ABSTRACT
Many casual observers typecast Drosophila melanogaster as a stationary pest that lurks around fruit and wine. However, the omnipresent fruit fly, which thrives even in desert habitats, likely established and maintained its cosmopolitan status via migration over large spatial scales. To perform long-distance dispersal, flies must actively maintain a straight compass heading through the use of external orientation cues, such as those derived from the sky. In this Review, we address how D. melanogaster accomplishes long-distance navigation using celestial cues. We focus on behavioral and physiological studies indicating that fruit flies can navigate both to a pattern of linearly polarized light and to the position of the sun – the same cues utilized by more heralded insect navigators such as monarch butterflies and desert ants. In both cases, fruit flies perform menotaxis, selecting seemingly arbitrary headings that they then maintain over time. We discuss how the fly's nervous system detects and processes this sensory information to direct the steering maneuvers that underlie navigation. In particular, we highlight recent findings that compass neurons in the central complex, a set of midline neuropils, are essential for navigation. Taken together, these results suggest that fruit flies share an ancient, latent capacity for celestial navigation with other insects. Furthermore, they illustrate the potential of D. melanogaster to help us to elucidate both the cellular basis of navigation and mechanisms of directed dispersal on a landscape scale.
KEY WORDS: Polarized light, Sun compass, Central complex, Dispersal, Insects, Migration
Summary: In this Review, we describe how the fruit fly, Drosophila melanogaster, uses the position of the sun and the pattern of polarized skylight to maintain a constant heading during long-distance dispersal flights.
Introduction
If asked to think of a migrating animal, you probably do not picture a fruit fly. Although it is a noted model organism for genetics, development and cell biology, Drosophila melanogaster may seem an odd choice for a review on navigation, as the tiny fly is known more for hovering over fruit bowls than for traipsing across continents. However, this notion of a fruit fly as an unadventurous homebody is unjust for several reasons. First, whereas virtuoso migrators such as locusts (Homberg, 2015) and monarch butterflies (Mouritsen et al., 2013) seem exceptional, these species might merely be more noticeable because of their large size and population density. In a thought-provoking paper based on observations at a mountain pass in the Pyrenees (Lack and Lack, 1951), ornithologists David and Elizabeth Lack argued that many migratory animals escape our observation largely because they are too small and their flight pathways too broad. The Lacks observed many species simply because the narrow mountain pass funneled the flight path of the migrants. More recent evidence, including measurements with entomological radar, supports the basic premise that long-distance migration is more common among insects than it might appear upon cursory observation. For example, the silver Y moth, Autographa gamma, migrates seasonally in Europe on a massive scale with respect to both population numbers and distances (Chapman et al., 2008). This migration is behaviorally impressive and ecologically important because it constitutes an enormous redistribution of biomass across continents (Chapman et al., 2011). Because of the scarce coverage of entomological radar, only a relatively small number of species has been observed with this method, implying that the silver Y moth is more likely a rule than an exception. In short, we should not be surprised if tiny D. melanogaster migrate, given that so many other insects do (Chapman et al., 2004). Rather than a rare specialization of virtuosos, migration may be part of an ancient set of behavioral modules that evolved early in insect evolution (Dickinson, 2014).
Another reason we should anticipate the migratory abilities of D. melanogaster is their historical persistence in regions that are seasonally inhospitable. Although recently adapted to exploit human agricultural products grown for alcohol production, D. melanogaster is a non-diapausing species adapted to dry, temperate environments including deserts (Powell, 1997). In southwestern North America, where deserts are subject to winter freezing and summer baking, populations of Drosophila species crash at inauspicious times of year but rebound dramatically a few months later (Heed, 1978). The only means by which flies can persist in regions that are seasonally inhospitable is via migration; the seeds of seasonal population booms must come from somewhere.
Indirect evidence that Drosophila migrate has emerged from studies of gene frequency in distant populations. For example, what appeared to be geographically separated populations of Drosophila pseudoobscura exhibited similar frequencies of an esterase allele (Jones et al., 1981). How could such tiny flies travel tens of kilometers to effect the requisite gene flow between locations? Initial mark–recapture experiments suggested dispersal distances of tens to hundreds of meters, which could not explain the paradox (Dobzhansky and Powell, 1974). In the late 1970s, however, a series of more ambitious mark–recapture experiments conducted in Death Valley, CA, USA, sought to test whether individual flies could travel over kilometer scales (Coyne et al., 1982; Jones et al., 1981). In one experiment (Coyne et al., 1982), researchers released ∼100,000 flies – a mixture of fluorescently marked species including D. pseudoobscura, D. melanogaster and D. simulans – and set up collection buckets filled with yeast and mashed bananas in two oases, 6.7 and 14.6 km away. Small squadrons of flies, which included ‘yellow flies’ (a mixture of D. melanogaster and D. simulans), found their way to the traps in the oases, probably making the flight in a few hours. These experiments demonstrate convincingly that even small Drosophila can make epic journeys of 3 million body lengths without refueling. Such feats are entirely consistent with flies' metabolic capacity; a well-fed D. melanogaster can fly for 2 h (Götz, 1987; Lehmann and Dickinson, 1997) and can cruise outdoors at speeds of 6.5 km h−1 (Combes et al., 2012; M.H.D., personal observation).
Metabolic capacity is not, however, the only prerequisite for long-distance migration; an animal must also possess the navigational capabilities to maintain a constant heading. Even humans walk in circles when deprived of visual landmarks (Souman et al., 2009). Flies that could not fly straight after taking off in a desert would be doomed to meander aimlessly unless lucky enough to encounter an attractive odor plume. Over short distances, flies can maintain a straight path by using their eyes and halteres to detect and minimize angular velocity (Dickinson and Muijres, 2016; Ristroph et al., 2010). Such reflexes are subject to drift, however, making them unsuitable for path stabilization over larger scales. One means of flying straight is to fix a visual landmark. When flying in closed-loop flight simulators, flies will orient continuously towards a dark stripe for many hours, a behavior called stripe fixation (Götz, 1987). Although it is possible that flies can use large distant landmarks, free-flight experiments suggest that object fixation is largely a transient phenomenon in which animals briefly orient towards a nearby feature such as a tree or fallen fruit (Censi et al., 2013). Each fixation bout ends as the fly either lands or turns away from the object as it expands on its retina (van Breugel and Dickinson, 2012). Continuous stripe fixation is therefore likely an artifact of laboratory conditions in which an animal sees, but never reaches, a visual target, much like a donkey that perpetually trots towards a carrot dangled in front of its cart.
All evidence suggests that D. melanogaster maintains an extended straight flight path using global reference cues available in the sky. In this Review, we focus on the use of these celestial features, in particular the position of the sun and the pattern of polarized skylight. We refer to insects' directed travel using these cues as celestial navigation, employing the term ‘navigation’ in the broad sense of maintaining a desired course via an external reference, rather than the narrower sense of heading towards a specific goal. Because a capacity for celestial navigation is shared by many insects, the recent findings in D. melanogaster reviewed here should inform a general view that extends to other species.
Orientation to polarized light
The polarization of light describes the directional distribution of the orthogonal electric and magnetic fields that oscillate in planes perpendicular to the direction of propagation (Maxwell, 1865). In unpolarized light, the oscillation directions are uniformly distributed, whereas in linearly polarized light, the electric and magnetic fields oscillate in specific, orthogonal axes. By convention, the direction of linearly polarized light is denoted by the electric field vector (e-vector) orientation. In the sky, Rayleigh scattering of sunlight by particles in the atmosphere produces a characteristic pattern of linearly polarized light that can be detected on the Earth's surface (Brines and Gould, 1982; Chandrasekhar, 1960; Strutt, 1871). This polarization pattern, which rotates with the sun's position, provides a potential navigation cue for organisms that can see it. Advantages of the polarization pattern, compared with discrete celestial objects such as the sun or moon, are that it can be detected in small patches of sky and is visible when the sun is just below the horizon (Cronin and Marshall, 2011; von Frisch, 1948). Furthermore, although polarization magnitude is highest under a clear sky, the basic pattern of e-vector orientation is detectable even under cloud cover (Hegedüs et al., 2007; Pomozi et al., 2001).
Karl von Frisch's discovery that bees utilize e-vector orientation to indicate direction to a food source provided initial evidence of animals' capacity to detect polarized light and use it as a navigational cue (von Frisch, 1949). Since this initial study, complementary behavioral, anatomical and physiological investigations have shown that many insects, including D. melanogaster, share a capacity to perceive polarized light and use it as a reference (Dacke et al., 2003; Mappes and Homberg, 2004; Rossel, 1993). Insects detect polarized skylight primarily via a specialized region of the eye called the dorsal rim area (DRA) (Fig. 1A; Wehner and Strasser, 1985). Retinal chromophores are intrinsically sensitive to polarization; they absorb light maximally when aligned with e-vectors (Israelachvili and Wilson, 1976). In most photoreceptors, however, the microvilli containing the tightly packed opsins twist along the long axis of the cell (like the bristles of a bottle brush), cancelling out any net polarization sensitivity (Wehner et al., 1975). In contrast, the microvilli of some photoreceptors, including the central R7/R8 photoreceptors in the DRA, do not twist (like the bristles of a toothbrush), endowing them with polarization sensitivity (Fig. 1B; Wernet et al., 2012; Wunderer and Smola, 1982). Using intracellular recordings, Hardie (1984) demonstrated that R7/R8 photoreceptors in the flies Calliphora erythrocephala and Musca domestica are strongly tuned to e-vector orientation, with the direction of tuning matching microvillar orientation. Furthermore, the microvilli of the R7 and R8 photoreceptors (which are stacked in series within the ommatidium) have a precisely orthogonal orientation, suggesting that the two cells might function in a simple opponency circuit (Hardie, 1984). Such an opponent coding scheme would help circumvent the intrinsic ambiguity in single photoreceptor responses, in which changes in e-vector orientation are not dissociable from variation in light intensity (Labhart, 2016; Weir et al., 2016). A recent study confirmed the opponent coding model in D. melanogaster by recording from photoreceptor terminals in the medulla using a genetically encoded Ca2+ indicator (Weir et al., 2016). Consistent with larger flies, the photoreceptors exhibited an inhibition in response to flashes of light that were polarized 90 deg from their preferred direction, suggesting a mutually inhibitory interaction between the R7 and R8 photoreceptors (Fig. 1C). A similar pattern of reciprocal inhibition has been observed between the color-encoding pairs of R7/R8 photoreceptors (Schnaitmann et al., 2013, 2018). This similarity between color and polarization processing makes sense, as it is likely that the polarized light pathway in insects was co-opted from the color pathway, or vice versa (Wernet et al., 2003).
Fig. 1.
The dorsal rim area (DRA) is specialized for the detection of linearly polarized light. (A) Scanning electron micrograph of Drosophila
melanogaster eye with DRA ommatidia colored purple (modified from Hardie, 2012). (B) Transmission electron micrograph of DRA showing rhabdomeres containing R1–R7 photoreceptors. The central rhabdomere containing R7 photoreceptors sits above the R8 photoreceptors (not visible). Parallel microvilli are visible within rhabdomeres (orientation of R1 and R7 microvilli shown with white lines; R7 microvilli enlarged in bottom right inset). (C) Ca2+ responses of R7/R8 DRA photoreceptor terminals in response to rotation of linearly polarized light. Top panel: mean fluorescence response relative to baseline (%Ft− /
/ ) of Ca2+ indicator GCaMP6f at a particular polarizer orientation (denoted in the top left corner). Indicator was expressed in both R7 and R8 photoreceptors. Bottom panel: responses for R7/R8 photoreceptors in three specific regions (i, ii and iii in top panel) at different e-vector angles. Paired R7/R8 photoreceptors exhibit opponent responses; the e-vector angles evoking peak responses in R7/R8 cells (arrowheads) shift linearly across receptor pairs. (D) Although neighboring regions of the DRA sample different sky regions, photoreceptors collectively sample all e-vector orientations. Top panel: optical axes (arrows) of DRA photoreceptors at distinct locations on the eye. Bottom panel: R7 photoreceptors are tuned to the full range of e-vector angles. Gray dots indicate microvillar orientations of R7 photoreceptors at distinct optical axes. Blue lines show preferred e-vector angle, measured via Ca2+ imaging. Adapted from Weir et al. (2016).
) of Ca2+ indicator GCaMP6f at a particular polarizer orientation (denoted in the top left corner). Indicator was expressed in both R7 and R8 photoreceptors. Bottom panel: responses for R7/R8 photoreceptors in three specific regions (i, ii and iii in top panel) at different e-vector angles. Paired R7/R8 photoreceptors exhibit opponent responses; the e-vector angles evoking peak responses in R7/R8 cells (arrowheads) shift linearly across receptor pairs. (D) Although neighboring regions of the DRA sample different sky regions, photoreceptors collectively sample all e-vector orientations. Top panel: optical axes (arrows) of DRA photoreceptors at distinct locations on the eye. Bottom panel: R7 photoreceptors are tuned to the full range of e-vector angles. Gray dots indicate microvillar orientations of R7 photoreceptors at distinct optical axes. Blue lines show preferred e-vector angle, measured via Ca2+ imaging. Adapted from Weir et al. (2016).
Although these studies suggest opponent coding in R7/R8 photoreceptors, the unambiguous detection of e-vector orientation and color requires comparison of at least three sensors (Labhart, 2016; Weir et al., 2016). Because of their position in the eye, the photoreceptors of the DRA observe a narrow band of the sky that runs from one horizon to the other. The orientation of the microvillar axes of the R7 and R8 cells rotates systematically from one end of the DRA to the other, collectively covering ∼180 deg (Fig. 1D; Weir et al., 2016). However, as in the rest of the eye, each of the DRA ommatidia views its own narrow region of the sky, making it impossible for the fly to determine the e-vector at a single point via simultaneous comparison of ommatidia. It is therefore more likely that the entire DRA functions as a spatial filter that detects a broad pattern of polarization across the sky (Labhart, 2016; Rossel and Wehner, 1986).
A study on the orientation behavior of walking flies suggests that additional photoreceptors in the ventral portion of the eye contribute to the detection of polarized light (Wernet et al., 2012). Normal wild-type flies align themselves with the e-vector direction when polarized light is presented either dorsally or ventrally. Complementary genetic silencing and rescue experiments showed that for a dorsal stimulus, the R7/R8 receptors in the DRA were necessary for behavioral responses as expected (Wernet et al., 2012). For a ventral stimulus, however, combinations of R7 and R1–R6 receptors were required for orientation to light polarized in UV wavelengths, whereas combinations of R8 and R1–R6 were required for orientation to polarized light in green wavelengths. Ultrastructural analyses of microvilli in the ventral eye revealed subsets of R4–R6 cells and R7/R8 cells with reduced microvilli twisting, which could explain the sensitivity to polarized light. Polarized light detection in the ventral eye is likely of limited relevance to celestial navigation, although it could potentially help flies orient relative to light reflected from water or other smooth surfaces (Heinloth et al., 2018).
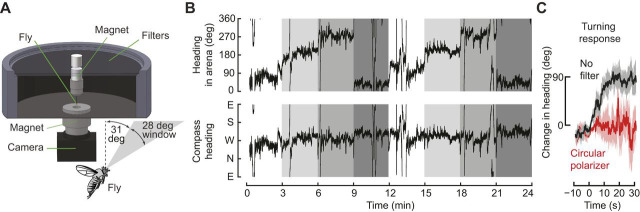
Although there have been several reports of transient orientation to polarized light in walking and flying flies (Heisenberg, 1972; Stephens et al., 1953; Wernet et al., 2012), the first conclusive evidence that D. melanogaster could orient to polarized light was provided by Wolf et al. (1980). They constructed a closed-loop apparatus that allowed flies to control their heading relative to a dorsal field of polarized light via changes in yaw torque. In this device, many flies maintained the polarization axis at a stable heading. A subsequent study using an outdoor apparatus showed that flies could orient to an actual pattern of skylight polarization, in which the magnitude of polarization is lower and e-vector direction more variable (Weir and Dickinson, 2012). In these experiments, flies could freely rotate about their yaw axis to choose an azimuthal orientation but could not translate forward (Fig. 2A). With only a small swath of blue sky visible, flies tended to maintain a straight course for many minutes (Fig. 2B). Moreover, when the entire apparatus was rotated, flies compensated to maintain their prior heading in global coordinates, indicating they were navigating relative to some celestial feature (Fig. 2B). If the transmission of linearly polarized light was blocked with a circular polarizer, flies no longer compensated for rotations of the apparatus, indicating that linearly polarized light was indeed required for the orientation behavior (Fig. 2C). Although experiments with other species of flies suggest that the R7/R8 DRA photoreceptors are narrowly tuned to ultraviolet light (Hardie, 1984), D. melanogaster maintain their navigation abilities when polarized light is restricted to visible wavelengths (Warren et al., 2018; Weir and Dickinson, 2012). This suggests that either the spectral sensitivity of R7/R8 photoreceptors in the DRA of D. melanogaster is broader than in other species or that other photoreceptors contribute to the detection of polarized light (Wernet et al., 2012; Wolf et al., 1980).
Fig. 2.
Flies maintain a straight flight course using polarized skylight. (A) Flies were glued to a steel pin and placed in a magnetic tether in which they were free to rotate about their yaw axis. During flight, they could see a 28 deg patch of sky but not the sun or visual landmarks. (B) An example of a fly actively rotating its flight orientation to maintain a straight heading in global coordinates (i.e. relative to the sky). During a 24 min flight, the arena was rotated by 90 deg every 3 min (changes in shading). The fly adjusted its local heading in arena coordinates (top panel) by ∼90 deg to compensate for each rotation (bottom panel). (C) When a circular polarizer was placed over the arena (red trace), flies no longer adjusted for arena rotations as they did with no filter (black trace). Adapted from Weir and Dickinson (2012).
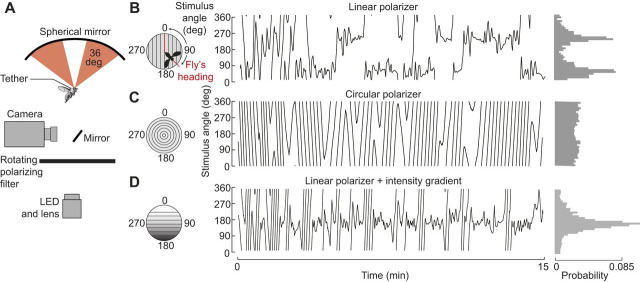
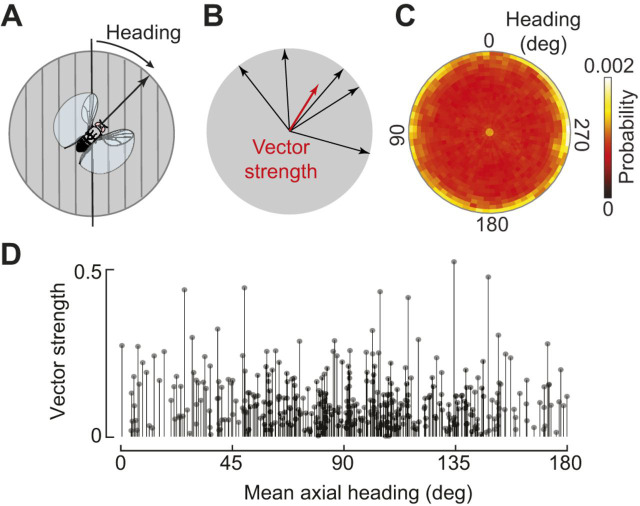
A fundamental step in understanding the sensory-to-motor transformation involved in polarized light navigation is determining how flies adopt their headings. Flying and walking flies respond to many stimuli in a highly stereotyped fashion, reliably positioning sensory cues either in front or behind, symmetrical with their longitudinal body axis. For example, flies steer to keep a dark vertical stripe straight ahead (Götz, 1987) and turn away from aversive odors such as benzaldehyde (Wasserman et al., 2012). An analogous stereotyped orientation reflex to a polarized light stimulus would be to align the e-vector either parallel or perpendicular to the longitudinal body axis. Indeed, a bias of this sort was reported by Wolf et al. (1980), although some flies did maintain oblique headings with respect to the e-vector orientation. However, always navigating towards or away from celestial cues, which are not feasible destinations themselves, does not have an obvious ethological function. An alternative possibility is that insects could travel straight by maintaining an arbitrary orientation relative to a celestial cue, a general behavior termed menotaxis (Heisenberg and Wolf, 1984; Jander, 1960). To distinguish between these possibilities, a recent study examined the heading distribution within a very large population of flies (Warren et al., 2018), employing a closed-loop flight apparatus (Fig. 3) similar to that used by Wolf et al. (1980). Across almost 100 h of flight in 372 individuals, a broad distribution of headings was observed, supporting the hypothesis that flies can maintain arbitrary flight orientations relative to e-vector direction (Fig. 4). The observed heading variability was not explained by factors such as time of day or initial heading at the start of flight. Taken together, these findings indicate D. melanogaster can perform menotaxis to a pattern of polarized light.
Fig. 3.
A flight simulator for studying polarized light navigation in tethered, head-fixed flies. (A) Schematic diagram of the apparatus (after Warren et al., 2018). The difference in wing stroke amplitude was coupled to the angular velocity of a polarized light stimulus, permitting head-fixed flies to steer. Stroke amplitude was monitored via an infrared camera. Linearly polarized light was generated by a rotating polarizer beneath the fly and then reflected onto the eyes via an overhead spherical mirror. (B) Example of an individual's flight orientation relative to the polarization axis. Left: polarizer orientation over the 15 min flight; 0 and 180 deg reflect headings where the axis of polarization is aligned with the longitudinal body axis. Right: distribution of headings during flight. The distinct peaks at 50 and 230 deg reflect axial symmetry of the stimulus. (C) Example control data with circular polarizer. (D) Example data with combined intensity cue and linear polarizer. Here, 0 deg is heading with the bright portion of the stimulus in front of the fly. In this example, the fly stabilizes the stimulus at a single heading of 168 deg. Adapted from Warren et al. (2018).
Fig. 4.
Drosophila perform menotaxis relative to a polarized light cue. (A) A fly's heading is defined as the angle between its longitudinal body axis and the polarization axis. At headings of 0 deg/180 deg, these two axes are aligned. (B) To calculate vector strength, a measure of heading consistency, flight headings are converted to unit vectors and summed. Vector strength ranges from 0 to 1, with 1 corresponding to a fly that precisely maintained the same heading. (C) A polar histogram showing the relative proportion of data at distinct combinations of heading and vector strength (denoted by angular and radial location in plot). Vector strength is computed over a 30 s time window to capture short-term stimulus stabilization. At the highest local vector strengths, flight data were distributed broadly across headings (N=372 flies; 93 h of flight data). (D) Mean axial heading for each flight (N=372) plotted against overall vector strength. Although the overall distribution was broad, there was a slight bias towards headings in which the polarization axis was perpendicular to the longitudinal body axis. Adapted from Warren et al. (2018).
Over the course of a 15 min closed-loop flight, flies' fidelity to their chosen polarization heading gradually increases (Warren et al., 2018). Heading fidelity is typically quantified as vector strength, the magnitude of the average of instantaneous unit heading vectors (Fig. 4B). This gradual rise in vector strength persists, even if flies are exposed to a rotating pattern of polarized light stimulus for 10 min prior to the start of flight, suggesting that the phenomenon is not the result of sensory facilitation, but rather that the flies' heading choice requires a few minutes to solidify. According to this notion, flies initiate flight without a clear travel direction and then gradually converge on a particular random heading. One prediction of this model is that if a fly's heading choice is already determined, no gradual rise in vector strength will occur. This prediction was tested by initiating a second flight following a short, 5 min interruption (Warren et al., 2018). In this second flight, flies tended to maintain their preference for the original heading and exhibited a much faster rise in vector strength than in the first flight. These data suggest that a fly's heading preference might crystallize at the start of one flight and then be recalled at the start of the second flight.
Polarized light is an intrinsically ambiguous reference cue because of the axial symmetry of e-vectors. This ambiguity is readily apparent in flight simulator experiments, in which flies switch between two headings 180 deg apart (Fig. 3B; Warren et al., 2018). In order not to flip back and forth between opposite headings, insects must combine directional information provided by the polarized light pattern with other cues (e.g. the sun itself, or intensity/chromatic gradients). When presented with a polarization pattern that was linked to an intensity cue mimicking the natural gradient in the sky, flies adopted a unimodal, non-axial heading (Fig. 3D; Warren et al., 2018). Moreover, the flies' capacity to navigate improved as measured via an increase in vector strength. These results suggest D. melanogaster can integrate multiple celestial cues.
Orientation to sun position
Although the polarization pattern of skylight has some unique attributes as an orientation cue, discrete celestial objects such as the sun or moon are potentially more robust references when visible. For example, a contrast-based sun stimulus can be detected throughout the eye (not just in the DRA), requires no opponent decoding and has no directional ambiguities. Despite these advantages of sun-based orientation, navigation using polarized light has received more attention (Homberg, 2015) – a bias that may reflect the appeal of studying a sensory-motor behavior in which the peripheral receptors (i.e. the R7/8 cells in the DRA) are well characterized (Fent, 1986; Labhart and Meyer, 1999; Wehner and Strasser, 1985). There are, however, many documented examples of insects using the sun to navigate, including von Frisch's pioneering study of the bee waggle dance (von Frisch, 1949). Monarch butterflies use sun position, along with other cues, to migrate between northern North American summering grounds and overwintering sites in Mexico (Reppert et al., 2010, 2016). Over shorter scales, desert ants use sun position to return straight back to their nest during foraging excursions (Lebhardt and Ronacher, 2014; Müller and Wehner, 2007) and dung beetles use the cue to roll their balls in straight paths away from conspecific competitors (Byrne et al., 2003). Thus, across diverse insect taxa with varying ecologies, the sun compass is an important component of the general navigational toolkit.
A recent study provides the first demonstration that D. melanogaster can also use the position of a simulated sun to fly straight (Giraldo et al., 2018). Tethered flies were placed in a flight arena and presented with a small, bright spot (Fig. 5A). As in the closed-loop experiments with patterns of polarized light, the fly's wing movements determined the azimuthal angular velocity of the spot. Flies adopted arbitrary headings with respect to the ersatz sun stimulus (Fig. 5B,D), and the response was distinct from their behavior towards a vertical stripe, which they always fixed frontally (Fig. 5C,D). To test whether individuals remember their headings during subsequent flight bouts, flies were allowed to orient using the sun stimulus, then rested for a variable period before being flown a second time. Flies resumed flying with roughly the same heading following inter-flight gaps of 5 min, 1 h, 2 h or even 6 h (Fig. 5E). A heading memory of a few hours would enable flies to maintain a straight course for approximately as long as they could sustain flight before needing to refuel (Götz, 1987), or as long as their normal crepuscular activity peaks at dawn and dusk.
Fig. 5.
Flies perform menotaxis to a sun stimulus and retain their heading preference over time. (A) Tethered flies were placed in a closed-loop LED flight arena and presented with a 2.3 deg bright spot or a 15 deg dark stripe. (B) When presented with a sun stimulus, an individual fly adopted a straight heading of 92 deg (dotted red line) within the first 90 s of flight. (C) A fly maintained a stripe frontally. (D) Population data are shown in polar plots, with each individual represented by a single line. Mean heading is indicated by angle and length represents vector strength. From left to right, plots show population responses to the sun stimulus, to a stripe or in the dark, with means and 95% confidence intervals in red. Points on the outside of each gray circle show a histogram of headings for all individuals. (E) Flies flew to the sun stimulus twice, with a gap of 5 min, 2 h or 6 h between flights. Plots show mean heading±circular variance, with variance multiplied by an arbitrary scale factor of 36 for visibility. The black diagonal line indicates perfect correspondence of headings in the first and second flight. The blue diagonal line shows where the second flight heading would be if heading were time compensated. Data are repeated on the ordinate to emphasize the circular nature of headings. Adapted from Giraldo et al. (2018).
Over durations longer than a few hours, accurate navigation along any arbitrary heading using a sun compass requires a means of compensating for the azimuthal motion of the sun through the sky, which is roughly 15 deg h−1 in temperate latitudes. Experiments suggest that bees and butterflies do utilize a time-compensated sun compass (Lindauer, 1960; Mouritsen and Frost, 2002; Perez et al., 1997), which might be mediated by the inputs from the clock circuit to the central complex (Merlin et al., 2009). To examine whether D. melanogaster exhibit a similar mechanism, researchers compared predictions of a fixed-memory model with a time-compensation model using data from the time-gap experiments described above (Giraldo et al., 2018). For gaps up to 1 h, there is little difference between the predictions of the models, given the small azimuthal motion of the sun over this period. After 2 or 6 h, however, the fixed-memory model better describes the data than the time-compensation model. The lack of a time-compensation sun compass in D. melanogaster makes ethological sense. Whereas time compensation is critical for a bee that must accurately communicate the location of a patch of flowers throughout the day, or a migrating butterfly that must maintain a constant course over a day-long flight, it is not necessary for an animal that uses the sun compass to maintain a fixed course for just a few hours.
Neural basis of celestial navigation
What are the neural mechanisms that allow flies to navigate using a celestial compass? This question has been examined more extensively in locusts (Schistocerca gregaria) and has focused on the pathways linking vision to the central complex (CX). Visual stimuli are detected by the eyes and information is conveyed through the optic lobes to several central brain neuropils, including the anterior optic tubercle (AOTU; el Jundi et al., 2011; Pfeiffer and Homberg, 2007). From there, neurons send axons to the bulb, a structure with projections to the CX (Homberg et al., 2003; Pegel et al., 2018; Träger et al., 2008). The CX is a set of mostly unpaired midline neuropils that is highly conserved among arthropods (Strausfeld, 2012) and has been recognized for its role in navigation and locomotion (Pegel et al., 2018; Seelig and Jayaraman, 2015; Strauss, 2002; Wehner, 2003). It is composed of four neuropils – the protocerebral bridge, the fan-shaped body (central body upper in other insects), the ellipsoid body (central body lower) and the noduli. The system consists of an elaborate network of tangential and columnar neurons that connect the various neuropil regions (Pfeiffer and Homberg, 2014; Wolff et al., 2015). Many excellent descriptions detailing the neuroanatomical structure of the CX in locusts, dung beetles and monarch butterflies can be found elsewhere (Heinze, 2017; Pfeiffer and Homberg, 2014; Webb and Wystrach, 2016).
Using 2-photon functional imaging, several recent studies (Omoto et al., 2017; Seelig and Jayaraman, 2013; Seelig and Jayaraman, 2015) have identified neurons that are responsive to visual objects and are likely part of the fly sun compass network. In such experiments, flies that express a genetically encoded Ca2+ indicator are tethered to a stage, the brain bathed in physiological saline and the fly presented with a variety of visual stimuli. AOTU-bulb neurons, homologous to tubercle–lateral accessory lobe neurons in locusts, respond to small, bright spots (Omoto et al., 2017). Out of two developmentally distinct classes of these neurons, one lineage (containing TuBus and TuBua neurons) is retinotopically organized with receptive fields that reflect an object's azimuth and elevation. These neurons likely synapse onto ring neurons, some of which are tuned to vertically oriented visual features (Seelig and Jayaraman, 2013). A parallel pathway containing TuBui neurons projects from the inferior bulb and likely synapses onto distinct classes of ring neurons (R3; Omoto et al., 2017). In locusts, there are similarly two parallel tracts from upper and lower regions of the AOTU to the CX (Homberg et al., 2003).
There are many similarities in the putative sky compass pathways in D. melanogaster and S. gregaria, suggesting that the circuits for processing sun and polarization information may be broadly conserved (Homberg et al., 2011). In flies, ring neurons (TL2/TL3 in locusts; Heinze and Homberg, 2009) likely synapse onto columnar neurons in the CX that have postsynaptic terminals in the ellipsoid body and project to the protocerebral bridge and a region adjacent to the CX called the gall (hence the name E-PG neurons; Wolff et al., 2015). When presented with a moving visual object, the array of E-PG neurons function as a ring attractor; the fly's heading relative to the object is encoded as a ‘bump’ of activity around the ring (Seelig and Jayaraman, 2015). If the object is removed, the network maintains its compass-like activity for some time using self-motion cues as the animal walks (Seelig and Jayaraman, 2015) or flies (Kim et al., 2017). This ability to integrate both visual and self-motion cues exhibited by E-PG cells is highly reminiscent of head-direction cells in mammals (Taube, 2007).
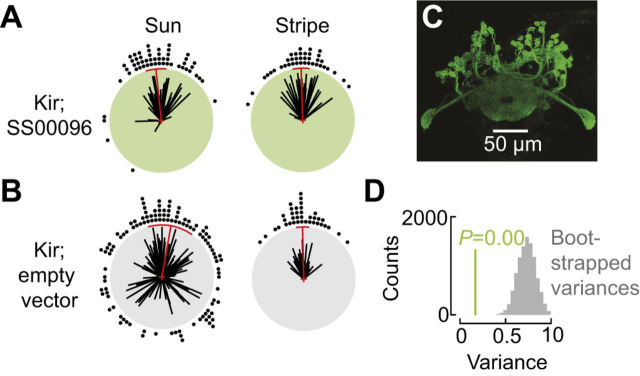
The activity of E-PG neurons is qualitatively similar whether flies are orienting to a sun stimulus or a vertical stripe (Giraldo et al., 2018). This suggests that the E-PG bump reflects the fly's estimate of its own heading, derived using any available cue. Genetically silencing these E-PG cells alters flies' responses to a sun stimulus but not to a stripe (Fig. 6D). With E-PG cells silenced, instead of selecting arbitrary headings (i.e. menotaxis), flies reliably position the sun in front (Fig. 6A,B,D). This suggests that the heading estimate encoded in the E-PG bump is required for sun menotaxis but not for a simpler phototaxis reflex. Stripe fixation is unaffected by E-PG silencing (Fig. 6A,B), consistent with a simple model suggesting that this reflex can occur independently of the CX (Fenk et al., 2014).
Fig. 6.
Compass neurons are necessary for sun menotaxis. (A) When E-PG neurons were silenced using the inwardly rectifying potassium channel Kir2.1 (Baines et al., 2001), flies no longer adopted arbitrary headings in response to a sun stimulus. (B) Control flies that possessed a copy of Kir2.1 and an empty vector that does not produce GAL4 protein (Hampel et al., 2015) did perform menotaxis. Stripe fixation was unaffected by E-PG silencing. (C) Expression pattern of the split-GAL4 line (Kim et al., 2017) used in these experiments. (D) Flies with silenced E-PG neurons had a smaller heading variance than the control flies. Headings observed in control flies were subsampled (N=50) 10,000 times to produce a bootstrapped distribution of variances (gray histogram). All bootstrapped variance values were larger than the variance observed for flies with silenced E-PG neurons (green line). Adapted from Giraldo et al. (2018).
In D. melanogaster, the circuits responsible for polarized light beyond the primary photoreceptors are largely unexplored. In other insects, however, the circuitry underlying polarized light processing has been studied extensively (reviewed in Heinze, 2017; Homberg et al., 2011; Webb and Wystrach, 2016). The processing pathways for polarized light and sun position appear to be similar, suggesting that neural representations of distinct celestial stimuli might converge on a common, shared orientation map (Pegel et al., 2018). In S. gregaria, polarization-sensitive neurons in the medulla project to the AOTU (Fig. 7A; el Jundi et al., 2011; Homberg et al., 2003), where the same neurons respond to multiple celestial cues (Fig. 7A; Kinoshita et al., 2007). Further processing occurs in the CX, where polarization sensitivity has been observed in numerous cell types (Homberg et al., 2011; Pegel et al., 2018), leading to a hypothesis that the CX is a crucial locus for polarized light navigation (Fig. 7B). In D. melanogaster, however, a recent study reported that responses to rotating polarized light were weak in all four major neuropils of the CX (Weir and Dickinson, 2015). One caveat in interpreting these data is that the behavioral responses to the polarized light stimulus were quite small, raising the possibility that there would be more robust neural responses if the stimuli were stronger or the flies were more engaged in a navigation task. In future studies, head-fixed paradigms for polarized light navigation (Warren et al., 2018; Wolf et al., 1980) should allow for elaboration of underlying cellular mechanisms via simultaneous monitoring of neural activity and behavior.
Fig. 7.
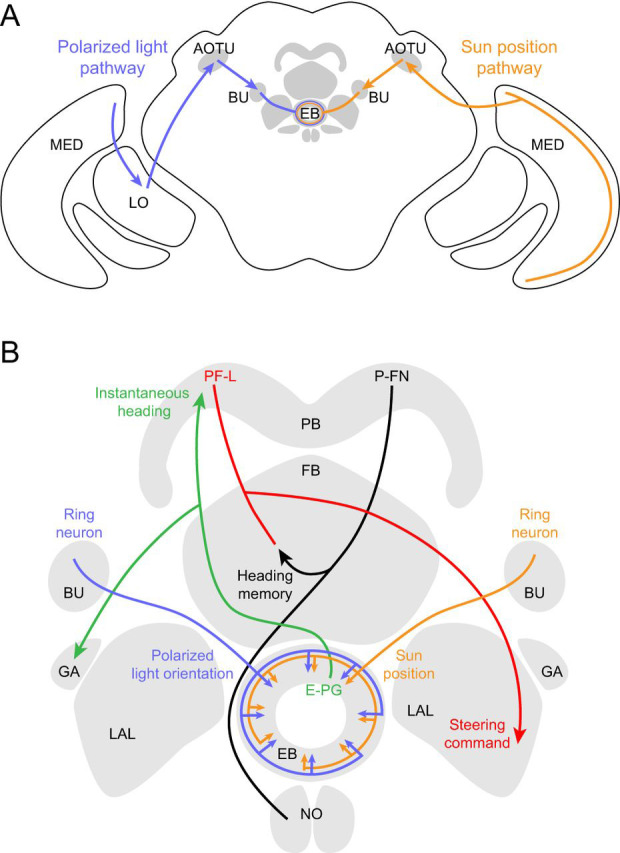
Proposed neural pathways for polarized light and sun position. (A) Polarized light (blue) and sun position (orange) are likely processed along similar pathways, illustrated here for convenience on different sides of the brain. Pathways are based on Schistocerca gregaria and, when available, D. melanogaster. Polarized light is detected by DRA photoreceptors which project to the medulla (Fortini and Rubin, 1991; Weir et al., 2016). In locusts, there are projections from the medulla to the anterior lobe of the lobula and then the lower unit of the anterior optic tubercle (AOTU; Homberg et al., 2003). From the AOTU, neurons project to the bulb (BU, the lateral triangle and median olive in locusts) and synapse onto neurons that terminate in the ellipsoid body (EB, central body lower in locusts). In flies, the polarized light pathway has not been directly characterized beyond the optic lobe but is assumed to be similar. For the sun position pathway (orange), classes of medulla neurons (TIM1, TML1) respond to a bright spot in locusts (el Jundi et al., 2011). In flies, there is a direct pathway from the medulla to the AOTU (Omoto et al., 2017), from where there are projections to the BU and then to the EB. (B) Celestial cue information is processed in the central complex and associated neuropils. Here, we indicate each columnar cell type with a single example for clarity. Ring neurons project from the BU to the EB, likely synapsing with E-PG cells (Omoto et al., 2017). E-PGs encode the fly's instantaneous heading (Seelig and Jayaraman, 2015) and respond to a sun stimulus (Giraldo et al., 2018). The E-PGs tile the EB and 16 medial glomeruli of the protocerebral bridge (PB) and project to the gall (GA), a putative output region (Seelig and Jayaraman, 2015; Wolff et al., 2015). According to an anatomically based model and electrophysiology in bees (Stone et al., 2017), flies might compare their instantaneous heading with the memory of their desired heading using P-FN cells. These P-FN neurons likely integrate input from the E-PGs and visual odometry cells (not shown) and in turn synapse onto PF-L cells (Wolff et al., 2015), which are predicted to control steering through projections to descending motor neurons (Stone et al., 2017). The outline of neuropil regions is modified from Wolff et al. (2015).
Discussion
Do flies possess a matched filter for the sky?
Flies' capacity to use either the pattern of polarized light or the position of the sun to navigate raises the possibility that their brains expect the two cues to be aligned as they would be in the actual sky. Alternatively, polarized light and sun position might be processed independently. In the former model, the fly's visual system creates a matched filter of the sky that makes use of all available information; in the latter model, the fly uses independent sensory channels, perhaps arranged in some hierarchy, with no expectation of the natural alignment. The simplest way to test these hypotheses would be to present flies with a sun stimulus and polarization pattern that are misaligned relative to the actual sky pattern. If the fly's brain contains a matched filter for the sky, such an artificial alignment should evoke weak behavioral responses. Alternatively, if flies process the sun and polarization cues independently, they should disregard the alignment of stimuli and either pay attention to the most salient cue or sum the input from both channels. There is evidence for both of these models in other species. Dung beetles process the sun and polarization channels separately according to a species-specific hierarchy (Dacke et al., 2004; el Jundi et al., 2016). In contrast, desert ants and locusts seem to integrate sun position and e-vector direction to create a matched filter of the sky (el Jundi et al., 2014; Lebhardt and Ronacher, 2014; Wehner, 1997).
As of yet, no one has presented flies with a sun and polarization stimulus to determine whether one stimulus is more salient and whether the fly is confused by unnatural alignments of sun position and polarization pattern. However, flies have been presented with a stimulus in which a polarization pattern was coupled to a linear intensity gradient such that one celestial hemisphere was brighter than the other (Warren et al., 2018). Under these conditions, the flies tended to fly toward the darker hemisphere and did not orient in randomly chosen directions as when presented with the polarization cue alone. This result suggests that strong intensity cues are preeminent over polarization cues. However, navigation performance as measured by vector strength was stronger when the intensity cue was coupled with linearly polarized light than when coupled with circularly polarized light. This would suggest that even if the intensity gradient is a more salient navigation cue, flies still process the polarized light signal if present.
How do flies choose their heading preferences?
All evidence from experiments with both polarized light and a sun stimulus suggests that flies choose a heading at random and then maintain that orientation over time, but how they acquire their initial preference is enigmatic. Based on evidence from other insects, the possible explanations cluster around two extremes: (1) flies possess a developmentally or environmentally pre-programmed heading preference, or (2) flies choose a heading during the first few minutes of flight. Neither of these two hypotheses is entirely consistent with the experiments that we have reviewed thus far. Flies remember their heading preferences from one flight bout to the next for both patterns of polarized light and sun position (e.g. Fig. 5E); however, this result is consistent with both the pre-programmed and choice models, assuming that once a fly selects a heading, it remembers that choice for several hours. The observation that bout-to-bout heading fidelity for sun orientation is maintained for up to 6 h (Fig. 5E) seems to lend weight to the pre-programmed hypothesis. However, datasets for 5 min and 1 h gaps show a closer correspondence between first and second flights than datasets with longer inter-trial intervals. Whether this is reflective of a gradual memory decay or a consequence of the longer durations that flies remained tethered will require additional experiments – for example, testing flies repeatedly over many hours. As noted above, vector strength increases gradually at the beginning of a first closed-loop experiment with a pattern of polarized light – a phenomenon that might reflect a reinforcement process as a fly chooses its heading (Warren et al., 2018). However, this gradual rise in vector strength is not observed at the start of closed-loop experiments using a sun stimulus (Y.M.G., unpublished data). This difference could reflect important distinctions between the sun position and polarization pathways or might merely indicate more mundane differences in the two experimental paradigms. Although it seems imprudent to entirely rule out the pre-programmed hypothesis, the choice model is attractive because it is consistent with a recently described ‘snapshot’ mechanism by which dung beetles acquire their heading preference just before they begin to roll a newly formed dung ball (Baird et al., 2012; el Jundi et al., 2016).
Whether the memory is preprogrammed or quickly chosen at the onset of flight, it must be stored somewhere in the animal's brain. Recently, Stone et al. (2017) published an elaborate model of path integration that provides insight into a likely locus for this orientation memory. In their model of path integration, memories for homing are stored during the search phase of a foraging excursion in the columnar arrays of CPU4 neurons in the fan-shaped body, which are probably homologous with classes of P-FN neurons (protocerebral bridge to fan-shaped body and noduli) in D. melanogaster (Fig. 7B). During an outbound flight, clusters of CPU4 neurons are posited to receive convergent input from the compass neurons and cells encoding visual odometry, allowing the network to store a memory of the path required to return to the nest. In their model, steering commands are executed by columnar CPU1 neurons that have both direct and indirect connections with CPU4 neurons in the fan-shaped body (Stone et al., 2017). CPU1 neurons project from the protocerebral bridge and the fan-shaped body to the lateral accessory lobe (PF-L cells; Hanesch et al., 1989; Wolff et al., 2015); although in Drosophila, this pathway appears to be polysynaptic (Franconville et al., 2018). Given the wiring of the PF-L neurons, their activity is hypothesized to reflect the difference between current heading and goal direction, thus enabling them to generate corrective steering commands that are transmitted to descending motor neurons (Stone et al., 2017). Although this model was developed to explain path integration, its authors suggest that the CPU4 network might be co-opted in migratory animals to store a fixed orientation for menotaxis to sun position (Stone et al., 2017), the pattern of polarization, or a visual landmark (Heinze, 2017). If true, one would expect to record a static pattern of activity across the columns of the P-FN neurons in flies during sun menotaxis (or polarized light orientation), with the phase of activity within the array of P-FN clusters encoding the azimuthal target heading at which the animal maintains the stimulus. Large flight responses observed in visually responsive fan-shaped body neurons are consistent with the importance of cells in this neuropil for visually guided flight behavior (Weir and Dickinson, 2015; Weir et al., 2014). In the future, it should be possible to test this model in flies by performing functional imaging experiments under a 2-photon microscope.
Conclusions
Although the fruit fly's small size and ubiquity as a laboratory model have perhaps obscured its capacity for long-distance migration, the findings reviewed here demonstrate that the fruit fly shares core navigational abilities with more celebrated migrants such as monarch butterflies. The burgeoning tools available for cell-type specific neural monitoring and manipulation during behavior will undoubtedly continue to make D. melanogaster a useful organism for probing cellular mechanisms of navigation. The possibility of studying fruit fly navigation in the field is also promising, as the fly's small size and genetic accessibility make it well suited for large-scale mark–recapture experiments as well as quantitative population genetics. Experiments of this sort could test predictions derived from laboratory experiments: that flies assume arbitrary headings relative to the sun and polarized light, that these preferences develop at different rates depending on stimulus type, that flies can remember headings following an interruption and they can travel long distances using multiple cues. In addition to revealing general, shared principles of insect navigation, studies within the Drosophila genus, with ecologically distinct species and subpopulations, have the potential to identify how differences in migratory behavior emerge among individuals and across taxa.
Acknowledgements
We thank Peter Weir for comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was funded by grants from the National Science Foundation (IOS 1547918), National Institutes of Health (U19NS104655) and the Simons Foundation (71582123) to M.H.D., as well as a National Institutes of Health NRSA postdoctoral fellowship (F32GM109777) to Y.M.G. Deposited in PMC for release after 12 months.
References
- Baines R. A., Uhler J. P., Thompson A., Sweeney S. T. and Bate M. (2001). Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 21, 1523-1531. 10.1523/JNEUROSCI.21-05-01523.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird E., Byrne M. J., Smolka J., Warrant E. J. and Dacke M. (2012). The dung beetle dance: an orientation behaviour? PLoS ONE 7, e30211 10.1371/journal.pone.0030211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M. L. and Gould J. L. (1982). Skylight polarization patterns and animal orientation. J. Exp. Biol. 96, 69-91. [Google Scholar]
- Byrne M., Dacke M., Nordström P., Scholtz C. and Warrant E. (2003). Visual cues used by ball-rolling dung beetles for orientation. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 189, 411-418. 10.1007/s00359-003-0415-1 [DOI] [PubMed] [Google Scholar]
- Censi A., Straw A. D., Sayaman R. W., Murray R. M. and Dickinson M. H. (2013). Discriminating external and internal causes for heading changes in freely flying Drosophila. PLoS Comput. Biol. 9, e1002891 10.1371/journal.pcbi.1002891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar S. (1960). Radiative Transfer. Dover Publications. [Google Scholar]
- Chapman J. W., Reynolds D. R., Smith A. D., Smith E. T. and Woiwod I. P. (2004). An aerial netting study of insects migrating at high altitude over England. Bull. Entomol. Res. 94, 123-136. 10.1079/BER2004287 [DOI] [PubMed] [Google Scholar]
- Chapman J. W., Reynolds D. R., Mouritsen H., Hill J. K., Riley J. R., Sivell D., Smith A. D. and Woiwod I. P. (2008). Wind selection and drift compensation optimize migratory pathways in a high-flying moth. Curr. Biol. 18, 514-518. 10.1016/j.cub.2008.02.080 [DOI] [PubMed] [Google Scholar]
- Chapman J. W., Klaassen R. H. G., Drake V. A., Fossette S., Hays G. C., Metcalfe J. D., Reynolds A. M., Reynolds D. R. and Alerstam T. (2011). Animal orientation strategies for movement in flows. Curr. Biol. 21, R861-R870. 10.1016/j.cub.2011.08.014 [DOI] [PubMed] [Google Scholar]
- Combes S. A., Rundle D. E., Iwasaki J. M. and Crall J. D. (2012). Linking biomechanics and ecology through predator–prey interactions: flight performance of dragonflies and their prey. J. Exp. Biol. 215, 903-913. 10.1242/jeb.059394 [DOI] [PubMed] [Google Scholar]
- Coyne J. A., Boussy I. A., Prout T., Bryant S. H., Jones J. S. and Moore J. A. (1982). Long-distance migration of Drosophila. Am. Nat. 119, 589-595. 10.1086/283936 [DOI] [Google Scholar]
- Cronin T. W. and Marshall J. (2011). Patterns and properties of polarized light in air and water. Philos. Trans. R. Soc. B Biol. Sci. 366, 619-626. 10.1098/rstb.2010.0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacke M., Nilsson D.-E., Scholtz C. H., Byrne M. and Warrant E. J. (2003). Animal behaviour: insect orientation to polarized moonlight. Nature 424, 33 10.1038/424033a [DOI] [PubMed] [Google Scholar]
- Dacke M., Byrne M. J., Scholtz C. H. and Warrant E. J. (2004). Lunar orientation in a beetle. Proc. R. Soc. B 271, 361-365. 10.1098/rspb.2003.2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson M. H. (2014). Death valley, Drosophila, and the Devonian toolkit. Annu. Rev. Entomol. 59, 51-72. 10.1146/annurev-ento-011613-162041 [DOI] [PubMed] [Google Scholar]
- Dickinson M. H. and Muijres F. T. (2016). The aerodynamics and control of free flight manoeuvres in Drosophila. Philos. Trans. R. Soc. B 371, 20150388 10.1098/rstb.2015.0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. and Powell J. R. (1974). Rates of dispersal of Drosophila pseudoobscura and its relatives. Proc. R. Soc. Lond. B 187, 281-298. 10.1098/rspb.1974.0075 [DOI] [PubMed] [Google Scholar]
- el Jundi B., Pfeiffer K. and Homberg U. (2011). A distinct layer of the medulla integrates sky compass signals in the brain of an insect. PLoS ONE 6, e27855 10.1371/journal.pone.0027855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Jundi B., Pfeiffer K., Heinze S. and Homberg U. (2014). Integration of polarization and chromatic cues in the insect sky compass. J. Comp. Physiol. A 200, 575-589. 10.1007/s00359-014-0890-6 [DOI] [PubMed] [Google Scholar]
- el Jundi B., Foster J. J., Khaldy L., Byrne M. J., Dacke M. and Baird E. (2016). A snapshot-based mechanism for celestial orientation. Curr. Biol. 26, 1456-1462. 10.1016/j.cub.2016.03.030 [DOI] [PubMed] [Google Scholar]
- Fenk L. M., Poehlmann A. and Straw A. D. (2014). Asymmetric processing of visual motion for simultaneous object and background responses. Curr. Biol. 24, 2913-2919. 10.1016/j.cub.2014.10.042 [DOI] [PubMed] [Google Scholar]
- Fent K. (1986). Polarized skylight orientation in the desert ant Cataglyphis. J. Comp. Physiol. A 158, 145-150. 10.1007/BF01338557 [DOI] [Google Scholar]
- Fortini M. E. and Rubin G. M. (1991). The optic lobe projection pattern of polarization-sensitive photoreceptor cells in Drosophila melanogaster. Cell Tissue Res. 265, 185-191. 10.1007/BF00318153 [DOI] [PubMed] [Google Scholar]
- Franconville R., Beron C. and Jayaraman V. (2018). Building a functional connectome of the Drosophila central complex. eLife 7, e37017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo Y. M., Leitch K. J., Ros I. G., Warren T. L., Weir P. T. and Dickinson M. H. (2018). Sun navigation requires compass neurons in Drosophila. Curr. Biol. 28, P2845-2852.E4 10.1016/j.cub.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz K. G. (1987). Course-control, metabolism and wing interference during ultralong tethered flight in Drosophila melanogaster. J. Exp. Biol. 128, 35-46. [Google Scholar]
- Hampel S., Franconville R., Simpson J. H. and Seeds A. M. (2015). A neural command circuit for grooming movement control. eLife 4, e08758 10.7554/eLife.08758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanesch U., Fischbach K.-F. and Heisenberg M. (1989). Neuronal architecture of the central complex in Drosophila melanogaster. Cell Tissue Res. 257, 343-366. 10.1007/BF00261838 [DOI] [Google Scholar]
- Hardie R. C. (1984). Properties of photoreceptors R7 and R8 in dorsal marginal ommatidia in the compound eyes of Musca and Calliphora. J. Comp. Physiol. A 154, 157-165. 10.1007/BF00604981 [DOI] [Google Scholar]
- Hardie R. C. (2012). Polarization vision: Drosophila enters the arena. Curr. Biol. 22, R12-R14. 10.1016/j.cub.2011.11.016 [DOI] [PubMed] [Google Scholar]
- Heed W. B. (1978). Ecology and genetics of Sonoran desert Drosophila. In Ecological Genetics: The Interface, pp. 109-126. Springer. [Google Scholar]
- Hegedüs R., Åkesson S. and Horváth G. (2007). Polarization patterns of thick clouds: overcast skies have distribution of the angle of polarization similar to that of clear skies. JOSA A 24, 2347-2356. 10.1364/JOSAA.24.002347 [DOI] [PubMed] [Google Scholar]
- Heinloth T., Uhlhorn J. and Wernet M. F. (2018). Insect responses to linearly polarized reflections: orphan behaviors without neural circuits. Front. Cell. Neurosci. 12, 50 10.3389/fncel.2018.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S. (2017). Unraveling the neural basis of insect navigation. Curr. Opin. Insect Sci. 24, 58-67. 10.1016/j.cois.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S. and Homberg U. (2009). Linking the input to the output: new sets of neurons complement the polarization vision network in the locust central complex. J. Neurosci. 29, 4911-4921. 10.1523/JNEUROSCI.0332-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. (1972). Comparative behavioral studies on two visual mutants of Drosophila. J. Comp. Physiol. 80, 119-136. 10.1007/BF00696485 [DOI] [Google Scholar]
- Heisenberg M. and Wolf R. (1984). Menotaxis. In Vision in Drosophila - Genetics of Microbehaviour, pp. 146-157. Berlin: Springer. [Google Scholar]
- Homberg U. (2015). Sky compass orientation in desert locusts—evidence from field and laboratory studies. Front. Behav. Neurosci. 9, 346 10.3389/fnbeh.2015.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg U., Hofer S., Pfeiffer K. and Gebhardt S. (2003). Organization and neural connections of the anterior optic tubercle in the brain of the locust, Schistocerca gregaria. J. Comp. Neurol. 462, 415-430. 10.1002/cne.10771 [DOI] [PubMed] [Google Scholar]
- Homberg U., Heinze S., Pfeiffer K., Kinoshita M. and el Jundi B. (2011). Central neural coding of sky polarization in insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 680-687. 10.1098/rstb.2010.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J. N. and Wilson M. (1976). Absorption characteristics of oriented photopigments in microvilli. Biol. Cybern. 21, 9-15. 10.1007/BF00326667 [DOI] [PubMed] [Google Scholar]
- Jander R. (1960). Menotaxis und Winkeltransponieren bei Köcherfliegen (Trichoptera). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 43, 680-686. [Google Scholar]
- Jones J., Bryant S., Lewontin R., Moore J. and Prout T. (1981). Gene flow and the geographical distribution of a molecular polymorphism in Drosophila pseudoobscura. Genetics 98, 157-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. S., Rouault H., Druckmann S. and Jayaraman V. (2017). Ring attractor dynamics in the Drosophila central brain. Science 356, 849-853. 10.1126/science.aal4835 [DOI] [PubMed] [Google Scholar]
- Kinoshita M., Pfeiffer K. and Homberg U. (2007). Spectral properties of identified polarized-light sensitive interneurons in the brain of the desert locust Schistocerca gregaria. J. Exp. Biol. 210, 1350-1361. 10.1242/jeb.02744 [DOI] [PubMed] [Google Scholar]
- Labhart T. (2016). Can invertebrates see the e-vector of polarization as a separate modality of light? J. Exp. Biol. 219, 3844-3856. 10.1242/jeb.139899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labhart T. and Meyer E. P. (1999). Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc. Res. Tech. 47, 368-379. [DOI] [PubMed] [Google Scholar]
- Lack D. and Lack E. (1951). Migration of insects and birds through a Pyrenean pass. J. Anim. Ecol. 20, 63-67. 10.2307/1644 [DOI] [Google Scholar]
- Lebhardt F. and Ronacher B. (2014). Interactions of the polarization and the sun compass in path integration of desert ants. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 200, 711-720. 10.1007/s00359-013-0871-1 [DOI] [PubMed] [Google Scholar]
- Lehmann F. O. and Dickinson M. H. (1997). The changes in power requirements and muscle efficiency during elevated force production in the fruit fly Drosophila melanogaster. J. Exp. Biol. 200, 1133-1143. [DOI] [PubMed] [Google Scholar]
- Lindauer M. (1960). Time-compensated sun orientation in bees. In Cold Spring Harbor Symposia on Quantitative Biology, pp. 371-377. Cold Spring Harbor Laboratory Press. [DOI] [PubMed] [Google Scholar]
- Mappes M. and Homberg U. (2004). Behavioral analysis of polarization vision in tethered flying locusts. J. Comp. Physiol. A 190, 61-68. 10.1007/s00359-003-0473-4 [DOI] [PubMed] [Google Scholar]
- Maxwell J. C. (1865). VIII. A dynamical theory of the electromagnetic field. Philos. Trans. R. Soc. Lond. 155, 459-512. 10.1098/rstl.1865.0008 [DOI] [Google Scholar]
- Merlin C., Gegear R. J. and Reppert S. M. (2009). Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies. Science 325, 1700-1704. 10.1126/science.1176221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H. and Frost B. J. (2002). Virtual migration in tethered flying monarch butterflies reveals their orientation mechanisms. Proc. Natl Acad. Sci. USA 99, 10162-10166. 10.1073/pnas.152137299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H., Derbyshire R., Stalleicken J., Mouritsen O. Ø., Frost B. J. and Norris D. R. (2013). An experimental displacement and over 50 years of tag-recoveries show that monarch butterflies are not true navigators. Proc. Natl. Acad. Sci. USA 110, 7348-7353. 10.1073/pnas.1221701110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. and Wehner R. (2007). Wind and sky as compass cues in desert ant navigation. Naturwissenschaften 94, 589-594. 10.1007/s00114-007-0232-4 [DOI] [PubMed] [Google Scholar]
- Omoto J. J., Keleş M. F., Nguyen B.-C. M., Bolanos C., Lovick J. K., Frye M. A. and Hartenstein V. (2017). Visual input to the Drosophila central complex by developmentally and functionally distinct neuronal populations. Curr. Biol. 27, 1098-1110. 10.1016/j.cub.2017.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegel U., Pfeiffer K. and Homberg U. (2018). Integration of celestial compass cues in the central complex of the locust brain. J. Exp. Biol. 221, jeb171207 10.1242/jeb.171207 [DOI] [PubMed] [Google Scholar]
- Perez S. M., Taylor O. R. and Jander R. (1997). A sun compass in monarch butterflies. Nature 387, 29 10.1038/387029a0 [DOI] [Google Scholar]
- Pfeiffer K. and Homberg U. (2007). Coding of azimuthal directions via time-compensated combination of celestial compass cues. Curr. Biol. 17, 960-965. 10.1016/j.cub.2007.04.059 [DOI] [PubMed] [Google Scholar]
- Pfeiffer K. and Homberg U. (2014). Organization and functional roles of the central complex in the insect brain. Annu. Rev. Entomol. 59, 165-184. 10.1146/annurev-ento-011613-162031 [DOI] [PubMed] [Google Scholar]
- Pomozi I., Horváth G. and Wehner R. (2001). How the clear-sky angle of polarization pattern continues underneath clouds: full-sky measurements and implications for animal orientation. J. Exp. Biol. 204, 2933-2942. [DOI] [PubMed] [Google Scholar]
- Powell J. R. (1997). Progress and Prospects in Evolutionary Biology: The Drosophila Model. Oxford University Press. [Google Scholar]
- Reppert S. M., Gegear R. J. and Merlin C. (2010). Navigational mechanisms of migrating monarch butterflies. Trends Neurosci. 33, 399-406. 10.1016/j.tins.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert S. M., Guerra P. A. and Merlin C. (2016). Neurobiology of monarch butterfly migration. Annu. Rev. Entomol. 61, 25-42. 10.1146/annurev-ento-010814-020855 [DOI] [PubMed] [Google Scholar]
- Ristroph L., Bergou A. J., Ristroph G., Coumes K., Berman G. J., Guckenheimer J., Wang Z. J. and Cohen I. (2010). Discovering the flight autostabilizer of fruit flies by inducing aerial stumbles. Proc. Natl. Acad. Sci. USA 107, 4820-4824. 10.1073/pnas.1000615107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossel S. (1993). Navigation by bees using polarized skylight. Comp. Biochem. Physiol. A Physiol. 104, 695-708. 10.1016/0300-9629(93)90146-U [DOI] [Google Scholar]
- Rossel S. and Wehner R. (1986). Polarization vision in bees. Nature 323, 128 10.1038/323128a0 [DOI] [Google Scholar]
- Schnaitmann C., Garbers C., Wachtler T. and Tanimoto H. (2013). Color discrimination with broadband photoreceptors. Curr. Biol. 23, 2375-2382. 10.1016/j.cub.2013.10.037 [DOI] [PubMed] [Google Scholar]
- Schnaitmann C., Haikala V., Abraham E., Oberhauser V., Thestrup T., Griesbeck O. and Reiff D. F. (2018). Color processing in the early visual system of Drosophila. Cell 172, 318-330.e18. 10.1016/j.cell.2017.12.018 [DOI] [PubMed] [Google Scholar]
- Seelig J. D. and Jayaraman V. (2013). Feature detection and orientation tuning in the Drosophila central complex. Nature 503, 262-266. 10.1038/nature12601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig J. D. and Jayaraman V. (2015). Neural dynamics for landmark orientation and angular path integration. Nature 521, 186-191. 10.1038/nature14446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souman J. L., Frissen I., Sreenivasa M. N. and Ernst M. O. (2009). Walking straight into circles. Curr. Biol. 19, 1538-1542. 10.1016/j.cub.2009.07.053 [DOI] [PubMed] [Google Scholar]
- Stephens G. C., Fingerman M. and Brown F. A. Jr (1953). The orientation of Drosophila to plane polarized light. Ann. Entomol. Soc. Am. 46, 75-83. 10.1093/aesa/46.1.75 [DOI] [Google Scholar]
- Stone T., Webb B., Adden A., Weddig N. B., Honkanen A., Templin R., Wcislo W., Scimeca L., Warrant E. and Heinze S. (2017). An anatomically constrained model for path integration in the bee brain. Curr. Biol. 27, 3069-3085.e11. 10.1016/j.cub.2017.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld N. J. (2012). Arthropod Brains: Evolution, Functional Elegance, and Historical Significance. Cambridge: Belknap Press of Harvard University Press. [Google Scholar]
- Strauss R. (2002). The central complex and the genetic dissection of locomotor behaviour. Curr. Opin. Neurobiol. 12, 633-638. 10.1016/S0959-4388(02)00385-9 [DOI] [PubMed] [Google Scholar]
- Strutt J. W. (1871). XV. On the light from the sky, its polarization and colour. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 41, 107-120. 10.1080/14786447108640452 [DOI] [Google Scholar]
- Taube J. S. (2007). The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci. 30, 181-207. 10.1146/annurev.neuro.29.051605.112854 [DOI] [PubMed] [Google Scholar]
- Träger U., Wagner R., Bausenwein B. and Homberg U. (2008). A novel type of microglomerular synaptic complex in the polarization vision pathway of the locust brain. J. Comp. Neurol. 506, 288-300. 10.1002/cne.21512 [DOI] [PubMed] [Google Scholar]
- van Breugel F. , and Dickinson M. H. (2012). The visual control of landing and obstacle avoidance in the fruit fly Drosophila melanogaster. J. Exp. Biol. 215, 1783-1798. 10.1242/jeb.066498 [DOI] [PubMed] [Google Scholar]
- von Frisch K. (1948). Gelöste und ungelöste Rätsel der Bienensprache. Naturwissenschaften 35, 12-23. 10.1007/BF00626624 [DOI] [Google Scholar]
- von Frisch K. (1949). Die Polarisation des Himmelslichtes als orientierender Faktor bei den Tänzen der Bienen. Experientia 5, 142-148. 10.1007/BF02174424 [DOI] [PubMed] [Google Scholar]
- Warren T. L., Weir P. T. and Dickinson M. H. (2018). Flying Drosophila melanogaster maintain arbitrary but stable headings relative to the angle of polarized light. J. Exp. Biol. 221, jeb177550 10.1242/jeb.177550 [DOI] [PubMed] [Google Scholar]
- Wasserman S., Lu P., Aptekar J. W. and Frye M. A. (2012). Flies dynamically anti-track, rather than ballistically escape, aversive odor during flight. J. Exp. Biol. 215, 2833-2840. 10.1242/jeb.072082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B. and Wystrach A. (2016). Neural mechanisms of insect navigation. Curr. Opin. Insect Sci. 15, 27-39. 10.1016/j.cois.2016.02.011 [DOI] [PubMed] [Google Scholar]
- Wehner R. (1997). The ant's celestial compass system: spectral and polarization channels. In Orientation and Communication in Arthropods (ed. M. Lehrer), pp. 145-185. Basel: Springer. [Google Scholar]
- Wehner R. (2003). Desert ant navigation: how miniature brains solve complex tasks. J. Comp. Physiol. A 189, 579-588. 10.1007/s00359-003-0431-1 [DOI] [PubMed] [Google Scholar]
- Wehner R. and Strasser S. (1985). The POL area of the honey bee's eye: behavioural evidence. Physiol. Entomol. 10, 337-349. 10.1111/j.1365-3032.1985.tb00055.x [DOI] [Google Scholar]
- Wehner R., Bernard G. D. and Geiger E. (1975). Twisted and non-twisted rhabdoms and their significance for polarization detection in the bee. J. Comp. Physiol. 104, 225-245. 10.1007/BF01379050 [DOI] [Google Scholar]
- Weir P. T. and Dickinson M. H. (2012). Flying Drosophila orient to sky polarization. Curr. Biol. 22, 21-27. 10.1016/j.cub.2011.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir P. T. and Dickinson M. H. (2015). Functional divisions for visual processing in the central brain of flying Drosophila. Proc. Natl. Acad. Sci. USA 112, E5523-E5532. 10.1073/pnas.1514415112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir P. T., Schnell B. and Dickinson M. H. (2014). Central complex neurons exhibit behaviorally gated responses to visual motion in Drosophila. J. Neurophysiol. 111, 62-71. 10.1152/jn.00593.2013 [DOI] [PubMed] [Google Scholar]
- Weir P. T., Henze M. J., Bleul C., Baumann-Klausener F., Labhart T. and Dickinson M. H. (2016). Anatomical reconstruction and functional imaging reveal an ordered array of skylight polarization detectors in Drosophila. J. Neurosci. 36, 5397-5404. 10.1523/JNEUROSCI.0310-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet M. F., Labhart T., Baumann F., Mazzoni E. O., Pichaud F. and Desplan C. (2003). Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell 115, 267-279. 10.1016/S0092-8674(03)00848-1 [DOI] [PubMed] [Google Scholar]
- Wernet M. F., Velez M. M., Clark D. A., Baumann-Klausener F., Brown J. R., Klovstad M., Labhart T. and Clandinin T. R. (2012). Genetic dissection reveals two separate retinal substrates for polarization vision in Drosophila. Curr. Biol. 22, 12-20. 10.1016/j.cub.2011.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R., Gebhardt B., Gademann R. and Heisenberg M. (1980). Polarization sensitivity of course control in Drosophila melanogaster. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 139, 177-191. 10.1007/BF00657080 [DOI] [Google Scholar]
- Wolff T., Iyer N. A. and Rubin G. M. (2015). Neuroarchitecture and neuroanatomy of the Drosophila central complex: a GAL4-based dissection of protocerebral bridge neurons and circuits. J. Comp. Neurol. 523, 997-1037. 10.1002/cne.23705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderer H. and Smola U. (1982). Fine structure of ommatidia at the dorsal eye margin of Calliphora erythrocephala Meigen (Diptera: Calliphoridae): an eye region specialised for the detection of polarized light. Int. J. Insect Morphol. Embryol. 11, 25-38. 10.1016/0020-7322(82)90035-6 [DOI] [Google Scholar]