Abstract
Standard evaluation and management of the patient with suspected or proven cardiovascular complications of coronavirus disease-2019 (COVID-19), the disease caused by severe acute respiratory syndrome related-coronavirus-2 (SARS-CoV-2), is challenging. Routine history, physical examination, laboratory testing, electrocardiography, and plain x-ray imaging may often suffice for such patients, but given overlap between COVID-19 and typical cardiovascular diagnoses such as heart failure and acute myocardial infarction, need frequently arises for advanced imaging techniques to assist in differential diagnosis and management. This document provides guidance in several common scenarios among patients with confirmed or suspected COVID-19 infection and possible cardiovascular involvement, including chest discomfort with electrocardiographic changes, acute hemodynamic instability, newly recognized left ventricular dysfunction, as well as imaging during the subacute/chronic phase of COVID-19. For each, the authors consider the role of biomarker testing to guide imaging decision-making, provide differential diagnostic considerations, and offer general suggestions regarding application of various advanced imaging techniques.
Key Words: COVID-19, myocardial injury, myocarditis, stress cardiomyopathy
Abbreviations and Acronyms: ACS, acute coronary syndrome; BNP, B-type natriuretic peptide; CAD, coronary artery disease; CMR, cardiac magnetic resonance imaging; CT, computed tomography; CTA, computed tomography angiography; ECG, electrocardiogram; cTn, cardiac troponin; EMB, endomyocardial biopsy; ICU, intensive care unit; LV, left ventricular; LVD, left ventricular dysfunction; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MPI, myocardial perfusion imaging; MRI, magnetic resonance imaging; NT-proBNP, N-terminal pro–B-type natriuretic peptide; PET, positron emission tomography; POCUS, point of care ultrasound; RV, right ventricular; STEMI, ST-segment elevation myocardial infarction
Central Illustration
Imaging is essential in the diagnosis and risk stratification of disease and in guiding management. The traditional “5-fingered approach” of history, physical examination, blood tests, electrocardiogram (ECG), and imaging is still relevant, but in the coronavirus disease-2019 (COVID-19) era, there are additional challenges due to significant overlap between coronavirus and typical cardiac presentations. For example, among those with COVID-19, chest discomfort is common, and ECG and cardiac troponins are frequently abnormal in the absence of obstructive coronary artery disease (CAD), especially among those with severe infections requiring intensive care unit (ICU) admission (1, 2, 3). As well, dyspnea is among the most common symptoms in COVID-19, and B-type natriuretic peptide and chest x-ray are also frequently abnormal in affected patients. Therefore, there is a need for guidance on discriminating significant cardiac pathology from findings that may be associated with COVID-19 infection.
The purpose of this document is to provide a summary of the evidence regarding the prevalence and significance of abnormal findings that may suggest the need for cardiac imaging and to provide expert guidance on incorporating advanced imaging into clinical pathways in suspected or confirmed COVID-19 patients.
Methodology
This guidance document was commissioned by the leadership of the American College of Cardiology Cardiovascular Imaging Leadership Council. The writing group for this document was organized to ensure diversity of perspectives and expertise including multimodality cardiovascular imaging, critical care cardiology, heart failure, interventional cardiology, and general cardiology. The work of the writing group was supported exclusively by the American College of Cardiology and specialty imaging societies without commercial support.
In order to maximize the clinical utility of this guidance document, descriptions of imaging findings and relative utility of imaging tests are organized by the most common clinical scenarios requiring expert cardiac consultation described to date. The writing group realizes that these clinical scenarios are not static and many overlap, but this format allowed to describe better the differential diagnoses and the evolving role of noninvasive imaging tests in guiding management of patients affected by COVID-19. The writing group also recognizes that some of these initial recommendations will likely evolve with increasing knowledge regarding the pathobiology of cardiac complications from severe acute respiratory syndrome related-coronavirus-2 (SARS-CoV-2) infection and the potential impact of novel therapies that are currently being evaluated on the natural history of the disease. It is also important to recognize that this guidance regarding the relative utility of imaging tests in specific clinical scenarios should be interpreted and adopted in the context of local availability and expertise.
COVID-19 Testing and Implications for Imaging
Diagnostic testing to identify persons infected with SARS-CoV-2 plays a crucial role in protection of health care personnel and guiding disinfection of imaging rooms and equipment used in patients with suspected or confirmed COVID-19 infection. The diagnostic sensitivity of reverse transcriptase polymerase chain reaction (RT-PCR)–based assays of respiratory specimens is reported to be between 89% and 96% (range 78% to 100%) depending on the sample source (4). The information has important implications for protection of health care personnel involved in imaging studies and for disinfection of imaging rooms and equipment after their used in patients who are confirmed COVID-19 positive and those who are RT-PCR negative but still considered high risk for COVID-19. Strict adherence to local infection control policies and procedures is required to minimize exposure and transmission of SARS-CoV-2 to health care staff and patients without COVID-19 referred for imaging. Specific recommendations regarding the use of personal protective equipment have been provided by all subspecialty imaging societies (5, 6, 7, 8).
Role of Biomarkers to Inform Imaging Decision-Making in COVID-19
Measurement of circulating biomarkers to support clinical judgment in patients with COVID-19 has grown because many circulating biomarkers reflecting end-organ stress/injury, inflammation, hypoperfusion, and pathway activation of thrombosis/hemostasis have been found to have prognostic value in patients with COVID-19 (Table 1 ), predicting longer hospital stay, need for ICU admission, onset of adult respiratory distress syndrome, and risk for death (2,9, 10, 11, 12, 13, 14, 15, 16, 17, 18). However, despite a prognostic role, almost none of the widely measured biomarkers represent a specific trigger for imaging outside of that supported by clinical judgment.
Table 1.
Frequently Measured Prognostic Circulating Biomarkers in COVID-19
| General chemistry | Alanine/aspartate aminotransferase Bilirubin Creatinine Lactate Lactate dehydrogenase |
| Cell counts | Leukocyte count (leukocytosis with lymphopenia) Platelet count (thrombocytopenia) |
| Inflammatory/acute phase markers | C-reactive protein Ferritin Interleukin-6 Procalcitonin |
| Thrombosis/hemostasis | D-dimer |
| Cardiac markers | B-type natriuretic peptide N-terminal pro–B-type natriuretic peptide Troponin T Troponin I |
When elevated or rising, these biomarkers provide incremental information regarding outcome, but may not necessarily identify an actionable process.
Cardiac-derived biomarkers reflecting myocardial stress (B-type natriuretic peptide [BNP] and N-terminal pro-BNP [NT-proBNP]) and myocardial injury (cardiac troponin [cTn]) are both strongly prognostic in COVID-19 (2,10, 11, 12, 13,15,16), particularly in patients with rising values during hospitalization and in those with marked biomarker elevation (12). Importantly, clinicians should be aware that most patients with abnormal BNP/NT-proBNP or cTn do not have acute heart failure or myocardial infarction, and rise in concentration of either class of biomarker presumably reflects complex processes including direct myocardial stress/injury related to systemic illness. To the extent cardiac dysfunction is a major adverse prognostic finding in COVID-19, links between BNP/NT-proBNP and cTnT or cTnI with myocardial dysfunction make it tempting to regard them as a valuable tool for decision-making regarding need for imaging.
Accordingly, when interpreted within the context of the entire clinical picture, low–modest and nonrising concentrations of either class of biomarker may help to exclude the need for imaging. In those with marked elevation (e.g., severe heart failure, myocardial infarction [MI], or myocarditis), selective imaging might be considered if clinical judgment dictates. Of course, both classes of biomarkers retain their usefulness for diagnosis of acute heart failure or type 1 MI in patients with concomitant acute cardiac issues and comorbid COVID-19.
Clinical Presentations
A typical presentation of the COVID-19 positive or possible patient is dyspnea with a chest x-ray demonstrating interstitial or airspace infiltrates suggestive of pneumonia (19). The clinician may be consulted to rule in or rule out a cardiogenic component of pulmonary abnormalities in this symptomatic patient population. Clinicians will rely on history, physical exam, ECG and biomarkers, and recent cardiac imaging tests if available. Underlying cardiac history including CAD, cardiomyopathy, heart failure, and arrhythmia should be sought, and frequent contributors to decompensation should be eliminated (20). As discussed in the following text, the additional use of cardiac imaging may be helpful in the evaluation of cardiac complications from COVID-19. However, given the higher risk of exposure of health care personnel, imaging studies should be used carefully and only in clinical situations where their use can inform a change in patient management. The writing group agreed that either a point of care ultrasound (POCUS) or a formal limited echocardiogram may be considered as the initial evaluation of positive or suspected COVID-19 patients with possible cardiac injury (21). Advanced imaging techniques may also play an important role, which will be discussed under the specific clinical scenarios discussed in the following text.
Clinical Scenario 1: Chest Pain and Abnormal ECG
Typical findings and differential diagnosis
Chest discomfort is a common symptom among patients with active COVID-19 infection and may result from a large number of etiologies (Table 2 ).
Table 2.
Clinical Scenarios and Differential Diagnosis
| Clinical Scenario | Differential Diagnosis |
|---|---|
| Chest pain with abnormal ECG | Pneumonia Myocarditis Pericarditis Pulmonary embolism Stress cardiomyopathy Myocardial injury related to hypoxemia and tachycardia (severe illness) Acute coronary syndrome |
| Hemodynamic instability | Coronary ischemia, with or without acute myocardial infarction Viral myocarditis Stress cardiomyopathy Cytokine-mediated cardiomyopathy Pulmonary embolism Pericardial effusion and tamponade RV dysfunction in the setting of high positive end-expiratory pressure (PEEP) with mechanical ventilation Arrhythmia Mixed shock in patients with septic shock and an inability to compensate due to chronic cardiac dysfunction |
| New left ventricular dysfunction without hemodynamic instability | Chronic ischemic heart disease Chronic nonischemic cardiomyopathy Acute coronary syndrome Acute/fulminant viral myocarditis, Stress cardiomyopathy Cytokine-mediated cardiomyopathy Tachycardia-mediated cardiomyopathy Other forms of cardiomyopathy including toxin and infiltrative |
| Subacute/chronic presentation | Heart failure from volume overload (e.g., from fluid resuscitation during the inpatient stay) or due to decompensation of their new or pre-existing cardiomyopathy Ischemic heart disease from progressive coronary or microvascular disease Myocarditis Pulmonary embolism Thromboembolic disease |
ECG = electrocardiogram; RV = right ventricular.
Initial diagnostic approach
The initial diagnostic evaluation of patients presenting with chest pain is illustrated in Figure 1 . The 12-lead ECG and biomarkers play a key role. In addition to concern for traditional acute coronary syndromes (ACS) (ST-segment elevation ACS or non–ST-segment elevation ACS), patients with active COVID-19 infection have been reported to present with ECG abnormalities later shown to be related to 1 or more of the conditions outlined in Table 2 (22,23). In patients with atypical symptoms for traditional ACS or nontypical ECG changes, rapid access to cardiovascular imaging may provide important diagnostic information to guide management.
-
•
Patients with chest pain and clinical concern for ST-segment elevation ACS or high clinical risk for in-hospital mortality (e.g., cardiogenic shock, left ventricular [LV] ejection fraction [LVEF] <40% felt due to non–ST-segment elevation MI [non-STEMI], dynamic ST-segment changes) should be referred for emergent coronary angiography and reperfusion therapy (24) (Figure 2).
-
•
In patients with equivocal symptoms, atypical or equivocal ECG abnormalities, or late presentation, clinicians may consider POCUS or limited echocardiogram to assess for regional wall motion abnormalities and LVEF and/or coronary computed tomography (CT) angiography (CTA) as discussed in the following text (8,24).
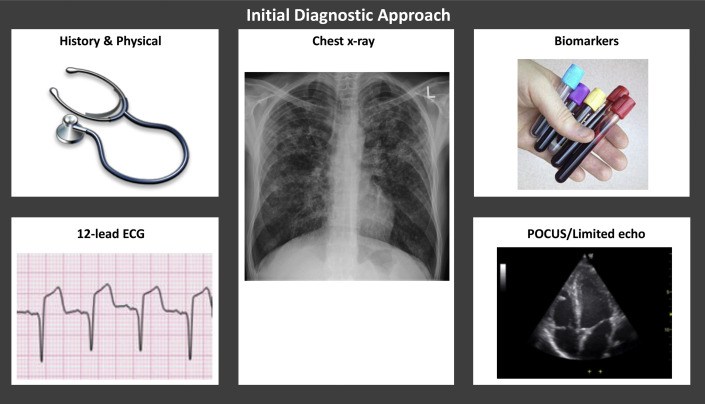
Figure 1.
Initial Diagnostic Approach
The initial diagnostic workup includes history and physical exam, ECG, chest x-ray, and biomarkers. POCUS or a limited echocardiogram should also be considered in selected clinical presentations. ECG = electrocardiogram; POCUS = point of care ultrasound.
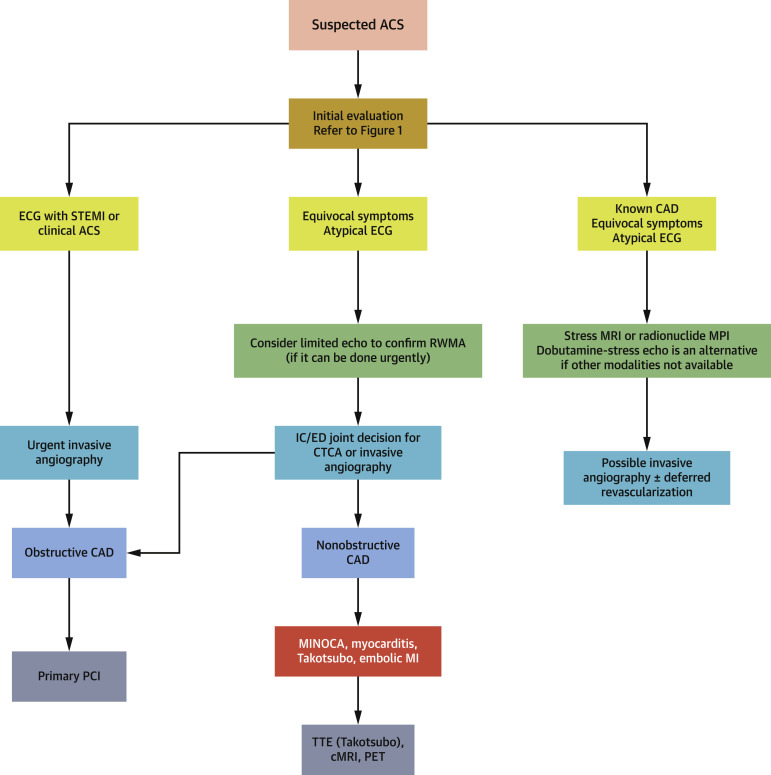
Figure 2.
Role of Cardiac Imaging in Patients Presenting With Chest Pain and Suspected ACS
Patients with chest pain and clinical concern for ST-segment elevation ACS or high clinical risk for in-hospital mortality should be referred for emergent coronary angiography and reperfusion therapy. In patients with equivocal symptoms, atypical or equivocal ECG abnormalities, or late presentation, clinicians may consider POCUS or limited echocardiogram to assess for regional wall motion abnormalities and LVEF and/or coronary CTA to rule out ACS and point to alternate diagnoses discussed of acute cardiac injury. In patients with known CAD and equivocal ECG changes, stress imaging may be helpful. ACS = acute coronary syndrome; CAD = coronary artery disease; cMRI = cardiac magnetic resonance imaging; CTA = computed tomography angiography; CTCA = computed tomography coronary angiography; IC/ED = intensive care/emergency department; LVEF = left ventricular ejection fraction; MI = myocardial infarction; MINOCA = myocardial infarction, normal or nonobstructive coronary arteries; MPI = myocardial perfusion imaging; MRI = magnetic resonance imaging; PCI = percutaneous coronary intervention; PET = positron emission tomography; RWMA = regional wall motion abnormality; TTE = transthoracic echocardiography; other abbreviations as in Figure 1.
Role of advanced imaging techniques
In patients with chest pain and ST-segment elevation without clear evidence of STEMI, coronary CTA is preferred as an initial advanced imaging study in order to rule out ACS (25) and point to alternate diagnoses discussed in clinical scenarios 2 and 3, especially in patients in whom a diagnostic quality study can be obtained. Patients need to be able to participate in breath holding and achieve reasonable heart rate control, and should not have contraindications to iodinated contrast administration. Coronary CTA is particularly useful in patients without previously established CAD or severe coronary artery calcification.
In patients with a clinical presentation of acute MI, normal or nonobstructive coronary arteries (MINOCA), which may present as STEMI or non-STEMI, with normal or abnormal LV systolic function, cardiac magnetic resonance imaging (CMR) can help confirm the diagnosis of MI or provide alternate diagnosis including myocarditis, stress cardiomyopathy, and embolic infarction (26) (Table 2, Figure 2, Supplemental Table 1). A patient without evidence of obstructive CAD by angiography and an echocardiogram demonstrating a pattern consistent with stress cardiomyopathy may not need additional imaging, particularly if a repeat study shows resolution of dysfunction. However, when the presentation is atypical or there is diagnostic uncertainty, CMR can help identify the mechanism of myocardial dysfunction.
In hemodynamically stable patients with previously established CAD presenting with chest pain of unclear etiology in whom a non–ST-segment elevation ACS is a consideration, pharmacological stress imaging is the preferred noninvasive approach (Figure 2), although in low-risk ACS, testing might be deferred until after COVID-19 resolution.
There are several important considerations when selecting among available functional stress imaging tests. Exercise stress testing, including exercise echocardiography (5), should be generally avoided in patients with confirmed or suspected active COVID-19 infection due to the potential risk of aerosolizing droplets. Vasodilator stress myocardial perfusion imaging (MPI) with single-photon emission CT, positron emission tomography (PET), or MRI are all options to consider. If available, stress MPI with PET or MRI, or stress-first approach with single-photon emission CT especially in patients with body mass index <30 and without known CAD would be preferred because of the short protocol times (generally 20 to 45 min), which limit exposure to staff. Dobutamine-stress echocardiography is an option if radionuclide imaging and MRI are not available.
Clinical Scenario 2: Hemodynamic Instability (Shock or Hypotension)
Typical findings and differential diagnosis
Patients with COVID-19 may manifest with hemodynamic instability with a wide range of abnormalities, including LV and/or right ventricular (RV) dysfunction with regional or global abnormalities, with or without myocardial injury, and with or without evidence of myocarditis (1,22,27, 28, 29, 30). Imaging findings typically associated with chronic cardiac conditions, such as LV hypertrophy and LV dilatation, have been well described in acute cardiac presentations of COVID-19 patients, have demonstrated significant reversibility, and may not be reliable markers to judge chronicity of cardiac dysfunction (31).
Although the pulmonary manifestations predominate in most patients with COVID-19, involvement of other organs is common with acute cardiac injury occurring in up to 28% and venous thrombotic events in up to 67% (32, 33, 34). Cardiogenic shock has been reported as the first manifestation of COVID-19 in the absence of respiratory symptoms (1). As such, clinicians should have a high degree of suspicion for COVID-19 in the setting of hemodynamic instability even if the patient is not presenting with its classic constellation of symptoms.
Hemodynamic instability includes shock, which is classically divided into several physiological categories: distributive, obstructive, hypovolemic, and cardiogenic (35). Cardiogenic shock can further be subdivided into classic cardiogenic shock, defined by low cardiac output, high filling pressures, and high systemic vascular resistance, and mixed (or vasodilatory) cardiogenic shock. Distributive or septic shock was observed commonly (∼20% of hospitalized COVID-19 patients) in early reports from China (19). Although the incidence of heart failure or cardiogenic shock is not well defined, early estimates described rates as high as 30% to 50% in patients requiring ICU care or in nonsurvivors (19,27).
The potential mechanisms of direct myocardial dysfunction leading to hemodynamic instability in patients with COVID-19 are numerous (Table 2) (32). The underlying pathological mechanisms of the clinical syndrome of myocarditis remain unclear and may include direct viral toxicity, microvascular dysfunction due to microthrombosis, vasculitis, vascular injury, or lymphocytic infiltration (30,32).
Initial diagnostic approach
Initial evaluation of the patient with hemodynamic instability should include all elements outlined in Figure 1. The physical examination should focus on an assessment of congestion, perfusion and be geared towards identification of cardiogenic or mixed shock. Initial testing should include markers of perfusion (e.g., lactate, liver function tests, renal function), and may include measurement of BNP/NT-proBNP, cTn, coagulation, and inflammatory markers, and an assessment of cardiac output.
In the setting of a clear STEMI with hemodynamic instability, it is recommended to proceed directly to coronary angiography and reperfusion therapy without the need of additional imaging (Figure 3 ), as described in Clinical Scenario 1 (24).
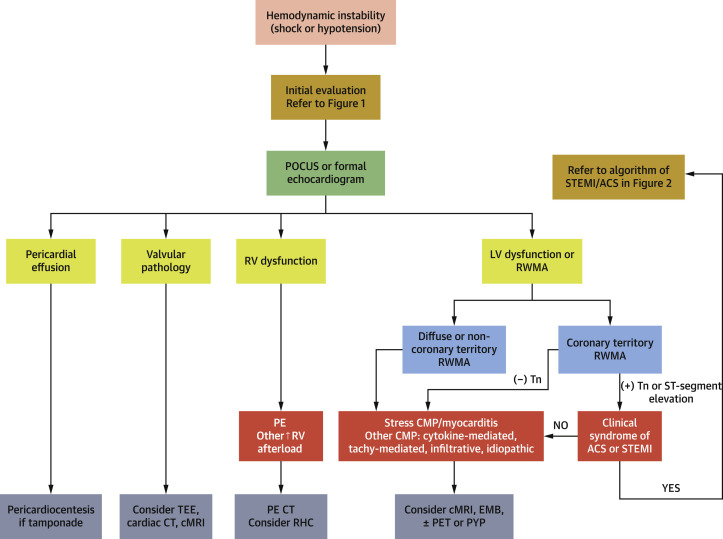
Figure 3.
Role of Cardiac Imaging in Patients Presenting With Hemodynamic Instability
After an initial evaluation, patients with clear STEMI with hemodynamic instability should be referred to coronary angiography and reperfusion therapy without additional imaging. In patients with evidence of significant/worsening myocardial injury, or ECG abnormalities without clear evidence of STEMI, assessment with POCUS or formal echocardiogram is recommended to help exclude pericardial effusion/tamponade, valvular pathology and RV dysfunction. Coronary CTA may be useful to exclude an ACS in patients with equivocal ECG changes and abnormal LV function. Cardiac MRI can help differentiate myocarditis from stress cardiomyopathy and can be considered if it is likely to lead to a change in patient management. CMP = cardiomyopathy; CT = computed tomography; EMB = endomyocardial biopsy; LV = left ventricular; PE = pulmonary embolism; PYP = pyrophosphate; RHC = right heart catheterization; RV = right ventricle; STEMI = ST-segment elevation myocardial infarction; TEE = transesophageal echocardiography; Tn = troponin; other abbreviations as in Figures 1 and 2.
In patients with hemodynamic instability, evidence of significant/worsening myocardial injury, or ECG abnormalities without clear evidence of STEMI, assessment with POCUS is recommended (Figure 3, Supplemental Tables 1 to 3). This may help guide the decision on whether to proceed with coronary evaluation, although it is noteworthy that myocarditis and myocardial injury may also present with regional wall motion abnormalities. In the absence of a high pre-test probability of acute MI (type 1), coronary CTA may be useful to minimize staff exposure (8,24).
Role of advanced imaging techniques
If acute epicardial CAD is ruled out or the suspicion for ACS is low, it is reasonable to consider further evaluation for myocarditis or stress cardiomyopathy (Figure 3, Supplemental Table 1), if such information may lead to a change in patient management.
There should be a high index of suspicion for pulmonary embolism in confirmed or suspected COVID-19 patients, particularly in those with hemodynamic instability, unexplained sinus tachycardia, or evidence of RV strain by ECG or echocardiogram or rising D-dimer. A contrast-enhanced chest CT is the imaging modality of choice (Figure 3). RV dysfunction is nonspecific and may also be observed in other high RV afterload states, such as with hypoxic pulmonary vasoconstriction or high PEEP in mechanically ventilated patients.
The role of endomyocardial biopsy (EMB) in COVID-19–associated cardiogenic shock remains unclear. Recommendations for the use of EMB in patients presenting with unexplained acute cardiomyopathy (36) are based on clinical presentations in which EMB results would change treatment or assist in prognostication (37,38). Given that no clear therapy exists for COVID-19–related myocarditis and the risks of personnel exposure during the procedure, use of EMB should be reserved for patients in whom such results would change management course.
Clinical Scenario 3: New LV Dysfunction Without Shock or Hypotension
Typical findings and differential diagnosis
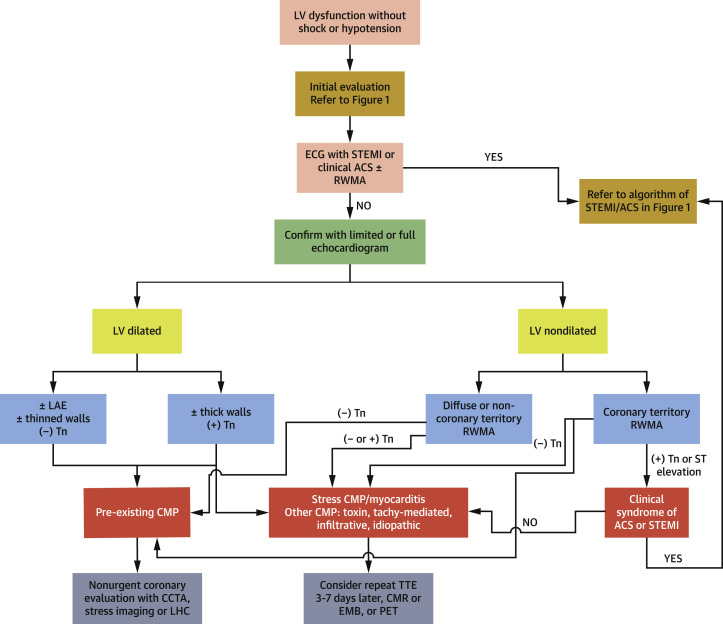
As noted in the preceding text, myocardial stress and injury, as reflected by abnormal concentrations of BNP/NT-proBNP or cTn, are common in patients with COVID-19 (27,39). The clinical presentation of LV dysfunction (LVD) in COVID-19 is quite heterogeneous ranging from incidentally discovered LVD with negative biomarkers to mild symptomatic LVD to a clinical picture of fulminant myocarditis and shock (Table 2). Systolic dysfunction can present as either regional wall motion abnormalities that may or may not fit a coronary artery distribution to various forms of stress cardiomyopathy including classic and atypical patterns to severe global systolic dysfunction with hemodynamic instability (Figure 4 ).
Figure 4.
Role of Cardiac Imaging in Patients Presenting With New LVD Without Hemodynamic Instability
Acute coronary syndrome should be considered in patients presenting with typical clinical and ECG features and, if present, should be referred to emergent coronary angiography and reperfusion therapy. Distinguishing between acute and chronic LVD in COVID-19 in patients without ACS can be difficult as multiple features overlap. Echocardiography, coronary CTA, CMR, and ischemia testing can be considered depending on initial clinical findings. CMR = cardiac magnetic resonance imaging; LAE = left atrial enlargement; LHC = left heart catheterization; LVD = left ventricular dysfunction; other abbreviations as in Figures 1, 2, and 3.
ACS should be considered in patients presenting with typical clinical, ECG, and echocardiographic features. Distinguishing between acute and chronic LVD in COVID-19 can be difficult because multiple features overlap (Figure 4). The presence of a nondilated LV on echocardiography, particularly in the context of significantly elevated/rising biomarker concentrations, profound systolic dysfunction and/or LV hypertrophy and significant heart failure or hemodynamic instability suggests an acute pathophysiology. By contrast, presentation of a severely dilated LV, left atrial enlargement, thinned or akinetic walls and low or stable cTn suggest a pre-existing chronic cardiomyopathy. The presence of myocardial inflammation/edema by CMR or PET can be useful in distinguishing acute from chronic LVD.
COVID-19 myocarditis diagnosed by combination of CMR and myocardial injury has been described both in patients with normal (40) or abnormal LV function (1). A thickened LV wall that regresses within 4 to 7 days is a common anecdotal finding described in case reports (1,28,29), which may represent extensive myocardial edema (1,29) and frequently associated with low voltage on the ECG.
Initial diagnostic approach
Initial evaluation should include all elements outlined in Figure 1. In addition to standard chest x-ray and ECG, biomarker testing may be useful to identify acute myocardial stress or injury as discussed in the preceding text and noted in Table 1 and Figure 4. The use of limited versus full echocardiography may depend on the differential diagnoses that are being considered (Table 2) and level of diagnostic information needed (Supplemental Table 2).
Role of advanced imaging techniques
In patients with known or suspected structural heart disease during the initial POCUS, additional echocardiographic imaging should be considered to further characterize such findings (Figure 4, Supplemental Tables 1 and 2). Transesophageal echocardiography is an aerosol-generating procedure and should be avoided if possible during the acute phase of COVID-19 (5). In selected cases, cardiac CT may be useful and minimize exposure of health care personnel (8).
The use of advanced imaging tests to evaluate for suspected ACS, chronic CAD, or other forms of myocardial injury in patients presenting with LVD should follow recommendations discussed in Clinical Scenarios 1 and 2 (Figure 4). When there is a suspicion for myocarditis, and CMR is equivocal or cannot be performed, cardiac PET may provide information on the presence of cardiac inflammation (41). As discussed in the preceding text, EMB may be considered in selected patients presenting with Clinical Scenarios 2 or 3 (Figure 4).
Clinical Scenario 4: Subacute/Chronic Phase
Typical findings and differential diagnosis
Many of the subacute and chronic findings in COVID-19 cardiovascular disease are nonspecific and may include symptoms such as dyspnea, fatigue, weakness, or cough. More concerning cardiac symptoms include chest pain, syncope, pre-syncope, or palpitations, and new signs of heart failure. The long-term consequences and prognosis of myocardial damage incurred during COVID-19 are unknown. However, following the initial SARS-CoV-1 epidemic in 2002, chronic cardiac and pulmonary manifestations were reported after recovery (18,42), and it is reasonable to expect the same with SARS-CoV-2.
The differential diagnosis should include COVID-19–related and non–COVID-19–related etiologies (Table 2). Non–COVID-19–related etiologies should be evaluated in standard fashion. COVID-19–related etiologies may include deconditioning, recovery from illness, residual lung disease, and manifestations of various forms of cardiovascular disease. Cardiovascular sequelae from acute COVID-19 may include arrhythmias, left and right ventricular dysfunction and heart failure, or ischemic heart disease. Both supraventricular and ventricular arrhythmias may appear in the subacute and chronic phases and may present as palpitations, heart failure, chest pain, presyncope, or syncope.
Initial diagnostic approach
Initial evaluation should include all elements outlined in Figure 1. The utility of serum biomarkers is undefined in the subacute/chronic phase. It is reasonable to suspect that elevated concentrations of BNP/NT-proBNP in a dyspneic post–COVID-19 patient might be helpful to understand presence and severity of cardiac dysfunction or clinical heart failure, and such biomarkers should be used in accordance with published clinical practice guidelines (43).
Role of advanced imaging techniques
New signs of heart failure/persistent dyspnea out of proportion to lung disease
The evaluation of patients presenting persisting or new dyspnea should follow the same considerations outlined in Clinical Scenario 3. If there is any concern for development of pulmonary hypertension, particularly chronic thromboembolic pulmonary hypertension, the patient should be referred to an appropriate specialist, and a right heart catheterization can be considered.
Known LVD (during acute phase)
If the patient was diagnosed with new LVD in the hospital, repeat echocardiogram (or MRI) at 2 to 6 months following discharge to assess for myocardial recovery may be considered. Guideline-directed medical therapy should be optimized (43).
New syncope/significant palpitations
These patients should undergo evaluation of syncope and arrhythmias as defined in current guidelines, which include consideration of advanced imaging to assess for CAD, scar, and ischemia (44).
Chest discomfort
These patients should undergo evaluation of ischemic heart disease as defined in current guidelines, which include consideration of advanced noninvasive imaging and coronary angiography (45,46).
Summary and Conclusions
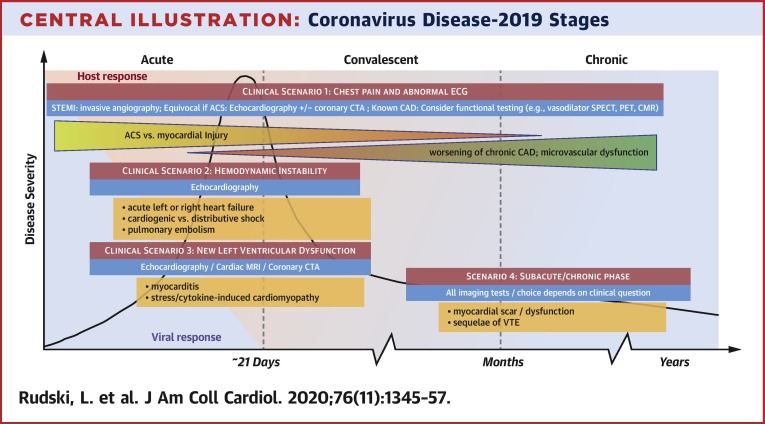
The management of cardiovascular complications in patients with COVID-19 presents substantial diagnostic and therapeutic challenges. Selective use of advanced cardiac imaging offers powerful qualitative and quantitative information that can help with patient management (Central Illustration ). In the following text are the key summary points from this expert panel guidance regarding the use of multimodality imaging in COVID-19 patients:
-
•
Transthoracic echocardiography is usually the initial cardiovascular imaging modality used to guide management. This may be in the form of an urgent POCUS or a limited study initially. More complete studies are guided by the clinical question and evolving patient condition.
-
•
Clear or suspected STEMI usually mandates emergent cardiac catheterization with limited role for noninvasive imaging. Less typical presentations of potential acute coronary syndrome may benefit from a POCUS and coronary CTA. In patients with MINOCA, CMR can be useful in distinguishing the etiology of myocardial injury.
-
•
In patients with known CAD with suspected low-risk ACS, vasodilator stress MRI or radionuclide MPI, especially PET if available, can be considered.
-
•
In patients with new LV systolic dysfunction, consideration of underlying CAD should be investigated. For those without evidence of CAD, CMR or PET can provide important insights into the etiology of myocardial dysfunction.
-
•
In the subacute phase, surveillance of previously detected abnormalities is important to follow for potential recovery (as in takotsubo cardiomyopathy or myocarditis) or progressive disease.
-
•
In all scenarios, consideration of risk to the health care workers is an integral part of the decision-making process. Decision to pursue additional imaging should be based on the assumption that the results will change patient management.
-
•
Individual institutions must rely on local expertise and resources to determine access to testing, and imaging must be consistent with goals of treatment.
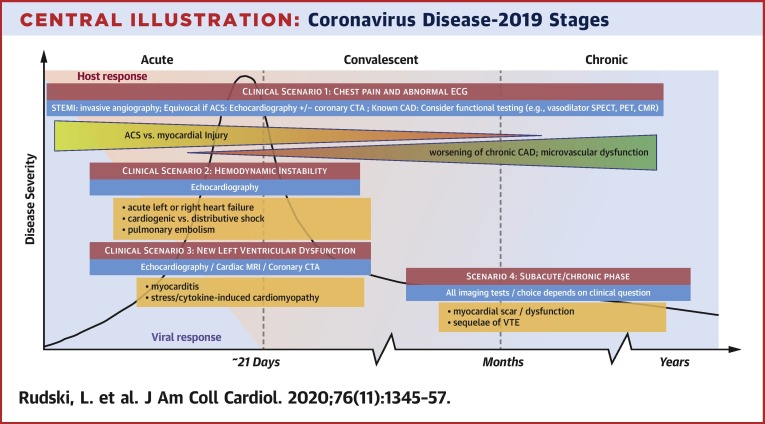
Central Illustration.
Coronavirus Disease-2019 Stages
This figure shows the various imaging questions that may present in various stages of coronavirus disease-2019 (COVID-19). The x-axis depicts time. As the disease progress, patient may evolve from having acute disease to a convalescent phase and then chronic disease. The 4 red boxes highlight the various clinical scenarios. The orange boxes list some of the pathological processes that are being evaluated in each scenario. ACS = acute coronary syndrome; CAD = coronary artery disease; CMR = cardiac magnetic resonance imaging; CTA = computed tomography angiography; ECG = electrocardiogram; MRI = magnetic resonance imaging; PET = positron emission tomography; SPECT = single-photon emission computed tomography; STEMI = ST-segment elevation myocardial infarction; VTE = venous thromboembolism.
Footnotes
The views expressed in this paper by the American College of Cardiology’s Cardiovascular Imaging Leadership Council do not necessarily reflect the views of the Journal of the American College of Cardiology or the American College of Cardiology.
Dr. Bucciarelli-Ducci is supported by the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the UK National Institute for Health Research or the UK Department of Health and Social Care.
Dr. Rudski has minor stock holdings in General Electric outside of a managed portfolio. Dr. Januzzi is a trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Novartis Pharmaceuticals and Abbott Diagnostics; has received consulting income from Abbott Diagnostics, Janssen, Novartis, and Roche Diagnostics; and has participated in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, CVRx, Janssen, and Takeda. Dr. Bohula has received institutional grant support from Amgen, Novartis, AstraZeneca, Eisai, and The Medicines Company; and has consulted for Kowa, Novo Nordisk, Amgen, and Medscape. Dr. Blankstein has received research support from Amgen Inc. and Astellas Inc. Dr. Patel received research grants from General Electric and Philips. Dr. Vorovich has served on the Speakers Bureau for Abiomed. Dr. Rao has received institutional research grants from Svelte Inc., Shockwave Medical, and Bayer. Dr. Beanlands has received research grants and consulting honoraria from Lantheus Medical Imaging, Jubilant DraxImage, and GE Healthcare. Dr. Di Carli has received institutional grant support from Gilead Sciences and Spectrum Dynamics; and has received consulting income from Janssen and Bayer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Daniel S. Berman, MD, served as Guest Associate Editor for this paper. P.K. Shah, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1096. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akhmerov A., Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu Y.P., Jennings R., Hart B., et al. Swabs collected by patients or health care workers for SARS-CoV-2 testing. N Engl J Med. 2020;383:494–496. doi: 10.1056/NEJMc2016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkpatrick J., Mitchell C., Taub C., Kort S., Hung J., Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. J Am Soc Echocardiogr. 2020;33:648–653. doi: 10.1016/j.echo.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y., Chen T., Bryant J., et al. Society for Cardiovascular Magnetic Resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn Reson 2020. 2020;22:26. doi: 10.1186/s12968-020-00628-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skali H., Murthy V.L., Al-Mallah M.H., et al. Guidance and best practices for nuclear cardiology laboratories during the coronavirus disease 2019 (COVID-19) pandemic: an information statement from ASNC and SNMMI. J Nucl Cardiol. 2020;27:1022–1029. doi: 10.1007/s12350-020-02123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi A.D., Abbara S., Branch K.R., et al. Society of Cardiovascular Computed Tomography guidance for use of cardiac computed tomography amidst the COVID-19 pandemic. J Cardiovasc Comput Tomogr. 2020;14:101–104. doi: 10.1016/j.jcct.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L., Jiang D., Wen X.S., et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21:83. doi: 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han H., Xie L., Liu R., et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92:819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 15.Li J.W., Han T.W., Woodward M., et al. The impact of 2019 novel coronavirus on heart injury: A Systematic review and Meta-analysis. Prog Cardiovasc Dis. 2020 Apr 16 doi: 10.1016/j.pcad.2020.04.008. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoso A., Pranata R., Wibowo A., Al-Farabi M.J., Huang I., Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2020 Apr 19 doi: 10.1016/j.ajem.2020.04.052. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thachil J., Tang N., Gando S., et al. Laboratory haemostasis monitoring in COVID-19. J Thromb Haemost. 2020 Apr 23 doi: 10.1111/jth.14866. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L., Yan X., Fan Q., et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollenberg S.M., Warner Stevenson L., Ahmad T., et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966–2011. doi: 10.1016/j.jacc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Spencer K.T., Kimura B.J., Korcarz C.E., Pellikka P.A., Rahko P.S., Siegel R.J. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567–581. doi: 10.1016/j.echo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with Covid-19: a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1286. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Mahmud E., Dauerman H.L., Welt F.G., et al. Management of acute myocardial infarction during the COVID-19 pandemic. J Am Coll Cardiol. 2020 Apr 21 doi: 10.1016/j.jacc.2020.04.039. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linde J.J., Kelbaek H., Hansen T.F., et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2020;75:453–463. doi: 10.1016/j.jacc.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Tamis-Holland J.E., Jneid H., Reynolds H.R., et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 27.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16 doi: 10.1093/eurheartj/ehaa190. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sala S., Peretto G., Gramegna M., et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zagrosek A., Wassmuth R., Abdel-Aty H., Rudolph A., Dietz R., Schulz-Menger J. Relation between myocardial edema and myocardial mass during the acute and convalescent phase of myocarditis--a CMR study. J Cardiovasc Magn Reson. 2008;10:19. doi: 10.1186/1532-429X-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atri D., Siddiqi H.K., Lang J., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. J Am Coll Cardiol Basic Trans Science. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Apr 10 doi: 10.1016/j.thromres.2020.04.013. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llitjos J.F., Leclerc M., Chochois C., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Diepen S., Katz J.N., Albert N.M., et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 36.Kociol R.D., Cooper L.T., Fang J.C., et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 37.Bennett M.K., Gilotra N.A., Harrington C., et al. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000-2009. Circ Heart Fail. 2013;6:676–684. doi: 10.1161/CIRCHEARTFAILURE.112.000087. [DOI] [PubMed] [Google Scholar]
- 38.Bozkurt B., Colvin M., Cook J., et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134:e579–e646. doi: 10.1161/CIR.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 39.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nensa F., Kloth J., Tezgah E., et al. Feasibility of FDG-PET in myocarditis: comparison to CMR using integrated PET/MRI. J Nucl Cardiol. 2018;25:785–794. doi: 10.1007/s12350-016-0616-y. [DOI] [PubMed] [Google Scholar]
- 42.Ong K.C., Ng A.W., Lee L.S., et al. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur Respir J. 2004;24:436–442. doi: 10.1183/09031936.04.00007104. [DOI] [PubMed] [Google Scholar]
- 43.Yancy C.W., Jessup M., Bozkurt B., et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91–e220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 45.Fihn S.D., Blankenship J.C., Alexander K.P., et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929–1949. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 46.Knuuti J., Wijns W., Saraste A., et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.