INTRODUCTION
Heparin-induced thrombocytopenia (HIT) is an immune-mediated adverse drug reaction caused by IgG antibodies to complexes of platelet factor 4 (PF4) and heparin which can activate platelets, causing the release of prothrombotic platelet-derived microparticles that promote both arterial and venous thrombosis1. Delayed-onset HIT is characterised by thrombocytopenia that begins or worsens several days after stopping heparin2. These patients can be challenging to manage because of the very high titres of IgG antibodies to PF4/heparin complexes and higher IgG-induced heparin-dependent and heparin-independent platelet activation that causes life-threatening thrombosis and profound thrombocytopenia1. First-line treatment of “classical” HIT includes stopping heparin and “switching” to a non-heparin anticoagulant, such as argatroban or bivalirudin1,2. However, even in the absence of randomised trials, fondaparinux has been used worldwide to treat HIT, as the risk of HIT is thought to be negligible with this medication3. In this paper, we present a case of in vivo and in vitro cross-reactivity to fondaparinux in an ischaemic stroke patient with IgG-PF4/heparin antibody-negative delayed-onset HIT.
CASE REPORT
A 67-year old male was admitted to hospital due to confusion and left-sided hemiplegia. The patient had been diagnosed six months earlier with myelodysplastic syndrome with excess blasts, type 2 (MDS-EB-2, as defined by the 2016 World Health Organization criteria), as he had dysplastic changes in all three blood cell lineages with 18% of myeloblasts in the bone marrow (BM). He started treatment with azacitidine and was scheduled for BM re-evaluation and allogeneic haematopoietic stem cell transplant from a matched unrelated donor in case of a favourable response. He was otherwise healthy and had no history of thrombosis or HIT, major surgical interventions, or known previous exposure to heparin or other anticoagulants. His concomitant medications were acyclovir and cotrimoxazole for the prevention of herpes virus and Pneumocystis infections, respectively. During the follow up, he was transfusion-independent; haemoglobin levels remained >100×109/L and platelets >100×109/L. Fourteen days earlier, the patient had suffered a non-ST segment elevation myocardial infarction (NSTEMI). He received bolus intravenous unfractionated heparin (5,000 IU/L) and was transferred for coronary angiography.
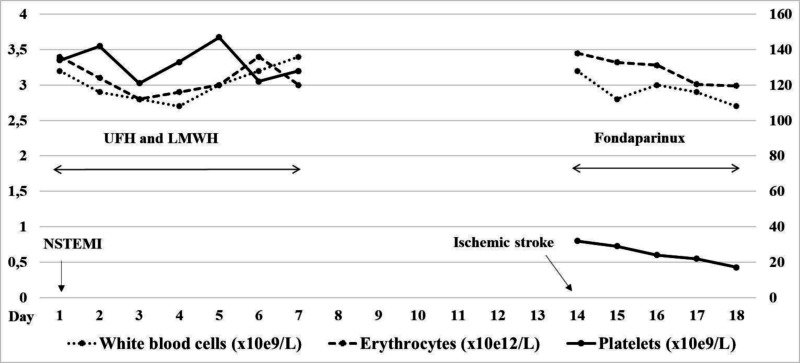
However, coronary angiography was unremarkable, and the patient was conservatively treated with low-molecular weight heparin (dalteparin sodium) twice daily for a total of seven days, together with with dual antiaggregation therapy (acetylsalicylic acid and clopidogrel). During that hospital stay, platelets were in the range of 121–147×109/L, and the patient was in good health when he was discharged from hospital. At last admission (fourteen days after NSTEMI), the patient was normotensive, afebrile, and there were no signs of bleeding diathesis. White blood cell count was 3.2×109/L (with no myeloblasts in the peripheral blood smear), erythrocyte count was 3.45×1012/L, and haemoglobin level was 108 g/L; however, his platelets were unexpectedly low (32×109/L). Brain magnetic resonance imaging revealed acute right-sided parieto-occipital ischaemic stroke (Figure 1). There were no signs of haemolysis or disseminated intravascular coagulation (DIC), his renal function was preserved, and shistocytes were absent in the peripheral blood smear. Serological screening for autoimmune disorders were negative, as were the serological tests for antiphospholipid syndrome. Direct and indirect antiglobulin tests were negative. Auto-antibodies to platelet glycoproteins IIb/IIIa, Ia/IIa and Ib/IX/V were also negative (Xmap technology for unbound IgG antibodies; Luminex xMap bead-based multiplexed immunoassay, BioRad, CA, USA). Even though there were no signs of haemolysis, and shistocytes were absent from the peripheral blood smear, there was also a possibility of acquired thrombotic thrombocytopenic purpura. However, auto-antibodies to von Willebrand factor (vWF) cleaving enzyme (ADAMTS13) were not measured, as the PLASMIC4 pre-test probability of severe ADAMTS13 deficiency was 0% (score of 2), which prompted us to consider an alternative diagnosis. BM examination revealed no progression of MDS and/or evolution to acute leukaemia; there were 7% of myeloblasts, and megakaryocytes were abundant. Due to previous exposure to heparin, there was a possibility of delayed-onset HIT. Enzyme-linked immunosorbent assay (ELISA PF4 IgG Kit, GTI Diagnostics, Waukesha, WI, USA) on the second day of admission was negative (OD [450 nM] 0.065; reference range <0.400). However, when calculating the 4T risk score2, which estimates the likelihood of HIT using the four variables (degree of thrombocytopenia, timing of thrombocytopenia, presence of thrombosis, and other potential causes of thrombocytopenia), the patient was still considered to be “high risk” for HIT development, as his 4T score was 6: a >50% fall in platelet count −2 points, timing of thrombocytopenia >10 days −1 point, arterial thrombosis −2 points, and other possible causes of thrombocytopenia −1 point). Heparin-induced platetet activation assay (HIPA) was performed the same day and showed platelet aggregation in the presence of heparin. The patient was then started on fondaparinux (7.5mg daily). On the fifth day of treatment with fondaparinux, platelets were 17×109/L and the patient showed signs of clinical deterioration. Computerised tomography of the brain did not reveal signs of haemorrhagic transformation. Laboratory assays for HIT antibodies were repeated the same day; IgG-PF4/heparin antibodies were again negative. However, HIPA was repeated again and demonstrated platelet activation in the presence of both heparin and fondaparinux, as well as in vitro spontaneous platelet activation. Unfortunately, the patient succumbed to the ischaemic stroke before an alternative anticoagulant or intravenous immunoglobulins (IVIG) could be introduced. Patient managment, starting with admission for NSTEMI, including information on blood cell counts during the treatment with UFH, LMWH and fondaparinux, is summarised in Figure 2.
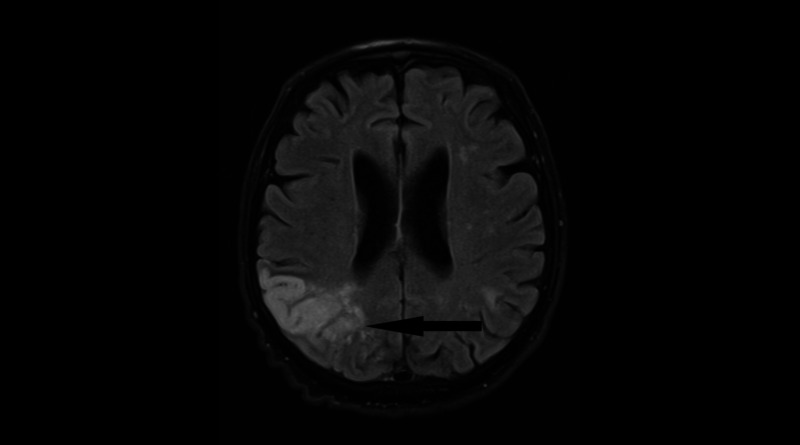
Figure 1.
Magnetic resonance of the brain (axial flair image) revealed well demarcated giriform T2 lesion corticosubcortically in the irrigation area of posterior cerebral artery, consistent with ischaemia
Figure 2.
Blood cell count dynamics during the treatment with unfractionated heparin (UFH), low-molecular weight heparin (LWMH) and fondaparinux, starting with the admission for non-ST segment elevation myocardial infarction (NSTEMI)
DISCUSSION
In our patient, the timing of thrombocytopenia led to a suspicion of delayed-onset HIT2. In vitro platelet activation in the presence of both heparin and fondaparinux, as well as in vitro spontaneous platelet activation, confirmed the clinical diagnosis. Interestingly, IgG-PF4/heparin antibodies in our patient were repeatedly negative, which might be an extremely rare event in delayed-onset HIT, due to its “autoimmune” nature with typically high titres of IgG-PF4/heparin antibodies and IgG-induced heparin-dependent and heparin-independent platelet activation. This observation might have several explanations. First, immunoassays may be negative in the early course of disease5. However, we re-tested our patient three days later and the results were again negative. Second, antigens other than PF4/heparin complexes (i.e. antibodies directed against the chemokine interleukin-8 or neutrophil-activating peptide 2) have been shown, on rare occasions, to be involved in the pathogenesis of HIT5. Furthermore, antibodies of the IgA and IgM class to PF4/heparin complexes have also been described. However, it is generally accepted that the pathogenesis in HIT is primarily based on IgG antibody-mediated platelet activation5. Third, rare cases of “spontaneous” HIT have been reported, in which different inf lammatory disorders generate antibodies to PF4/heparin complexes without prior heparin exposure6. In addition, antibody-negative HIT has been described in patients on immunosupresants, during plasmapheresis or with massive transfusions, but our patient did not receive any of these therapies5. Furthermore, a substantial number of MDS patients have been shown to present with antecedent or concomitant autoimmune diseases and poorly defined systemic inf lammatory syndromes7. In this perspective, it is possible that some other auto-antibodies, in the context of systemic inf lammation accompanying NSTEMI or MDS-EB-2, might have caused platetet activation with a clinical picture resembling delayed-onset HIT. Finally, there is also a possibility that treatment with azacitidine and platelet consumption during the ischaemic stroke, in concert with decreased platelet production in the BM due to MDS-EB-2, might have been responsible for the profound thrombocytopenia. However, our patient had maintained normal platelet counts (>100×109/L) during the haematologic follow up, and thrombocytopenia of this degree twenty days after the fourth cycle of azacitidine would be an unexpected finding. Moreover, haematologic re-evaluation revealed an abundance of megakaryocytes in the BM and the patient responded well to azacitidine treatment, as he had a low percentage of myeloblasts (7%) in the BM. On the other hand, our patient, at “high risk” for HIT development, had a history of recent exposure to heparin, and, even though immunoassays were negative, HIPA was repeatedly positive in the presence of both heparin and fondaparinux. These observations indicate that the pathological antigen in this case most probably involved a complex of heparin and a molecule other than PF4. Due to the current unavailability of argatroban and bivalirudin in our country, we treated our patient with fondaparinux. Fondaparinux is a smaller molecule than heparin and has a lower risk of promoting antibody binding to PF4. In addition, IgG-PF4/heparin antibodies usually fail to react against PF4 in the presence of fondaparinux3. However, cases have already been published describing HIT associated with fondaparinux9, and even a disorder resembling HIT, but without previous exposure to heparin10. Delayed-onset HIT can be very difficult to manage, as it often presents with extensive life-threatening thromboses and DIC1. Furthermore, stroke patients with HIT have been shown to have particularly poor outcomes11. Emerging evidence suggests that IVIG and/or treatment with direct oral anticoagulants could be useful12,13. However, due to the rapid clinical deterioration of our patient, we were unable to “switch” fondaparinux to an alternative anticoagulant or to administer IVIG.
The limitation of this report is that only one immunological and one functional test (HIPA) were performed. However, this situation is common in resource limited settings, and both of these assays were performed twice, which reduced the possibility of a laboratory error. Moreover, different functional assays (HIPA, flow cytometry, serotonine release assay, aggregometry) offer similar sensitivity and specificity for the detection of HIT5. Unfortunately, the patient died before we could perform other functional tests.
In conclusion, this case illustrates that, on rare occasions, delayed-onset HIT might present with IgG/PF4-antibody negativity. Clinicians should be aware of this phenomenon and functional platetet assays should be ordered when facing a patient with an “intermediate/high” probability of delayed-onset HIT and negative IgG/PF4 antibodies1,2,5. In addition, when failure of fondaparinux anticoagulation is observed, alternative anticoagulants together with IVIG should be initiated12,13.
Footnotes
ETICHS
The patient’s family gave their informed consent for this publication. The General Hospital of Šibenik-Knin County does not require ethical approval for reporting individual cases or case series.
AUTHORS’ CONTRIBUTIONS
IK and VG-K treated the patient. GT interpreted the laboratory assays. JŠ interpreted the radiological imaging. IK, GT and DP wrote the first draft of the manuscript. All Authors revised and approved the final version of the manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360–92. doi: 10.1182/bloodadvances.2018024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warkentin TE, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med. 2001;135:502–6. doi: 10.7326/0003-4819-135-7-200110020-00009. [DOI] [PubMed] [Google Scholar]
- 3.Warkentin TE. Fondaparinux: does it cause HIT? Can it treat HIT? Expert Rev Hematol. 2010;3:567–81. doi: 10.1586/ehm.10.54. [DOI] [PubMed] [Google Scholar]
- 4.Bendapudi PK, Hurwitz S, Fry A, et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017;4:e157–e164. doi: 10.1016/S2352-3026(17)30026-1. [DOI] [PubMed] [Google Scholar]
- 5.Leo A, Winteroll S. Laboratory Diagnosis of heparin-induced thrombocytopenia and monitoring of alternative anticoagulants. Clin Diagn Lab Immunol. 2003;10:731–40. doi: 10.1128/CDLI.10.5.731-740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warkentin TE, Basciano PA, Knopman J, Bernstein RA. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood. 2014;123:3651–54. doi: 10.1182/blood-2014-01-549741. [DOI] [PubMed] [Google Scholar]
- 7.Grignano E, Jachiet V, Fenaux P, et al. Autoimmune manifestations associated with myelodysplastic syndromes. Ann Hematol. 2018;97:2015–23. doi: 10.1007/s00277-018-3472-9. [DOI] [PubMed] [Google Scholar]
- 8.Rota E, Bazzan M, Fantino G. Fondaparinux-related thrombocytopenia in a previous low-molecular-weight heparin (LMWH)-induced heparin-induced thrombocytopenia (HIT) Thromb Haemost. 2008;99:779–81. doi: 10.1160/TH07-09-0573. [DOI] [PubMed] [Google Scholar]
- 9.Tvito A, Bakchoul T, Rowe JM, et al. Severe and persistent heparin-induced thrombocytopenia despite fondaparinux treatment. Am J Hematol. 2015;90:675–78. doi: 10.1002/ajh.23971. [DOI] [PubMed] [Google Scholar]
- 10.Warkentin TE, Maurer BT, Aster RH. Heparin-induced thrombocytopenia associated with fondaparinux. N Engl J Med. 2007;356:2653–55. doi: 10.1056/NEJMc070346. [DOI] [PubMed] [Google Scholar]
- 11.LaMonte MP, Brown PM, Hursting MJ. Stroke inpatients with heparin-induced thrombocytopenia and the effect of argatroban therapy. Crit Care Med. 2004;32:976–80. doi: 10.1097/01.ccm.0000119426.34340.e2. [DOI] [PubMed] [Google Scholar]
- 12.Warkentin TE. High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: a review. Expert Rev Hematol. 2019;12:685–98. doi: 10.1080/17474086.2019.1636645. [DOI] [PubMed] [Google Scholar]
- 13.Skelley JW, Kyle JA, Roberts RA. Novel oral anticoagulants for heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2016;42:172–78. doi: 10.1007/s11239-016-1365-0. [DOI] [PubMed] [Google Scholar]