Serratia marcescens is a bacterium frequently found in the environment, but over the last several decades it has evolved into a concerning clinical pathogen, causing fatal bacteremia. To establish such infections, pathogens require specific nutrients; one very limited but essential nutrient is iron. We sought to characterize the iron acquisition systems in S. marcescens isolate UMH9, which was recovered from a clinical bloodstream infection. Using RNA sequencing (RNA-seq), we identified two predicted siderophore gene clusters (cbs and sch) that were regulated by iron.
KEYWORDS: Serratia marcescens, bacteremia, iron, serratiochelin, siderophores
ABSTRACT
Serratia marcescens is a bacterium frequently found in the environment, but over the last several decades it has evolved into a concerning clinical pathogen, causing fatal bacteremia. To establish such infections, pathogens require specific nutrients; one very limited but essential nutrient is iron. We sought to characterize the iron acquisition systems in S. marcescens isolate UMH9, which was recovered from a clinical bloodstream infection. Using RNA sequencing (RNA-seq), we identified two predicted siderophore gene clusters (cbs and sch) that were regulated by iron. Mutants were constructed to delete each iron acquisition locus individually and in conjunction, generating both single and double mutants for the putative siderophore systems. Mutants lacking the sch gene cluster lost their iron-chelating ability as quantified by the chrome azurol S (CAS) assay, whereas the cbs mutant retained wild-type activity. Mass spectrometry-based analysis identified the chelating siderophore to be serratiochelin, a siderophore previously identified in Serratia plymuthica. Serratiochelin-producing mutants also displayed a decreased growth rate under iron-limited conditions created by dipyridyl added to LB medium. Additionally, mutants lacking serratiochelin were significantly outcompeted during cochallenge with wild-type UMH9 in the kidneys and spleen after inoculation via the tail vein in a bacteremia mouse model. This result was further confirmed by an independent challenge, suggesting that serratiochelin is required for full S. marcescens pathogenesis in the bloodstream. Nine other clinical isolates have at least 90% protein identity to the UMH9 serratiochelin system; therefore, our results are broadly applicable to emerging clinical isolates of S. marcescens causing bacteremia.
INTRODUCTION
Historically, Serratia marcescens has been considered an environmental pathogen due to its ability to infect insects and survive in soil and aquatic reservoirs. However, over the last several decades, S. marcescens has emerged as a major opportunistic pathogen in humans, causing deadly infections that include bacteremia and endocarditis (1). Antibiotic-resistant isolates of this Gram-negative pathogen continue to arise (1), and emergent strains have been found to carry multiple virulence factors, including a hemolysin, various proteases and nucleases, and a polysaccharide capsule (2, 3). Despite these observations, it is clear that significant research is still required to advance our understanding of the pathogenesis of S. marcescens.
Iron is essential to many cellular processes in bacteria and therefore is critical to pathogenesis (4). Humans harbor approximately 3 to 4 g of total iron within their bloodstream and tissues (5); however, most of this iron is not readily available, as it is sequestered by host proteins, including transferrin, ferritin, ferroportin, lactoferrin, myoglobin, and hemoglobin (6–8). Consequently, free iron concentrations in serum are less than 10−24 M at physiological pH, requiring microbes to have developed specific strategies to access iron sequestered within host proteins to enable survival (9). For example, heme acquisition systems use dedicated cell surface receptors in concert with the TonB complex to bind and internalize host heme, releasing the iron molecule into the bacterial cytoplasm (10). In addition, siderophores, small organic molecules with high affinities for iron (Kd [dissociation constant] = 10−52 M) (11), are synthesized by the bacterium and exported to the extracellular space to scavenge iron from host proteins, including transferrin, lactoferrin, and ferritin. Outer membrane TonB-dependent receptors then facilitate the import of iron-loaded siderophores into the bacterial cytoplasm (7).
Iron acquisition has been studied extensively in the context of other Gram-negative pathogens, especially Escherichia coli (12). For example, uropathogenic E. coli mutants lacking receptors for specific siderophores or heme import show decreased fitness in both the bladder and kidney in a mouse model of ascending urinary tract infection, establishing the importance of iron uptake during infections of the bladder and kidneys (13). Additionally, hypervirulent strains of Klebsiella pneumoniae encode up to four siderophore systems (14). These systems have been shown to induce host inflammatory cytokines and facilitate bacterial dissemination during pneumonia (15).
Despite the importance of iron acquisition to the pathogenesis of other Gram-negative bacteria, comparatively little is known about the contribution of S. marcescens iron uptake systems to its pathogenesis in mammalian hosts. Nevertheless, there has been some work defining the molecular mechanisms of S. marcescens iron acquisition with particular focus on heme uptake, characterizing two systems, Hem and Has (16). The hem operon encodes an outer membrane receptor, HemR, that imports iron by stripping the metal directly from heme (10, 17). The has operon encodes a proteinaceous hemophore, HasA, that extracts iron from heme molecules, and then the hemophore-iron complex is bound and internalized by the HasR receptor (18, 19). The contribution of the Hem or Has system to S. marcescens virulence was not explored by these studies. The only studies defining S. marcescens siderophores have been performed in nonmammalian systems. A transposon library constructed in S. marcescens Db11, initially described as a Drosophila melanogaster pathogen, was used to identify a mutation within a predicted siderophore biosynthetic gene that displayed reduced virulence in a Caenorhabditis elegans model of infection (20). Previous studies have shown several potential siderophores produced by Serratia species. For example, Serratia liquefaciens and Serratia ficaria produce the siderophore aerobactin, whereas S. marcescens does not. Many isolates, however, typically encode an aerobactin receptor (21, 22). S. marcescens W225 synthesizes another siderophore, enterobactin, and can utilize ferric citrate as an iron source as well (22).
A siderophore designated serratiochelin is also commonly produced by the S. marcescens and Serratia plymuthica species. Biochemical studies have been undertaken to analyze serratiochelin synthesis. The results allowed the authors to hypothesize that serratiochelin is a molecular hybrid of enterobactin and vibriobactin, based on gene similarity and mutagenesis experiments (23). Other studies have shown that S. marcescens also produces a low-affinity siderophore, chrysobactin, first identified in Dickeya chrysanthemi (24). Genes associated with the synthesis of either serratiochelin or chrysobactin have been found in the sequenced genomes of other S. marcescens strains. For example, a clinical isolate of S. marcescens SM6 is predicted to produce serratiochelin and chrysobactin based on genetic identity to the schF (nonribosomal peptide synthetase [NRPS] in S. plymuthica V4) and cbsF (NRPS in Dickeya dadantii 3937) genes, respectively (25).
In this study, we identified the iron-regulated transcriptome of Serratia marcescens bloodstream isolate UMH9 by RNA sequencing (RNA-seq). Two gene clusters, upregulated under iron-limited conditions, were predicted to encode biosynthetic machinery for siderophore production. Two putative siderophore operons, cbs and sch, previously predicted in silico, encode production of chrysobactin and serratiochelin, respectively (25). Mutants lacking sch lost iron-chelating ability quantified by the chrome azurol S (CAS) assay, compared to iron chelation observed in the wild type (WT) and cbs mutant. Mass spectrometry (MS) analysis identified the chelating siderophore as serratiochelin. Mutants lacking serratiochelin were significantly outcompeted during cochallenge by wild-type UMH9 in the kidneys and spleen in a bacteremia mouse model. This result was further confirmed by independent challenge, demonstrating that serratiochelin is required for full S. marcescens pathogenesis in the bloodstream.
RESULTS
Identification of the S. marcescens transcriptome during iron limitation.
To identify iron-dependent genome-wide changes in transcription, differential gene expression of S. marcescens strain UMH9 under iron-rich and iron-limited conditions was quantified using RNA-seq. Three concentrations (125 μM, 200 μM, and 250 μM) of dipyridyl, an iron chelator, were added to LB medium to empirically find subinhibitory levels of iron limitation (Fig. 1). Transcripts isolated from UMH9 cultured in LB medium and LB medium supplemented with 200 μM dipyridyl were sequenced and analyzed to identify differentially regulated genes. A total of 114 genes were significantly upregulated (see Table S1 in the supplemental material) and 46 genes were significantly downregulated (Table S2) during iron limitation. Upregulated genes related to iron uptake were grouped into systems and revealed the heme uptake systems (hem and has), genes involved in the ferrous iron uptake system (sitABCD), and ferric citrate uptake system (fecARI). The results also identified two putative siderophore operons. Seven additional orphan TonB-dependent transporters were upregulated as well, suggesting that UMH9 can import siderophores through multiple transporters or bring in xenosiderophores (Table 1).
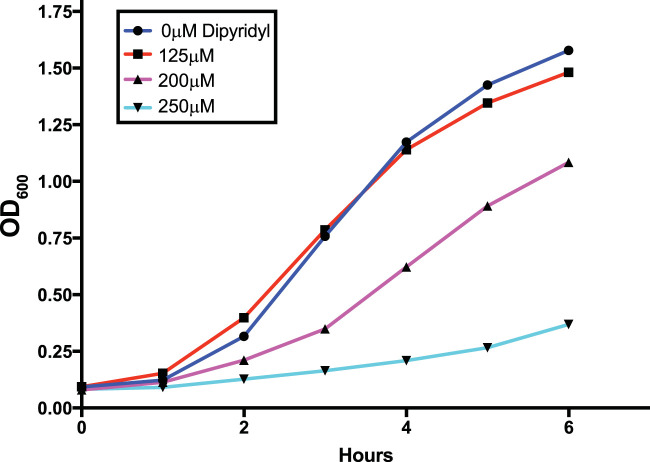
FIG 1.
Serratia marcescens UMH9 growth is abrogated by increasing concentrations of dipyridyl. Dipyridyl was added at 0, 125, 200, and 250 μM to S. marcescens UMH9 cultured in LB medium. Bacterial growth was measured by monitoring the OD600 every hour as cultures were incubated with aeration at 37°C. A concentration of 200 μM dipyridyl was used to achieve iron-limiting conditions for RNA sequencing.
TABLE 1.
Upregulated iron acquisition systems in Serratia marcescens UMH9 during iron-limited RNA-seq
| Function | Locus tag BVG96_RS… | Gene | Description | Log2 FCa |
|---|---|---|---|---|
| Heme uptake | 01515 | hasI | RNA polymerase sigma factor | 3.38 |
| 01520 | hasS | Anti-sigma factor | 3.09 | |
| 01525 | hasR | TonB-dependent receptor | 5.25 | |
| 01530 | hasA | Hemophore | 6.64 | |
| 01535 | hasD | Type I secretion system permease/ATPase | 4.04 | |
| 01540 | hasE | Periplasmic adaptor subunit | 3.90 | |
| 01545 | tonB/hasB | Energy transducer | 3.03 | |
| 07350 | hemV | Heme ABC transporter ATP-binding protein | 5.70 | |
| 07355 | hemU | Hemin ABC transporter permease | 6.54 | |
| 07360 | hemT | Periplasmic hemin-binding protein | 6.25 | |
| 07365 | hemS | Hemin-degrading factor | 6.89 | |
| 07370 | hemR | TonB-dependent receptor | 7.77 | |
| 07375 | hemP | Hemin uptake protein | 7.48 | |
| 07380 | Glutathione peroxidase | 3.97 | ||
| Ferrous iron uptake | 07170 | sitD | Iron ABC transporter, inner membrane permease | 2.18 |
| 07175 | sitC | Iron ABC transporter, inner membrane permease | Not represented | |
| 07180 | sitB | Iron ABC transporter, ATP-binding protein | 2.87 | |
| 07185 | sitA | Iron ABC transporter, periplasmic-binding protein | 3.36 | |
| 11025 | efeB | Iron transport peroxidase | 2.75 | |
| 11030 | efeO | Iron transport periplasmic protein | 2.79 | |
| 11035 | efeU | Iron transport permease | 2.77 | |
| Ferric citrate transport | 07970 | fecA | TonB-dependent receptor | 5.45 |
| 07975 | fecR | Operon regulator | 6.02 | |
| 07980 | fecI | RNA polymerase sigma factor | 6.16 | |
| Putative siderophore biosynthesis and transport | 08795 | Siderophore exporter | 3.89 | |
| 08800 | Nonribosomal peptide synthetase | 6.30 | ||
| 08805 | Chaperone | 6.74 | ||
| 08810 | Esterase | 6.86 | ||
| 08815 | TonB-dependent receptor | 6.28 | ||
| 21965 | Condensation protein | 5.12 | ||
| 21970 | Adenylation protein | 5.96 | ||
| 21975 | Glutamate racemase | 6.05 | ||
| 21980 | TonB-dependent receptor | 7.62 | ||
| 21985 | Condensation protein | 6.65 | ||
| 21990 | Hypothetical protein | 6.47 | ||
| 21995 | TonB-dependent receptor | 4.41 | ||
| 22000 | Esterase | 5.47 | ||
| 22005 | Chaperone | 5.80 | ||
| 22010 | Nonribosomal peptide synthetase | 4.60 | ||
| 22015 | Siderophore exporter | 3.61 | ||
| 22020 | Isochorismate synthase | 5.32 | ||
| 22025 | DHB adenylate synthase-AMP ligase | 5.59 | ||
| 22030 | Isochorismatase | 5.62 | ||
| 22035 | Hypothetical protein | 5.55 | ||
| Additional iron transporters | 12820 | TonB-dependent siderophore receptor | 7.03 | |
| 01005 | iutA | Aerobactin siderophore receptor | 4.04 | |
| 01485 | TonB-dependent siderophore receptor | 5.88 | ||
| 11645 | TonB-dependent receptor | 4.51 | ||
| 14005 | TonB-dependent siderophore receptor | 5.54 | ||
| 14175 | TonB-dependent receptor | 5.74 | ||
| 14360 | TonB-dependent siderophore receptor | 3.91 |
FC, fold change.
Characterization of two putative siderophore operons.
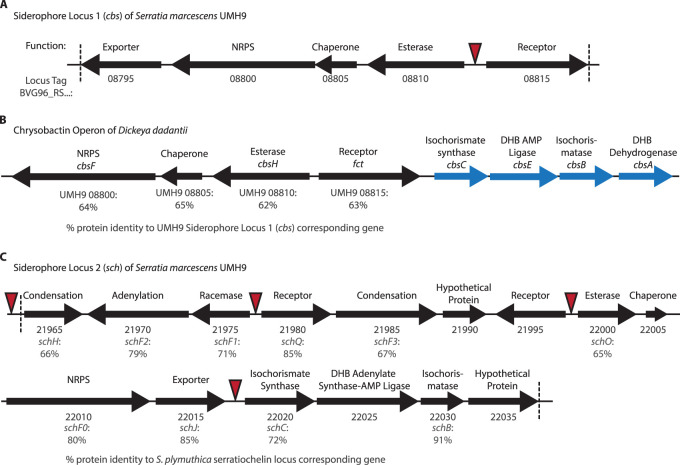
The two identified siderophore gene loci are organized in potential operons and appear distinct, as they contain unique gene compositions relative to one another and are located in different regions of the chromosome. The first putative operon (referred to as cbs) is 13,859 bp and has fewer genes than what is typical of a pathogen-associated siderophore locus (26). cbs encodes a TonB-dependent receptor (gene tag: BVG96_RS08815) as well as an exporter (BVG96_RS08795), presumably for the molecule it produces. The majority of this operon is comprised of a single predicted nonribosomal peptide synthetase (NRPS) (BVG96_RS08800)-encoding gene (Fig. 2A). Genes within the cbs operon have similarity (four corresponding genes have >60% amino acid identity) to the chrysobactin locus of Dickeya dadantii 3937, and previous studies have reported that certain S. marcescens strains produce chrysobactin (Fig. 2B) (24). However, this operon lacks several essential genes in the chrysobactin biosynthesis pathway, such as genes for an isochorismate synthase, a 2,3-dihydroxybenzoate-AMP ligase, an isochorismatase, and a 2/3-dihydro-2,3-dihydroxybenzoate dehydrogenase. These genes are encoded elsewhere in the UMH9 genome, several of which are found in the second identified operon (26).
FIG 2.
Siderophore locus 1 (cbs) is similar to the chrysobactin operon in Dickeya dadantii, while siderophore locus 2 (sch) shows similarity to Serratia plymuthica serratiochelin genes. (A) The UMH9 cbs locus has an organization similar to that of the chrysobactin operon found in Dickeya dadantii (B). Four genes (in blue) are missing from the UMH9 cbs locus that are present in the D. dadantii chrysobactin operon. However, these genes, involved in the production of chrysobactin precursor molecules, are found elsewhere in the UMH9 genome. The percentages reported underneath the genes in panel B are the protein percent identity to the UMH9 siderophore locus 1 corresponding gene in panel A. (C) The UMH9 sch locus contains genes predicted to be necessary for siderophore biosynthesis and transport. The percentages reported under the genes are the amino acid percent identity to the S. plymuthica serratiochelin locus corresponding gene. The gene function is noted above each arrow. The UMH9 locus tag number (BVG96_RS…) is referenced below each arrow for panels A and C. Red triangles indicate the locations of putative ferric uptake regulator (Fur) binding sequences in the UMH9 genome. The genes located between the vertical hashed lines in panels A and C were deleted in the cbs::kan and sch::kan mutants, respectively, as well as in the cbs::cam sch::kan mutant together.
The second putative operon (referred to as sch) is much larger than the first (27,686 bp) and appears to contain all genes predicted for the complete biosynthetic pathway of an NRPS-dependent siderophore, including genes required to encode enzymes that could synthesize siderophore precursor molecules, and the obligate NRPS (27). Additionally, sch encodes two TonB-dependent receptors as well as an exporter. The composition of the sch genetic region is more typical of a pathogen-associated siderophore and displays similarity to components of the serratiochelin biosynthetic operon of S. plymuthica (10 genes have over 65% identity) (Fig. 2C). Serratiochelin, a Serratia-specific siderophore, was first identified in S. marcescens TW (24). The biosynthesis pathway has been described for Serratia V4 by predicting evolutionary mixing of the vibriobactin and enterobactin biosynthetic operons to produce serratiochelin (23). While the UMH9 sch operon is not identical to the operon in Serratia V4, we nevertheless predict that UMH9 uses this similar biosynthetic pathway to produce serratiochelin. Interestingly, some of the biosynthetic genes missing from cbs are present in sch, suggesting that the ability of cbs to encode synthesis of a siderophore may be dependent on sch (Fig. 2). Due to the similarity of these genetic regions to reported chrysobactin and serratiochelin loci, the putative siderophore operons are heretofore referred to as cbs and sch, respectively.
Loss of sch abrogates iron chelation in S. marcescens.
Each siderophore locus was disrupted individually (cbs::kan [Δcbs] and sch::kan [Δsch]) and in combination (cbs::cam sch::kan [Δcbs/sch]) in the parent strain, UMH9 (WT). In each case, the entire siderophore-encoding locus was deleted and replaced with a kanamycin or chloramphenicol resistance cassette. To determine how each locus contributes to siderophore production, the wild-type strain and each construct were cultured overnight in iron-depleted M9 minimal medium or M9 medium supplemented with 36 μM FeSO4. Culture supernatants were used in a soluble CAS assay the following day to quantify siderophore synthesis. As expected, the WT strain and deletion constructs showed no siderophore production under iron-replete conditions (Fig. 3). Conversely, the WT showed the greatest amount of CAS activity under iron limitation. CAS activity comparable to that of the WT was observed for the Δcbs mutant. However, significant loss of iron chelation activity was observed in the Δsch and Δcbs/sch mutants. These results suggested that under iron limitation, UMH9 produces a siderophore that can chelate iron from the ferri-CAS complex, and the sch locus is necessary for the production of the chelating activity.
FIG 3.
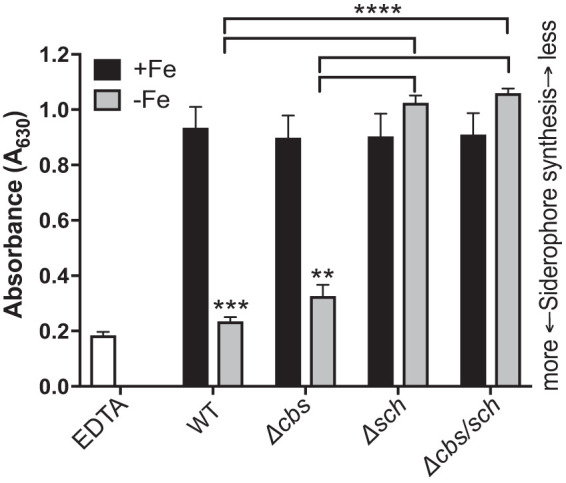
The sch locus is responsible for detectable siderophore production. Siderophore production was quantified by liquid chrome azurol S (CAS) assay on bacterial supernatants. Bacteria were cultured overnight in M9 minimal medium with or without 36 μM FeSO4. EDTA (10 μM) served as the positive control for iron limitation. Low absorbance (A630) values indicate more siderophore production. Data are the means of three independent replicates, and error bars represent standard errors of the means. Significant differences (**, P < 0.01; ***, P < 0.001; ****, P < 0.0001) were determined using a two-tailed unpaired t test.
Mass spectrometry reveals that the sch operon produces serratiochelin A.
Because the siderophore produced by the sch locus is responsible for detectable CAS activity, we wanted to definitively identify the siderophore being produced by the sch locus using mass spectrometry (MS). Wild-type UMH9 and the Δcbs, Δsch, and Δcbs/sch siderophore mutants were cultured overnight in both iron-depleted M9 minimal medium and M9 medium supplemented with 36 μM FeSO4. MS was performed on bacterial supernatants (Fig. 4). Based on previous literature and the similarity of the putative sch locus to serratiochelin-producing operons, we used fragmentation patterns matching that of serratiochelin as a reference. MS/MS spectra obtained from bacterial extracts of the wild type and mutants were first analyzed using a global natural product search (GNPS)-based DEREPLICATOR+ algorithm for probing the presence of serratiochelin (28, 29). The algorithm compared our data set to all spectra in the GNPS database, enabling high-throughput identification of known natural products present in the bacterial extract.
FIG 4.
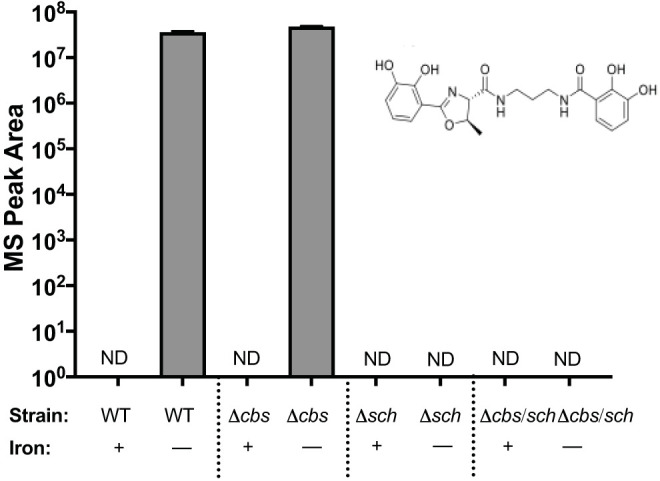
Serratiochelin A production is lost in an sch mutant. An intensity plot of serratiochelin A was generated using mass spectrometry performed on the supernatants of the indicated strains after overnight culture in M9 minimal medium with and without iron (where indicated). A directed approach identified serratiochelin A with a retention time of 4.84 min to be associated with the sch operon. The molecular structure of serratiochelin A is shown. ND, not detected.
To analyze our samples using DEREPLICATOR+, we opted for a stringent score threshold of a 0% false discovery rate (FDR) and removed the metabolites present in blank samples that consisted of M9 growth medium only. The analysis clearly showed the presence of an [M+H]+ ion at 430.1723 m/z and an [M+Na]+ ion at 452.1470 m/z (Fig. S1) with the molecular formula C21H23N3O7, unambiguously identifying the presence of serratiochelin A in WT and Δcbs samples without iron and the absence of serratiochelin A in Δsch and Δcbs/sch samples both with and without iron (Fig. 4).
Serratiochelin A is necessary for growth under iron limitation in vitro.
Mass spectrometry results indicated that the sch locus encodes enzymes required to synthesize serratiochelin A, and serratiochelin is most likely responsible for iron chelation, as indicated by the CAS assay. A very low concentration of chrysobactin was present in wild-type supernatants as determined by mass spectrometry, associated with the cbs locus (data not shown). Unlike the case with the sch locus, chrysobactin did not demonstrate any notable iron chelation by CAS assay, likely due to its low concentration. Based on these results, we hypothesized that in vitro growth under iron-limiting conditions would be contingent on the presence of the sch locus, which encodes enzymes required for serratiochelin A production.
To test this, increasingly iron-limited conditions were established by titrating dipyridyl into LB. The UMH9 WT, Δcbs mutant, Δsch mutant, and Δcbs/sch double mutant all grew similarly in LB medium without dipyridyl (Fig. 5A). As dipyridyl concentrations increased, all strains exhibited less growth, as expected (Fig. 5). The WT and the Δcbs mutant had comparable growth in 200 μM dipyridyl, while the Δsch and Δcbs/sch mutants displayed noticeably inhibited growth (Fig. 5B). At 250 μM (Fig. S2A) and 300 μM (Fig. 5C) dipyridyl, the Δsch and Δcbs/sch mutants showed notably decreased growth compared to that of the WT and Δcbs mutant. The Δcbs mutant had the most robust growth under these conditions, while the WT had mildly decreased growth compared to that of the Δcbs mutant. The Δsch and Δcbs/sch mutants failed to grow at dipyridyl concentrations of 350 μM (Fig. S2B) and 400 μM (Fig. 5D). The Δcbs mutant continued to display the best growth under these concentrations. The WT had abrogated growth at 350 μM dipyridyl and minimal growth at 400 μM dipyridyl.
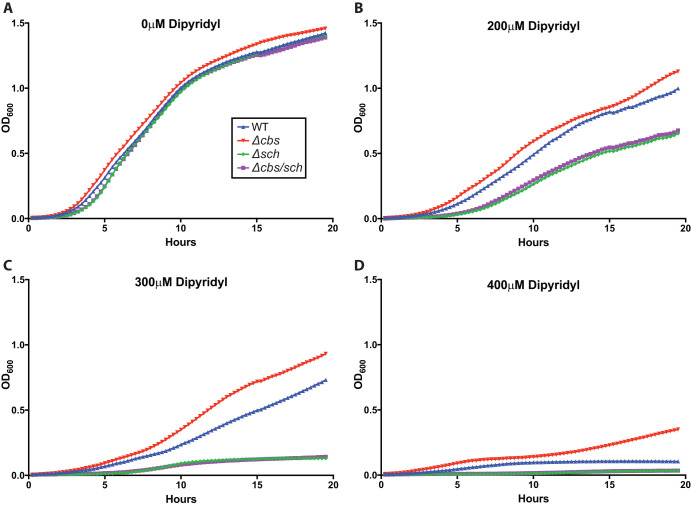
FIG 5.
UMH9 growth under iron limitation is dependent upon presence of the sch locus. The S. marcescens UMH9 wild type, cbs locus mutant (Δcbs), sch locus mutant (Δsch), and cbs/sch double mutant (Δcbs/sch) were inoculated into LB medium containing 0 μM (A), 200 μM (B), 300 μM (C), or 400 μM (D) dipyridyl after culture overnight in LB medium with 250 μM dipyridyl. Cultures were incubated with aeration at 37°C, and OD600 readings were taken every 15 min to measure bacterial growth. The means of three independent replicates are shown.
These results suggested that the sch locus is essential for growth under these iron-limited conditions. Without sch, UMH9 is much more inhibited by dipyridyl iron limitation than the WT strain. Interestingly, the absence of cbs appears to provide a growth advantage in UMH9. Additionally, because the Δcbs/sch mutant shows severely inhibited growth comparable to that seen in the Δsch mutant, the inhibited growth related to the loss of sch cannot be overcome by the growth advantage related to losing the cbs locus under iron limitation.
Serratiochelin is required for growth in human serum.
Although both cbs and sch affect growth in LB culture with dipyridyl, the importance of these genes in vivo remained to be determined. To explore their role under a physiologically relevant iron-limited condition, we first assessed growth of the wild type and mutants in 100% heat-inactivated human serum (Fig. 6). Iron bound to transferrin is the principal iron carrier in the blood and the main source of iron in human serum (30); therefore, we hypothesized that the ability to produce a siderophore would be advantageous to growth in this medium.
FIG 6.
Serratiochelin mutants grow poorly in human serum. The S. marcescens UMH9 wild type and Δcbs, Δsch, and Δcbs/sch mutants were cultured in 100% heat-inactivated human serum at 37°C with aeration. OD600 readings were taken every 15 min to measure bacterial growth. Data represent the means from three independent replicates.
The WT strain and the three mutants displayed an initial decrease in optical density, likely due to bacterial aggregation during the lag phase (Fig. 6). Eventually, the bacterial aggregates dispersed and logarithmic growth was observed. The WT began to grow after nearly 8 h, while the Δcbs, Δsch, and Δcbs/sch mutants showed growth at 10 h. After initial growth at 10 h, the Δsch and Δcbs/sch mutants reached the stationary phase at approximately 16 h, whereas the WT and Δcbs strains grew beyond 20 h. The final optical density at 600 nm (OD600) was significantly higher in the WT and Δcbs strains than in the Δsch and Δcbs/sch mutants (Fig. S3).
These results suggest that the sch locus contributes to the growth of S. marcescens in human serum. Taking growth in serum with the growth in the presence of dipyridyl together, the cbs operon appears to be dispensable for growth under iron limitation and does not contribute significantly to growth even under physiologically relevant iron-limited conditions.
Mutants lacking sch are attenuated during bacteremia.
Siderophores have been found to contribute significantly to virulence in murine models of infection (13). UMH9 was isolated from a bacteremic patient; therefore, we wanted to determine if its siderophores contributed to its pathogenesis in vivo. Since serratiochelin was essential for growth in low-iron medium in vitro, we hypothesized that it would contribute to its pathogenesis in the bloodstream. Using a murine model of bacteremia adapted from use with E. coli (31), we evaluated the fitness of the siderophore mutants in the bloodstream using competition experiments. We prepared a 1:1 mixture of each siderophore mutant with WT UMH9 and then coinoculated mice with 5 × 107 CFU of this suspension via the tail vein. Competitive indices (CI) of mutant and wild-type bacteria were calculated for the kidneys, spleen, and liver 24 h postinfection (hpi). The Δsch and Δcbs/sch mutants displayed a significant competitive disadvantage (log CI, <0) in the kidneys (Fig. 7A) and spleen (Fig. 7B), with a modest disadvantage observed in the liver (Fig. 7C). The Δcbs mutant competed equally with wild type in the spleen and liver (log CI, 0) (Fig. 7B and C), but a competitive advantage was observed in the kidneys (log CI, >0), suggesting that the loss of the cbs locus was advantageous to kidney colonization (Fig. 7A). These results suggest that serratiochelin is necessary for complete fitness in the bloodstream.
FIG 7.
Fitness of UMH9 siderophore mutants in a murine model of bacteremia. CBA/J mice were infected with 5 × 107 CFU/mouse in a 1:1 mixture of the wild type and the indicated mutant strain via the tail vein. Mice were euthanized at 24 h and the kidneys (A), spleen (B), and liver (C) were collected from each mouse. Organs were homogenized. Homogenates were diluted and selectively plated. The relative fitness for each mutant compared to that of the wild type was calculated as a competitive index (CI). The dotted line represents a CI of 1.0, meaning that the two strains competed equally. Solid lines represent median CI. Statistics were calculated using the Wilcoxon signed-rank test with a theoretical median of 0 (*, P < 0.05; **, P < 0.01).
Although the entire sch gene cluster, including the serratiochelin receptor gene, was deleted, we hypothesized that serratiochelin produced by the wild-type strain may have partially cross-complemented the mutant strains, since the mutants retained genes that were annotated as encoding putative orphan siderophore receptors. Therefore, we performed independent infections with each strain, enumerating bacterial burdens in the kidneys, spleen, and liver at 24 hpi. Mutants lacking sch (Δsch and Δcbs/sch) had significantly fewer CFU recovered from the kidney than the wild type and the Δcbs mutant (P < 0.0007), suggesting that mutants unable to produce serratiochelin are attenuated in the kidney (Fig. 8A). The Δcbs/sch mutant colonized the spleen significantly less well than the WT (Fig. 8B). The presence or absence of serratiochelin did not appear to affect colonization in the liver (Fig. 8C). Since sch mutants were attenuated in both cochallenge and independent-challenge experiments, this rules out that the wild-type strain contributed to any significant cross-feeding of the sch mutant during cochallenge. Together, these data indicate that serratiochelin is required for full pathogenicity in the bloodstream.
FIG 8.
Independent infections of UMH9 siderophore mutants in a murine model of bacteremia. CBA/J mice were independently inoculated with the indicated strain via the tail vein. Mice were euthanized 24 h postinoculation. Kidneys (A), spleens (B), and livers (C) were collected and homogenized. Homogenates were diluted and plated. The dotted line indicates the limit of detection. Significance, determined by the Mann-Whitney test, is indicated with asterisks (*, P < 0.05; ***, P < 0.001; ****, P < 0.0001).
The characterized siderophore operons are conserved across clinical isolates of S. marcescens.
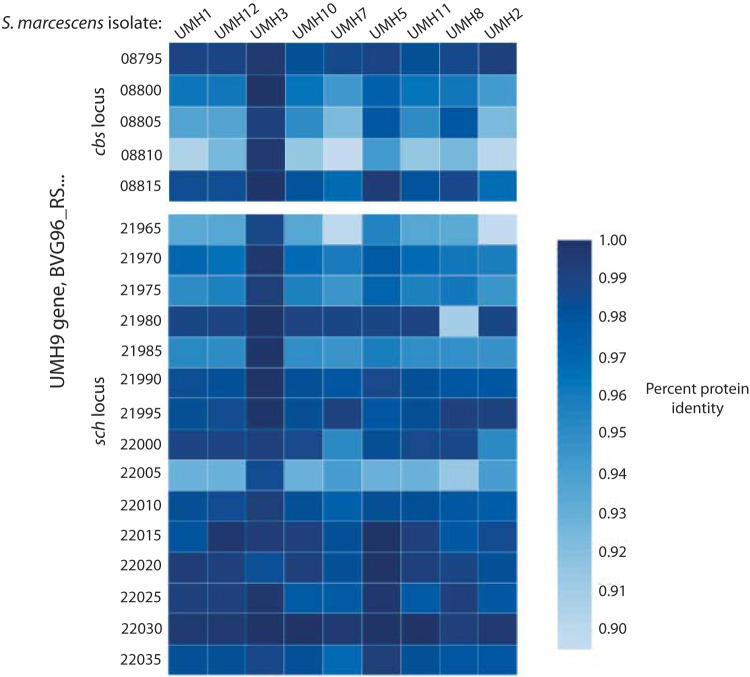
S. marcescens is a diverse species with a core genome of 3,372 genes and an accessory pangenome consisting of 10,215 genes (32). To determine whether the two putative siderophore loci are conserved among S. marcescens strains, we used PATRIC to compare predicted protein sequence similarity of nine clinical bloodstream isolates of S. marcescens to that of UMH9 (33). We found that genes within the cbs and sch loci are conserved and individual predicted proteins typically have more than 90% amino acid sequence identity to UMH9 proteins (Fig. 9).
FIG 9.
The cbs and sch genetic regions are conserved among clinical isolates of Serratia marcescens. The amino acid sequences of the UMH9 siderophore loci were compared to the siderophore loci of nine other bacteremic isolates of Serratia marcescens. Using PATRIC’s online protein identity platform, we determined the conservation of these loci among other S. marcescens clinical strains.
DISCUSSION
The contribution of siderophores to infection has been well established for a large number of bacterial pathogens (4, 34–36). However, because S. marcescens was thought to largely reside in environmental reservoirs, we aimed to determine the importance of Serratia siderophores to infection within the mammalian host. Prior to this work, S. marcescens siderophore systems had not been described in bacteremia infection models relevant to human disease. Two identified siderophore operons had been reported previously; however, this is the first study detailing their upregulation under iron-limited conditions as well as their contribution to S. marcescens pathogenesis. We demonstrate that serratiochelin A is produced by the sch locus and sch is necessary for S. marcescens UMH9 to grow under iron-limiting conditions both in vitro (Fig. 5 and 6) and in vivo (Fig. 7 and 8). The cbs locus is dispensable for infection and does not significantly contribute to growth under iron limitation. Indeed, under some conditions, the expression of cbs seems to limit UMH9 growth. However, despite the different contributions to fitness between the two siderophore loci under the conditions tested in this study, both are highly conserved among other sequenced clinical isolates.
S. marcescens occupies broad environmental niches beyond the mammalian host, as it also infects insects and survives in aquatic environments (1). Because the cbs locus is dispensable in the mammalian model of infection, we speculate that cbs might be important for S. marcescens survival in the environment rather than within the host. The UMH9 cbs locus encodes proteins that share close amino acid similarity to those encoded by the chrysobactin operon in the plant pathogen Erwinia chrysanthemi (Dickeya dadantii). Chrysobactin, a virulence factor for E. chrysanthemi, is responsible for pathogen dissemination throughout the host plant (37). Therefore, cbs may contribute to pathogenesis in environmental niches and chrysobactin production may be dependent on an unknown environmental cue; however, it does not significantly contribute to pathogenesis within the mammalian host.
Additionally, despite the increased expression of genes in the cbs locus under iron limitation, there is little detectable chrysobactin. In fact, the cbs mutant conferred a growth advantage under some conditions compared to wild-type growth. We do not believe that this growth advantage is related to increased serratiochelin production when the cbs locus is deleted because the mass spectrometry results showed comparable serratiochelin levels in wild-type and cbs mutant supernatants in M9 minimal medium. We expect that this is likely the case in LB medium with dipyridyl as well, although it has not been tested. We hypothesize that synthesis of the enzymes encoded by cbs could be energetically costly, resulting in increased growth of the cbs mutant during iron limitation. Interestingly, chrysobactin synthesis may be biosynthetically dependent on several genes within the sch locus. sch genes BVG96_RS22020, BVG96_RS22025, and BVG96_RS22030 have similarity to chrysobactin biosynthetic genes cbsC, cbsE, and cbsB, respectively. These genes can be found in the D. dadantii chrysobactin operon but are absent in the S. marcescens UMH9 cbs locus. These genes are involved in both serratiochelin and chrysobactin precursor production according to previously published biosynthetic pathways of these molecules (23, 26). In strain UMH9, once these precursor molecules are produced, they are most likely used to produce serratiochelin. These products can also be used for chrysobactin synthesis, but given the small amount of chrysobactin identified in WT UMH9 supernatants, this most likely occurs to a lower extent. Additionally, there are possibly hurdles in efficiently coordinating small-molecule biosynthesis across differentially regulated operons. The low level of chrysobactin production could be explained by this inefficiency. However, further research is needed to explore possible interactions between the sch and cbs loci.
Gram-negative pathogens can utilize siderophores made by other bacteria, a strategy often used in polymicrobial settings (38). S. marcescens possesses a receptor for aerobactin, IutA, but does not produce aerobactin itself. It also has a receptor with similarity to FepA, an enterobactin transporter. Additionally, UMH9 has several orphan hypothetical siderophore receptors outside of the sch and cbs siderophore gene clusters, suggesting an ability to import other potential siderophores that it does not itself produce. Therefore, considering these observations along with S. marcescens being a known opportunist, it is likely that S. marcescens depends on xenosiderophores in polymicrobial infections, especially since it possesses only one siderophore that contributes to pathogenesis, whereas other Gram-negative pathogens typically have two or more (13, 14). Interestingly, it is unknown whether other Gram-negative pathogens can utilize serratiochelin or chrysobactin.
S. marcescens also acquires iron from heme using two well-known systems, Hem and Has. Given the high concentration of heme in the bloodstream, we hypothesize that these systems also play a role in iron acquisition during bacteremia. Previous literature has highlighted the redundancies between the major iron acquisition systems in other pathogens, which may explain why we see little to no decrease in colonization in the mouse spleen and liver when sch is deleted (13). In these environments, iron acquired from heme may be the dominant iron source, whereas in the kidney, siderophores play a larger role. S. marcescens also possesses a ferric citrate transport system that was upregulated during iron-limited RNA-seq experiments. Citrate is capable of binding and importing iron; however, its affinity for iron is weak compared to that of siderophores (34). Nonetheless, citrate has been shown to act as a siderophore and may play a redundant role in iron acquisition along with serratiochelin, chrysobactin, and heme uptake (39, 40). To fully understand iron import during bacteremia, future studies evaluating the contribution of the heme uptake systems and ferric citrate import should be performed. Additionally, it is possible that heme uptake can compensate for siderophores and vice versa; therefore, a mutant in which all siderophore systems, as well as heme systems, are deleted would be an interesting tool to evaluate the contribution of iron acquisition irrespective of specific systems.
We recognize that this study examined one clinical isolate of Serratia marcescens and that additional isolates could possess additional iron acquisition systems. Our lab has sequenced the genomes of nine clinical S. marcescens strains isolated from humans with bacteremia. Genome comparison studies show that other S. marcescens isolates also possess the cbs and sch loci and have a high amino acid identity to the operons found in UMH9. Therefore, our results may be considered broadly applicable to the virulence of bacteremic strains of S. marcescens.
In summary, we have defined the iron-regulated transcriptome of S. marcescens UMH9 to identify the full repertoire of iron acquisition systems and components within this important opportunistic pathogen. We characterized the expression of two well-conserved putative siderophore systems (sch and cbs), identified the molecular products synthesized, and implicated the sch locus product, serratiochelin A, as contributing to the pathogenesis in the murine bloodstream. However, the cbs locus has a yet-undetermined role in pathogenesis and does not appear to contribute to S. marcescens UMH9 virulence in mice. These findings clearly show that the sch product, serratiochelin, is a bona fide virulence factor during bacteremia.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. marcescens strains and plasmids used in this study are listed in Table 2. Bacteria were routinely cultured in lysogeny broth (LB) medium (10 g/liter of tryptone, 5 g/liter of yeast extract, 0.5 g/liter of NaCl). The following antibiotics were added to cultures when appropriate: kanamycin (25 μg/ml), chloramphenicol (30 μg/ml), and spectinomycin (100 μg/ml). 2,2′-Dipyridyl (Sigma-Aldrich), an iron chelator, was added to LB cultures at the desired concentrations to achieve iron limitation. M9 defined medium (0.1 mM CaCl2, 1 mM MgSO4, 0.4% glucose) was used to culture bacteria for mass spectrometry and chrome azurol S (CAS) experiments (41). M9 was treated with Chelex 100 to remove iron, creating iron-limited conditions. FeSO4 (36 μM) was added back to M9 cultures where indicated.
TABLE 2.
S. marcescens strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Description | Reference or source |
|---|---|---|---|
| S. marcescens strains | |||
| UMH9 | Wild type | Bloodstream infection isolate | 3 |
| Siderophore 1 (cbs) mutant | cbs::kan (Δcbs) | Full operon deletion from gene BVG96_RS08795 to BVG96_RS08815, replaced with kanamycin resistance cassette from pKD4 | This study |
| Siderophore 2 (sch) mutant | sch::kan (Δsch) | Full operon deletion from gene BVG96_RS21965 to BVG96_RS22035, replaced with kanamycin resistance cassette from pKD4 | This study |
| Siderophore 1 and siderophore 2 (cbs/sch) mutant | cbs::cam, sch::kan (Δcbs/sch) | Double mutant with both full operon deletions replaced with the indicated antibiotic resistance cassette | This study |
| Plasmids | |||
| pKD4 | Source of kanamycin resistance gene | 44 | |
| pKD3 | Source of chloramphenicol resistance gene | 44 | |
| pSIM19 | Recombineering plasmid | 45 |
RNA purification and sequencing.
S. marcescens strain UMH9 was cultured overnight at 37°C in LB medium. The following day, bacteria were collected by centrifugation and washed three times with LB medium containing 200 μM dipyridyl. Washed bacteria were diluted 1:100 into either LB medium or LB medium plus 200 μM dipyridyl and incubated at 30°C with shaking until an optical density at 600 nm (OD600) between 0.4 and 0.5 was reached. RNA was stabilized using RNAprotect (Qiagen). Bacteria were lysed by incubation for 10 min at room temperature with 15 mg/ml of lysozyme (Sigma-Aldrich) and 20 mg/ml of proteinase K (Qiagen). The RNAeasy minikit (Qiagen) was used to purify RNA according to the directions of the manufacturer. Turbo DNase (Thermo Fisher) was used to deplete genomic DNA contamination. RNA was repurified using the RNEasy minikit (Qiagen). rRNA removal, library preparation, and sequencing were performed by the University of Michigan Sequencing Core using the ScriptSeq Complete kit (bacteria) library kit (Illumina). cDNA libraries were sequenced using Illumina HiSeq 2500 (single-end, 50-bp read length). Detailed methods outlining the RNA-seq analysis can be found in the supplemental material.
Construction of UMH9 mutants.
Single and double mutations of the cbs and sch loci were constructed using the lambda red recombinase system (42). PCR amplification of the kanamycin and chloramphenicol resistance cassettes from pKD4 and pKD3, respectively, was performed using primers with ∼50 bp of homology at the 5′ end to the siderophore locus and ∼20 bp of homology to pKD3/4 at the 3′ end of each primer. Primers for mutant construction are listed in Table 3. PCR products were digested with DpnI (New England BioLabs) and then transformed into UMH9 cells expressing lambda red recombinase from pSIM19 after heat treatment for 20 min at 42°C. pSIM19 was removed from the mutant strains as described previously (42). Mutants were confirmed by PCR and Sanger sequencing (Table 3).
TABLE 3.
Primers for mutant constructiona
| Locus | Forward primer | Reverse primer |
|---|---|---|
| Siderophore 1 (cbs) | CGCCGCTGAGGTTGCCGGCGCGGGCTCATCGTGCTCGACCAAGGCGTTCGGTGTAGGCTGGAGCTGCTTC | TGACCGAAGCGATGATGCTGCGGCCGCTGCCGTAGAAACAGGCGTACTCGATGGGAATTAGCCATGGTCC |
| Siderophore 2 (sch) | CGCTTTGGCCTCTGAATGGTTGCCGCTGGCGCCAGCGCAGCTTGATTTCTGTGTAGGCTGGAGCTGCTTC | GCGGATCACGTCGTCCGGCGCGGTGGCTTCTTTCTCGCGGCTGGAAGCCTATGGGAATTAGCCATGGTCC |
Primer sequences are listed 5′ to 3′. Underlined sequences in mutant construction primers are identical to regions at the 5′ to 3′ ends of the locus to be disrupted.
Growth of S. marcescens strains.
Bacterial strains were cultured with aeration overnight in LB medium at 37°C. The following day, bacteria were collected by centrifugation and washed twice with LB medium containing 250 μM dipyridyl. Bacteria were suspended in LB plus 250 μM dipyridyl, and 30 μl was used to inoculate 3 ml of LB plus 250 μM dipyridyl for overnight cultures. A sample of culture (3 μl) was used to inoculate each well of a 100-well growth plate containing 300 μl of LB medium with dipyridyl to an OD600 of 0.01. Growth was also measured in 100% heat-inactivated human serum. Bacteria were cultured and loaded into the serum-containing growth plate as described above. Growth curves were performed in biological and technical triplicates. Plates were incubated at 37°C with continuous shaking, and the OD600 was measured every 15 min for 21 h using a BioScreenC plate reader. For technical reasons, blank serum measurements were not subtracted from growth measurements, accounting for the higher starting OD600.
Murine model of bacteremia.
Overnight cultures of WT and mutant constructs were harvested by centrifugation (3,500 × g for 30 min at 4°C) and resuspended in phosphate-buffered saline (PBS) to a density of 5 × 108 CFU/ml. Four groups of 10 female CBA/J mice (Jackson 000656) aged 6 to 8 weeks were independently inoculated with either UMH9 WT, Δcbs, Δsch, or Δcbs/sch constructs. An inoculum (100 μl) of 5 × 108 CFU/ml was injected into the tail vein. Infection was allowed to progress for 24 h or until mice appeared moribund. Mice were euthanized by anesthetic overdose. Spleen, kidneys, and liver were collected and homogenized in PBS. Homogenates were diluted and plated onto LB agar or LB agar containing the appropriate antibiotics using an Autoplate 4000 spiral plater (Spiral Biotech) and incubated overnight at 37°C. Bacterial colonies were counted the following day using a QCount (Chemopharm). For cochallenge experiments, the following modifications were made. Each mutant strain was combined in a 1:1 mixture with the UMH9 WT strain at 5 × 108 CFU/ml. Competitive indices were calculated using the following equation: log10 [(CFU of mutant/CFU of wild type) output/(CFU of mutant/CFU of wild type) input]. All animal experiments were conducted using protocols approved by the Institutional Animal Care and Use Committee.
Extraction and sample preparation for mass spectrometry.
S. marcescens UMH9 WT and mutant constructs were cultured overnight in LB medium with the appropriate antibiotics. The following day, cultures were centrifuged and pellets were washed twice in M9 minimal medium. Cultures were diluted 1:100 into M9 minimal medium with and without supplemented FeSO4 (36 μM) and then cultured overnight. Bacterial pellets and supernatants were collected by centrifugation (5,000 × g, 7 min, and 4°C) the next day and then delivered to the University of Michigan Natural Products Discovery Core. Detailed methods outlining the mass spectrometry protocol and analysis can be found in the supplemental material.
Detection of siderophore production.
Bacteria were cultured overnight in LB medium with aeration at 37°C and harvested by centrifugation the next day. Bacterial pellets were washed twice with M9 minimal medium and then diluted 1:100 into M9 minimal medium for overnight cultures with and without 36 μM FeSO4. The following day, cultures were adjusted to an OD600 of 1.5 and then centrifuged (5,000 × g, 7 min, and 4°C). Supernatants were removed. One hundred microliters of chrome azurol S (CAS) shuttle solution was added to 100 μl of siderophore-containing supernatant (43). EDTA (10 μM) was used as a positive control. The CAS-supernatant mixture was allowed to develop for 20 min at room temperature. A visible color change from blue to red indicates siderophore production. This color change was measured using a MicroQuant spectrophotometer (Bio-Tek) at an absorbance of 630 nm. Samples were measured in biological and technical triplicates. Final absorbance was calculated by dividing the average absorbance of triplicate samples by the absorbance of the M9 solution alone. Standard errors of the means were calculated. Statistical significance was determined using an unpaired t test in Prism v.14.
Data availability.
All RNA-seq data in this publication have been deposited in NCBI’s Gene Expression Omnibus repository under accession no. GSE145587.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI134731 from the National Institutes of Health.
We acknowledge support from the Bioinformatics Core of the University of Michigan Medical School’s Biomedical Research Core Facilities.
We thank Christopher Alteri for his intellectual contributions to the work presented here.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Mahlen SD. 2011. Serratia infections: from military experiments to current practice. Clin Microbiol Rev 24:755–791. doi: 10.1128/CMR.00017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hines DA, Saurugger PN, Ihler GM, Benedik MJ. 1988. Genetic analysis of extracellular proteins of Serratia marcescens. J Bacteriol 170:4141–4146. doi: 10.1128/jb.170.9.4141-4146.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson MT, Mitchell LA, Zhao L, Mobley H. 2017. Capsule production and glucose metabolism dictate fitness during Serratia marcescens bacteremia. mBio 8:e00740-17. doi: 10.1128/mBio.00740-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratledge C, Dover LG. 2000. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol 54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- 5.Iron Disorders Institute. 2009. How much iron is in the body. http://www.irondisorders.org/how-much-iron-is-in-the-body/. Accessed 5 February 2020.
- 6.Crichton RR. 2001. Inorganic biochemistry of iron metabolism: from molecular mechanisms to clinical consequences. John Wiley & Sons, New York, NY. [Google Scholar]
- 7.Faraldo-Gómez JD, Sansom MSP. 2003. Acquisition of siderophores in gram-negative bacteria. Nat Rev Mol Cell Biol 4:105–116. doi: 10.1038/nrm1015. [DOI] [PubMed] [Google Scholar]
- 8.Ward DM, Kaplan J. 2012. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta 1823:1426–1433. doi: 10.1016/j.bbamcr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raymond KN, Dertz EA, Kim SS. 2003. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci U S A 100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wandersman C, Stojiljkovic I. 2000. Bacterial heme sources: the role of heme, hemoprotein receptors and hemophores. Curr Opin Microbiol 3:215–220. doi: 10.1016/s1369-5274(00)00078-3. [DOI] [PubMed] [Google Scholar]
- 11.Carrano CJ, Raymond KN. 1979. Ferric ion sequestering agents. 2. Kinetics and mechanism of iron removal from transferrin by enterobactin and synthetic tricatechols. J Am Chem Soc 101:5401–5404. doi: 10.1021/ja00512a047. [DOI] [Google Scholar]
- 12.Robinson AE, Heffernan JR, Henderson JP. 2018. The iron hand of uropathogenic Escherichia coli: the role of transition metal control in virulence. Future Microbiol 13:745–756. doi: 10.2217/fmb-2017-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia EC, Brumbaugh AR, Mobley HL. 2011. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect Immun 79:1225–1235. doi: 10.1128/IAI.01222-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choby JE, Howard-Anderson J, Weiss DS. 2020. Hypervirulent Klebsiella pneumoniae—clinical and molecular perspectives. J Intern Med 287:283–300. doi: 10.1111/joim.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holden VI, Breen P, Houle S, Dozois CM, Bachman MA. 2016. Klebsiella pneumoniae siderophores induce inflammation, bacterial dissemination, and HIF-1alpha stabilization during pneumonia. mBio 7:e01397-16. doi: 10.1128/mBio.01397-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benevides-Matos N, Biville F. 2010. The Hem and Has haem uptake systems in Serratia marcescens. Microbiology 156:1749–1757. doi: 10.1099/mic.0.034405-0. [DOI] [PubMed] [Google Scholar]
- 17.Letoffe S, Delepelaire P, Wandersman C. 2008. Functional differences between heme permeases: Serratia marcescens HemTUV permease exhibits a narrower substrate specificity (restricted to heme) than the Escherichia coli DppABCDF peptide-heme permease. J Bacteriol 190:1866–1870. doi: 10.1128/JB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Letoffe S, Ghigo JM, Wandersman C. 1994. Iron acquisition from heme and hemoglobin by a Serratia marcescens extracellular protein. Proc Natl Acad Sci U S A 91:9876–9880. doi: 10.1073/pnas.91.21.9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wandersman C, Delepelaire P. 2012. Haemophore functions revisited. Mol Microbiol 85:618–631. doi: 10.1111/j.1365-2958.2012.08136.x. [DOI] [PubMed] [Google Scholar]
- 20.Kurz CL, Chauvet S, Andres E, Aurouze M, Vallet I, Michel GP, Uh M, Celli J, Filloux A, De Bentzmann S, Steinmetz I, Hoffmann JA, Finlay BB, Gorvel JP, Ferrandon D, Ewbank JJ. 2003. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J 22:1451–1460. doi: 10.1093/emboj/cdg159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbonell GV, Vidotto MC. 1992. Virulence factors in Serratia marcescens: cell-bound hemolysin and aerobactin. Braz J Med Biol Res 25:1–8. [PubMed] [Google Scholar]
- 22.Angerer A, Klupp B, Braun V. 1992. Iron transport systems of Serratia marcescens. J Bacteriol 174:1378–1387. doi: 10.1128/jb.174.4.1378-1387.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seyedsayamdost MR, Cleto S, Carr G, Vlamakis H, Joao Vieira M, Kolter R, Clardy J. 2012. Mixing and matching siderophore clusters: structure and biosynthesis of serratiochelins from Serratia sp. V4. J Am Chem Soc 134:13550–13553. doi: 10.1021/ja304941d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehlert G, Taraz K, Budzikiewicz H. 1994. Serratiochelin, a new catecholate siderophore from Serratia marcescens. Z Naturforsch C 49:11–17. doi: 10.1515/znc-1994-1-203. [DOI] [Google Scholar]
- 25.Khilyas IV, Tursunov KA, Shirshikova TV, Kamaletdinova LK, Matrosova LE, Desai PT, McClelland M, Bogomolnaya LM. 2019. Genome sequence of pigmented siderophore-producing strain Serratia marcescens SM6. Microbiol Resour Announc 8:e00247-19. doi: 10.1128/MRA.00247-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reitz ZL, Sandy M, Butler A. 2017. Biosynthetic considerations of triscatechol siderophores framed on serine and threonine macrolactone scaffolds. Metallomics 9:824–839. doi: 10.1039/c7mt00111h. [DOI] [PubMed] [Google Scholar]
- 27.Crosa JH, Walsh CT. 2002. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev 66:223–249. doi: 10.1128/mmbr.66.2.223-249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, Nguyen DD, Watrous J, Kapono CA, Luzzatto-Knaan T, Porto C, Bouslimani A, Melnik AV, Meehan MJ, Liu W-T, Crüsemann M, Boudreau PD, Esquenazi E, Sandoval-Calderón M, Kersten RD, Pace LA, Quinn RA, Duncan KR, Hsu C-C, Floros DJ, Gavilan RG, Kleigrewe K, Northen T, Dutton RJ, Parrot D, Carlson EE, Aigle B, Michelsen CF, Jelsbak L, Sohlenkamp C, Pevzner P, Edlund A, McLean J, Piel J, Murphy BT, Gerwick L, Liaw C-C, Yang Y-L, Humpf H-U, Maansson M, Keyzers RA, Sims AC, Johnson AR, Sidebottom AM, Sedio BE, Klitgaard A, Larson CB, P CAB, Torres-Mendoza D, Gonzalez DJ, Silva DB, Marques LM, Demarque DP, Pociute E, O'Neill EC, Briand E, Helfrich EJN, Granatosky EA, Glukhov E, Ryffel F, Houson H, Mohimani H, Kharbush JJ, Zeng Y, Vorholt JA, Kurita KL, Charusanti P, McPhail KL, Nielsen KF, Vuong L, Elfeki M, Traxler MF, Engene N, Koyama N, Vining OB, Baric R, Silva RR, Mascuch SJ, Tomasi S, Jenkins S, Macherla V, Hoffman T, Agarwal V, Williams PG, Dai J, Neupane R, Gurr J, Rodríguez AMC, Lamsa A, Zhang C, Dorrestein K, Duggan BM, Almaliti J, Allard P-M, Phapale P, Nothias L-F, Alexandrov T, Litaudon M, Wolfender J-L, Kyle JE, Metz TO, Peryea T, Nguyen D-T, VanLeer D, Shinn P, Jadhav A, Müller R, Waters KM, Shi W, Liu X, Zhang L, Knight R, Jensen PR, Palsson BO, Pogliano K, Linington RG, Gutiérrez M, Lopes NP, Gerwick WH, Moore BS, Dorrestein PC, Bandeira N. 2016. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat Biotechnol 34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohimani H, Gurevich A, Shlemov A, Mikheenko A, Korobeynikov A, Cao L, Shcherbin E, Nothias LF, Dorrestein PC, Pevzner PA. 2018. Dereplication of microbial metabolites through database search of mass spectra. Nat Commun 9:4035. doi: 10.1038/s41467-018-06082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haurowitz F, Koshland D. Protein: Proteins in the blood serum, Encyclopaedia Britannica, Encyclopaedia Britannica, Inc. https://www.britannica.com/science/protein/proteins-of-the-blood-serum.
- 31.Smith SN, Hagan EC, Lane MC, Mobley HL. 2010. Dissemination and systemic colonization of uropathogenic Escherichia coli in a murine model of bacteremia. mBio 1:e00262-10. doi: 10.1128/mBio.00262-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moradigaravand D, Boinett CJ, Martin V, Peacock SJ, Parkhill J. 2016. Recent independent emergence of multiple multidrug-resistant Serratia marcescens clones within the United Kingdom and Ireland. Genome Res 26:1101–1109. doi: 10.1101/gr.205245.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wattam AR, Davis JJ, Assaf R, Boisvert S, Brettin T, Bun C, Conrad N, Dietrich EM, Disz T, Gabbard JL, Gerdes S, Henry CS, Kenyon RW, Machi D, Mao C, Nordberg EK, Olsen GJ, Murphy-Olson DE, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Vonstein V, Warren A, Xia F, Yoo H, Stevens RL. 2017. Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res 45:D535–D542. doi: 10.1093/nar/gkw1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miethke M, Marahiel MA. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saha R, Saha N, Donofrio RS, Bestervelt LL. 2013. Microbial siderophores: a mini review. J Basic Microbiol 53:303–317. doi: 10.1002/jobm.201100552. [DOI] [PubMed] [Google Scholar]
- 36.Bullen JJ, Griffiths E. 1999. Iron and infection: molecular, physiological, and clinical aspects. John Wiley and Sons, New York, NY. [Google Scholar]
- 37.Rauscher L, Expert D, Matzanke BF, Trautwein AX. 2002. Chrysobactin-dependent iron acquisition in Erwinia chrysanthemi. Functional study of a homolog of the Escherichia coli ferric enterobactin esterase. J Biol Chem 277:2385–2395. doi: 10.1074/jbc.M107530200. [DOI] [PubMed] [Google Scholar]
- 38.Harrison F, McNally A, da Silva AC, Heeb S, Diggle SP. 2017. Optimised chronic infection models demonstrate that siderophore ‘cheating’ in Pseudomonas aeruginosa is context specific. ISME J 11:2492–2509. doi: 10.1038/ismej.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parrow NL, Fleming RE, Minnick MF. 2013. Sequestration and scavenging of iron in infection. Infect Immun 81:3503–3514. doi: 10.1128/IAI.00602-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagegg W, Braun V. 1981. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein FecA. J Bacteriol 145:156–163. doi: 10.1128/JB.145.1.156-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 42.Thomason LC, Sawitzke JA, Li X, Costantino N, Court DL. 2014. Recombineering: genetic engineering in bacteria using homologous recombination. Curr Protoc Mol Biol 106:1.16.1–1.16.39. doi: 10.1002/0471142727.mb0116s106. [DOI] [PubMed] [Google Scholar]
- 43.Schwyn B, Neilands JB. 1987. Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 44.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Datta S, Costantino N, Court DL. 2006. A set of recombineering plasmids for gram-negative bacteria. Gene 379:109–115. doi: 10.1016/j.gene.2006.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All RNA-seq data in this publication have been deposited in NCBI’s Gene Expression Omnibus repository under accession no. GSE145587.