Abstract
Background:
Transcatheter aortic valve replacement (TAVR) has increased in volume as an alternative to surgical aortic valve replacement (SAVR). Comparisons of total episode expenditures, while largely ignored thus far, will be key to the value proposition for payers.
Methods:
We evaluated 6,359 Blue Cross Blue Shield of Michigan and Medicare fee-for-service beneficiaries undergoing TAVR (17 hospitals, n=1,655) or SAVR (33 hospitals, n=4,704) in Michigan between 2012 and 2016. Payments through 90 post-discharge days between TAVR and SAVR were price-standardized and risk-adjusted. Centers were divided into terciles of procedural volume separately for TAVR and SAVR, and payments were compared between lowest and highest terciles.
Results:
Payments (± standard deviation) were higher for TAVR than SAVR ($69,388 ± $22,259 vs. $66,683 ± $27,377, p<0.0001), while mean hospital length of stay was shorter for TAVR (6.2 ± 5.6 days vs. 10.2 + 7.5, p<0.0001). Index hospitalization payments were $4,374 higher for TAVR (p<0.0001), while readmission and post-acute care payments were $1,150 (p=0.0007) and $739 (p=0.004) lower, respectively, and professional payments were similar. For SAVR, high volume centers had lower episode payments (difference: 5.0%, $3,255; p=0.01) and shorter length of stay (10.0 ± 7.5 vs. 11.1 ± 7.9 days, p=0.002) than low volume centers. In contrast, we found no volume-payment relationship among TAVR centers.
Conclusions:
Episode payments were higher for TAVR, despite shorter length of stay. While not a driver for TAVR, center SAVR volume was inversely associated with payments. These data will be increasingly important to address value-based reimbursement in valve replacement surgery.
Classifications: Aortic valve, replacement, Health economics, Heart valve replacement, percutaneous, Heart valve prosthesis
INTRODUCTION
Severe aortic stenosis affects millions of Americans, with an incidence rate of 4.9% per year and exponential increase in prevalence with age [1–4]. If not treated with surgical intervention, severe aortic stenosis carries a 50% mortality rate within 2 years [5–7]. Transcatheter aortic valve replacement (TAVR) has emerged as a clinically viable, though more expensive alternative to surgical aortic valve replacement (SAVR), gaining regulatory approval [8–10] and Centers for Medicare and Medicaid Services coverage [11] for use in patients at extreme, high, and intermediate risk for open surgical replacement.
In Michigan, TAVRs are now performed more frequently than SAVRs. Published series using hospital charges have reported higher hospital costs associated with TAVR [12–15]. However, total episode payments are a better reflection of the payers’ (and society’s) perspective on cost, as they reflect the actual realized cost of the operation and its associated postoperative care and are thus more relevant to considerations of the impact of national payment reform such as bundled payments and value-based referral. While not performed thus far, financial viability (from the perspective of hospitals and payers) of TAVR, relative to SAVR, requires further evaluation of administrative costs, as assessed through real-world reimbursement data over an episode of care. Because most work to date has focused on clinical outcomes, less is known concerning differences in episode payments. Additionally, while prior analyses [12,16] have found increased SAVR volume with the introduction of TAVR and improved outcomes in higher volume centers for both TAVR [17–20] and SAVR [21–22], there is little evidence on the relationship between procedure volume and economic outcomes.
We compared total and component 90-day episode payments for TAVR and SAVR in Michigan and evaluated the relationship between hospital procedure volume and payments.
PATIENTS AND METHODS
This study was approved by the Institutional Review Board of the University of Michigan Health System (HUM00130122), notice of determination of “not regulated” status.
Patient Population
The Michigan Value Collaborative (MVC), which now involves 79 acute care hospitals, began in 2013 with a goal of helping Michigan hospitals achieve their best possible patient outcomes at the lowest reasonable cost. Working in conjunction with many specialty-specific Collaborative Quality Improvement programs in Michigan, MVC also aims to understand variation in healthcare use, identify best practices, and lead interventions for improving care before, during, and after hospitalization. The MVC is a partnership between Michigan hospitals and the state’s largest commercial payer (Blue Cross and Blue Shield of Michigan: BCBSM). MVC developed and maintains a validated claims-based registry that provides detailed information regarding payments and utilization surrounding an episode of care for both BCBSM preferred provider organizations (PPO) and Medicare fee-for-service (FFS) patients [23].
We included MVC payment data from patients who underwent SAVR or TAVR at any of the 33 non-federal hospitals performing cardiac surgery in the state of Michigan between January 1, 2012 and December 31, 2016 whose procedure was reimbursed by BCBSM PPOs or Medicare FFS insurance programs.
Measures
For MVC payment data, TAVR was defined using CPT procedure codes 33361–33369, while SAVR was defined with codes 33405–33406 and 33410–33412.
The primary outcome for this analysis was 90-day price standardized episode payments. Payments were quantified for 90-day episodes of care and were disaggregated into index hospitalization, professional, readmission, and post-acute care payments [24]. Payments incurred 6 months prior to the index operation were also analyzed. We collected patient clinical demographic data for these episodes using International Classification of Diseases 9 (ICD-9) codes and additional MVC variables such as hospital length of stay (LOS) and readmission rate.
Hospital procedural volume data were extracted from the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (MSTCVS-QC) database to capture the total TAVR and SAVR experience at each of 33 hospitals practicing cardiac surgery in Michigan during the same period. To determine SAVR operative experience, SAVR with and without coronary artery bypass (CAB) cases were counted for each hospital. Hospitals were divided into terciles by total number of procedures performed over the study period, separately for TAVR and SAVR. Three centers with less than 10 TAVRs performed were excluded from the analysis.
Statistical Analysis
Categorical variables were presented as percentages and continuous variables as mean (± standard deviation) for univariate analyses. Parametric two-sample t-tests and chi-square tests were used to test for statistical significance. P-values of less than 0.05 (2-tailed) were considered statistically significant.
Payments were price standardized, according the methods developed by the Dartmouth Atlas of Health Care, [25] using average Medicare payments in the state of Michigan to account for payer-type, inflation, regional variation, and contractual differences. Therefore, payments can be considered a measure of overall healthcare utilization.
Total and component payments between TAVR and SAVR were risk-adjusted using a two-step regression model, adjusting for patient characteristics, co-morbidities, and payments 6 months prior to index procedure. Risk adjustment was performed using observed/expected (O/E) ratios. Condition and component-specific expected payments are based on a statistical model that uses a combination of required and non-required variables. The required variables include payer, age, sex, and high 6-month prior spending. Non-required variables include 79 hierarchical condition category (HCC) comorbidities as well as whether a coronary artery bypass grafting (CABG) procedure was performed concurrently. These variables are selected using a model specification technique that occurs in two steps. First, all candidate variables are tested using a univariate regression model to see if they predict payment (p<0.10). Second, all retained variables are included in a multivariable regression model to determine which are included in the final model (p<0.05).
Hospitals were divided into terciles by procedural volume separately for TAVR and SAVR using MSTCVS-QC data, and payments from MVC data were compared between lowest and highest terciles. We used data from 12,403 patients (TAVR, n=3,640; SAVR, n=8,763) in the 33 MSTCVS-QC hospitals to establish SAVR and TAVR volume terciles. Of the 8,763 SAVR patients, 5,313 underwent isolated SAVR and 3,450 underwent SAVR + CAB. We included 33 centers that performed SAVR while 17 centers performed TAVR. An additional analysis was performed for TAVR centers in which high volume was defined as >100 annual cases and low volume if ≤50 annual cases.
All analyses were conducted using SAS Version 9.4M3.
RESULTS
In total, 6,359 BCBSM PPO and Medicare FFS beneficiaries underwent TAVR (17 hospitals, n=1,655) or SAVR (33 hospitals, n=4,704). Patient characteristics and comorbidities are shown in Table 1. Overall TAVR patients were older and sicker than SAVR patients, with higher rates of stage IV-V CKD (12% versus 5%, p<0.0001), COPD (42% versus 29%, p<0.0001), prior stroke (7% versus 5%, p=0.001), diabetes (48% versus 44%, p=0.001), and congestive heart failure (87% versus 57%, p<0.0001).
Table 1.
TAVR and SAVR patient characteristics.
| Variable | Overall (%) n=6,359 | TAVR (%) n=1,655 | SAVR (%) n=4,704 | P value |
|---|---|---|---|---|
| Male sex | 3,673 (57.76) | 805 (48.64) | 2,868 (60.97) | <0.0001 |
| Age (mean ± standard deviation) in years | 75 ± 10 | 82 ± 8 | 72 ± 10 | <0.0001 |
| Medicare FFS insurance | 5,571 (87.61) | 1,618 (97.76) | 3,953 (84.03) | <0.0001 |
| Stage IV-V CKD | 443 (6.97) | 198 (11.96) | 245 (5.21) | <0.0001 |
| Prior CVA | 361 (5.68) | 120 (7.25) | 241 (5.12) | 0.0013 |
| COPD | 2,043 (32.13) | 701 (42.36) | 1,342 (28.53) | <0.0001 |
| CHF | 4,150 (65.26) | 1,448 (87.49) | 2,702 (57.44) | <0.0001 |
| Diabetes | 2,861 (44.99) | 802 (48.46) | 2,059 (43.77) | 0.0010 |
| Vascular Disease | 3,597 (56.57) | 1,276 (77.10) | 2,321 (49.34) | <0.0001 |
| Respiratory Dysfunction | 465 (7.31) | 177 (10.69) | 288 (6.12) | <0.0001 |
| Neurologic Disorder | 410 (6.45) | 152 (9.18) | 258 (5.48) | <0.0001 |
| Psychiatric Disorder | 222 (3.49) | 49 (2.96) | 173 (3.68) | 0.1717 |
| Prior Cancer | 791 (12.44) | 252 (15.23) | 539 (11.46) | <0.0001 |
| Liver Disease | 130 (2.04) | 53 (3.20) | 77 (1.64) | 0.0001 |
CKD, chronic kidney disease; CVA, cerebrovascular accident; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disorder.
Adjusted for patient characteristics, co-morbidities, and payments made 6 months prior to index procedure, 90-day episode payments (± standard deviation) were $69,388 ± $22,259 for TAVR versus $66,683 ± $27,377 for SAVR (p<0.0001), while mean index hospital length of stay (LOS) was shorter for TAVR (6.2 ± 5.6 days vs. 10.2 ± 7.5, p<0.0001). Mean index hospitalization payments were higher for TAVR ($51,472 ± $9,430 vs. $47,098 ± $16,005, p<0.0001), while readmission and post-acute care payments were lower, and professional payments were similar (Table 2).
Table 2.
Adjusted mean 90-day episode payments by valve replacement procedure type
| Measure | Procedure Type | P-Value | |
|---|---|---|---|
| SAVR (n=4,704) | TAVR (n=1,655) | ||
| 90-Day Episode | $66,683 ± $27,377 | $69,388 ± $22,259 | <0.0001 |
| Index Hospitalization | $47,098 ± $16,005 | $51,472 ± $9,430 | <0.0001 |
| Readmission | $4,698 ± $14,781 | $3,548 ± $10,669 | 0.0007 |
| Post-Acute Care | $6,968 ± $9,066 | $6,229 ± $9,020 | 0.004 |
| Professional | $7,398 ± $3,083 | $7,243 ± $3,812 | 0.227 |
| Index Length of Stay (days) | 10.2 ± 7.5 | 6.2 ± 5.6 | <0.0001 |
| Readmission Rate (%) | 22.7 | 23.1 | 0.77 |
All payment and length of stay data are reported as mean ± standard deviation.
Case volume was lower for TAVR (mean: 215, median: 38) than SAVR (mean: 269, median: 43). Ninety-day episode, index hospitalization, professional, readmission, and post-acute care payments for TAVR were similar between high and low volume centers (Table 3). Patients at high volume centers had higher index hospital LOS (6.6 ± 5.7 days vs. 5.3 ± 5.3, p=0.002), while similar readmission rates. Patients at the highest (versus lowest) SAVR tercile volume centers had lower 90-day episode payments ($65,483 ± $26,737 vs. $68,738 ± $25,046, p=0.01) [Table 4]. Relative to low volume centers, patients at high volume centers had lower post-acute care ($6,528 ± $8,799 vs. $8,127 ± $9,624, p=0.0005) and professional ($7,216 ± $2,954 vs. $7,977 ± $3,149, p<0.0001) payments, in addition to a shorter index hospital LOS (10.0 days ± 7.5 vs. 11.1 ± 7.9, p=0.002) [Figure].
Table 3.
TAVR adjusted mean 90-day episode payments by volume
|
Measure |
Procedural Volume |
P-Value |
|
|---|---|---|---|
| Low Volume Hospitals (n=238) | High Volume Hospitals (n=1,072) | ||
| Index Hospitalization | $51,566 ± $10,271 | $51,288 ± $9,320 | 0.71 |
| Professional | $7,279 ± $3,884 | $7,389 ± $3,888 | 0.69 |
| Readmission | $2,732 ± $6,802 | $3,785 ± $10,437 | 0.053 |
| Post-Acute Care | $5,967 ± $8,069 | $6,487 ± $9,380 | 0.38 |
| 90-Day Episode | $68,074 ± $20,717 | $69,764 ± $21,725 | 0.27 |
| Index Length of Stay (days) | 5.3 ± 5.3 | 6.6 ± 5.7 | 0.002 |
| Readmission Rate (%) | 23.5 | 23.9 | 0.91 |
All payment and length of stay data are reported as mean + standard deviation.
Table 4.
SAVR adjusted mean 90-day episode payments by volume
|
Measure |
Procedural Volume |
P-Value |
|
|---|---|---|---|
| Low Volume Hospitals (n=512) | High Volume Hospitals (n=2,944) | ||
| Index Hospitalization | $48,267 ± $14,477 | $46,555 ± $16,046 | 0.015 |
| Professional | $7,977 ± $3,149 | $7,216 ± $2,954 | <0.0001 |
| Readmission | $4,689 ± $13,249 | $4,474 ± $14,545 | 0.74 |
| Post-Acute Care | $8,127 ± $9,624 | $6,528 ± $8,799 | 0.0005 |
| 90-Day Episode | $68,738 ± $25,046 | $65,483 ± $26,737 | 0.01 |
| Index Length of Stay (days) | 11.1 ± 7.9 | 10.0 ± 7.5 | 0.002 |
| Readmission Rate (%) | 24.8 | 21.6 | 0.11 |
All payment and length of stay data are reported as mean + standard deviation.
Figure.
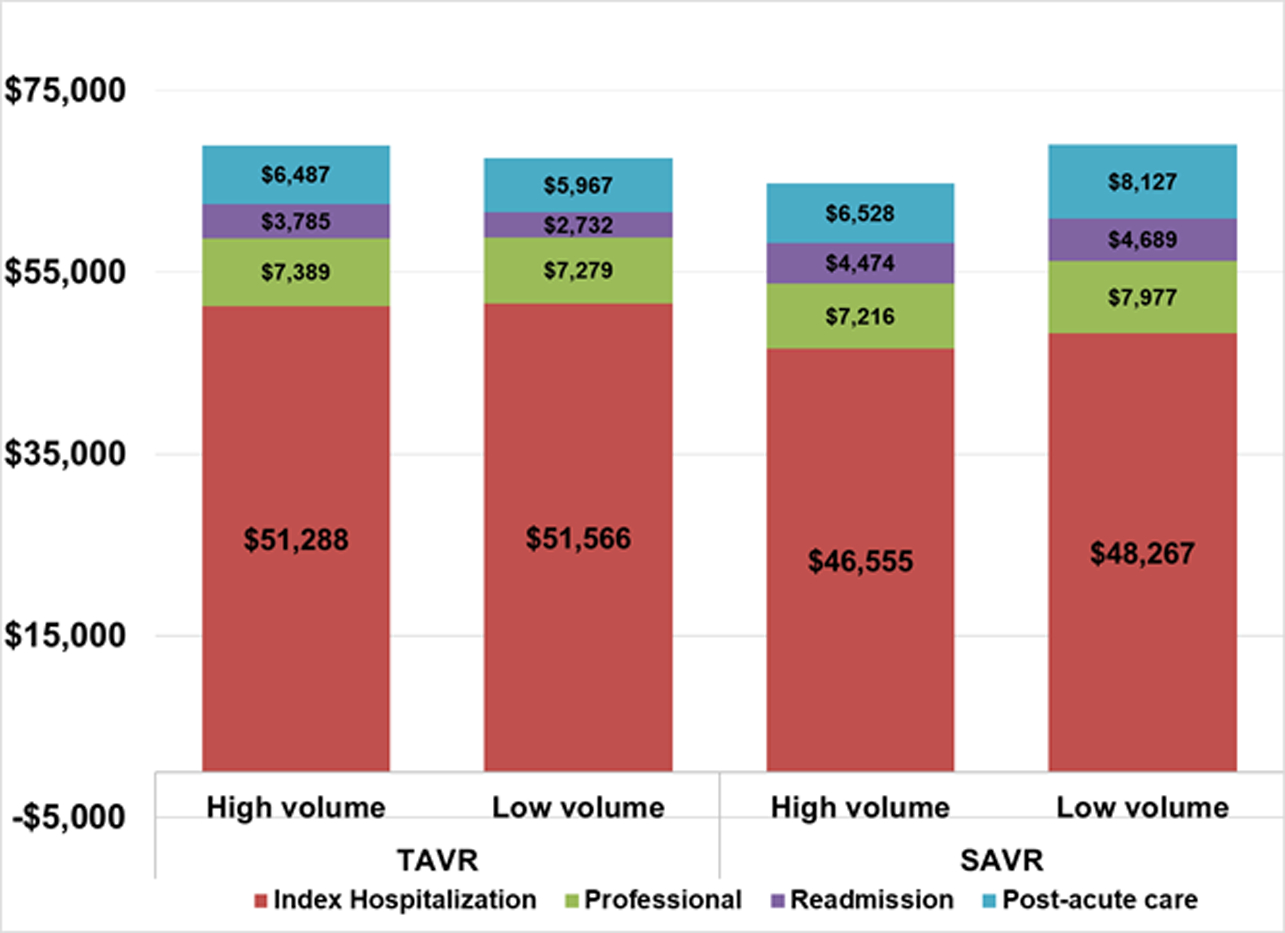
Adjusted mean 90-day episode payments by case volume: TAVR versus SAVR comparison.
COMMENT
We used data from two multi-institutional statewide quality improvement collaboratives to evaluate differences in episode payments between TAVR and SAVR and explore volume-payment relationships between the two procedures. Ninety-day episode payments were higher for TAVR than SAVR, despite a nearly 4-day shorter average hospital stay. Higher TAVR episode payments were driven predominantly by higher index hospitalization payments, while readmission and post-acute care payments were lower for TAVR. Professional payments were similar between TAVR and SAVR. We noted a volume-payment relationship among SAVR centers, as patients treated in high (versus low) volume tercile hospitals had lower overall and component payments, as well as a shorter length of stay. This volume-payment relationship was not apparent for TAVR procedures.
Previous work evaluating the value of TAVR and SAVR has focused on hospital charges, rather than reimbursement [12–15]. While hospital charges provide an estimate of in-hospital spending, they are an unreliable indicator of actual payments made, and include costs during the index hospitalization only. In contrast, episode payments more fully represent the realized costs of care provided. In studies using cost-to-charge ratios to estimate financial outcomes, patients undergoing TAVR are found to incur significantly higher charges compared to SAVR [14–15]. Nevertheless, TAVR could be a more cost-effective option for many patients overall, if the subsequent care needs in the rest of the episode are decreased. A systematic review by Indraratna et al. concluded TAVR may be justified medically and economically compared to medical therapy for “inoperable” patients, but that evidence is currently insufficient to economically justify TAVR over SAVR [26]. Because these studies utilized trial data with specific inclusion/exclusion criteria, they may be poorly generalizable to every day practices [27–32].
In reporting reimbursed payments, our data informs value-based reimbursement, which has become increasingly important for payers. While TAVR could be less expensive to hospitals due to lower costs incurred from a shorter length of stay and less expensive readmissions, we have found TAVR is more expensive to the payer attributed in part to higher Medicare diagnosis-related group (DRG) index payments. As the volume of TAVRs continues to increase, evaluations that include both administrative and clinical data are warranted to more fully assess treatment tradeoffs for both patients and payers.
The Virginia experience from 2002 to 2015 by Hawkins et al. [12] has detailed how the estimated cost (derived from institutional cost-to-charge ratios) of SAVR has increased across the duration of TAVR adoption (i.e. pre-TAVR to early TAVR to commercial TAVR eras). The authors found that isolated aortic valve replacement costs consistently increased over their study period. Our findings, which derive from a different methodological approach given we use true payments, reveal that the overall 90-day reimbursement payments for TAVR are higher than SAVR in Michigan. If the trend of increased resource utilization continues for SAVR, the value proposition for both hospitals and payers could drastically change in the setting of potential bundled payments and the volume of overall TAVR procedures surpassing that of SAVR. Future analyses should consider complementing reimbursement payment data (as presented here) with hospital technical revenues and expenditures to fully evaluate the financial viability of these two treatment options given the rapid rise of TAVR volume in the commercial era.
Several studies have evaluated volume-outcome effects for TAVR and SAVR [17–22, 33]. In this present analysis, we found that high procedural volume was associated with lower payments for SAVR but not for TAVR (Table 3), a finding that persisted at different volume thresholds. Notably, 11 of our 17 TAVR centers were in the 1st tercile for SAVR volume, while five were 2nd tercile. The Center for Medicare and Medicaid Services (CMS) currently has hospital volume criteria for establishing a TAVR program (e.g. ≥ 50 SAVRs in the year prior to forming a program). It is possible that the lack of a volume-payment effect for TAVRs in the present analysis may be attributed in part to most of the low volume TAVR centers also operating as intermediate or high volume SAVR centers, with established heart teams and effective processes of care and in place for aortic valve interventions.
Whereas SAVR had seven major DRGs for reimbursement, hospitals only had two primary DRGs for TAVR. As intermediate risk patients were not approved to electively undergo TAVR until the end of our study period [10], the majority of TAVR patients in our analysis were extreme/inoperable or high risk and thus more likely to have the higher index DRG payments. While readmission rates were similar for the two procedures, readmission payments were almost 25% lower for TAVR. The most common cause of readmission (overall: 129/926 readmissions, 6.3%; TAVR: 48/225, 8.9%; SAVR: 81/701, 5.4%) was heart failure and shock with major complication or comorbidity. The combination of these payments and the nearly 4-day shorter average hospital stay for TAVR patients provides ample areas for targeted improvement to maximize value.
Additionally, the relative uniformity in payments between high and low volume centers may indicate that TAVR procedures are more amenable to protocolized care pre-, intra-, and post-procedure as compared to SAVR. As the volume of TAVR procedures continues to rise, optimizing hospital processes of care and payments will become important for low volume SAVR hospitals, to maintain SAVR as a viable alternative to TAVR and for survival of these programs, especially as their low SAVR volume provides an impediment to establishing a TAVR program.
We acknowledge several limitations to our study. First, while our payment data do not include TAVR and SAVR patients reimbursed by every payer, our data did include our state’s largest public (Medicare) and private (BCBSM) payers. Second, our data are limited to the state of Michigan, though we believe that a statewide experience provides a more generalizable and real-world cohort relative to traditional reports utilizing data from randomized trials. Third, while we risk-adjusted and price standardized our data, we cannot rule out unmeasured confounding. Fourth, while we report payment data through a 90-day episode, we recognize we are not able to capture all relevant expenditures (e.g. Medicare Part D, patient out-of-pocket costs). Fifth, we cannot fully account for payment differences between procedural approach absent linking detailed clinical data to payment data at a patient level. Sixth, we did not have access to data that would enable us to explore whether TAVR procedure location (e.g. catheterization lab, operating room, or hybrid room) and corresponding differences in support staff requirements could contribute to differences in payments across procedures.
In conclusion, 90-day episode payments were higher for TAVR than SAVR across Michigan hospitals, despite a significantly shorter average length of hospital stay. Additionally, our data suggest an inverse volume-payment relationship for SAVR, although not for TAVR. These findings will be important for hospitals and payers as they address areas for maximizing value for both SAVR as well as the increasing proportion of patients undergoing TAVR.
DISCLOSURE:
Dr. Likosky is supported in part by grant number R01HS022535 from the Agency for Healthcare Research and Quality (AHRQ). The opinions expressed in this document are those of the authors and do not reflect the official position of the AHRQ or the U.S. Department of Health and Human Services.
Jim Dupree, Scott Regenbogen, Michael Thompson, and John Syrjamaki receive salary support from Blue Cross Blue Shield of Michigan for their roles with the Michigan Value Collaborative.
Support for the MSTCVS Quality Collaborative and MVC is provided by the Blue Cross and Blue Shield of Michigan (BCBSM) and Blue Care Network as part of the BCBSM Value Partnerships program.
Footnotes
Publisher's Disclaimer: DISCLAIMER:
Publisher's Disclaimer: Although BCBSM works collaboratively with MSTCVS-QV and MVC, the opinions, beliefs and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs and viewpoints of BCBSM or any of its employees.
Poster presentation: Society of Thoracic Surgeons 54th Annual Meeting, Fort Lauderdale, FL, January 27-31, 2018.
REFERENCES
- 1.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 368 (2006), pp. 1005–1011. [DOI] [PubMed] [Google Scholar]
- 2.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol, 21 (1993), pp. 1220–1225. [DOI] [PubMed] [Google Scholar]
- 3.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol, 29 (1997), pp. 630–634. [DOI] [PubMed] [Google Scholar]
- 4.Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromso study. Heart, 99 (2013), pp. 396–400. [DOI] [PubMed] [Google Scholar]
- 5.Ross J Jr., Braunwald E. Aortic stenosis. Circulation, 1968;38(1 Suppl):61–7. [DOI] [PubMed] [Google Scholar]
- 6.Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcomes. Circulation 1997;95:2262–70. [DOI] [PubMed] [Google Scholar]
- 7.Schueler R, Hammerstingl C, Sinning JM, et al. Prognosis of octogenarians with severe aortic valve stenosis at high risk for cardiovascular surgery. Heart 2010;96:1831–6. [DOI] [PubMed] [Google Scholar]
- 8.SAPIEN Transcatheter Heart Valve, model 9000TFX. Approval for use in inoperable patients by Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA). 2 November 2011. <https://www.accessdata.fda.gov/cdrh_docs/pdf10/P100041A.pdf>
- 9.SAPIEN Transcatheter Heart Valve, model 9000TFX. Approval for use in high risk patients by Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA). 13 June 2012. <https://www.accessdata.fda.gov/cdrh_docs/pdf11/P110021b.pdf>
- 10.Edwards SAPIEN 3 Transcatheter Heart Valve, model 9600TFX. Approval for use in intermediate risk patients by Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA). 18 August 2016. < https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140031S010a.pdf>
- 11.National Coverage Determination for Transcatheter Aortic Valve Replacement to receive Medicare coverage. 1 May 2012. <https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=355&ncdver=1&bc=AAAAgAAAAAAAAA%3d%3d&>
- 12.Hawkins RB, Downs EA, Johnston LE, et al. Impact of Transcatheter Technology on Surgical Aortic Valve Replacement Volume, Outcomes, and Cost. Ann Thorac Surg 2017. June;103(6): 1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds MR, Lei Y, Wang K, et al. CoreValve US High Risk Pivotal Trial Investigators. Cost-Effectiveness of Transcatheter Aortic Valve Replacement With Self-Expanding Prosthesis Versus Surgical Aortic Valve Replacement. J Am Coll Cardiol. 2016. January 5;67(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ailawadi G, LaPar DJ, Speir AM, et al. Contemporary Costs Associated With Transcatheter Aortic Valve Replacement: A Propensity-Matched Cost Analysis. Ann Thoracic Surg. 2016. January;101(1):154–60. [DOI] [PubMed] [Google Scholar]
- 15.Osnabrugge RL, Head SJ, Genders TS, et al. Costs of transcatheter versus surgical aortic valve replacement in intermediate-risk patients. Ann Thorac Surg. 2012. December;94(6):1954–60. [DOI] [PubMed] [Google Scholar]
- 16.Patel HJ, Herbert MA, Paone G, et al. The Midterm Impact of Transcatheter Aortic Valve Replacement on Surgical Aortic Valve Replacement in Michigan. Ann Thorac Surg. 2016. September;102(3): 728–734. [DOI] [PubMed] [Google Scholar]
- 17.Carroll JD, Vemulapalli S, Dai D et al. Procedural Experience for Transcatheter Aortic Valve Replacement and Relation to Outcomes: The STS/ACC TVT Registry. J Am Coll Cardiol. 2017. July 4;70(1):29–41. [DOI] [PubMed] [Google Scholar]
- 18.Khera S, Kolte D, Gupta T, et al. Association Between Hospital Volume and 30-Day Readmissions Following Transcatheter Aortic Valve Replacement. JAMA Cardiol 2017. July;2(7):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma DR, Pershad Y, Lazkani M, et al. Volume-outcome relationships for transcatheter aortic valve replacement-risk-adjusted and volume stratified analysis of TAVR outcomes. Indian Heart J 2017. Nov-Dec;69(6):700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Biasi AR, Paul A, Nasar A, et al. National analysis of short-term outcomes and volume-outcome relationships for transcatheter aortic valve replacement in the era of commercialization. Cardiology 2016;133(1):58–68. [DOI] [PubMed] [Google Scholar]
- 21.Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg 2013. November;96(5):1560–5. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez AA, Dimick JB, Birkmeyer JD, et al. Understanding the volume-outcome effect in cardiovascular surgery: the role of failure to rescue. JAMA Surg 2014. February;149(2):119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellimoottil C, Syrjamaki JD, Voit B, Guduguntla V, Miller DC, Dupree JM. Validation of a claims-based algorithm to characterize episodes of care. Am J Manag Care 2017;23:e382–e386. [PubMed] [Google Scholar]
- 24.Birkmeyer JD, Gust C, Baser O, et al. Medicare Payments for Common Inpatient Procedures: Implications for Episode-Based Payment Bundling. Health Serv Res 2010;45(6 Pt 1):1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottlieb DJ, Zhou W, Song Y, Andrews KG, Skinner JS, Sutherland JM. Prices don’t drive regional Medicare spending variations. Health Aff (Millwood);29:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Indraratna P, Ang SC, Gada H, et al. Systematic review of the cost-effectiveness of transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2014;148:509–14. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds M, Magnuson E, Wang K, Lei Y, Vilain K, Walczak J, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis. Circulation. 2012;125:1102–9. [DOI] [PubMed] [Google Scholar]
- 28.Watt M, Mealing S, Eaton J, Piazza N, Moat N, Brasseur P, et al. Cost-effectiveness of transcatheter aortic valve replacement in patients ineligible for conventional aortic valve replacement. Heart. 2012;98:370–6. [DOI] [PubMed] [Google Scholar]
- 29.Neyt M, Van Brabandt H, Devriese S, Van De Sande S. A cost-utility analysis of transcatheter aortic valve implantation in Belgium: focusing on a well-defined and identifiable population. BMJ Open. 2012;2:e001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doble B, Blackhouse G, Goeree R, Xie F. Cost-effectiveness of the Edwards SAPIEN transcatheter heart valve compared with standard management and surgical aortic valve replacement in patients with severe symptomatic aortic stenosis: a Canadian perspective. J Thorac Cardiovasc Surg. 2013;146:52–60.e3. [DOI] [PubMed] [Google Scholar]
- 31.Gada H, Kapadia S, Tuzcu E, Svensson L, Marwick T. Markov model for selection of aortic valve replacement versus transcatheter aortic valve implantation (without replacement) in high-risk patients. Am J Cardiol. 2012;109:1326–33. [DOI] [PubMed] [Google Scholar]
- 32.Gada H, Agarwal S, Marwick T. Perspective on the cost-effectiveness of transapical aortic valve implantation in high-risk patients: outcomes of a decision analytic model. Ann Cardiothorac Surg. 2012;1:145–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regenbogen SE, Gust C, Birkmeyer JD. Hospital surgical volume and the cost of inpatient surgery in the elderly. Journal of the American College of Surgeons 2012;215(6):758–765. [DOI] [PubMed] [Google Scholar]


