Staphylococcus aureus fatty acid kinase FakA is necessary for the incorporation of exogenous fatty acids into the lipid membrane. We previously demonstrated that the inactivation of fakA leads to decreased α-hemolysin (Hla) production but increased expression of the proteases SspAB and aureolysin in vitro, and that the ΔfakA mutant causes larger lesions than the wild type (WT) during murine skin infection. As expected, necrosis is Hla dependent in the presence or absence of FakA, as both hla and hla ΔfakA mutants are unable to cause necrosis of the skin.
KEYWORDS: FakA, Hla, MRSA, Staphylococcus aureus, immune response, protease
ABSTRACT
Staphylococcus aureus fatty acid kinase FakA is necessary for the incorporation of exogenous fatty acids into the lipid membrane. We previously demonstrated that the inactivation of fakA leads to decreased α-hemolysin (Hla) production but increased expression of the proteases SspAB and aureolysin in vitro, and that the ΔfakA mutant causes larger lesions than the wild type (WT) during murine skin infection. As expected, necrosis is Hla dependent in the presence or absence of FakA, as both hla and hla ΔfakA mutants are unable to cause necrosis of the skin. At day 4 postinfection, while the ΔfakA mutant maintains larger and more necrotic abscesses, bacterial numbers are similar to those of the WT, indicating the enhanced tissue damage of mice infected with the ΔfakA mutant is not due to an increase in bacterial burden. At this early stage of infection, skin infected with the ΔfakA mutant has decreased levels of proinflammatory cytokines, such as interleukin-17A (IL-17A) and IL-1α, compared to those of WT-infected skin. At a later stage of infection (day 7), abscess resolution and bacterial clearance are hindered in ΔfakA mutant-infected mice. The paradoxical findings of decreased Hla in vitro but increased necrosis in vivo led us to investigate the role of the proteases regulated by FakA. Utilizing Δaur and ΔsspAB mutants in both the WT and fakA mutant backgrounds, we found that the absence of these proteases in a fakA mutant reduced dermonecrosis to levels similar to those of the WT strain. These studies suggest that the overproduction of proteases is one factor contributing to the enhanced pathogenesis of the ΔfakA mutant during skin infection.
INTRODUCTION
From 1999 to 2009, the incidence of Staphylococcus aureus infections increased, as did the percentage of hospitalizations resulting from skin and soft-tissue infections (SSTIs) (1, 2). More recently, a report analyzing S. aureus bloodstream infections from 2012 to 2017 revealed that while progress has been made toward decreasing these infections, progress toward reducing community-associated (CA) methicillin-resistant Staphylococcus aureus (CA-MRSA) cases has slowed but CA methicillin-susceptible S. aureus (CA-MSSA) infections have increased (3). Considering these trends and the rise in antibiotic-resistant strains, S. aureus remains a global health threat, and it is now more important than ever to understand the underlying mechanisms of S. aureus infections. Our goal through these studies is to better understand how the host and S. aureus interact to cause disease.
Staphylococcus aureus is the most common cause of SSTIs (4), which are also the most common type of S. aureus infection (5). During an SSTI, there is a complex interplay between the host and S. aureus. The bacterium secretes virulence factors to establish infection, and the host immune system responds with a combination of innate and adaptive immune factors. One key component for combating S. aureus skin infections is the production of interleukin-17A/F (IL-17A/F) by subsets of T cells, including γδ T cells (6, 7). Pattern recognition receptors (PRRs), such as TLR2 (8–10), TLR9 (11), and NOD2 (9, 12), recognize S. aureus and promote an effective immune response by stimulating the release of several cytokines and chemokines. Cytokines such as IL-17 (6), IL-1α, and IL-1β (13) have been shown to be important players in the clearance of S. aureus skin infections due to their ability to recruit neutrophils, which are necessary for eradicating S. aureus (14). To combat the potent defense of the immune system, S. aureus produces a myriad of virulence factors, including a variety of lytic toxins, such as the leukocidins, hemolysins, and phenol-soluble modulins (PSMs). In particular, α-hemolysin (Hla) is a crucial toxin for the generation of surface-exposed necrotic lesions and enhanced bacterial titers in the skin (15–17). This pathogenesis revolves around the concentration-dependent ability of Hla to activate the proteinase ADAM10 and the NLRP3 inflammasome and to lyse a multitude of cell types, culminating in tissue destruction and dermonecrosis (18–21). Additionally, S. aureus produces several proteases that can degrade host proteins, such as complement protein C3 (22), proteins contributing to epidermal cell junctions (23), and all classes of human immunoglobulins (24), as well as modulate levels of members of the S. aureus extracellular proteome (25). Importantly, these proteases, like Hla, also play a role in S. aureus SSTI (25).
In a previous report, we identified the S. aureus fatty acid kinase A (FakA; formerly known as VfrB) as a contributor to virulence factor regulation. FakA, along with FakB1 and FakB2, compose a system that activates exogenous fatty acids (exoFAs) so that they can be used for lipid generation. In the absence of FakA, S. aureus cannot utilize exoFAs and has increased resistance to unsaturated fatty acids (26, 27) and an altered metabolism (28). In the initial report of FakA, we identified the altered regulation of key virulence factors in the ΔfakA mutant and demonstrated that the ΔfakA mutant is more virulent than wild-type (WT) S. aureus in a mouse model of SSTI (29). The mechanism by which FakA alters virulence factor production was later clarified by identifying that FakA positively regulates the activity of the SaeRS two-component system (30, 31). Specifically, the kinetic expression of Hla is altered in the ΔfakA mutant (30), and there is increased production of the staphylococcal serine protease A (SspA or V8), SspB, and aureolysin (Aur) proteases (29). While Hla is known to lyse cells and disrupt barriers by facilitating the cleavage of cell junction proteins, both SspA and SspB proteases have also been shown in vitro to degrade cell junctions between keratinocytes without causing keratinocyte cell damage (32, 33). Additionally, SspA and SspB can degrade other components of the host’s extracellular matrix, which may contribute to invasive S. aureus infections (34, 35). Furthermore, others have shown that skin infections formed by ΔsspA and ΔsspB mutants have decreased numbers of CFU compared to those of skin abscesses formed by WT S. aureus (36), suggesting the importance of SspA and SspB in limiting bacterial clearance. However, whether the dysregulation of Hla or proteases contributes to the enhanced virulence of the ΔfakA mutant in vivo remains unknown.
We hypothesized that in the absence of FakA, the expression and/or function of several S. aureus virulence factors would be altered, leading to the increased virulence displayed by the ΔfakA mutant in vivo. In this study, we found that Hla, as expected, is necessary for both the S. aureus WT and ΔfakA mutant to form necrotic surface lesions in a mouse model of SSTI. Compared to SSTI caused by WT S. aureus, infection with the ΔfakA mutant causes decreased local levels of IL-17A and IL-1α at day 4 postinoculation (p.i.) but an increased abundance of other proinflammatory cytokines at day 7 p.i. Importantly, our in vivo studies suggest that S. aureus proteases are involved in promoting the hypervirulent nature of the ΔfakA mutant. Therefore, we postulate that enhanced protease production is a contributing factor that allows the ΔfakA mutant to cause increased pathogenesis in a murine model of S. aureus SSTI.
RESULTS
ΔfakA mutant is hypervirulent through resolution of infection.
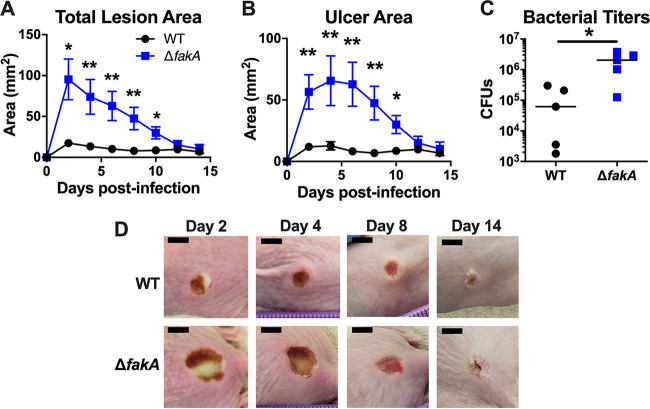
Previously, we showed that the ΔfakA mutant causes larger and more necrotic skin infections than WT S. aureus for up to 3 days postinfection (p.i.), despite a similar bacterial burden (29). To expand our studies, we tested whether this phenotype persists throughout the course of infection. We infected outbred SKH1 mice with either WT or ΔfakA mutant S. aureus and monitored the infection for fourteen days. As expected, the WT S. aureus-infected mice developed a blanched lesion (total lesion area) on day 1 p.i. that became necrotic (ulcer area) and reached maximal size by day 4 p.i. (Fig. 1); we deem this the peak of infection. As is typical of these infections, the ulcer dominated the lesion area between days 2 and 4 p.i. Mice infected with the ΔfakA mutant maintained larger total lesion and ulcer areas, which did not resolve to WT levels until day 12 (Fig. 1). Despite similar time to lesion resolution, mice infected with the ΔfakA mutant had higher local bacterial titers at day 14 p.i. (Fig. 1C). These data demonstrate that SSTIs caused by the S. aureus ΔfakA mutant show increased virulence compared to that of WT infection, observed as significantly larger necrotic lesions 2 to 10 days p.i. with increased bacterial burden at the time of lesion resolution (day 14 p.i.), suggesting that FakA plays a role in mitigating WT infections. We hypothesize that this is due to altered regulation of virulence factors, such as Hla and proteases in ΔfakA mutant infection.
FIG 1.
ΔfakA mutant maintains enhanced virulence throughout infection. Mice were infected with 2 × 107 CFU of WT or ΔfakA mutant S. aureus. Total lesion (A) and ulcer (B) areas were measured every other day. (C) On day 14 postinfection (p.i.), mice were euthanized and local bacterial titers (CFU) were determined. (D) Representative images of WT or ΔfakA mutant infection sites on days 2, 4, 8, and 14 p.i. Scale bars, 5 mm. *, P < 0.05; **, P < 0.01 by Mann-Whitney test. Data shown are means ± standard errors of the means (SEM) (n = 5) for panels A and B. The bars in panel C represents the medians.
ΔfakA mutant infections maintain higher bacterial titers at resolution initiation.
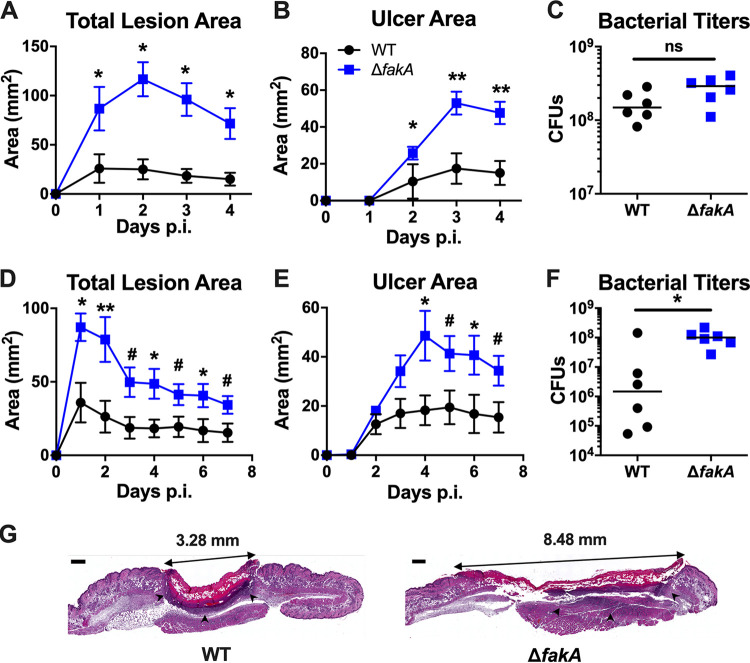
Because we observed that necrosis peaked at ∼4 days p.i. in both WT S. aureus- and ΔfakA mutant-infected mice with the initiation of resolution at day 7 p.i., we selected these two time points for more detailed analysis. As expected, the ΔfakA mutant-infected mice had larger total lesions (Fig. 2A and D) and ulcers (Fig. 2B and E) than those infected with WT S. aureus. Consistent with our previous study (29), at 3 days p.i., there was no statistical difference between WT and ΔfakA mutant bacterial titers at 4 days p.i. (Fig. 2C), again demonstrating that the increase in ulcer area during ΔfakA mutant infections is not due to increased bacterial numbers. In contrast, at 7 days p.i., the ΔfakA mutant-infected mice still had bacterial burdens similar to day 4 levels, while the WT had a 4-log variance in numbers of CFU, indicating varied clearance in each animal (Fig. 2F). This suggests that in contrast to the ΔfakA mutant, by day 7 p.i., WT S. aureus infections are beginning to be cleared. The finding that these relative differences between the WT and ΔfakA mutant in bacterial burden are also seen at day 14 p.i. (Fig. 1C) suggests a decreased ability of the host to clear the ΔfakA mutant infection.
FIG 2.
ΔfakA mutant infections maintain higher bacterial titers at point of resolution initiation. Mice were infected with 1 × 107 to 3 × 107 CFU of WT or ΔfakA mutant S. aureus. Total lesion and ulcer areas were measured through day 4 (A and B) or day 7 (D and E) p.i. The numbers of CFU were determined on day 4 (C) or day 7 (F) p.i. (G) H&E staining of infection site skin on day 4 p.i. (scale bar, 0.5 mm; arrowheads indicate the neutrophilic boundary). *, P < 0.05; **, P < 0.01; #, P = 0.06; ns, not significant; all by Mann-Whitney test. Data shown are means ± SEM (n = 6) for panels A, B, D, and E. The bars in panels C and F represent the medians.
To visualize total lesion architecture, beyond the two-dimensional surface area, we performed hematoxylin and eosin (H&E) staining of day 4 p.i. skin sections. As shown in Fig. 2G, the WT-infected mouse lesion (left) is beginning to contract, a sign of healing initiation (37), as seen by the concave shape of the lesion. In contrast, the ΔfakA mutant-infected lesion (right) remains elongated with no sign of contraction. Additionally, compared to the well-defined neutrophilic abscess boundary (indicated by arrowheads) of the lesion formed by WT S. aureus, the neutrophilic boundary formed by the ΔfakA mutant is less well-defined. Taken together, these data demonstrate that mice infected with the ΔfakA mutant have increased tissue damage despite similar numbers of bacteria through peak infection. Moreover, bacterial clearance and wound resolution are delayed in ΔfakA mutant-infected mice. These results suggest that the enhanced pathogenicity of the ΔfakA mutant over WT S. aureus, despite similar bacterial burdens, results from altered virulence factor expression and/or an altered host immune response.
Infection by the ΔfakA mutant causes altered cytokine expression in the host.
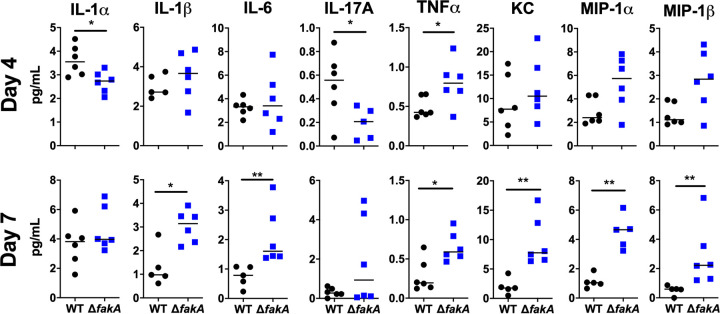
In our initial infections using the ΔfakA mutant, we identified locally increased skin levels of the proinflammatory cytokines IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α) compared to those of WT infections at day 3 p.i. (29). To build upon these studies, we expanded our cytokine analysis with the quantification of key chemokines and cytokines at both 4 and 7 days p.i., time points of peak infection (peak ulcer size) and initiation of the resolution of the lesion, respectively. IL-1α and IL-1β, which are recognized by IL-1R (38), have been shown to play a role in mitigating S. aureus skin infections (13). IL-1α is mainly produced by keratinocytes in the uppermost layer of the skin, whereas IL-1β is made by keratinocytes as well as various leukocytes located deeper within the skin (13, 39). IL-1β is key to combatting S. aureus during a subdermal infection, as performed here (13). While IL-6 has not been studied extensively in the context of S. aureus skin infections, it is important in the defense against other Gram-positive bacteria, such as Streptococcus pneumoniae (40) and Streptococcus pyogenes (41). The level of IL-6 also is elevated in the serum of patients presenting with S. aureus USA300 SSTIs (42). IL-17 is a key cytokine in the clearance of S. aureus during an SSTI (6, 13). IL-17 is produced by several T cell types in the skin and is important for neutrophil recruitment (43). Neutrophils play a vital/critical role in S. aureus clearance. Keratinocytes also produce TNF-α (44), to prime neutrophils to kill the bacteria (45), as well as keratinocyte chemoattractant (KC; also known as CXCL1), to further recruit neutrophils (46). Macrophage inflammatory proteins 1α (MIP-1α) and MIP-1β are chemokines produced by macrophages upon stimulation with several bacterial molecules, including Gram-positive lipoteichoic acid as well as IL-1β (47, 48). These chemokines are important in recruiting other leukocytes and lymphocytes to the site of infection. Additionally, we measured the production of the anti-inflammatory cytokine IL-10.
At peak infection (day 4), the ΔfakA mutant caused significantly increased levels of TNF-α compared to those of WT infection (Fig. 3, top). Additionally, while not statistically significant, the proinflammatory cytokines MIP-1α and MIP-1β trended toward increased abundance in mice infected with the ΔfakA mutant compared to those infected with WT S. aureus. At this same time point, ΔfakA mutant infections had decreased levels of IL-1α and IL-17A (Fig. 3, top), which is significant considering IL-1α leads to the production of IL-17, an important cytokine for clearing S. aureus infections (6). The mechanism behind the decreased production of IL-17A in infections caused by the ΔfakA mutant is unknown and will be a subject of further investigations.
FIG 3.
ΔfakA mutant causes altered cytokine expression in the host. Mice were subcutaneously infected with 1 × 107 to 3 × 107 CFU of WT or ΔfakA mutant S. aureus. Mice were sacrificed 4 or 7 days p.i. Infection site skin was collected and cytokine levels measured in homogenate. Dots indicate an individual mouse (n = 6), whereas bars indicate the median. *, P < 0.05; **, P < 0.01 by Mann-Whitney test. KC, keratinocyte chemoattractant. MIP, macrophage inflammatory protein.
During resolution (day 7), we observed increased levels of several cytokines, including IL-1β, IL-6, TNF-α, KC (CXCL1), MIP-1α, and MIP-1β, in ΔfakA mutant-infected mice (Fig. 3, bottom), with no difference in IL-1α or IL-10 levels (Fig. 3, bottom; see also Fig. S1 in the supplemental material). There was also a trend toward increased LIX (CXCL5) and IL-17A, a reversal of our day 4 p.i. findings (Fig. 3, bottom, and Fig. S1). Together, our data demonstrate that infection by the ΔfakA mutant leads to decreased local levels of IL-17A around the peak of the infection (day 4 p.i.) but an increase of proinflammatory cytokines and chemokines when the infection begins to resolve (day 7 p.i.).
Enhanced lesion formation by the ΔfakA mutant is Hla dependent.
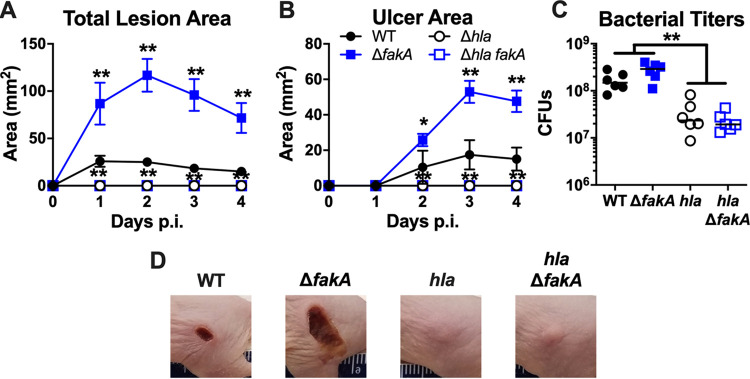
Previous studies have shown that Hla is an important virulence factor in SSTI because it leads to the formation of necrotic tissue in murine models (15, 17, 49). In addition, we have previously reported that the ΔfakA mutant has altered Hla expression in vitro (29, 30). Therefore, given the increased lesion size caused by ΔfakA mutant SSTI in mice, we asked whether Hla contributed to the pathogenesis of the ΔfakA mutant. We infected mice with WT, ΔfakA, hla, or hla ΔfakA mutant strains. As expected, mice inoculated with either strain lacking Hla failed to exhibit skin ulceration (Fig. 4A, B, and D). In agreement with our other data, there was no difference in bacterial burden present at the site of infection between wild-type- and ΔfakA mutant-infected mice (Fig. 4C). However, infection with strains lacking Hla (hla and hla ΔfakA mutant strains) resulted in significantly lower (∼1-log) bacterial burdens than those of their respective parent strains. Clearly, the production of Hla is necessary for surface lesion formation and bacterial survival in infections by both WT S. aureus and the ΔfakA mutant.
FIG 4.
Enhanced lesion formation by ΔfakA mutant is dependent on Hla. Mice were subcutaneously infected with 1 × 107 to 3 × 107 CFU of WT, ΔfakA, hla, or hla ΔfakA mutant S. aureus. Total lesion (A) and ulcer (B) areas were measured daily. (C) Numbers of CFU were measured on day 4 p.i. (D) Representative infection site images taken on day 4 p.i. *, P < 0.05; **, P < 0.01 by Mann-Whitney test. Data shown in panels A and B are means ± SEM (n = 6). Bars in panel C indicate the medians.
Proteases contribute to increased ΔfakA mutant-mediated skin lesion formation.
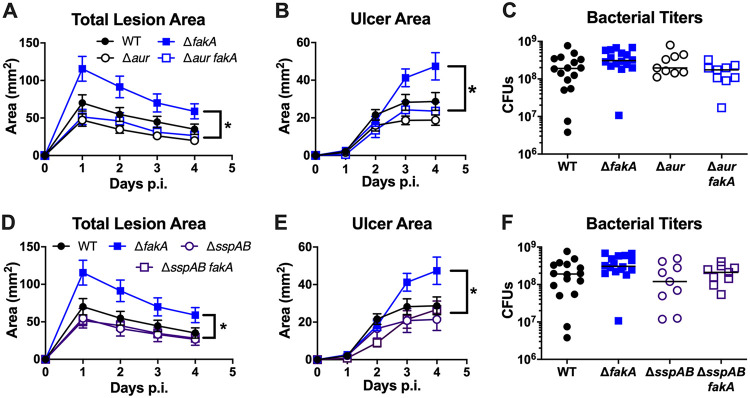
We have previously shown that the ΔfakA mutant has increased protease expression and activity in vitro (29). Specifically, increased transcription of aur and sspABC by the ΔfakA mutant corresponds to increased protease activity, as measured by β-casein zymography. Aureolysin (Aur) is at the top of the S. aureus extracellular protease cascade (50). Pro-aureolysin self-cleaves to produce active aureolysin, which in turn cleaves pro-SspA, creating the serine protease SspA (50, 51). SspA will then go on to cleave pro-SspB, forming the cysteine protease SspB (33). It was previously shown that a 7-day skin infection with a mutant lacking SspA and SspB resulted in reduced bacterial burden compared to that of WT infection, and the mutant lacking Aur also had decreased virulence compared to that of the WT (although the difference was not significant) (36). Therefore, it was possible that the overproduction of proteases Aur, SspA, and SspB could enhance the virulence of the ΔfakA mutant. To elucidate the role proteases play during a subcutaneous infection, we infected mice with single protease mutants or those mutations in combination with fakA. While measures of infection with the Δaur mutant did not differ from WT infection, infection with the Δaur fakA mutant resulted in significantly reduced lesion size and necrosis compared to that of ΔfakA mutant infection (Fig. 5A and B). These findings demonstrate that Aur is necessary for the full virulence potential of the ΔfakA mutant. A slight but nonsignificant decrease in bacterial titer was observed in mice infected with the Δaur fakA mutant compared to that of its ΔfakA mutant parent strain (Fig. 5C). Because Aur is important for the activation of SspA and SspB, the phenotype we observe in the Δaur fakA mutant could be due to inactive SspA and SspB. Therefore, to discern the roles of Aur and SspAB during infection, we infected mice with the ΔsspAB mutant. Again, infection with the ΔsspAB mutant had little impact on total lesion size, ulcer area, or local bacterial burden compared to those of WT S. aureus-infected mice (Fig. 5D to F). However, consistent with S. aureus Δaur fakA mutant infection, mice infected with the ΔsspAB fakA mutant showed reduced overall lesion size and ulcer area compared to that of ΔfakA mutant infection. Consistent with the surface measurements, H&E staining of individual protease mutants did not demonstrate altered lesion formation compared to that of the WT. However, the ΔfakA and protease combination mutants largely resembled the ΔfakA mutant. Thus, while the absence of a specific protease decreases the surface area of lesions, we did not see a marked difference in the subsurface tissue architecture (data not shown). Together, these data demonstrate that the Aur and SspAB proteases all are necessary for increased pathogenesis in the absence of FakA.
FIG 5.
Proteases contribute to increased ΔfakA mutant lesion formation. Mice were subcutaneously infected with 1 × 107 to 3 × 107 CFU of WT, ΔfakA, Δaur, ΔsspAB, fakA Δaur, or fakA ΔsspAB mutant S. aureus. (A to F) Total lesion (A and D) and ulcer (B and E) areas were measured daily. (C and F) Numbers of CFU were measured on day 4 p.i. *, P < 0.05 between fakA and corresponding protease mutant after calculating the area under the curve. Data shown for panels A, B, D, and E are means ± SEM of n = 16 for WT and ΔfakA mutant infections and n = 11 for Δaur, ΔsspAB, fakA Δaur, and fakA ΔsspAB mutant infections. Bars in panels C and F indicate the medians.
DISCUSSION
With S. aureus being the leading cause of SSTIs, it is imperative that we understand the underlying mechanisms of this host-pathogen interaction. We have shown previously that the absence of the fatty acid kinase A (FakA) protein leads to a more virulent infection in a murine SSTI model, although the mechanism has not been elucidated. In this work, we hypothesized that the altered virulence factor expression by the ΔfakA mutant causes differences in tissue destruction and the immune system response to infection compared to that of WT S. aureus. From these experiments, we have discovered that the virulence phenotype of the ΔfakA mutant is observable to 10 days p.i. (Fig. 1), bacterial burden is increased at late time points (Fig. 1C and 2F), host cytokine profiles are altered (Fig. 3), Hla is required (Fig. 4), and proteases play an important role in the formation of lesions in the absence of FakA and contribute to the hypervirulence of the ΔfakA mutant (Fig. 5). These studies have provided new insight into how the ΔfakA mutant causes enhanced tissue damage in the skin (summarized in Fig. 6).
FIG 6.
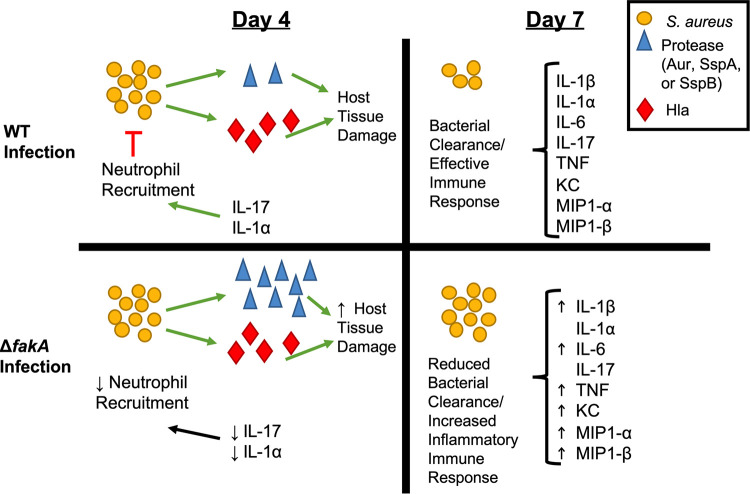
Model of WT and ΔfakA mutant infections at days 4 and 7. During WT infection, proteases and Hla induce host tissue damage. Several S. aureus factors induce production of IL-1α and IL-17, leading to the recruitment of neutrophils, which, over time, leads to an effective clearance of bacteria and an effective immune response. During the ΔfakA mutant infection, the increase in protease production potentially leads to an increase in host tissue damage along with damage caused by Hla. Through an unknown mechanism, the ΔfakA mutant does not induce the production of IL-1α and IL-17, which then does not allow for the recruitment of neutrophils and leads to an altered immune response and decreased ability for the host to effectively clear the bacteria (as seen at days 7 and 14 p.i.). The increased tissue damage would likely also cause an increase in damage-associated molecular patterns (DAMPs), which would then cause an increased immune response (as seen in the increase in proinflammatory cytokines produced at day 7).
As mentioned above, the absence of FakA during S. aureus SSTI results in an altered inflammatory cytokine response prior to differences in bacterial burden, suggesting an impact on the host innate response. At the peak of infection, 4 days p.i., we saw increased lesion size and ulcer formation in mice infected with the ΔfakA mutant compared to those of WT S. aureus. This increased pathology was not due to fitness differences, since bacterial titers were equivalent in mice infected with either strain (Fig. 2C). It is known that IL-1 signaling leads to the production of IL-17, a key cytokine for neutrophil recruitment and bacterial clearance during S. aureus SSTIs (6). Correlating with enhanced pathology, during the initial stage of infection, we observed a decrease in abundance of local IL-1α and IL-17A in mice infected with the ΔfakA mutant versus the WT. This suggests that the increased pathology at this early time point is due to bacterial components rather than immune factors, a possibility that will have to be further explored. At day 4 p.i., the primary cells producing cytokines in the abscesses would likely include innate immune cells, such as neutrophils, macrophages, and Langerhans cells. Keratinocytes could also be producing cytokines, such as IL-1α, that would lead to immune cell recruitment (13, 52). The production of cytokines by keratinocytes could result from cell layer disruption induced by several S. aureus-secreted factors, including Hla and SspAB (32–35, 53). Thus, reduced levels of IL-1α and IL-17A may cause a decrease in neutrophil recruitment, which would reduce the immune response to infection with the ΔfakA mutant. Future studies will determine the mechanism driving altered immune signaling during ΔfakA mutant skin infection.
As the immune response changes over the course of infection, we predicted that tissue damage would correlate with the cytokine response. On day 7 p.i., the beginning of resolution, we observed the continuation of the increased overall and necrotic lesion sizes caused by the ΔfakA mutant (Fig. 2). However, at this time point, we began to see the clearance of the wild-type bacteria but not the ΔfakA mutant. Consistent with the observed enhanced damage in ΔfakA mutant infection at day 7 p.i., there is a significant increase in proinflammatory cytokines IL-1β, IL-6, TNF-α, KC (CXCL1), MIP-1α, and MIP-1β and a trending increase in LIX (CXCL5) and IL-17A (Fig. 3; see also Fig. S1 in the supplemental material). SKH1 mice are immunocompetent but are outbred, and this may account for some of the variability in the results; however, such outbred mouse strains may better represent the human population than inbred mouse strains. We speculate that the early increase (day 4) of IL-17A and IL-1α production in the wild type-infected mice aids in controlling the infection early, leading to more rapid resolution and bacterial clearance. In contrast, ΔfakA mutant-infected mice are delayed in the production of these key cytokines; thus, the infections are less controlled, and this delays resolution and clearance. While it is tempting to speculate which cells are responsible for altered cytokine abundance in response to ΔfakA mutant infections, we plan on investigating this directly in future studies.
Previous studies have confirmed that Hla is required for S. aureus to produce a necrotic lesion in a murine SSTI model (17, 49). Therefore, it was not surprising that we found that Hla is required for the ΔfakA mutant to cause enhanced necrosis during SSTI. We first identified FakA (previously called VfrB) due to reduced hemolytic activity on blood agar and from bacterial supernatant (29). Later, we and others went on to show that FakA is a key contributor to the activation of the SaeRS two-component system, leading to altered kinetic expression of hla at the transcriptional level (30, 31). The results from this study further demonstrate that the production of Hla is essential for skin necrosis and suggest that the requirement for FakA to fully induce Hla production is not as simple as that seen in vitro. This can be explained by two important previous results. First, unlike other Sae-dependent promoters, like Pcoa, in broth culture the ΔfakA mutant produces Hla during the transition from exponential to stationary phase (30). Second, the need for FakA to induce hla expression can be bypassed by high SaeS activity (30). Together, these results suggest that in vivo, Sae activity is adequate for Hla production independent of the need for FakA.
The role proteases play during an S. aureus SSTI is largely unknown. Two groups have identified a slight decrease in bacterial burden during S. aureus SSTI in the absence of protease expression (25, 36). However, one study used a total protease knockout strain (25), and the other study utilized a high-dose, low-virulence strain (36), which could explain our observed lack of bacterial burden reduction. Here, we add to this knowledge by reporting the effect of specific proteases in a clinically relevant strain on skin pathology and extend this to the context of ΔfakA mutant infection. In this study, we show that without the protease Aur or SspAB, the enhanced ΔfakA mutant phenotype is reversed (Fig. 5). The exact reason for this is not clear and is likely multifactorial. Considering these proteases are upregulated in the ΔfakA mutant in vitro, one possibility is that enhanced protease production in vivo affects the levels of Hla. However, at least in vitro, the absence of proteases leads to the increased abundance of secreted virulence factors (25). We have previously shown that the fakA mutant has decreased hemolysis of rabbit red blood cells. However, at least on blood agar plates, this was not due to proteases (29). Therefore, if in vitro conditions translate to in vivo conditions, one can hypothesize that the ΔfakA mutant overproducing proteases would consequently lead to reduced abundance of other virulence proteins during infection, causing less host tissue damage. However, our data are not consistent with a model where the overproduction of proteases in the ΔfakA mutant leads to decreased levels of virulence factors because we see increased virulence, unless the tissue-damaging virulence factors are indeed the proteases themselves. An additional biological function for S. aureus proteases is to degrade host factors, such as complement and immune cell receptors. Aur has been shown to cleave several different immunological proteins, such as complement protein C3 (22) and LL-37 peptide (54). The serine protease SspA can cleave junctions between keratinocytes (32) and all classes of human immunoglobulins (24). The staphopain protease SspB can also degrade junctions between keratinocytes (33) and can cleave CD31, a repulsion signal, on the surface of neutrophils (55). Because SspA and SspB proteases can disrupt the integrity of the epithelial layer, this could allow for greater infiltration of S. aureus into the skin and may be the reason the ΔfakA mutant causes more necrotic lesions with less defined boundaries (Fig. 2). Lastly, Aur and SspB have recently been shown to play a role in nutrient acquisition during skin infection, allowing S. aureus to acquire peptides (56). This is also unlikely to be the mechanism by which the ΔfakA mutant causes enhanced disease, since we do not see a fitness advantage (i.e., higher bacterial numbers) at either 3 or 4 days p.i. (Fig. 5) (29). Thus, identifying a role for proteases in enhanced tissue damage during ΔfakA mutant infection sheds new light on the factors contributing to enhanced virulence. However, given the multifactorial roles of proteases, further studies will be necessary to elucidate the mechanistic details. It is interesting that the need for Hla is dominant over other potential factors, such as the overproduction of proteases in the ΔfakA mutant. This could be due to the absolute need of Hla to lyse cells or stimulate the immune response. It is also possible that Hla’s role in tight-junction degradation is key for the penetration of cells or other virulence factors into tissues.
Taking these findings together, we show that the ΔfakA mutant is hypervirulent during skin infection and that the hypervirulence is dependent on Hla. In addition, proteases are contributing factors, although the exact mechanism for this is uncertain. Building on our previous study (29), we have now observed that the inability of S. aureus to use fatty acids leads to enhanced pathogenesis, alterations in the host immune response, and a less controlled infection in the skin. To cause disease, S. aureus possesses a multitude of virulence factors that must be tightly regulated for optimal expression. In the fakA mutant, based on our data, these systems become dysregulated, leading to changes in virulence factor production. The consequence of this during infection is a decrease of key cytokines early during infection that are needed to resolve infection appropriately. As a result, the ΔfakA mutant causes larger necrotic lesions with higher bacterial titers in later stages of infection. Whether this is due to direct tissue damage by the toxins and proteases themselves or dysfunction of the immune response is unclear. However, it is clear that a combination of Hla and proteases are key players in the pathogenesis of the ΔfakA mutant. Together, the results of these studies shed new light on the hypervirulence of the ΔfakA mutant as well as the contribution of proteases during skin infection.
MATERIALS AND METHODS
Generating strains.
The stains used in the study are shown in Table 1. AH1824 (ΔsspAB mutant) and AH1358 (Δaur mutant) were provided by Alex Horswill at the University of Colorado Anschutz Medical Campus. To construct the fakA mutant version of these strains, the fakA::ΦNΣ mutation from NE229 was transferred by phi11-based transduction (57).
TABLE 1.
Strains used in this study
| Strain name | Descriptiona | Reference or source |
|---|---|---|
| AH1263 | USA300 CA-MRSA strain LAC lacking LAC-p03, wild-type strain | 58 |
| AH1358 | AH1263 Δaur | A. Horswill |
| AH1824 | AH1263 ΔsspAB | 59 |
| JLB2 | AH1263 ΔfakA | 29 |
| JLB11 | AH1263 ΔsspAB fakA::ΦNΣ | This study |
| JLB12 | AH1263 Δaur fakA::ΦNΣ | This study |
| JLB24 | AH1263 hla::ΦΝΣ | 29 |
| JLB25 | AH1263 hla::ΦΝΣ ΔfakA | 29 |
| NE229 | Source of fakA::ΦNΣ | 60 |
Strains with ΦNΣ contain the bursa aurealis transposon and encode erythromycin resistance.
Bacterial growth conditions for inocula.
For all mouse experiments, bacteria were cultured from −80°C freezer stocks and grown overnight with shaking at 250 rpm at 37°C in tryptic soy broth (TSB). Overnight cultures were then diluted 1:100 into 10 ml TSB in a 50-ml conical tube and grown for 3 to 5 h at 37°C with shaking at 250 rpm. Bacteria were pelleted by centrifugation at 3,000 × g for 5 min, washed with phosphate-buffered saline (PBS) or Dulbecco’s PBS, and resuspended to yield 1 × 107 to 3 × 107 CFU per 50-μl injection, which was confirmed by dilution plating.
Subcutaneous infections.
All studies were conducted in strict accordance with approved protocols at The University of New Mexico and The University of Kansas Medical Center. Female SKH1 mice (Charles River, Wilmington, MA) between 8 and 12 weeks of age were used for all experiments. Infections were carried out as previously described (29). Briefly, mice were anesthetized using isoflurane gas and were inoculated subcutaneously with 50 μl of bacteria diluted in PBS into the flank of mice using a 27-gauge needle. Mice were weighed, and the site of inoculation was photographed either daily or every other day. On the final day of the experiment, mice were euthanized using CO2 inhalation per the institutional protocol. We then excised 2.25 cm2 of skin around the inoculation site and homogenized the samples in Hank’s balanced salt solution (HBSS; no cations) plus 0.2% (wt/vol) human serum albumin (A5843; Sigma Aldrich, St. Louis, MO) plus 10 mM HEPES using 2.3 mm zirconia/silica beads (BioSpec, Bartlesville, OK) in a Mini-Beadbeater-16 (BioSpec) or with lysing matrix H and a Fastprep-24 5G (MP Biomedicals), according to the manufacturers’ protocols for skin. Homogenized skin samples were diluted 1:10 in PBS plus 0.1% (vol/vol) Triton X-100 and further diluted in PBS for plating and CFU enumeration on 5% (vol/vol) sheep blood agar (BD and Co., Franklin Lakes, NJ) or TSB. Remaining homogenate was clarified by centrifugation at 16,000 × g for 10 min and stored at −80°C for future cytokine analysis.
Cytokine analysis.
Cytokine levels in clarified homogenates were measured using a custom MILLIPLEX kit (Millipore, Burlington, MA) according to the manufacturer’s recommendations. Clarified homogenates were thawed and diluted 1:5 in assay buffer. Samples were tested in triplicate for the following cytokines: IL-1β, IL-6, IL-10, IL-17A, TNF-α, KC (CXCL1), MIP-1α, MIP-2, IL-1α, MIP-1β, and LIX (CXCL5).
Histology.
Mice were infected with WT and ΔfakA mutant S. aureus as previously described, and after a 4-day infection, a 2.25-cm2 area of skin surrounding the inoculation site was excised and placed onto filter paper in a sectioning cassette. Each cassette was incubated separately in 30 ml of 10% (wt/vol) formalin for 24 h and then transferred to a single container of 70% (vol/vol) ethanol. Tissues were paraffin embedded, sectioned (4 μm), hematoxylin and eosin stained, and imaged by the Human Tissue Repository and Tissue Analysis Shared Resource at the University of New Mexico.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism, version 8. See individual figure legends for details.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alex Horswill for providing strains used in this study and Mary A. Markiewicz for her insightful comments and suggestions.
This work was supported by a Pilot Project under NIGMS grant P20GM112117 and NIAID grant AI121073 to J.L.B. as well as NIAID grants AI131098 and AI145324 to P.R.H. Additionally, P.R.H. was supported in part by the Autophagy, Inflammation, and Metabolism (AIM) Center of Biomedical Research Excellence (CoBRE), supported by NIH P20 GM121176. The research in this paper was also supported by the Human Tissue Repository and Tissue Analysis Shared Resource, funded by the Department of Pathology, The University of New Mexico Comprehensive Cancer Center, and NCI 2P30CA118100, as well as the CTSC Translational Clinical Laboratory, funded by UL1TR001449 from the National Center for Advancing Translational Sciences.
J.L.B. serves on the Scientific Advisory Board and is a consultant for Azitra, Inc. These activities did not financially support and are unrelated to the current manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Klein E, Smith DL, Laxminarayan R. 2007. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis 13:1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suaya JA, Mera RM, Cassidy A, O’Hara P, Amrine-Madsen H, Burstin S, Miller LG. 2014. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect Dis 14:296. doi: 10.1186/1471-2334-14-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kourtis AP, Emerging Infections Program MRSA Author Group, Hatfield K, Baggs J, Mu Y, See I, Epson E, Nadle J, Kainer MA, Dumyati G, Petit S, Ray SM, Ham D, Capers C, Ewing H, Coffin N, McDonald LC, Jernigan J, Cardo D. 2019. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb Mortal Wkly Rep 68:214–219. doi: 10.15585/mmwr.mm6809e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. 2007. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis 57:7–13. doi: 10.1016/j.diagmicrobio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Tognetti L, Martinelli C, Berti S, Hercogova J, Lotti T, Leoncini F, Moretti S. 2012. Bacterial skin and soft tissue infections: review of the epidemiology, microbiology, aetiopathogenesis and treatment: a collaboration between dermatologists and infectivologists. J Eur Acad Dermatol Venereol 26:931–941. doi: 10.1111/j.1468-3083.2011.04416.x. [DOI] [PubMed] [Google Scholar]
- 6.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. 2010. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Investig 120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molne L, Corthay A, Holmdahl R, Tarkowski A. 2003. Role of gamma/delta T cell receptor-expressing lymphocytes in cutaneous infection caused by Staphylococcus aureus. Clin Exp Immunol 132:209–215. doi: 10.1046/j.1365-2249.2003.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullaly SC, Kubes P. 2006. The role of TLR2 in vivo following challenge with Staphylococcus aureus and prototypic ligands. J Immunol 177:8154–8163. doi: 10.4049/jimmunol.177.11.8154. [DOI] [PubMed] [Google Scholar]
- 9.Müller-Anstett MA, Müller P, Albrecht T, Nega M, Wagener J, Gao Q, Kaesler S, Schaller M, Biedermann T, Götz F. 2010. Staphylococcal peptidoglycan co-localizes with Nod2 and TLR2 and activates innate immune response via both receptors in primary murine keratinocytes. PLoS One 5:e13153. doi: 10.1371/journal.pone.0013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opitz B, Schroder NW, Spreitzer I, Michelsen KS, Kirschning CJ, Hallatschek W, Zahringer U, Hartung T, Gobel UB, Schumann RR. 2001. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kappaB translocation. J Biol Chem 276:22041–22047. doi: 10.1074/jbc.M010481200. [DOI] [PubMed] [Google Scholar]
- 11.Parker D, Prince A. 2012. Staphylococcus aureus induces type I IFN signaling in dendritic cells via TLR9. J Immunol 189:4040–4046. doi: 10.4049/jimmunol.1201055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inohara N, Ogura Y, Chen FF, Muto A, Nunez G. 2001. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem 276:2551–2554. doi: 10.1074/jbc.M009728200. [DOI] [PubMed] [Google Scholar]
- 13.Cho JS, Zussman J, Donegan NP, Ramos RI, Garcia NC, Uslan DZ, Iwakura Y, Simon SI, Cheung AL, Modlin RL, Kim J, Miller LS. 2011. Noninvasive in vivo imaging to evaluate immune responses and antimicrobial therapy against Staphylococcus aureus and USA300 MRSA skin infections. J Investig Dermatol 131:907–915. doi: 10.1038/jid.2010.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigby KM, DeLeo FR. 2012. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, DeLeo FR. 2011. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis 204:937–941. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tkaczyk C, Hamilton MM, Datta V, Yang XP, Hilliard JJ, Stephens GL, Sadowska A, Hua L, O'Day T, Suzich J, Stover CK, Sellman BR. 2013. Staphylococcus aureus alpha toxin suppresses effective innate and adaptive immune responses in a murine dermonecrosis model. PLoS One 8:e75103. doi: 10.1371/journal.pone.0075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi SD, Malachowa N, DeLeo FR. 2015. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol 185:1518–1527. doi: 10.1016/j.ajpath.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craven RR, Gao X, Allen IC, Gris D, Wardenburg JB, McElvania-TeKippe E, Ting JP, Duncan JA. 2009. Staphylococcus aureus α-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muñoz-Planillo R, Franchi L, Miller LS, Núñez G. 2009. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol 183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berube BJ, Bubeck Wardenburg J. 2013. Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins (Basel) 5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laarman AJ, Ruyken M, Malone CL, van Strijp JAG, Horswill AR, Rooijakkers S. 2011. Staphylococcus aureus metalloprotease aureolysin cleaves complement C3 to mediate immune evasion. J Immunol 186:6445–6453. doi: 10.4049/jimmunol.1002948. [DOI] [PubMed] [Google Scholar]
- 23.Hirasawa Y, Takai T, Nakamura T, Mitsuishi K, Gunawan H, Suto H, Ogawa T, Wang XL, Ikeda S, Okumura K, Ogawa H. 2010. Staphylococcus aureus extracellular protease causes epidermal barrier dysfunction. J Investig Dermatol 130:614–617. doi: 10.1038/jid.2009.257. [DOI] [PubMed] [Google Scholar]
- 24.Prokesova L, Potuznikova B, Potempa J, Zikan J, Radl J, Hachova L, Baran K, Porwit-Bobr Z, John C. 1992. Cleavage of human immunoglobulins by serine proteinase from Staphylococcus aureus. Immunol Lett 31:259–265. doi: 10.1016/0165-2478(92)90124-7. [DOI] [PubMed] [Google Scholar]
- 25.Kolar SL, Antonio Ibarra J, Rivera FE, Mootz JM, Davenport JE, Stevens SM, Horswill AR, Shaw LN. 2013. Extracellular proteases are key mediators of Staphylococcus aureus virulence via the global modulation of virulence-determinant stability. MicrobiologyOpen 2:18–34. doi: 10.1002/mbo3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krute CN, Ridder MJ, Seawell NA, Bose JL. 2019. Inactivation of the exogenous fatty acid utilization pathway leads to increased resistance to unsaturated fatty acids in Staphylococcus aureus. Microbiology 165:197–207. doi: 10.1099/mic.0.000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alnaseri H, Kuiack RC, Ferguson KA, Schneider JET, Heinrichs DE, McGavin MJ. 2018. DNA binding and sensor specificity of FarR, a novel TetR family regulator required for induction of the fatty acid efflux pump FarE in Staphylococcus aureus. J Bacteriol 201:e00602-18. doi: 10.1128/JB.00602-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeMars ZR, Bose JL. 2018. Redirection of metabolism in response to fatty acid kinase in Staphylococcus aureus. J Bacteriol 200:e00345-18. doi: 10.1128/JB.00345-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bose JL, Daly SM, Hall PR, Bayles KW. 2014. Identification of the Staphylococcus aureus vfrAB operon, a novel virulence factor regulatory locus. Infect Immun 82:1813–1822. doi: 10.1128/IAI.01655-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krute CN, Rice KC, Bose JL. 2017. VfrB is a key activator of the Staphylococcus aureus SaeRS two-component system. J Bacteriol 199:e00828-16. doi: 10.1128/JB.00828-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ericson ME, Subramanian C, Frank MW, Rock CO. 2017. Role of fatty acid kinase in cellular lipid homeostasis and SaeRS-dependent virulence factor expression in Staphylococcus aureus. mBio 8:e00988-17. doi: 10.1128/mBio.00988-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B, McHugh BJ, Qureshi A, Campopiano DJ, Clarke DJ, Fitzgerald JR, Dorin JR, Weller R, Davidson DJ. 2017. IL-1β–induced protection of keratinocytes against Staphylococcus aureus-secreted proteases is mediated by human β-defensin 2. J Investig Dermatol 137:95–105. doi: 10.1016/j.jid.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massimi I, Park E, Rice K, Muller-Esterl W, Sauder D, McGavin MJ. 2002. Identification of a novel maturation mechanism and restricted substrate specificity for the SspB cysteine protease of Staphylococcus aureus. J Biol Chem 277:41770–41777. doi: 10.1074/jbc.M207162200. [DOI] [PubMed] [Google Scholar]
- 34.Ohbayashi T, Irie A, Murakami Y, Nowak M, Potempa J, Nishimura Y, Shinohara M, Imamura T. 2011. Degradation of fibrinogen and collagen by staphopains, cysteine proteases released from Staphylococcus aureus. Microbiology 157:786–792. doi: 10.1099/mic.0.044503-0. [DOI] [PubMed] [Google Scholar]
- 35.Potempa J, Dubin A, Korzus G, Travis J. 1988. Degradation of elastin by a cysteine proteinase from Staphylococcus aureus. J Biol Chem 263:2664–2667. [PubMed] [Google Scholar]
- 36.Shaw L, Golonka E, Potempa J, Foster SJ. 2004. The role and regulation of the extracellular proteases of Staphylococcus aureus. Microbiology 150:217–228. doi: 10.1099/mic.0.26634-0. [DOI] [PubMed] [Google Scholar]
- 37.Wong VW, Sorkin M, Glotzbach JP, Longaker MT, Gurtner GC. 2011. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol 2011:969618. doi: 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosley B, Urdal DL, Prickett KS, Larsen A, Cosman D, Conlon PJ, Gillis S, Dower SK. 1987. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem 262:2941–2944. [PubMed] [Google Scholar]
- 39.Mizutani H, Black R, Kupper TS. 1991. Human keratinocytes produce but do not process pro-interleukin-1 (IL-1) beta. Different strategies of IL-1 production and processing in monocytes and keratinocytes. J Clin Investig 87:1066–1071. doi: 10.1172/JCI115067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. 1997. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis 176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 41.Diao H, Kohanawa M. 2005. Endogenous interleukin-6 plays a crucial protective role in streptococcal toxic shock syndrome via suppression of tumor necrosis factor alpha production. Infect Immun 73:3745–3748. doi: 10.1128/IAI.73.6.3745-3748.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alegre ML, Chen L, David MZ, Bartman C, Boyle-Vavra S, Kumar N, Chong AS, Daum RS. 2016. Impact of Staphylococcus aureus USA300 colonization and skin infections on systemic immune responses in humans. J Immunol 197:1118–1126. doi: 10.4049/jimmunol.1600549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, Yamada M, Kawamura N, Ariga T, Tsuge I, Karasuyama H. 2009. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med 206:1291–1301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aufiero B, Guo M, Young C, Duanmu Z, Talwar H, Lee HK, Murakawa GJ. 2007. Staphylococcus aureus induces the expression of tumor necrosis factor-alpha in primary human keratinocytes. Int J Dermatol 46:687–694. doi: 10.1111/j.1365-4632.2007.03161.x. [DOI] [PubMed] [Google Scholar]
- 45.Ferrante A, Martin AJ, Bates EJ, Goh DH, Harvey DP, Parsons D, Rathjen DA, Russ G, Dayer JM. 1993. Killing of Staphylococcus aureus by tumor necrosis factor-alpha-activated neutrophils. The role of serum opsonins, integrin receptors, respiratory burst, and degranulation. J Immunol 151:4821–4828. [PubMed] [Google Scholar]
- 46.Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl GM, Frei U, Jörres A. 2000. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol 165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 47.Wang ZM, Liu C, Dziarski R. 2000. Chemokines are the main proinflammatory mediators in human monocytes activated by Staphylococcus aureus, peptidoglycan, and endotoxin. J Biol Chem 275:20260–20267. doi: 10.1074/jbc.M909168199. [DOI] [PubMed] [Google Scholar]
- 48.Maurer M, von Stebut E. 2004. Macrophage inflammatory protein-1. Int J Biochem Cell Biol 36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 49.Castleman MJ, Pokhrel S, Triplett KD, Kusewitt DF, Elmore BO, Joyner JA, Femling JK, Sharma G, Hathaway HJ, Prossnitz ER, Hall PR. 2018. Innate sex bias of Staphylococcus aureus skin infection is driven by alpha-hemolysin. J Immunol 200:657–668. doi: 10.4049/jimmunol.1700810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nickerson NN, Joag V, McGavin MJ. 2008. Rapid autocatalytic activation of the M4 metalloprotease aureolysin is controlled by a conserved N-terminal fungalysin-thermolysin-propeptide domain. Mol Microbiol 69:1530–1543. doi: 10.1111/j.1365-2958.2008.06384.x. [DOI] [PubMed] [Google Scholar]
- 51.Drapeau GR. 1978. Role of metalloprotease in activation of the precursor of staphylococcal protease. J Bacteriol 136:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olaru F, Jensen LE. 2010. Staphylococcus aureus stimulates neutrophil targeting chemokine expression in keratinocytes through an autocrine IL-1alpha signaling loop. J Investig Dermatol 130:1866–1876. doi: 10.1038/jid.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ezepchuk YV, Leung DY, Middleton MH, Bina P, Reiser R, Norris DA. 1996. Staphylococcal toxins and protein A differentially induce cytotoxicity and release of tumor necrosis factor-alpha from human keratinocytes. J Investig Dermatol 107:603–609. doi: 10.1111/1523-1747.ep12583377. [DOI] [PubMed] [Google Scholar]
- 54.Sieprawska-Lupa M, Mydel P, Krawczyk K, Wójcik K, Puklo M, Lupa B, Suder P, Silberring J, Reed M, Pohl J, Shafer W, McAleese F, Foster T, Travis J, Potempa J. 2004. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother 48:4673–4679. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smagur J, Guzik K, Bzowska M, Kuzak M, Zarebski M, Kantyka T, Walski M, Gajkowska B, Potempa J. 2009. Staphylococcal cysteine protease staphopain B (SspB) induces rapid engulfment of human neutrophils and monocytes by macrophages. Biol Chem 390:361–371. doi: 10.1515/BC.2009.042. [DOI] [PubMed] [Google Scholar]
- 56.Lehman MK, Nuxoll AS, Yamada KJ, Kielian T, Carson SD, Fey PD. 2019. Protease-mediated growth of Staphylococcus aureus on host proteins is opp3 dependent. mBio 10:e02553-18. doi: 10.1128/mBio.02553-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krausz KL, Bose JL. 2016. Bacteriophage transduction in Staphylococcus aureus: broth-based method. Methods Mol Biol 1373:63–68. doi: 10.1007/7651_2014_185. [DOI] [PubMed] [Google Scholar]
- 58.Boles BR, Thoendel M, Roth AJ, Horswill AR. 2010. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 5:e10146. doi: 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wörmann ME, Reichmann NT, Malone CL, Horswill AR, Gründling A. 2011. Proteolytic cleavage inactivates the Staphylococcus aureus lipoteichoic acid synthase. J Bacteriol 193:5279–5291. doi: 10.1128/JB.00369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.