Abstract
Hydroxychloroquine (HCQ)-induced hyperpigmentation is uncommon but is increasingly recognized. To our knowledge, HCQ-induced hyperpigmentation has not been reported in the pediatric age group. Herein, we present the case of a 14-year-old girl with systemic lupus erythematosus, who developed hyperpigmentation on her shins and dorsum of the left foot, approximately 3 years after initiating treatment with HCQ. Physicians who treat children with HCQ for reasons such as rheumatologic disorders, dermatologic disorders and, more recently, coronavirus disease-19 should be aware of this less-known side effect of HCQ.
Keywords: chloroquine, COVID-19, dermatologic disorders, melanin, rheumatologic disorders
Introduction
Chloroquine-induced hyperpigmentation is well known.1 On the other hand, hydroxychloroquine (HCQ)-induced hyperpigmentation is an uncommon but increasingly recognized side effect of HCQ therapy.1 The hyperpigmentation may develop from a few months to a few years following the initiation of HCQ therapy.2,3 To our knowledge, HCQ-induced hyperpigmentation has not been reported in the pediatric age group. Herein, we report on the case of a 14-year-old girl with systemic lupus erythematosus (SLE), who developed hyperpigmentation on her shins and dorsum of the left foot after treatment with HCQ for 3 years. No review board approval was necessary and was therefore not obtained. Signed consent was obtained.
Case report
A 14-year-old Indigenous Canadian female with SLE presented with a 2-year history of asymptomatic hyperpigmentation on the bilateral shins and the dorsum of the left foot. The hyperpigmented patches first appeared over the bilateral shins and the dorsum of the left foot in areas of previous bruising. There was no history of trauma.
The patient’s symptoms began at the age of 9 years, when she presented with polyarthritis, low serum C3 and C4 levels, positive antinuclear antibodies, anti-double-stranded DNA antibodies, and anti-Smith antibodies. She was seen by a pediatric rheumatologist who diagnosed her with SLE based on the clinical and laboratory findings. The patient was treated with oral methotrexate, 20 mg, weekly and oral HCQ at a dose of 1800 mg per week (200 mg daily on weekdays and 400 mg daily on weekends, averaging 4.8 mg/kg/day). During the course of her illness, she was also treated with cyclophosphamide, corticosteroids, rituximab, and mycophenolate mofetil at various points in time. She did not have a known coagulopathy and was not on anticoagulation therapy. Physical examination revealed Fitzpatrick type IV skin with symmetrical, bluish-grey patches bilaterally on the pretibial areas and dorsum of the left foot (Figure 1). There was no pigmentation at other body sites including the oral mucosa and the nails. A clinical diagnosis of HCQ-induced hyperpigmentation was made based on the typical appearance of symmetrical, bluish-grey patches bilaterally on both shins. The HCQ was discontinued. However, the patient expired from complications of lupus prior to the observance of any effect.
Figure 1.
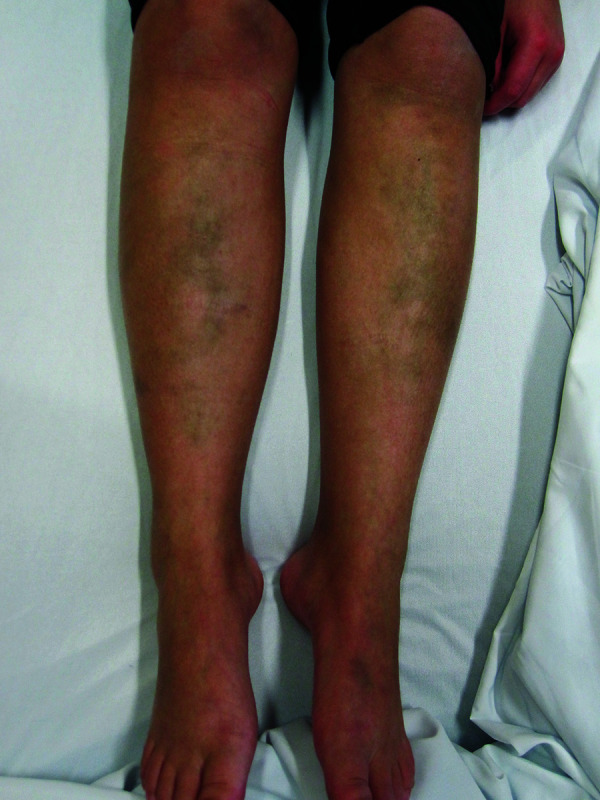
Bilateral bluish-grey patches on the shins and dorsum of the left foot.
Discussion
HCQ was first developed as an antimalarial agent, and is now also used in the treatment of SLE, juvenile idiopathic arthritis, rheumatoid arthritis, Sjogren syndrome, dermatomyositis, actinic lichen planus, oral lichen planus, and sarcoidosis owing to its anti-inflammatory and immune-modulating properties.1,4–7 Recently, HCQ has also been used in the treatment of coronavirus disease-19 (COVID-19).8–10 HCQ has also been suggested as a candidate prophylactic agent for COVID-19 in populations at high risk for COVID-19, such as high-risk groups in New York and Italy.11 HCQ is preferred to chloroquine because it is less toxic. For the treatment of COVID-19, HCQ works by blocking severe acute respiratory syndrome-related coronavirus (the causative virus for COVID-19) viral entry into host cells through inhibition of angiotensin-converting enzyme 2 receptor glycosylation, reducing viral replication, and blocking the export of newly constructed virions.8–10
HCQ has a relatively favorable safety profile, is generally well tolerated, and is readily available at relatively low cost.4 HCQ-induced hyperpigmentation of the skin is increasingly recognized as a side effect of HCQ therapy.12 Although infrequently reported in the literature, a recent cross-sectional study found that 29% of patients on HCQ developed HCQ-induced hyperpigmentation.2 In this regard, hyperpigmentation associated with chloroquine therapy is more common than that of HCQ.1,13 Typically, HCQ-induced hyperpigmentation presents as bluish, blue–grey macules/patches most commonly on the shins, but can also been seen on the arms, forearms, face, oral mucosa, trunk, nails, and axilla.2,4,6,14 There is no apparent predilection for sun-exposed areas and oral mucocutaneous hyperpigmentation also occurs.14,15 The involvement is typically bilateral,14 although unilateral involvement has rarely been reported.16 The differential diagnosis includes erythema nodosum, ecchymosis, minocycline dyspigmentation, and erysipelas.17 The color, chronicity, and associated HCQ use help to distinguish HCQ-induced hyperpigmentation from these other disorders. There are no formal criteria for HCQ-induced hyperpigmentation as the diagnosis is usually clinically apparent. A skin biopsy is often not required but can help confirm the diagnosis.
Our patient presented with symmetrical, poorly defined, grey patches bilaterally on the shins and the dorsum of the left foot. Cutaneous pigmentation occurs in 10–25% of patients after a few months to a few years of treatment with HCQ.2,3,6,18 In one study of 41 patients treated with HCQ, pigmentation appeared after a median duration of HCQ treatment of 32 months (range 6–108 months).2 There is no clear association with duration of treatment or cumulative dose of HCQ.19 In the present case, the child developed hyperpigmentation on the shins and dorsum of the right foot approximately 3 years after initiating HCQ therapy. To our knowledge, HCQ-induced hyperpigmentation of the skin has not been reported in children. As such, our patient represents the first case reported in the pediatric age group. HCQ-induced hyperpigmentation usually resolves within 6 months after HCQ has been discontinued6,13,20,21 but, occasionally, the hyperpigmentation may persist.22 If possible, HCQ should be substituted with another medication that has similar therapeutic effects. The use of a Q-switched 755-nm alexandrite laser may be considered for the treatment of persistent hyperpigmentation.
The exact pathogenesis for the development of HCQ-induced hyperpigmentation is not known. A strong association has been found with preceding ecchymosis or bruising, which suggests that the mechanism may be, at least in part, due to localized trauma.6 Other predisposing factors include the use of oral anticoagulants or antiplatelet agents, long-term use of corticosteroids, and antiphospholipid syndrome.2,3,13,18,19 HCQ-induced damage of dermal vessels with leakage of erythrocytes is another possibility.1 Histopathological examination of the hyperpigmented lesion shows yellow–brown granules within macrophages in the dermis, melanin in reticular dermis, and perivascular iron/hemosiderin on special staining.6,23 HCQ can accumulate within the skin and has a strong binding affinity to melanin.4,14,24 It has been suggested that the hyperproduction of melanin by epidermal melanocytes can be under the direct influence of HCQ.1 HCQ-induced hyperpigmentation is more common in dark-skinned individuals likely due to greater melanin levels in dark skin.2
Other HCQ-induced cutaneous manifestations include xerosis, pruritis, nail hyperpigmentation, hair discoloration, alopecia, urticarial and lichenoid skin rash, toxic epidermal necrolysis, Stevens–Johnson syndrome, and exacerbation of pre-existing psoriasis.1,6,25 Non-cutaneous adverse effects of HCQ include nausea, diarrhea, prolongation of QT-interval, corneal lens opacity, retinopathy, ciliary body dysfunction, posterior subcapsular lens opacity, myopathy, and ochronosis.5,11,26,27 Some authors suggest that HCQ-induced hyperpigmentation is a marker of increased risk of retinopathy.16,20,27
Conclusion
HCQ-induced hyperpigmentation of the skin is increasingly recognized as a side effect of HCQ therapy. Typically, HCQ-induced hyperpigmentation presents as bluish, blue–grey macules/patches most commonly on the shins. To our knowledge, the condition has not been described in the pediatric age group. HCQ has been used widely in the treatment of a variety of rheumatologic and dermatologic conditions and, recently, by some physicians in the prophylaxis and treatment of COVID-19. Physicians who use HCQ in children should be aware of this less-known side effect of HCQ so that an accurate diagnosis can be made, and unnecessary investigations and inappropriate treatment can be avoided.
Acknowledgements
None.
Footnotes
Contributions: Professor Alexander KC Leung wrote the first draft of the article and oversaw the manuscript creation. Dr McMillan, Dr Human and Dr Lam were involved with the care of this patient and helped with the draft and revision of this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: Professor Alexander KC Leung is an associate editor of Drugs in Context and confirms that this article has no conflicts of interest otherwise. This manuscript was sent out for independent peer review by the Editor-in-Chief. All other authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/07/dic.2020-5-8-COI.pdf
Funding declaration: Professor Leung, Dr McMillan, Dr Human, and Dr Lam disclose no relevant financial relationship. There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2020 Leung AKC, McMillan T, Human A, Lam JM. https://doi.org/10.7573/dic.2020-5-8. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Peer review comments to author: 12 June 2020\
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Cho EB, Kim BC, Park EJ, et al. Hydroxychloroquine-induced hyperpigmentation. J Dermatol. 2012;39(10):859–860. doi: 10.1111/j.1346-8138.2012.01591.x. [DOI] [PubMed] [Google Scholar]
- 2.Bahloul E, Jallouli M, Garbaa S, et al. Hydroxychloroquine-induced hyperpigmentation in systemic diseases: prevalence, clinical features and risk factors: a cross-sectional study of 41 cases. Lupus. 2017;26(12):1304–1308. doi: 10.1177/0961203317700486. [DOI] [PubMed] [Google Scholar]
- 3.Ivo R, Lopes CA, Reis R. Woman in grey: hydroxychloroquine-induced hyperpigmentation. BMJ Case Rep. 2018;11(1):e227305. doi: 10.1136/bcr-2018-227305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melikoglu MA, Melikoglu M, Gurbuz U, Budak BS, Kacar C. Hydroxychloroquine-induced hyperpigmentation: a case report. J Clin Pharm Ther. 2008;33(6):699–701. doi: 10.1111/j.1365-2710.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 5.Stokkermans TJ, Trichonas G. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020 Jan–2019 Jun 4. Chloroquine and hydroxychloroquine toxicity. [PubMed] [Google Scholar]
- 6.Thakur V, Dalla A, Kumar S, Kumaran MS, Aggarwal D, Radotra BD. Hydroxychloroquine induced cutaneous pigmentation: a unique pattern. Postgrad Med J. 2019;95(1121):169–170. doi: 10.1136/postgradmedj-2018-136377. [DOI] [PubMed] [Google Scholar]
- 7.Yeshurun A, Bergman R, Bathish N, Khamaysi Z. Hydroxychloroquine sulphate therapy of erosive oral lichen planus. Australas J Dermatol. 2019;60(2):e109–e112. doi: 10.1111/ajd.12948. [DOI] [PubMed] [Google Scholar]
- 8.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 9.Shen K, Yang Y, Wang T, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J Pediatr. 2020:1–9. doi: 10.1007/s12519-020-00343-7. . Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hon KL, Leung KKY, Leung AKC, et al. Coronavirus disease 2019 (COVID-19): latest developments in potential treatments. Drugs Context. 2020;9:2020-4-15. doi: 10.7573/dic.2020-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Principi N, Susanna Esposito S. Chloroquine or hydroxychloroquine for prophylaxis of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30296-6. S1473-3099(20)30296-6. . Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chacón-Dulcey V, López-Labady J, Villarroel-Dorrego M, et al. Oral manifestations associated with antimalarial therapy in patients with systemic lupus erythematosus. Lupus. 2020 doi: 10.1177/0961203320922620. 961203320922620. . Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Sawalha AH. Hydroxychloroquine-induced hyperpigmentation of the skin. J Rheumatol. 2015;42(1):135–136. doi: 10.3899/jrheum.140995. [DOI] [PubMed] [Google Scholar]
- 14.Adnan Mir A, Boyd KP, Meehan SA, McLellan B. Hydroxycholoroquine-induced hyperpigmentation. Dermatol Online J. 2013;19(12):20723. [PubMed] [Google Scholar]
- 15.Rood MJ, Vermeer MH, Huizinga TW. Hyperpigmentation of the skin due to hydroxychloroquine. Scand J Rheumatol. 2008;37(2):158. doi: 10.1080/03009740701769735. [DOI] [PubMed] [Google Scholar]
- 16.True DG, Bryant LR, Harris MD, Bernert RA. Clinical images: hydroxychloroquine-associated mucocutaneous hyperpigmentation. Arthritis Rheum. 2002;46(6):1698. doi: 10.1002/art.10278. [DOI] [PubMed] [Google Scholar]
- 17.Leung AKC, Leong KF, Lam JM. Erythema nodosum. World J Pediatr. 2018;14(6):548–554. doi: 10.1007/s12519-018-0191-1. [DOI] [PubMed] [Google Scholar]
- 18.Jallouli M, Francès C, Piette J-C, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case control study. JAMA Dermatol. 2013;149(8):935–940. doi: 10.1001/jamadermatol.2013.709. [DOI] [PubMed] [Google Scholar]
- 19.Coulombe J, Boccara O. Hydroxychloroquine-related skin discoloration. CMAJ. 2017;189(5):E212. doi: 10.1503/cmaj.150622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amichai B, Gat A, Grunwald MH. Cutaneous hyperpigmentation during therapy with hydroxychloroquine. J Clin Rheumatol. 2007;13(2):113. doi: 10.1097/01.rhu.0000260649.36417.09. [DOI] [PubMed] [Google Scholar]
- 21.Millard TP, Kirk A, Ratnavel R. Cutaneous hyperpigmentation during therapy with hydroxychloroquine. Clin Exp Dermatol. 2004;29(1):92–93. doi: 10.1111/j.1365-2230.2004.01412.x. [DOI] [PubMed] [Google Scholar]
- 22.Morrison LK, Nordlund JJ, Heffernan MP. Persistent cutaneous hyperpigmentation due to hydroxychloroquinone one year after therapy discontinuation. Dermatol Online J. 2009;15(12):15. [PubMed] [Google Scholar]
- 23.Puri PK, Lountzis NI, Tyler W, Ferringer T. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35(12):1134–1137. doi: 10.1111/j.1600-0560.2008.01004.x. [DOI] [PubMed] [Google Scholar]
- 24.Ings RM. The melanin binding of drugs and its implications. Drug Metab Rev. 1984;15(5–6):1183–1212. doi: 10.3109/03602538409033561. [DOI] [PubMed] [Google Scholar]
- 25.Sharma AN, Mesinkovska NA, Paravar T. Characterizing the adverse dermatologic effects of hydroxychloroquine: a systematic review. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.04.024. S0190-9622(20)30564-305648. [DOI] [PubMed] [Google Scholar]
- 26.Kwon JB, Kleiner A, Ishida K, Godown J, Ciafaloni E, Looney RJ., Jr Hydroxychloroquine-induced myopathy. J Clin Rheumatol. 2010;16(1):28–31. doi: 10.1097/RHU.0b013e3181c47ec8. [DOI] [PubMed] [Google Scholar]
- 27.Tekgöz E, Akıncıoğlu E, Çınar M, Yılmaz S. A case of exogenous ochronosis associated with hydroxychloroquine. Eur J Rheumatol. 2018;5(3):206–208. doi: 10.5152/eurjrheum.2018.17190. [DOI] [PMC free article] [PubMed] [Google Scholar]

