stem cells (Krishnamurthy et al. 2014) and that the STAT3 pathway mediates self-renewal in several stem cell types (Niwa et al. 1998; Raz et al. 1999; Kiger et al. 2001; Tulina and Matunis 2001; Chen et al. 2015), we studied here the impact of endothelial cell–IL-6 on STAT3 activation in dental pulp stem cells. We observed here that endothelial cell–derived IL-6 promotes the self-renewal of dental pulp stem cells through STAT3 signaling and induction of Bmi-1 expression. This provided a mechanistic explanation for the functional role of endothelial cell–secreted factors on the maintenance of stem cell pools within the perivascular niche.
We postulate here that dental pulp stem cells are capable of generating perivascular niches through asymmetric division, where some stem cells differentiate into endothelial cells while other stem cells remain in an undifferentiated state. Thus, while multipotency allows dental pulp stem cells to give rise to multiple cell types, including functional endothelium (Sakai et al. 2010), self-renewal allows for the maintenance of a stem cell pool in close proximity to newly formed blood vessels. As such, our results unveil a pivotal role for STAT3 signaling in the orchestration of cellular events that enable the generation of multicellular perivascular niches.
When we transplanted STAT3-silenced dental pulp stem cells into immunodeficient mice, we observed similar morphological features to those observed in control specimens, with dense connective tissues interspaced with areas of loose connective tissue. Generally, the denser areas of tissue presented perfused, mature blood vessels. The looser connective tissue area contained smaller blood vessels with fewer red blood cells. Despite similar morphologies, microscopic analyses showed fewer perivascular niches (i.e., blood vessels surrounded by stem cells) when STAT3-silenced dental pulp stem cells were transplanted into mice. We also observed that STAT3 silencing decreases the self-renewal and vasculogenic potential of dental pulp stem cells in vitro. These results suggest that STAT3 is a master regulator of the development and maintenance of perivascular niches in the dental pulp.
In summary, this study showed evidence of a functional crosstalk between endothelial cells and stem cells in dental pulp tissue. We demonstrated that this crosstalk is initiated by endothelial cell–secreted IL-6 that binds to IL-6R in dental pulp stem cells to activate STAT3 signaling and Bmi-1 expression. We also showed that this pathway is critical for the establishment and maintenance of perivascular niches in the dental pulp. As such, we propose that the function of endothelial cells in the dental pulp is not limited to enabling influx of oxygen and nutrients. Rather, these results suggest that the maintenance of pools of stem cells throughout the life of the dental pulp requires signaling events triggered by nearby vascular endothelial cells.
Author Contributions
M. Oh, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; Z. Zhang, A. Mantesso, A.E. Oklejas, contributed to data acquisition, critically revised the manuscript; J.E. Nör, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520925417 for Endothelial-Initiated Crosstalk Regulates Dental Pulp Stem Cell Self-Renewal by M. Oh, Z. Zhang, A. Mantesso, A.E. Oklejas and J.E. Nör in Journal of Dental Research
Acknowledgments
The authors thank Kristy Warner and Carolina Cucco for technical assistance and for their support throughout this project; Tatiana Botero, William Giannobile, Jan Hu, and Elizabeth Lawlor for their guidance and wisdom; Songtao Shi (University of Pennsylvania) for dental pulp stem cells used in this study; the Biomedical Research Microscopy Core (University of Michigan Medical School) and the Histology Core (University of Michigan School of Dentistry) for their expertise.
Footnotes
A supplemental appendix to this article is available online.
This work was supported by grants T32-DE007057 (M. Oh from David Kohn-PI), F30-DE025471 (M. Oh), and R01-DE021410 (J.E. Nör) from the National Institute of Dental and Craniofacial Research.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Data Sharing Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Biehs B, Hu JK, Strauli NB, Sangiorgi E, Jung H, Heber RP, Ho S, Goodwin AF, Dasen JS, Capecchi MR, et al. 2013. BMI-1 represses Ink4a/Arf and Hox genes to regulate stem cells in the rodent incisor. Nat Cell Biol. 15(7):846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Aksoy I, Gonnot F, Osteil P, Aubry M, Hamela C, Rognard C, Hochard A, Voisin S, Fontaine E, et al. 2015. Reinforcement of STAT3 activity reprogrammes human embryonic stem cells to naive-like pluripotency. Nat Commun. 6:7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Papadimitriou D, Donadoni C, Salani S, Del Bo R, Strazzer S, Bresolin N, Comi GP. 2006. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 24(4):975–985. [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. 2012. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 481(7382):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. 2003. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17(10):1253–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. 2007. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 1(5):555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. 2000. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 97(25):13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. 2001. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 294(5551):2542–2545. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, et al. 2006. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 12(6):657–664. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nör JE. 2010. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 70(23):9969–9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy S, Meyers K, Dong Z, Imai A, Ward B, Helman JI, Taichman R, Bellile EL, McCauley L, Polverini PJ, et al. 2014. Endothelial IL-6 defines the tumorigenic potential of human head and neck cancer stem-like cells. Stem Cells. 32(11):2845–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy S, Nör JE. 2013. Orosphere assay: a method for propagation of head and neck cancer stem cells. Head Neck. 35(7):1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. 2006. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 66(12):6063–6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CV, Passos ST, Campos TM, Bernardi L, Vilas-Bôas DS, Nör JE, Telles PD, Nascimento IL. 2016. The dental pulp stem cell niche based on aldehyde dehydrogenase 1 expression. Int Endod J. 49(8):755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. 2003. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 100(10):5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. 2003. BMI-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 425(6961):962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Iohara K, Murakami M, Nakamura H, Sato Y, Ariji Y, Matsushita K. 2017. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther. 8(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Reddi AH. 2003. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 21(9):1025–1032. [DOI] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith A. 1998. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 12(13):2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nör JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, Addison CL, Mooney DJ, Polverini PJ. 2001. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 81(4):453–463. [DOI] [PubMed] [Google Scholar]
- Oh M, Nör JE. 2015. The perivascular niche and self-renewal of stem cells. Front Physiol. 6:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R, Lee CK, Cannizzaro LA, d’Eustachio P, Levy DE. 1999. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci USA. 96(6):2846–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. 1992. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 255(5052):1707–1710. [DOI] [PubMed] [Google Scholar]
- Rosa V, Zhang Z, Grande RH, Nör JE. 2013. Dental pulp tissue engineering in full-length human root canals. J Dent Res. 92(11):970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, et al. 2007. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 131(2):324–336. [DOI] [PubMed] [Google Scholar]
- Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, Santos CF, Nör JE. 2010. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 89(8):791–796. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. 2003. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 18(4):696–704. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. 2006. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 25(6):977–988. [DOI] [PubMed] [Google Scholar]
- Tulina N, Matunis E. 2001. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 294(5551):2546–2549. [DOI] [PubMed] [Google Scholar]
- Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, et al. 2018. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 10(455). pii: eaaf3227. [DOI] [PubMed] [Google Scholar]
- Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, Chai Y. 2018. Secretion of Shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 23(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520925417 for Endothelial-Initiated Crosstalk Regulates Dental Pulp Stem Cell Self-Renewal by M. Oh, Z. Zhang, A. Mantesso, A.E. Oklejas and J.E. Nör in Journal of Dental Research