Abstract
Background
Patients’ demographic and epidemiological characteristics, local variations in clinicians’ knowledge and experience and types of surgery can influence peri-operative transfusion practices. Sharing data on transfusion practices and recipients may improve patients’ care and implementation of Patient Blood Management (PBM).
Materials and methods
This was a multicentre, prospective, observational, cross-sectional study that included 61 centres. Clinical and transfusion data of patients undergoing major elective surgery were collected; transfusion predictors and patients’ outcomes were analysed.
Results
Of 6,121 patients, 1,579 (25.8%) received a peri-operative transfusion. A total of 5,812 blood components were transfused: red blood cells (RBC), fresh-frozen plasma and platelets in 1,425 (23.3%), 762 (12.4%) and 88 (1.4%) cases, respectively). Pre-operative anaemia was identified in 2,019 (33%) patients. Half of the RBC units were used by patients in the age group 45–69 years. Specific procedures with the highest RBC use were coronary artery bypass grafting (16.9% of all units) and hip arthroplasty (14.9%). Low haemoglobin concentration was the most common indication for intra-operative RBC transfusion (57%) and plasma and platelet transfusions were mostly initiated for acute bleeding (61.3% and 61.1%, respectively). The RBC transfusion rate in study centres varied from 2% to 72%. RBC transfusion was inappropriate in 99% (n=150/151) of pre-operative, 23% (n=211/926) of intra-operative and 43% (n=308/716) of post-operative RBC transfusion episodes. Pre-operative haemoglobin, increased blood loss, open surgery and duration of surgery were the main independent predictors of intra-operative RBC transfusion. Low pre-operative haemoglobin concentration was independently associated with post-operative pulmonary complications.
Conclusions
These findings identified areas for improvement in peri-operative transfusion practice and PBM implementation in Turkey.
Keywords: Blood components, patient blood management, peri-operative, transfusion
INTRODUCTION
Transfusion practice for surgical patients has changed from replacing surgically lost blood with allogenic blood transfusions to implementing strategies that reduce transfusion requirements1,2. The new concept is Patient Blood Management (PBM), which is “the timely application of evidence-based medical and surgical concepts designed to maintain haemoglobin concentration, optimize haemostasis and minimize blood loss in an effort to improve patient outcome”3. In this context, the decision of transfusion has become an important aspect of peri-operative patient care4. Despite several but in part contradictory peri-operative transfusion guidelines, it is challenging for an anaesthesiologist to implement transfusion guidelines because of local variations in clinicians’ knowledge and experience and characteristics of surgery5–9. Analysing such factors in a representative sample of surgical transfusion recipients can elucidate areas for implementing restrictive transfusion guidelines and PBM4–11.
The Turkish Society of Anaesthesiology and Reanimation conducted the Turkish National Perioperative Transfusion Study (TULIP-TS) to determine the areas for improvements in transfusion practice, to define practice standards, to collect data on future transfusion requirements and for health care planning, and to form a scientific basis for future research.
The aim of the present study was to evaluate the peri-operative transfusion practices in patients undergoing elective major surgery; the incidence of and indications for peri-operative transfusion and the impact of transfusion on patients’ outcomes were analysed.
MATERIALS AND METHODS
The TULIP-TS was a multicentre, prospective, observational study involving patients undergoing major elective surgery. All patients received local standard care and no intervention was applied. The study was conducted in a 1-month period between April 2, 2018 and May 3, 2018. The ethics committee of the University of Health Sciences Diskapi Yildirim Beyazit Teaching Hospital approved the study (12/06/2017-39/12). Each participating centre provided local review board approval and consent was obtained from each patient. The study was registered at www.clinicaltrials.gov (NCT03468738).
Hospitals performing major surgery in at least one of the following specialties were invited to participate in the study: general, orthopaedic, urological, cardiovascular, thoracic, paediatric, gynaecological, obstetric, transplant surgery and neurosurgery. After initial evaluation of hospitals that voluntarily indicated their interest in taking part in the study, nine additional hospitals were invited to participate in order to achieve a geographical distribution across the country as well as to ensure hospital diversity.
Centres were selected on the basis of a pre-study survey. This survey included questions regarding the annual numbers of major surgical procedures and blood component utilisation in each study centre. The minimum expected enrolment rate of each centre was calculated accordingly.
Patients undergoing major elective surgical procedures were considered eligible. All age groups and both genders were included. The surgical procedures were predefined according to the Classification of Diagnostic, Therapeutic, and Surgical Procedures of the National Social Insurance Institution, in which every procedure has a unique code (Online_Supplementary_Content, Table SI)12. In order to avoid enrolment bias, all patients undergoing a certain surgical procedure were enrolled irrespectively of whether they received a transfusion. Emergency cases requiring surgery in less than 2 hours and patients admitted to the operating area through the emergency department were excluded.
The following data were collected: the patient’s characteristics, diagnosis, comorbidities, physiological parameters, surgical parameters, laboratory results (haemoglobin concentration, platelet count, and coagulation profile), anaesthesia management and monitoring, intra-operative PBM strategies (autologous blood donation, cell salvage, acute normovolaemic haemodilution, procoagulant drugs), transfusion-related data (indication [Online_Supplementary_Content, Table SII], blood components, and amount), estimated blood loss, urine output, intravenous fluids, unanticipated intensive care unit admission and prolonged duration of stay according to the local standard of care, post-operative adverse outcomes, and all-cause mortality at day 30 after surgery. Comorbidity, physiological and surgical risk scores were determined for each patient13–15. The haemoglobin concentration on admission to hospital was defined as the pre-operative haemoglobin; this value was used to define pre-operative anaemia (<12 g/dL in females; <13 g/dL in males; <11 g/dL in pregnancy)16. The last measured haemoglobin concentration before red blood cell (RBC) transfusion was defined as the pre-transfusion haemoglobin. For RBC transfusions, the pre-transfusion haemoglobin values were considered as a measure of transfusion trigger. Post-transfusion haemoglobin values were recorded. Blood components were RBC, fresh-frozen plasma (FFP), and platelets (1 Unit [U] of aphaeresis platelet equals 5 U of random platelets). Inappropriate RBC transfusion was defined as RBC transfusion in patients with a haemoglobin concentration ≥7 g/dL without active bleeding and/or the presence of co-morbid disease and/or physiological transfusion trigger6,7. Blood loss was estimated using both the blood absorbed in sponges and the blood aspirated into canisters during an operation. The European Perioperative Clinical Outcome definitions were used to define post-operative adverse outcomes including hypotension, new onset arrhythmia, angina, non-fatal cardiac arrest, acute myocardial infarction, congestive heart failure, cardiogenic pulmonary oedema, new onset anticoagulation, myocardial injury in non-cardiac surgery, acute kidney injury, thromboembolic-ischaemic events, infections, stroke, new neurological deficit, and pulmonary complications17. Mortality within 30 days of surgery was documented from the National Death Certification System. All patients were followed up for a 1-month period. The drop-out criterion was withdrawal of a patient’s consent.
The primary outcome was the incidence of peri-operative transfusion. Transfusion indications and the impact of transfusion on patients’ outcomes were the other outcomes.
Data collection
OpenClinica© open source software 3.3 (OpenClinica LLC, Waltham, MA, USA) was used for the data collection and management. Data were first collected on paper case report forms (CRF) and thereafter entered into the database. All CRF were completed by the study investigators. The investigators were given training regarding the study protocol, obtaining consent from patients, and using the database. Patient recruitment and data plausibility checks were performed daily. Independent query management, data cleaning, and source data verification provided high quality data. Enrolment bias was investigated by comparing the enrolment rate of each study centre with its minimum expected enrolment rate and by cross-checking the scheduled surgery lists of the study centre for eligible patients.
Statistical analysis
The Predictive Analytical Software statistics for Windows, version 18 (SPSS Inc., Chicago, IL, USA) was used for the analyses. Descriptive statistics were expressed as numbers (proportion) for categorical variables and as mean ± standard deviation, median (interquartile range) for numerical variables. In binary logistic regression multivariable analysis, potential predictive variables were included according to their clinical relevance and test requirements. The variables were checked for singularity and multicollinearity. Linearity assumption was checked by the Box-Tidwell test. The final model fit was tested using the Hosmer-Lemeshow test. Results were expressed as odds ratio (OR) and 95% confidence intervals (CI). All tests were two-tailed and an alpha significance level less than 0.05 was considered statistically significant.
Sample size
In 2017, the blood component utilisation throughout the country was 2,453,051 URBC, 1,263,462 UFFP, and 540,217 U platelets (unpublished data, Ministry of Health, Turkey). According to the pre-study survey, 25% of these components were issued to the hospitals included in this study (RBC: 467,321 U, FFP: 457,219 U, and platelet: 96,928 U). Furthermore, based on the annual numbers of major operations performed in the study centres, the expected number of patients to be enrolled was 6,000. The results of our pilot study revealed a transfusion rate of 28%; accordingly, we assumed we would be able to collect data from over 1,500 transfused patients18.
Although data were collected from all age groups, as patients aged <18 years have unique features and surgical characteristics as well as anaemia definitions and transfusion indications, only data from adult patients are analysed here.
RESULTS
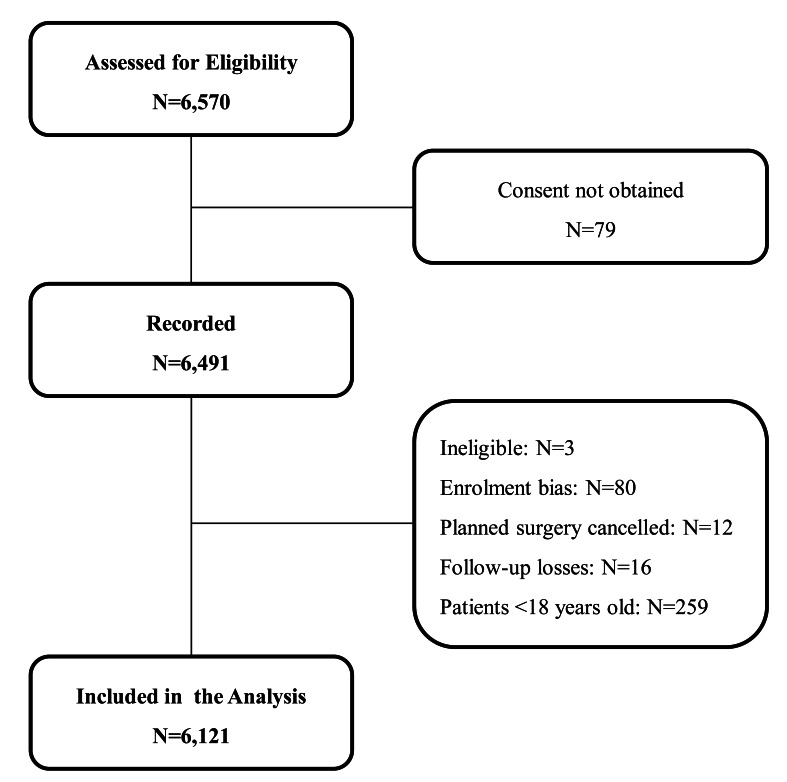
Sixty-one centres participated in the study: 32 university hospitals, 23 teaching hospitals, four private hospitals and two state hospitals. The hospital size ranged from 250 to 1,300 beds. During the study period, 6,570 patients were assessed for eligibility, 79 patients did not consent to participate in the study, and 6,491 patients were recorded. The planned operations were not completed in 12 patients and 16 patients were lost during follow-up. Three patients were considered ineligible because their operations were performed within less than 2 hours of hospital admission (diagnosis of epidural haematoma). Eighty patients were not included in the analysis because of protocol violations; the number of patients recorded was less than the minimum expected enrolment rate and only the patients in whom transfusion occurred were recruited, which was considered an enrolment bias. Thus, the final analyses was based on 6,121 adult patients (Figure 1). The annual number of blood transfusions performed in the study centres accounted for one quarter of the nationwide blood component utilisation in 2017 and it is, therefore, considered that the results of this study can reflect the peri-operative transfusion practice throughout the country. Blood components are prepared in accordance with the European Directorate for the Quality of Medicines and Healthcare19. This standardisation enables international comparison of the results.
Figure 1.
Flow chart of patients’ enrolment
Features of the study population are summarised in Table I. The median (interquartile range) pre-operative haemoglobin concentration was 12.9 (11.6–14.1) g/dL and pre-operative anaemia was identified in 2,019 (33%) patients; anaemia was more common in males than in females (34.7% vs 32.0 %, p=0.02) and the incidence increased with age.
Table I.
Characteristics, anaesthesia and surgical data of the patients transfused or not transfused in the peri-operative period
| Characteristics of the patients | Overall n=6,121 |
Transfused n=1,579 |
Non-transfused n=4,542 |
|---|---|---|---|
|
| |||
| Age, years | 57 (43–67) | 62 (52–70) | 55 (39–66) |
|
| |||
| Gender | |||
| Male | 2,262 (37%) | 747 (47.3%) | 1,515 (33.3%) |
| Female | 3,859 (63%) | 832 (52.6%) | 3,027 (66.6%) |
|
| |||
| BMI, kg.m2 | 27.7 (24.9–31.4) | 27.3 (24.2–30.7) | 28.1 (25.1–31.9) |
|
| |||
| ASA | |||
| I–II | 4,525 (74.2%) | 862 (54.7%) | 3,663 (81%) |
| ≥III | 1,576 (25.8%) | 714 (45.3%) | 862 (19%) |
|
| |||
| CCI | 0.67 ±1.11 | 0.95 ±1.27 | 0.57 ± 1.03 |
|
| |||
| P-POSSUM | 2.16 ± 5.9 | 4.87 ± 10.14 | 1.21 ± 2.76 |
|
| |||
| SRS | 2.38 ±2.71 | 3.92 ±3.83 | 1.85 ±1.92 |
|
| |||
| Coagulation disorder | 37 (0.6%) | 25 (1.6%) | 12 (0.3%) |
|
| |||
| Antiplatelet/Anticoagulant drug | 934 (15.3%) | 437 (27.8%) | 497 (11%) |
| Acetyl salicylic acid | 634 (68.3%) | 268 (61.6%) | 366 (74.2%) |
| Clopidogrel | 150 (16.2%) | 78 (17.9%) | 72 (14.6%) |
| Dual therapy | 12 (1.3%) | 4 (0.9%) | 8 (1.6%) |
| NOAC | 30 (3.2%) | 16 (3.7%) | 14 (2.8%) |
| Warfarin | 56 (6%) | 37 (8.5%) | 19 (3.9%) |
| Ticlopidine | 2 (0.2%) | 2 (0.5%) | 0 (0%) |
|
| |||
| Abnormal coagulation profile | 506 (8.4%) | 222 (14.2%) | 284 (6.4%) |
|
| |||
| Pre-operative haemoglobin, g/dL | 12.9 (11.6–14.1) | 12 (10.4–13.6) | 13 (11.9–14.2) |
|
| |||
| Pre-operative anaemia | 2019 (33%) | 870 (55.1%) | 1,152 (25.4%) |
| Male | 787 (34.7%) | 388 (51.9%) | 399 (26.4%) |
| Female | 1,232 (32.0%) | 481 (58.0%) | 751 (24.9%) |
| Pregnancy | 146 (21.3%) | 19 (70.4%) | 127 (19.3%) |
| Pre-operative anaemia by age groups | |||
| 18–44 yrs, n=1,672 | 459 (27.4%) | 324 (28.7%) | 324 (28.7%) |
| 45–69 yrs, n=1,609 | 1,009 (31.1%) | 554 (28%) | 554 (28%) |
| 70–79 yrs, n=942 | 353 (37.4%) | 156 (16.5%) | 156 (16.5%) |
| ≥80 yrs, n=251 | 157 (62.5%) | 76 (52.8%) | 76 (52.8%) |
|
| |||
| Anaemia investigations | |||
| Ferritin/Transferrin | 55 (0.8%) | 30 (1.8%) | 25 (0.5%) |
| BUN; Creatinin | 51 (0.8%) | 26 (1.6%) | 25 (0.5%) |
| CRP | 36 (0.5%) | 19 (1.2%) | 17 (0.3%) |
|
| |||
| Anaemia treatment | 79 (1.3%) | 18 (1.1%) | 61 (1.3%) |
| Oral iron | |||
| Intravenous iron | 8 (0.1%) | 2 (0.1%) | 6 (0.1%) |
| Vitamin B12, folic acid | 7 (0.1%) | 2 (0.1%) | 5 (0.1%) |
|
| |||
| Haemostatic agents | |||
| Tranexamic acid | 584 (9.5%) | 247 (17.3%) | 337 (7.4%) |
| Fibrinogen | 8 (1.3%) | 7 (2.5%) | 1 (0.3%) |
|
| |||
| Autologous transfusion | |||
| None | 5,956 (98.4%) | 1,479 (94.7%) | 4,477 (99.7%) |
| Acute normovolemic haemodilution | 58 (1%) | 56 (3.6%) | 2 (0%) |
| Pre-operative autologous donation | 22 (0.4%) | 13 (0.8%) | 9 (0.2%) |
| Cell salvage | 15 (0.2%) | 13 (0.8%) | 2 (0%) |
|
| |||
| Controlled hypotension | 626 (10.3%) | 241 (15.4%) | 385 (8.6%) |
|
| |||
| Surgical specialty | |||
| Orthopaedics | 1,408 (23.0%) | 401 (25.4%) | 1,007 (22.2%) |
| Gynaecology/obstetrics | 1,403 (23.0%) | 125 (7.9%) | 1,288 (28.4%) |
| General surgery | 973 (15.9%) | 230 (14.6%) | 743 (16.4%) |
| Neurosurgery | 935 (15.3%) | 169 (10.7%) | 766 (16.9%) |
| Cardiovascular/thoracic | 761 (12.4%) | 502 (31.8%) | 259 (5.7%) |
| Urology | 547 (8.9%) | 120 (7.6%) | 427 (9.4%) |
| Transplantation | 84 (1.4%) | 32 (2%) | 52 (1.1%) |
|
| |||
| Duration of surgery, min | 155.5 ± 102.6 | 214.9 (113.4 | 134.7 ±89.7 |
|
| |||
| * Intra-operative blood loss, mL | 373.8 ±485.4 | 704.4 ±775.3 | 262.9 ±253.1 |
Values are mean ± standard deviation, median (interquartile range) or numbers (proportion).
BMI: body mass index; ASA: American Society of Anaesthesiologists physical class; CCI: Carlson co-morbidity index; P-POSSUM: Portsmouth physiological and operative severity score; SRS: Surgical risk scale; NOAC: novel oral anticoagulants; BUN: blood urea nitrogen; CRP: C-reactive protein.
In non-cardiac surgery.
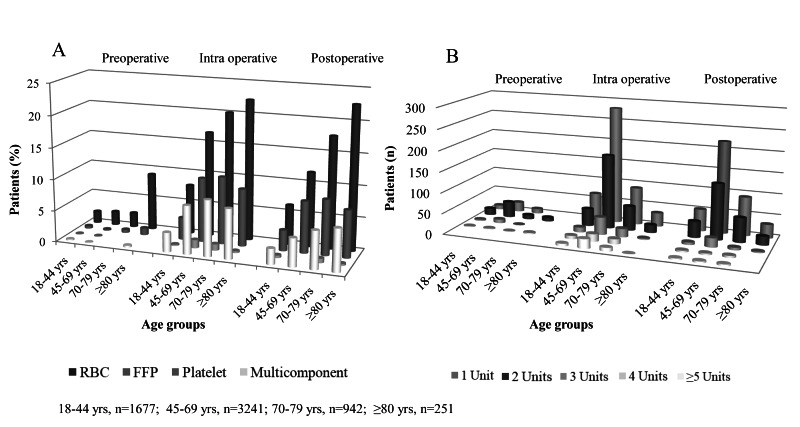
During the peri-operative course, 1,579 (25.8%) patients received at least 1 unit of a blood component; a total of 5,812 blood components were transfused (Table II). A transfusion was given to 154 (9.8%) patients pre-operatively, 1,057 (66.9%) patients intra-operatively, and 889 (56.3%) patients post-operatively. There were more female transfusion recipients (52.6% vs 47.3%). The median (interquartile range) age of the transfusion recipients was 62 (52–70) years and the rate of receiving a transfusion was the highest in patients >80 years old (144/251, 57.3%) (Table II). A median of 2 (1–3) U of RBC, 2 (1–4) U of FFP and 4 (4–8) U of platelets were transfused per patient. Among transfused patients 861/1,579 (54.5%) cases received >1 U of RBC; the numbers of patients receiving each blood component and numbers of transfused RBC units by age groups are displayed in Figure 2. Cardiovascular/thoracic surgery was the surgical specialty with the highest RBC, FFP and platelet transfusion rate, 31.3%, 62.5% and 65.0% respectively. The procedures with the highest RBC transfusion rate were coronary artery bypass grafting (n=313/359, 87.2%) and in non-cardiac surgery vertebrae instrumentation for diagnosis of a malignancy (n=37/81, 45.6%) (Table II).
Table II.
Peri-operative transfusion data and Red blood cell transfusion indications
| A. Peri-operative transfusion data of the study population | ||||
|---|---|---|---|---|
|
| ||||
| N=6,121 | Total | RBC | FFP | Platelets |
|
| ||||
| Transfused patients | ||||
| Peri-operative | 1,579 (25.8%) | 1,425 (23.3%) | 762 (12.4%) | 88 (1.4%) |
| Pre-operative | 154/1,579 (9.8%) | 151 (10.6%) | 25 (3.3%) | 5 (5.7%) |
| Intra-operative | 1.057/1,579 (66.9%) | 926 (65.0%) | 517 (67.8%) | 59 (67.0%) |
| Post-operative | 889/1,579 (56.3%) | 716 (50.2%) | 425 (55.8%) | 38 (43.2%) |
|
| ||||
| Patients transfused by gender | ||||
| Male, n=2,262 | 747 (47.3%) | 639 (28.2 %) | 417 (18.4%) | 60 (2.6%) |
| Female, n=3,859 | 832 (52.6%) | 786 (20.3 %) | 344 (8.9%) | 28 (0.7%) |
|
| ||||
| Transfused patients by age group | ||||
| 18–44 yrs, n=1,672 | 230 (13.7%) | 213 (12.7%) | 101 (6%) | 11 (1.8%) |
| 45–69 yrs, n=1,609 | 923 (57.3%) | 807 (50.1%) | 475 (29.5%) | 58 (3.6%) |
| 70–79 yrs, n=942 | 312 (33.1%) | 296 (31.4%) | 147 (15.6%) | 17 (1.8%) |
| ≥80 yrs, n=251 | 107 (42.7%) | 104 (41.4%) | 37 (14.7%) | 2 (0.7%) |
|
| ||||
| Blood components | ||||
| Units transfused | 5,812 (100.0%) | 3,137 (53.9%) | 2,092 (35.9%) | 583 (10.0%) |
| Units transfused per patient | 2 (1–4) | 2 (1–3) | 2 (1–4) | 4 (4–8) |
|
| ||||
| Transfused units by surgical specialty (n=5,812) | ||||
| Cardiovascular/thoracic | 2,670 (45.9%) | 982 (31.3%) | 1,309 (62.5%) | 379 (65.0%) |
| Orthopaedics | 850 (14.6%) | 719 (22.9%) | 126 (6.0%) | 5 (0.8%) |
| General surgery | 755 (12.9%) | 450 (14.3%) | 261 (12.4%) | 44 (7.5%) |
| Neurosurgery | 450 (7.7%) | 353 (11.2%) | 93 (4.4%) | 4 (0.6%) |
| Gynaecology/obstetrics | 425 (7.3%) | 278 (8.8%) | 115 (5.4%) | 32 (5.4%) |
| Urology | 334 (5.7%) | 236 (7.5%) | 83 (3.9%) | 15 (2.5%) |
| Transplantation | 328 (5.6%) | 119 (3.7%) | 105 (5.0%) | 104 (17.8%) |
|
| ||||
| Transfused patients by surgical procedure * | ||||
| CABG, n=359 | 296 (82.4%) | 313 (87.2%) | 249(69.5%) | 43(11.9%) |
| CABG + valve surgery, n=36 | 36 (100.0%) | 27 (75.0%) | 31(86.1%) | 1(2.7%) |
| Valve surgery, n=128 | 111 (86.7%) | 88 (68.7%) | 97 (52.3%) | 17(13.2%) |
| Vertebrae - Instrumentation - Malign, n:81 | 37(45.6%) | 37 (45.6%) | 25(30.8%) | |
| Hip arthroplasty - Fracture, n=266 | 127 (47.7%) | 114 (42.8%) | 12(4.5%) | 1(0.3%) |
| Hip arthroplasty - Coxarthrosis, n=265 | 110 (41.5%) | 112 (42.2%) | 26 (9.8%) | |
| Vertebrae - Instrumentation - Benign, n=207 | 59 (28.5) | 59 (28.5%) | 22(10.6%) | |
| Colectomy - Malign, n=285 | 85 29.8%) | 76 (26.6%) | 39(13.6%) | |
| Prostatectomy - Malign, n=136 | 31 (22.7%) | 30 (22.0%) | 11(8.0%) | |
|
| ||||
| B. Red blood cell transfusion indications reported by participants | ||||
|
| ||||
| Pre-operative | Intra-operative | Post-operative | ||
|
| ||||
| Haemoglobin trigger | 151 (100%) | 527 (57.3%) | 521 (72.8%) | |
| Haemoglobin <7 g/dL | 12 (7.9%) | 48 (9.5%) | 12 (2.4%) | |
| Haemoglobin 7–10 g/dL | 98 (64.9%) | 435 (86.1%) | 454 (91.2%) | |
| Haemoglobin >10 g/dL | 41 (27.1%) | 22 (4.4%) | 32 (6.4%) | |
| Surgical blood loss | - | 341 (37.1%) | 109 (15.2%) | |
| Hypotension | - | 295 (32.1%) | 103 (14.4%) | |
| Oozing | - | 189 (20.5%) | 175 (24.4%) | |
| Tachycardia | - | 140 (15.1%) | 46 (6.4%) | |
| Haemoglobin trigger and physiological trigger ** | - | 175 (19.0%) | 88 (12.3%) | |
| Haemoglobin trigger and blood loss | - | 124 (13.5%) | 49 (6.8%) | |
| Haemoglobin trigger, physiological trigger and blood loss | - | 73 (7.8%) | 6 (0.8%) | |
| Blood loss and physiological trigger | - | 109 (11.8%) | 20 (2.8%) | |
| Presence of a co-morbidity | - | 26 (2.8%) | 13 (1.8%) | |
| Decreased tissue oxygenation † | - | 11 (1.1%) | - | |
|
| ||||
| Inappropriate RBC transfusion ‡ | 150 (99%) | 211 (23%) | 308 (43%) | |
Values are mean ± standard deviation, median (interquartile range) or numbers (proportion).
The first seven procedures with the highest RBC transfusion rate are presented. There are patients in whom transfusion occurred in >1 period, with >1 component, and with >1 U.
Physiological trigger includes hypotension, tachycardia, acidosis, high lactate levels.
Decreased tissue oxygenation includes jugular venous oximetry and regional oxygen saturation.
Inappropriate transfusion = haemoglobin concentration ≥7 g/dL and without active bleeding and/or without the presence of comorbid disease and/or physiological transfusion trigger.
RBC: red blood cells; FFP: fresh-frozen plasma; CABG: coronary artery bypass grafting; U: unit.
Figure 2.
Transfusion data by age groups
A: Number of blood components transfused in each age group; B: Number of red blood cell units transfused in each age group.
RBC: red blood cells, FFP: fresh-frozen plasma.
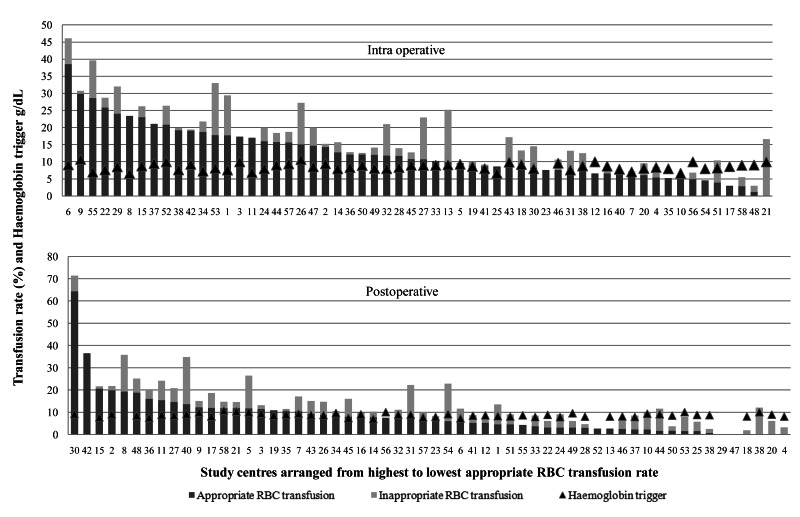
The most common RBC transfusion indication was a “haemoglobin trigger”, which was the case for all pre-operative RBC transfusions and 57.3% of intra-operative and 72.8% of post-operative RBC transfusions (Table II). The mean haemoglobin concentrations prior to a RBC transfusion were 9.2±1.4 g/dL pre-operatively, 8.9±1.9 g/dL, intra-operatively, and 8.8±1.3 g/dL post-operatively. The mean haemoglobin trigger in study centres varied from 6.6±0.9 to 10.7±1.2 g/dL intra-operatively and from 7.0±1.0 to 10.6±1.2 g/dL post-operatively (Figure 3). The mean haemoglobin concentrations after RBC transfusion were 10.3±1.2 g/dL pre-operatively, 9.8±1.8 g/dL intra-operatively, and 9.7±1.3 g/dL post-operatively. Haemoglobin concentrations were measured after each RBC transfusion for 81.0% of the RBC transfusions. Transfusion indications for FFP were hypotension (36.2%), oozing (32.6%); surgical blood loss (30.4%) and guided by point-of-care coagulation testing (1.4%) intra-operatively, and oozing (49.4%), hypotension (20.5%) and surgical blood loss (11.8%) post-operatively. Transfusion indications for platelets were oozing (39.7%), surgical blood loss (19.0%) and guided by point-of-care testing (1.7%) intra-operatively, and oozing (44.7%) and surgical blood loss (26.3%) post-operatively.
Figure 3.
Intra-operative and post-operative red blood cell transfusion rates and mean haemoglobin triggers in study centres
RBC: red blood cells.
The RBC transfusion rate in study centres varied from 3 to 46% intra-operatively and from 2 to 72% post-operatively. According to our predefined criteria, RBC transfusion was inappropriate in 150 (99%) patients pre-operatively, in 211 (23%) intra-operatively and in 308 (43%) post-operatively (Figure 3). The logistic regression analysis of the total RBC transfusions and inappropriate RBC transfusions in both the intra-operative and post-operative periods did not reveal an association between these (r=0.216, p=0.104 and r=0.207, p=0.125, respectively).
Patient-, procedure-, and anaesthesia-related factors associated with intra-operative RBC transfusions are presented in Table III. Independent risk factors for intra-operative RBC transfusion were age (OR: 1.025, 95% CI: 1.019–1.031; p<0.001), body mass index (OR: 0.982, 95% CI: 0.965–0.997; p=0.018), presence of coronary artery disease (OR 1.279, 95% CI: 1.019–1.606); p=0.034), presence of heart failure (OR 1.489, 95% CI: 1.066–2.101; p=0.023), low pre-operative haemoglobin concentration, per 1 g/dL decrease (OR: 1.471, 95% CI: 1.403–1.541; p<0.001), presence of an abnormal coagulation profile (OR 1.447, 95% CI: 1.101–1.901; p=0.008), blood loss >400 mL (OR: 8.677, 95% CI: 7.191–10.470; p<0.001), duration of surgery >180 min (OR: 2.985, 95% CI: 2.468–3.610; p<0.001), and open surgical technique (OR: 3.266, 95% CI: 2.064–5.167; p<0.001).
Table III.
Multivariate analyses
| A. Factors associated with intra-operative red blood cell transfusion | ||||
|---|---|---|---|---|
| Factors | Univariate analysis | Multivariate analysis | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Patient-related factors | ||||
| Gender (male vs female) | 1.747 (1.517–2.011) | <0.001 | 1.025 (1.019–1.031) | <0.001 |
| Age change per year increase | 1.025 (1.020–1.029) | <0.001 | 0.982 (0.965–0.997) | 0.018 |
| BMI | 0.961 (0.948–0.975) | <0.001 | ||
| ASA change per class increase | 1.954 (1.783–2.141) | <0.001 | ||
| CCI change per class increase | 1.644 (1.373–1.968) | <0.001 | ||
| SRS | 1.272 (1.241–1.304) | <0.001 | ||
| P-POSSUM change per class increase | 4.153 (3.569–4.840) | <0.001 | ||
| Coronary artery disease | 2.472 (2.080–2.938) | <0.001 | 1.279 (1.019–1.606) | 0.034 |
| Heart failure | 2.999 (2.305–3.903) | <0.001 | 1.489 (1.056–2.101) | 0.023 |
| Stroke | 1.921 (1.262–2.923) | 0.002 | - | 0.733 |
| Malignancy | 1.290 (1.020–1.630) | 0.033 | - | 0.121 |
| Pre-operative haemoglobin decrease per 1 g/dL | 1.329 (1.280–1.381) | <0.001 | 1.471 (1.403–1.541) | <0.001 |
| Pre-operative anaemia | 2.819 (2.444–3.251) | <0.001 | ||
| Presence of antiplatelet/anticoagulant drugs | 2.290 (1.937–2.707) | <0.001 | ||
| Presence of an abnormal coagulation profile* | 2.404 (1.952–2.960) | <0.001 | 1.447 (1.101–1.901) | 0.008 |
| Presence of a coagulation disorder | 4.839 (2.525–9.273) | <0.001 | ||
| Presence of pre-operative RBC transfusion | 2.703 (1.905–3,835) | <0.001 | ||
| Pre-operative RBC transfusion per 1 unit increase | 1.393 (1.184–1.638) | <0.001 | ||
| Procedure-related factors | ||||
| Amount of blood loss increase per 100 mL | 1.364 (1.333–1.395) | <0.001 | ||
| Blood loss more than 400 mL | 10.796 (9.206–12.260) | <0.001 | 8.677 (7.191–10.470) | <0.001 |
| Duration of surgery increase per 1 min | 1.008 (1.007–1.008) | <0.001 | ||
| Duration of surgery more than 180 min | 5.539 (4.778–6.421) | <0.001 | 2.985 (2.468–3.610) | <0.001 |
| Surgical technique (open vs other**) | 5.708 (3.776–8.629) | <0.001 | 3.266 (2.064–5.167) | <0.001 |
| Anaesthesia-related factors | ||||
| Use of haemostatic agents | 2.098 (1.716–2.098) | <0.001 | ||
| Anaesthesia method (general vs other†) | 2.229 (1.853–2.683) | <0.001 | ||
| Hospital-related factors | ||||
| Type of hospital (university-teaching vs other‡) | 1.335 (1.106–1.611) | 0.003 | −0.160 | |
| Size of hospital (>750 beds vs ≤750 beds) | 1.324 (1.142–1.534) | <0.001 | - | 0.057 |
| B. Factors associated with post-operative pulmonary complications | ||||
| Factors | Univariate analysis | Multivariate analysis | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Patient-related factors | ||||
| Gender (male vs female) | 2.132 (1.473–3.085) | <0.001 | 1.530 (1.003–2.253) | 0.048 |
| Age change per year increase | 1.039 (1.025–1.052) | <0.001 | 1.030 (1.016–1.045) | <0.001 |
| BMI | 0.997 (0.964–1.032) | 0.879 | ||
| ASA change per class increase | 1.920 (1.578–2.335) | <0.001 | ||
| CCI change per class increase | 2.106 (1.471–3.014) | <0.001 | ||
| SRS | 1,157 (1,117–1,198) | <0,001 | ||
| P-POSSUM change per class increase | 2.727 (2.163–3.437) | <0.001 | ||
| Coronary artery disease | 2.988 (2.002–4.459) | <0.001 | 1.581 (1.024–2.441) | 0.039 |
| Heart failure | 3.082 (1.739–5.464) | <0.001 | ||
| Stroke | 1.348 (0.422–4.304) | 0.614 | - | 0.652 |
| Malignancy | 1.254 (0.685–2.295) | 0.463 | - | 0.937 |
| Pre-operative haemoglobin decrease per 1 g/dL | 1.143 (1.040–1.256) | 0.006 | 1.171 (1.064–1.289) | 0.001 |
| Pre-operative anaemia | 1.800 (1.246–2.600) | 0.002 | ||
| Use of antiplatelet/anticoagulant drugs | 1.868 (1.220–2.860) | 0.004 | ||
| Presence of an abnormal coagulation profile* | 1.937 (1.148–3.267) | 0.013 | - | 0.250 |
| Presence of pre-operative RBC transfusion | 2.614 (1.196–5.713) | 0.016 | ||
| Pre-operative RBC transfusion per 1 unit increase | 1.542 (1.157–2.054) | 0.003 | ||
| Procedure-related factors | ||||
| Amount of blood loss increase per 100 mL | 1.042 (1.020–1.064) | <0.001 | ||
| Blood loss more than 400 mL | 2.934 (2.030–4.242) | <0.001 | 2.025 (1.347–3.044) | 0.001 |
| Duration of surgery increase per 1 min | 1.004 (1.003–1.006) | <0.001 | ||
| Duration of surgery more than 180 minutes | 3.207 (2.212–4.650) | <0.001 | 2.006 (1.321–3.045) | 0.001 |
| Surgical technique (open vs other†) | 1.396 (0.726–2.682) | 0.317 | - | 0.771 |
| Anaesthesia-related factors | ||||
| Presence of haemostatic agents | 0.993 (0.530–1.859) | 0.983 | ||
| Anaesthesia method (general vs other‡) | 2.280 (1.359–3.825) | 0.002 | ||
| Intra-operative crystalloid infusion (per liter) | 1,400 (1,258–1,558) | <0,001 | ||
| Intra-operative colloid infusion (per liter) | 2,519 (1,623–3,910) | <0,001 | ||
| Intra-operative blood transfusion (per unit) | 1,334 (1,185–1,501) | <0,001 | ||
| Use of intra-operative blood transfusion | 3,686 (2,520–5,392) | <0,001 | ||
Values are odds ratio and 95% confidence intervals.
Prothrombin time, activated prothrombin time, and international normalized ratio above institutional laboratory references.
Laparoscopic, robotic.
Neuro-axial block, peripheral block.
state, private.
ASA: American Society of Anesthesiologists; CCI: Carlson co-morbidity index; SRS: Surgical risk score; P-POSSUM: Portsmouth physiological and operative severity score for the enumeration of morbidity and mortality; RBC: red blood celsl; CI: confidence interval.
Adverse outcomes following surgery were observed in 371 (6.1%) patients (Table IV). The unadjusted rates of post-operative adverse outcomes and mortality were higher in patients who received a transfusion. The adverse outcome rate was 3.7% (n=229/6,121 patients) in transfused patients and 2.3% (n=142/6,121 patients) in non-transfused patients (p<0.001). The all-cause mortality rate at day 30 was 1.4% (n=58/1,579 transfused patients and n=27/4,542 non-transfused patients) (p<0.001). After adjusting for confounders, the regression models did not provide statistically significant explanatory power; a multivariate analysis could only be performed for the composite “post-operative pulmonary complications”. Factors associated with an increased risk of post-operative pulmonary complications are presented in Table III; pre-operative low haemoglobin concentration was associated with post-operative pulmonary complications whereas intra-operative RBC transfusion was not.
Table IV.
Univariate analysis of the post-operative outcomes of the transfused and non-transfused patients
| Overall | Transfused | Non-transfused | p | |
|---|---|---|---|---|
| Mortality | 85 (1.4%) | 58 (0.9%) | 27 (0.4%) | <0.001 |
| Unanticipated ICU admission | 34 (0.6%) | 19 (0.3%) | 15 (0.2%) | <0.001 |
| PLOS | 155 (2.5%) | 84 (1.3%) | 71 (0.1%) | <0.001 |
| Single organ outcomes | ||||
| Pneumonia | 39 (0.6%) | 26 (0.4%) | 13 (0.2%) | <0.001 |
| Pulmonary embolism | 13 (0.2%) | 5 (0.08%) | 8 (0.1%) | <0.001 |
| Acute kidney injury | 27 (0.4%) | 15 (0.2%) | 12 (0.1%) | <0.001 |
| Myocardial infarction | 8 (0.1%) | 6 (0.09%) | 2 (0.03%) | <0.001 |
| Stroke | 13 (0.2%) | 8 (0.1%) | 5 (0.08%) | <0.001 |
| Infection | 130 (2.1%) | 57 (0.9%) | 73 (1.1%) | <0.001 |
| Composite outcomes | ||||
| Major adverse cardiac event | 33 (0.5%) | 26 (0.4%) | 7 (0.1%) | <0.001 |
| Pulmonary complications | 116 (1.9 %) | 68 (1.1%) | 48 (0.7%) | <0.001 |
| Post-operative morbidity | 103 (1.7%) | 58 (0.9%) | 45 (0.7%) | <0.001 |
Values are numbers (proportion). ICU: intensive care unit; PLOS: prolonged length of stay according to local standard care;
Infection includes: infection source unknown, surgical site infection (superficial, deep, and organ/space), urinary tract infection, blood stream infection
Major adverse cardiac event includes: arrhythmia, angina, new requirement for anticoagulation, cardiogenic pulmonary oedema, non-fatal cardiac arrest, cardiac arrest, myocardial infarction, myocardial injury after non-cardiac surgery, congestive failure. Pulmonary complications include: pneumonia, pulmonary embolism, respiratory failure, respiratory infection, acute respiratory distress syndrome.
Post-operative morbidity includes: new requirement for oxygen or respiratory support, hypotension, ischaemia, arrhythmia, urinary catheter, increased creatinine, delirium, stroke, new neurological deficit, coma, thromboembolic event, ischaemic event.
DISCUSSION
This study provides detailed clinical data for a large number of surgical patients, comprising both transfusion recipients and non-recipients, and a description of transfusion practices and patients’ outcomes.
In the present study transfusion practice is evaluated throughout all three peri-operative periods because the decision to transfuse is made by different clinicians with presumably diverse indications in each stage. In general, anaesthesiologists are primary decision-makers during surgery, whereas surgeons make the decisions regarding transfusion before and after surgery.
We documented a high peri-operative incidence of transfusion. Even though the highest proportion of transfusions occurred intra-operatively; transfusions performed before and after surgery constituted almost an equal proportion of peri-operative transfusion.
One of the important results of the TULIP-TS is that one-third of the study population had pre-operative anaemia. We used the World Health Organisation definition to detect anaemia. This definition is considered deficient because females have lower circulating blood volume and, therefore, the same amount of blood loss during similar surgery leads to a higher proportion of lost circulating blood volume compared to that in men20. Using a definition of anaemia of <13 g/dL for both sexes, a higher incidence of pre-operative anaemia could have been documented in females.
The management of pre-operative anaemia is an integral aspect of PBM. Pre-operative anaemia increases the likelihood of RBC transfusion in surgical patients and is associated with adverse outcomes21. Iron deficiency is the most common cause of anaemia in surgical patients and can be treated with either oral or intravenous iron replacement depending on the timing of surgery20. Intravenous iron therapy is recommended prior to surgery as it both increases haemoglobin concentration and decreases the requirement for RBC transfusion22.
Unfortunately, in our study population pre-operative anaemia was either left untreated or RBC transfusions were given, with a low haemoglobin concentration being the reason for all the pre-operative RBC transfusions. The mean pre-operative haemoglobin concentration that we determined suggests that the “historical 10 g haemoglobin rule” is still accepted among surgeons.
According to our results intra-operative RBC transfusions were triggered primarily by a haemoglobin threshold. Current guidelines suggest that RBC transfusion is indicated when haemoglobin concentration is <7 g/dL, can be administered depending on co-morbidities, intravascular volume and blood loss when haemoglobin concentration is 7–10 g/dL and is unnecessary when haemoglobin concentration is >10 g/dL, and a target haemoglobin concentration of 7–9 g/dL is recommended during active bleeding5–9. Adapting low haemoglobin thresholds for transfusion reduces RBC utilization20,22. Nevertheless, indications for intra-operative RBC transfusion are not clearly defined and the 7–10 g/dL haemoglobin concentration range, in particular, is left to clinicians’ discretion5–11.
In the TULIP-TS the intra-operative haemoglobin trigger was mostly between 7–10 g/dL; however, a reason to justify all of these transfusions was not reported by the participants. These findings indicate that a restrictive transfusion strategy is not well adopted among anaesthesiologists.
The appropriateness of RBC transfusion was assessed on the basis of a haemoglobin trigger (<7 g/dL) and the presence of active bleeding and/or a co-morbidity and/or indicators of impaired oxygenation to rationalise higher haemoglobin triggers. Accordingly, over one-fifth of intra-operative RBC transfusion episodes were considered inappropriate.
We examined the potential predictors of intra-operative RBC transfusion. A low pre-operative haemoglobin concentration also appeared as an independent risk factor for intra-operative RBC transfusion; the probability of intra-operative RBC transfusion increased by 1.5-fold for each 1 g/dL decrease in pre-operative haemoglobin concentration. The analyses also included adjustments for the patients’ co-morbidity, physiological and surgical risk indices which include vital signs, laboratory values, drugs and the magnitude of surgery as parameters. The results show that not the severity of illness but the presence of coronary arterial disease and heart failure were independently associated with increased transfusion requirement. The study centres were included in this analysis according to their healthcare provision levels and size; we found that neither the type nor the size of the hospital was a predictor of intra-operative RBC transfusion. This result is in accordance with previous studies reporting wide variability between hospitals in blood component transfusion irrespective of hospital type or surgical case volume23.
Increased blood loss was the second most reported trigger of intra-operative RBC transfusion, and a physiological transfusion trigger -hypotension- was the subsequent reason for initiating a RBC transfusion. Physiological triggers based on signs of impaired global or regional oxygenation can be used to guide transfusion, provided that the volume status and anaesthesia is optimised24. Physiological triggers should replace arbitrary haemoglobin values to determine individual transfusion requirements24.
Post-operative RBC transfusions were also mainly triggered by a haemoglobin threshold. We evaluated inappropriate post-operative RBC transfusions separately and found a higher rate compared to that in the intra-operative period, suggesting that the surgeons are even more reluctant to use restrictive haemoglobin triggers.
Of particular interest with regards to inappropriate RBC use, we documented that inappropriate pre-operative RBC transfusions accounted for over 10% of all RBC transfusions; 23% of the intra-operative and 43% of the post-operative RBC transfusion episodes were considered inappropriate. These data show the potential extent of decrease in RBC use after implementation of PBM.
The main indication for FFP and platelet transfusions was “blood loss” and specifically “oozing” both in the intra-operative and post-operative periods. Although this type of blood loss suggests coagulopathy, the FFP and platelet transfusions were not accompanied by coagulation testing. Oozing and post-operative bleeding also suggests that bleeding disorders or drugs that compromise the coagulation pathway may be involved25. Our results did not support these assumptions; however, the results showed that the presence of an abnormal coagulation profile increased the risk of intra-operative RBC transfusion. Oozing itself is a subjective definition; blood transfusion because of oozing without coagulation testing actually shows that a clinician’s knowledge, experience and personal preferences affect transfusion decisions.
The utilisation of blood conservation strategies during the study period was very low. One exception to this observation was the use of tranexamic acid. Tranexamic acid was used in 9.5% of the overall study population; however, considering that the majority of the study population underwent orthopaedic procedures it could be commented that the usage is still low.
One of the main drivers of the change in transfusion practices is the relation of transfusion with adverse outcomes, morbidity and mortality26–29.
Our results show higher rates of mortality and other adverse outcomes except for infections in transfused patients. Despite these results, since the incidence of adverse outcomes in the study population was low, the explanatory power of multivariate analysis was not sufficient to document a relationship between transfusion and adverse outcomes. We did document an increased risk of post-operative pulmonary complications in patients with a low pre-operative haemoglobin concentration.
Previous studies on transfusion in surgical patients are limited to certain surgical procedures2,30. Our study is comparable to the European Transfusion Practice and Outcome Study, although only RBC-transfused patients were included and only intra-operative transfusion was evaluated in that study31. The TULIP-TS is more comprehensive since the results indicate that the rate of transfusions outside the operating theatre might approximate the rate of those performed intra-operatively.
The prospective design is a strength of this study which enabled transfusion indications to be evaluated in relation to all patients’ conditions, in contrast to registries which associate the transfusion indication with only one diagnosis32. Moreover, this study included all patients undergoing major surgery and, therefore, the case mix of this sample can be regarded as representative of the surgical population. We report data from both transfusion recipients an non-recipients. Despite providing data from a large number of surgical patients, the short duration of recruitment is a limitation because certain surgical procedures may have been unequally represented in the study period. Another limitation is that post-operative transfusion was only monitored on the first day following surgery; some patients in the study population may have received transfusions in the following days, which could have affected the outcomes. Finally post-transfusion haemoglobin concentration was not recorded after each transfusion so the intended goal of transfusion could not be determined.
In this comprehensive evaluation of current peri-operative transfusion practices in Turkey we identified blood use that is non-compliant with evidence-based transfusion practice. We also found that PBM strategies are only applied individually, and transfusion decisions are mainly affected by physicians’ preferences.
Implementing a nationwide PBM programme may improve our transfusion practice1. Specific areas for improvement pointed out by our results are: pre-operative anaemia management, adapting low haemoglobin thresholds, increasing the utilisation of autologous transfusion methods and tranexamic acid when appropriate and increasing the use of coagulation testing20,33–38. This study indicated that the priority specialties for implementing PBM strategies are cardiovascular surgery and orthopaedics.
We also detected wide variations between study centres, especially in the haemoglobin triggers, RBC transfusion rates and inappropriate RBC transfusion rates. We consider this variation as a result of transfusion decisions being made by a single and different clinician in each peri-operative period. There is clear need to reduce blood use variability among hospitals and clinicians. Our results indicate that a formal training plan for surgeons and anaesthesiologists should be developed. Also practice standards in accordance with local legislation and infrastructure are needed. Involving surgeons in these efforts may improve success39. The performance of hospitals and physicians should be audited according to these standards. Establishing clinical transfusion management committees at hospitals may solve problems regarding transfusions based on clinicians’ preferences.
Both the transfusion rates and the haemoglobin triggers that we have determined constitute a reference point and can be used to assess the effectiveness of our PBM programmes in future audits.
We plan to present these results to the Ministry of Health and pursue legal enactment of implementation of a national PBM programme, acceptance of PBM strategies for hospital quality assessments, and constitution of clinical transfusion committees.
CONCLUSIONS
In Turkey, 25.8% of the patients presenting for elective major surgery received blood transfusion. The indications for RBC transfusion included haemoglobin threshold, presence of bleeding, and subsequent physiological triggers of transfusion.
Pre-operative anaemia was detected in more than one-third of the patients presenting for surgery. A low pre-operative haemoglobin concentration was not only an independent risk factor for intra-operative RBC transfusion but was also associated with a higher risk of post-operative pulmonary complications.
Supplementary Information
ACKNOWLEDGEMENTS
We thank Dr. H. K. Gülkesen, Department of Biostatistics and Medical Informatics, Akdeniz University, School of Medicine, Antalya, Turkey. We also acknowledge the Department of Blood Organ Transplantation Services, Ministry of Health for providing the data on annual blood component utilisation in the country.
Footnotes
FUNDING AND RESOURCES
This study was funded by the Turkish Society of Anaesthesiology and Reanimation.
AUTHORSHIP CONTRIBUTIONS
DU, YS, RP, DRS, FT and NA designed the research study and wrote the paper; DU, YS and NA analysed the data; DU, YS, RP, DRS, FT and NA reviewed the clinical aspects of the analysis. All the Authors approved the final version of the paper.
CONFLICT OF INTEREST
DU has received travel support for consulting or lecturing from the Turkish Society of Anaesthesiology and Reanimation, Istanbul, Turkey; the Society of Anaesthesiology and Reanimation Specialists, Ankara, Turkey; and Abdi İbrahim Ilaç Pazarlama, Istanbul, Turkey; RP has received travel support for consulting or lecturing from the Turkish Society of Anaesthesiology and Reanimation, Istanbul, Turkey; DRS has received honoraria/travel support for consulting or lecturing from Danube University of Krems, Austria; US Department of Defense, Washington, USA; European Society of Anaesthesiology, Brussels, Belgium; Korean Society for Patient Blood Management, Seoul, Korea; Korean Society of Anaesthesiologists, Seoul, Kore; Baxter AG, Volketswil, Switzerland; Baxter S.p.A., Rome, Italy; Bayer AG, Zürich, Switzerland; Bayer Pharma AG, Berlin, Germany; B. Braun Melsungen AG, Melsungen, Germany; Boehringer Ingelheim GmbH, Basel, Switzerland; Bristol-Myers-Squibb, Rueil-Malmaison Cedex, France and Baar, Switzerland; CSL Behring GmbH, Hattersheim am Main, Germany and Berne, Switzerland; Celgene International II Sàrl, Couvet, Switzerland; Curacyte AG, Munich, Germany; Daiichi Sankyo AG, Thalwil, Switzerland; GlaxoSmithKline GmbH & Co. KG, Hamburg, Germany; Haemonetics, Braintree, MA, USA; Instrumentation Laboratory (Werfen), Bedford, MA, USA; LFB Biomédicaments, Courtaboeuf Cedex, France; Merck Sharp & Dohme, Kenilworth, NJ, USA; Octapharma AG, Lachen, Switzerland; Organon AG, Pfäffikon/SZ, Switzerland; PAION Deutschland GmbH, Aachen, Germany; Pharmacosmos A/S, Holbaek, Denmark; Photonics Healthcare B.V., Utrecht, the Netherlands; Pierre Fabre Pharma, Alschwil, Switzerland; Roche Diagnostics International Ltd, Reinach, Switzerland; Roche Pharma AG, Reinach, Switzerland; Sarstedt AG & Co., Sevelen, Switzerland and Nümbrecht, Germany; Schering-Plough International, Inc., Kenilworth, NJ, USA; Tem International GmbH, Munich, Germany; Verum Diagnostica GmbH, Munich, Germany; Vifor Pharma, Munich, Germany, Vienna, Austria and Villars-sur-Glâne, Switzerland; Vifor (International) AG, St. Gallen, Switzerland; and Zuellig Pharma Holdings, Singapore, Singapore. Dr. Spahn’s academic department has received grant support from the Swiss National Science Foundation, Berne, Switzerland; the Swiss Society of Anaesthesiology and Reanimation (SGAR), Berne, Switzerland; the Swiss Foundation for Anaesthesia Research, Zurich, Switzerland; and Vifor SA, Villars-sur-Glâne, Switzerland. Dr. Spahn is co-chair of the ABC-Trauma Faculty, sponsored by unrestricted educational grants from Novo Nordisk Health Care AG, Zurich, Switzerland; CSL Behring GmbH, Marburg, Germany; LFB Biomédicaments, Courtaboeuf Cedex, France; and Octapharma AG, Lachen, Switzerland; YS has received travel support for consulting or lecturing from the Turkish Society of Anaesthesiology and Reanimation, Istanbul, Turkey; FT has received travel support for consulting or lecturing from the Turkish Society of Anaesthesiology and Reanimation, Istanbul, Turkey; NA has received honoraria from MSD Turkey; Eczacıbaşı İlaç Pazarlama, Abbvie Turkey; and Gen İlaç, Medtronic Medikal Teknoloji, Turkey. Dr. Alkis has received travel support for consulting or lecturing from the Turkish Society of Anaesthesiology and Reanimation; Draeger Medikal Ticaret ve Servis A.Ş.; Medtronic Medikal Teknoloji; CSL Behring GmbH, Hattersheim am Main, Germany and Berne, Switzerland; Vifor (International) AG, St. Gallen, Switzerland; Abdi İbrahim Pharmaceuticals, Istanbul, Turkey; and Masimo Medikal Ürünler Ticaret Limited Sti. Turkey. Dr. Alkıs’s academic department has received grant support from Gen İlaç, Eczacıbaşı İlaç Pazarlama, Turkey.
Contributor Information
Alanoglu Zekeriyya, Department of Anaesthesiology and Reanimation, Ankara University Medical Faculty, Ankara, Turkey.
Aydinli Bahar, Department of Anaesthesiology and Reanimation, Mersin City Hospital, Mersin, Turkey.
Bermede Ahmet Onat, Department of Anaesthesiology and Reanimation, Ankara University Medical Faculty, Ankara, Turkey.
Bilgin Hulya, Department of Anaesthesiology and Reanimation, Bursa Uludag University Medical Faculty, Bursa, Turkey.
Buget Mehmet, Department of Anaesthesiology, Istanbul University Medical Faculty, Istanbul, Turkey.
Coskunfirat Nesil, Department of Anaesthesiology and Reanimation, Akdeniz University Medical Faculty, Antalya, Turkey.
Demir Asli, Department of Anaesthesiology and Reanimation, University of Health Sciences Ankara City Hospital, Ankara, Turkey.
Goren Suna, Department of Anaesthesiology and Reanimation, Bursa Uludag University Medical Faculty, Bursa, Turkey.
Guner Can Meltem, Department of Anaesthesiology and Reanimation, Acibadem Mehmet Ali Aydınlar University School of Medicine, Atakent Hospital, Istanbul, Turkey.
Orhan Mukadder, Department of Anaesthesiology, Istanbul University Medical Faculty, Istanbul, Turkey.
Senturk Mert, Department of Anaesthesiology, Istanbul University Medical Faculty, Istanbul, Turkey.
Tezcan Busra, Department of Anaesthesiology and Reanimation, University of Health Sciences Ankara City Hospital, Ankara, Turkey.
Toprak Huseyin Ilksen, Department of Anaesthesiology, Inonu University Liver Transplantation Institute, Malatya, Turkey.
Yildirim Guclu Cigdem, Department of Anaesthesiology and Reanimation, Ankara University Medical Faculty, Ankara, Turkey.
Abitagaoglu Suheyla, Department of Anaesthesiology and Reanimation, University of Health Sciences Fatih Sultan Mehmet Teaching Hospital, Istanbul, Turkey.
Abut Yesim, Department of Anaesthesiology and Reanimation, University of Health Sciences Istanbul Teaching Hospital, Istanbul, Turkey.
Akdaglı Ekici Arzu, Department of Anaesthesiology and Reanimation, Hitit University, School of Medicine, Corum Erol Olcok Teaching Hospital, Corum, Turkey.
Akdas Tekin Esra, Department of Anaesthesiology and Reanimation, University of Health Sciences Okmeydanı Teaching Hospital, Istanbul, Turkey.
Akdogan Ali, Department of Anaesthesiology and Reanimation, Karadeniz Technical University Medical Faculty Farabi Hospital, Trabzon, Turkey.
Akin Mine, Department of Anaesthesiology, University of Health Sciences Ankara Child Health and Diseases Hematology Oncology Teaching Hospital, Ankara, Turkey.
Akovali Nukhet, Department of Anaesthesiology and Reanimation, Baskent University Medical Faculty Ankara Hospital, Ankara, Turkey.
Aksoy Semsi Mustafa, Department of Anaesthesiology and Reanimation, Ankara Ataturk Teaching Hospital, Ankara, Turkey.
Alaygut Ergin, Department of Anaesthesiology and Reanimation, University of Health Sciences Izmir Tepecik Teaching Hospital, Izmir, Turkey.
Arar Makbule Cavidan, Department of Anaesthesiology and Reanimation, Namık Kemal University Medical Faculty, Tekirdag, Turkey.
Arican Sule, Department of Anaesthesiology and Reanimation, Necmettin Erbakan University Meram Medical Faculty, Konya, Turkey.
Arici Ayse Gulbin, Department of Anaesthesiology and Reanimation, Akdeniz University Medical Faculty, Antalya, Turkey.
Arik Emine, Department of Anaesthesiology and Reanimation, University of Health Sciences Diskapi Yildirim Beyazit Teaching Hospital, Ankara, Turkey.
Arik Esma, Department of Anaesthesiology and Reanimation, Gazi University Medical Faculty, Ankara, Turkey.
Arslan Mahmut, Department of Anaesthesiology and Reanimation, Sutcu Imam University Medical Faculty, Kahramanmaraş, Turkey.
Ay Necmiye, Department of Anaesthesiology and Reanimation, University of Health Sciences Kanuni Sultan Suleyman Teaching Hospital, Istanbul, Turkey.
Aykac Zuhal, Department of Anaesthesiology and Reanimation, Marmara University Pendik Teaching Hospital, Istanbul, Turkey.
Ayoglu Hilal, Department of Anaesthesiology and Reanimation, Zonguldak Bulent Ecevit University Medical Faculty Center for Health and Research, Zonguldak, Turkey.
Basaran Cumhur, Department of Anaesthesiology and Reanimation, University of Health Sciences Ankara Teaching Hospital, Ankara, Turkey.
Baytas Volkan, Department of Anaesthesiology and Reanimation, Ankara University Medical Faculty, Ankara, Turkey.
Bedirli Nurdan, Department of Anaesthesiology and Reanimation, Gazi University Medical Faculty, Ankara, Turkey.
Bestas Azize, Department of Anaesthesiology and Reanimation, Fırat University Medical Faculty, Elazıg, Turkey.
Bigat Zekiye, Department of Anaesthesiology and Reanimation, Akdeniz University Medical Faculty, Antalya, Turkey.
Bilgin Mehmet Ugur, Department of Anaesthesiology and Reanimation, University of Health Sciences Izmir Bozyaka Teaching Hospital, Izmir, Turkey.
Boran Omer Faruk, Department of Anaesthesiology and Reanimation, Sutcu Imam University Medical Faculty, Kahramanmaraş, Turkey.
Buyukcoban Sibel, Department of Anaesthesiology and Reanimation, Dokuz Eylul University Medical Faculty, Izmir, Turkey.
Cakar Turhan Sanem, Department of Anaesthesiology and Reanimation, Ankara University Medical Faculty, Ankara, Turkey.
Cakmak Meltem, Department of Anaesthesiology and Reanimation, University of Health Sciences Izmir Tepecik Teaching Hospital, Izmir, Turkey.
Cankaya Baris, Department of Anaesthesiology and Reanimation, Marmara University Pendik Teaching Hospital, Istanbul, Turkey.
Capar Ayse, Department of Anaesthesiology and Reanimation, University of Health Sciences Kayseri Teaching Hospital, Kayseri, Turkey.
Cebeci Zubeyir, Department of Anaesthesiology and Reanimation, Ordu University Medical Faculty, Ordu, Turkey.
Cetinkaya Ethemoglu Filiz Banu, Department of Anaesthesiology and Reanimation, Yenimahalle Teaching Hospital, Ankara, Turkey.
Cicekci Faruk, Department of Anaesthesiology and Reanimation, Selcuk University Medical Faculty, Konya, Turkey.
Colak Alkin, Department of Anaesthesiology and Reanimation, Trakya University Medical Faculty, Edirne, Turkey.
Colak Yusuf Ziya, Department of Anaesthesiology, Inonu University Liver Transplantation Institute, Malatya, Turkey.
Dagli Esra, Department of Anaesthesiology and Reanimation, University of Health Sciences Okmeydanı Teaching Hospital, Istanbul, Turkey.
Demir Hafize Fisun, Department of Anaesthesiology and Reanimation, Balikesir University Medical Faculty Teaching Hospital, Balıkesir, Turkey.
Derbent Abdurrahim, Department of Anaesthesiology and Reanimation, University of Health Sciences Kanuni Sultan Suleyman Teaching Hospital, Istanbul, Turkey.
Dumanlı Ozcan Ayca, Department of Anaesthesiology and Reanimation, Ankara Ataturk Teaching Hospital, Ankara, Turkey.
Ekinci Osman, Department of Anaesthesiology and Reanimation, University of Health Sciences Haydarpasa Numune Teaching Hospital, Istanbul, Turkey.
Erdogan Kayhan Gulay, Department of Anaesthesiology and Reanimation, Eskisehir Osmangazi University Medical Faculty, Eskisehir, Turkey.
Erturk Engin, Department of Anaesthesiology and Reanimation, Karadeniz Technical University Medical Faculty Farabi Hospital, Trabzon, Turkey.
Erus Ipek, Department of Anaesthesiology and Reanimation, University of Health Sciences Umraniye Teaching Hospital, Istanbul, Turkey.
Tekeli Arzu Esen, Department of Anaesthesiology and Reanimation, Van Yuzuncu yil University Medical Faculty Dursun Odabası Medical Center, Van, Turkey.
Gamli Mehmet, Department of Anaesthesiology and Reanimation, University of Health Sciences Yuksek Ihtisas Teaching Hospital, Bursa, Turkey.
Gulel Basak, Department of Anaesthesiology and Reanimation, University of Health Sciences Diskapi Yildirim Beyazit Teaching Hospital, Ankara, Turkey.
Gulgun Gamze, Department of Anaesthesiology and Reanimation, Tunceli State Hospital, Tunceli, Turkey.
Gunduz Emel, Department of Anaesthesiology and Reanimation, Akdeniz University Medical Faculty, Antalya, Turkey.
Gunes Isin, Department of Anaesthesiology and Reanimation, Erciyes University, Medical Faculty, Kayseri, Turkey.
Guven Aytac Betul, Department of Anaesthesiology, University of Health Sciences Etlik Zubeyde Hanim Gynecology and Obstetrics Teaching Hospital, Ankara, Turkey.
Hacibeyoglu Gulcin, Department of Anaesthesiology and Reanimation, Necmettin Erbakan University Meram Medical Faculty, Konya, Turkey.
Has Selmi Nazan, Department of Anaesthesiology and Reanimation, University of Health Sciences Ankara Numune Teaching Hospital, Ankara, Turkey.
Ilgaz Kocyigit Ozgen, Department of Anaesthesiology and Reanimation, Acıbadem Mehmet Ali Aydınlar University School of Medicine, Atakent Hospital, Istanbul, Turkey.
Ilksen Egilmez Ayse, Department of Anaesthesiology and Reanimation, University of Health Sciences Konya Teaching Hospital, Konya, Turkey.
Iyigun Muzeyyen, Department of Anaesthesiology and Reanimation, Acıbadem Mehmet Ali Aydınlar University School of Medicine, Atakent Hospital, Istanbul, Turkey.
Kara Inci, Department of Anaesthesiology and Reanimation, Selcuk University Medical Faculty, Konya, Turkey.
Karakaya Deniz, Department of Anaesthesiology and Reanimation, Ondokuz Mayis University Medical Faculty, Samsun, Turkey.
Karasu Derya, Department of Anaesthesiology and Reanimation, University of Health Sciences Yuksek Ihtisas Teaching Hospital, Bursa, Turkey.
Karaveli Arzu, Department of Anaesthesiology and Reanimation, University of Health Sciences Antalya Teaching Hospital, Antalya, Turkey.
Kavas Ayse Duygu, Department of Anaesthesiology and Reanimation, University of Health Sciences Umraniye Teaching Hospital, Istanbul, Turkey.
Kaya Mensure, Department of Anaesthesiology and Reanimation, University of Health Sciences Dr Abdurrahman Yurtaslan Ankara Oncology Teaching Hospital, Ankara, Turkey.
Kaya Suleyman, Department of Anaesthesiology and Reanimation, Ankara Ataturk Teaching Hospital, Ankara, Turkey.
Kazak Bengisun Zuleyha, Department of Anaesthesiology and Reanimation, Ufuk University School of Medicine, Ankara, Turkey.
Keskin Gulsen, Department of Anaesthesiology, University of Health Sciences Ankara Child Health and Diseases Hematology Oncology Teaching Hospital, Ankara, Turkey.
Kilci Oya, Department of Anaesthesiology and Reanimation, University of Health Sciences Ankara Numune Teaching Hospital, Ankara, Turkey.
Kilic Yeliz, Department of Anaesthesiology and Reanimation, Eskisehir Osmangazi University Medical Faculty, Eskisehir, Turkey.
Kirdemir Pakize, Department of Anaesthesiology and Reanimation, Suleyman Demirel University Medical Faculty, Isparta, Turkey.
Koc Zeynep, Department of Anaesthesiology and Reanimation, University of Health Sciences Kayseri Teaching Hospital, Kayseri, Turkey.
Koksal Ceren, Department of Anaesthesiology and Reanimation, University of Health Sciences Fatih Sultan Mehmet Teaching Hospital, Istanbul, Turkey.
Kozanhan Betul, Department of Anaesthesiology and Reanimation, University of Health Sciences Konya Teaching Hospital, Konya, Turkey.
Kucukguclu Semih, Department of Anaesthesiology and Reanimation, Dokuz Eylul University Medical Faculty, Izmir, Turkey.
Kucukosman Gamze, Department of Anaesthesiology and Reanimation, Zonguldak Bulent Ecevit University Medical Faculty Center for Health and Research, Zonguldak, Turkey.
Kupeli Ilke, Department of Anaesthesiology and Reanimation, Ministry of Health Erzincan University Mengucek Gazi Teaching Hospital, Erzincan, Turkey.
Kurtay Aysun, Department of Anaesthesiology and Reanimation, University of Health Sciences Kecioren Teaching Hospital, Ankara, Turkey.
Kurtipek Omer, Department of Anaesthesiology and Reanimation, Gazi University Medical Faculty, Ankara, Turkey.
Meco Basak Ceyda, Department of Anaesthesiology and Reanimation, Ankara University Medical Faculty, Ankara, Turkey.
Nalbant Burak, Department of Anaesthesiology and Reanimation, University of Health Sciences Diskapi Yildirim Beyazit Teaching Hospital, Ankara, Turkey.
Okyay Rahsan Dilek, Department of Anaesthesiology and Reanimation, Zonguldak Bulent Ecevit University Medical Faculty Center for Health and Research, Zonguldak, Turkey.
Omur Dilek, Department of Anaesthesiology and Reanimation, Dokuz Eylul University Medical Faculty, Izmir, Turkey.
Orak Yavuz, Department of Anaesthesiology and Reanimation, Sutcu Imam University Medical Faculty, Kahramanmaraş, Turkey.
Ounde Elif, Department of Anaesthesiology and Reanimation, University of Health Sciences Haydarpasa Numune Teaching Hospital, Istanbul, Turkey.
Özayar Esra, Department of Anaesthesiology and Reanimation, University of Health Sciences Kecioren Teaching Hospital, Ankara, Turkey.
Ozcelik Menekse, Department of Anaesthesiology and Reanimation, Ankara University Medical Faculty, Ankara, Turkey.
Ozden Eyup Sabri, Department of Anaesthesiology and Reanimation, Suleyman Demirel University Medical Faculty, Isparta, Turkey.
Ozer Yetkin, Department of Anaesthesiology and Reanimation, Anadolu Health Center, Istanbul, Turkey.
Ozgok Aysegul, Department of Anaesthesiology and Reanimation, University of Health Sciences Ankara City Hospital, Ankara, Turkey.
Ozhan Mehmet Ozgur, Department of Anaesthesiology and Reanimation, Private Cankaya Hospital, Ankara, Turkey.
Ozlu Onur, Department of Anaesthesiology, University of Economics and Technology, Medical Faculty Hospital, Ankara, Turkey.
Sagir Ozlem, Department of Anaesthesiology and Reanimation, Balikesir University Medical Faculty Teaching Hospital, Balıkesir, Turkey.
Saglik Arzu, Department of Anaesthesiology and Reanimation, University of Health Sciences Kayseri Teaching Hospital, Kayseri, Turkey.
Sagun Aslinur, Department of Anaesthesiology and Reanimation, Mersin University Medical Faculty, Mersin, Turkey.
Sahap Mehmet, Department of Anaesthesiology and Reanimation, University of Health Sciences Kecioren Teaching Hospital, Ankara, Turkey.
Sahin Cihan, Department of Anaesthesiology, University of Economics and Technology, Medical Faculty Hospital, Ankara, Turkey.
Sahiner Yeliz, Department of Anaesthesiology and Reanimation, Hitit University, School of Medicine, Corum Erol Olcok Teaching Hospital, Corum, Turkey.
Salman Nevriye, Department of Anaesthesiology and Reanimation, University of Health Sciences Ankara City Hospital, Ankara, Turkey.
Saracoglu Ayten, Department of Anaesthesiology and Reanimation, Marmara University Pendik Teaching Hospital, Istanbul, Turkey.
Saracoglu Kemal Tolga, Department of Anaesthesiology and Reanimation, University of Health Sciences Kartal Dr. Lutfi Kirdar Teaching Hospital, Istanbul, Turkey.
Sarizeybek Hilal, Department of Anaesthesiology and Reanimation, University of Health Sciences Derince Teaching Hospital, Kocaeli, Turkey.
Selcuk Sert Gokce, Department of Anaesthesiology and Reanimation, University of Health Sciences Ankara City Hospital, Ankara, Turkey.
Sen Betul, Department of Anaesthesiology and Reanimation, Istanbul Medeniyet University Goztepe Teaching Hospital, Istanbul, Turkey.
Sen Ozlem, Department of Anaesthesiology and Reanimation, University of Health Sciences Dr Abdurrahman Yurtaslan Ankara Oncology Teaching Hospital, Ankara, Turkey.
Sener Elif Bengi, Department of Anaesthesiology and Reanimation, Ondokuz Mayis University Medical Faculty, Samsun, Turkey.
Sengul Fatma Isil, Department of Anaesthesiology and Reanimation, University of Health Sciences Antalya Teaching Hospital, Antalya, Turkey.
Silay Emin, Department of Anaesthesiology and Reanimation, University of Health Sciences Kayseri Teaching Hospital, Kayseri, Turkey.
Subası Ferhunde Dilek, Department of Anaesthesiology and Reanimation, University of Health Sciences Haydarpasa Numune Teaching Hospital, Istanbul, Turkey.
Tarikci Kilic Ebru, Department of Anaesthesiology and Reanimation, University of Health Sciences Umraniye Teaching Hospital, Istanbul, Turkey.
Tas Nilay, Department of Anaesthesiology and Reanimation, Ordu University Medical Faculty, Ordu, Turkey.
Tekgul Zeki Tuncel, Department of Anaesthesiology and Reanimation, University of Health Sciences Izmir Bozyaka Teaching Hospital, Izmir, Turkey.
Tekgunduz Sibel, Department of Child Health and Diseases, University of Health Sciences Kecioren Teaching Hospital, Ankara, Turkey.
Tezcan Keles Gonul, Department of Anaesthesiology and Reanimation, Manisa Celal Bayar University Medical Faculty Hafsa Sultan Hospital, Manisa, Turkey.
Topcu Hulya, Department of Anaesthesiology and Reanimation, Hitit University, School of Medicine, Corum Erol Olcok Teaching Hospital, Corum, Turkey.
Tunay Abdurrahman, Department of Anaesthesiology and Reanimation, University of Health Sciences Istanbul Teaching Hospital, Istanbul, Turkey.
Ugun Fatih, Department of Anaesthesiology and Reanimation, Balikesir University Medical Faculty Teaching Hospital, Balıkesir, Turkey.
Un Canan, Department of Anaesthesiology and Reanimation, University of Health Sciences Ankara Numune Teaching Hospital, Ankara, Turkey.
Unal Petek, Department of Anaesthesiology and Reanimation, University of Health Sciences Haydarpasa Numune Teaching Hospital, Istanbul, Turkey.
Unver Suheyla, Department of Anaesthesiology and Reanimation, University of Health Sciences Dr Abdurrahman Yurtaslan Ankara Oncology Teaching Hospital, Ankara, Turkey.
Ural Sedef Gulcin, Department of Anaesthesiology and Reanimation, Osmaniye State Hospital, Osmaniye, Turkey.
Filiz Uzumcugil, Department of Anaesthesiology and Reanimation, Hacettepe University School of Medicine, Ankara, Turkey.
Yerebakan Akesen Selcan, Department of Anaesthesiology and Reanimation, Bursa Uludag University Medical Faculty, Bursa, Turkey.
Yesildal Kadir, Department of Anaesthesiology and Reanimation, University of Health Sciences Okmeydanı Teaching Hospital, Istanbul, Turkey.
Yildirim Mustafa, Department of Anaesthesiology and Reanimation, University of Health Sciences Diskapi Yildirim Beyazit Teaching Hospital, Ankara, Turkey.
Yildiz Altun Aysun, Department of Anaesthesiology and Reanimation, Fırat University Medical Faculty, Elazıg, Turkey.
Yildiz Munise, Department of Anaesthesiology and Reanimation, University of Health Sciences Konya Teaching Hospital, Konya, Turkey.
Yilmaz Erisen Hatice, Department of Anaesthesiology and Reanimation, University of Health Sciences Izmir Tepecik Teaching Hospital, Izmir, Turkey.
Yilmaz Hakan, Department of Anaesthesiology and Reanimation, Ufuk University School of Medicine, Ankara, Turkey.
Yilmaz Mehmet, Department of Anaesthesiology and Reanimation, University of Health Sciences Derince Teaching Hospital, Kocaeli, Turkey.
Yuzkat Nureddin, Department of Anaesthesiology and Reanimation, Van Yuzuncu Yil University Medical Faculty Dursun Odabası Medical Center, Van, Turkey.
REFERENCES
- 1.Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57:1347–58. doi: 10.1111/trf.14006. [DOI] [PubMed] [Google Scholar]
- 2.Spahn DR. Patient blood management: the new standard. Transfusion. 2017;57:1325–27. doi: 10.1111/trf.14095. [DOI] [PubMed] [Google Scholar]
- 3.Society for the Advancement of Blood Management (SABM) Who we are. [Accessed on 25/03/2020]. Available at: https://www.sabm.org/who-we-are/
- 4.Chen A, Trivedi AN, Jiang L, et al. Hospital blood transfusion patterns during major noncardiac surgery and surgical mortality. Medicine. 2015;94:e1342. doi: 10.1097/MD.0000000000001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozek-Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol. 2017;34:332–95. doi: 10.1097/EJA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 6.American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists task force on perioperative blood management. Anaesthesiology. 2015;122:241–75. doi: 10.1097/ALN.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 7.National Blood Authority (NBA) Patient Blood Management Guidelines: Module 2, Perioperative. 2012. [Accessed on 28/08/2019]. Available from: https://www.blood.gov.au/system/files/documents/pbm-module-2.pdf.
- 8.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party: Recommendations for the transfusion management of patients in the peri-operative period. II. The intra-operative period. Blood Transfus. 2011;9:189–217. doi: 10.2450/2011.0075-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party. Recommendations for the transfusion management of patients in the peri-operative period. II. The post-operative period. Blood Transfus. 2011;9:320–35. doi: 10.2450/2011.0076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Bartolomeo E, Merolle L, Marraccini C, et al. Patient Blood Management: transfusion appropriateness in the post-operative period. Blood Transfus. 2019;17:459–64. doi: 10.2450/2019.0035-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biggin K, Warner P, Prescott R, et al. A review of methods used in comprehensive, descriptive studies that relate red blood use to clinical data. Transfusion. 2010;50:711–8. doi: 10.1111/j.1537-2995.2009.02459.x. [DOI] [PubMed] [Google Scholar]
- 12.Republic of Turkey Social Insurance Institution Health Application Comunique. 2018. [Accessed on 28/08/2019]. Available from: http://www.resmigazete.gov.tr/eskiler/2018/07/20180705M1-1.htm.
- 13.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 14.Sutton R, Bann S, Brooks M, et al. The Surgical Risk Scale as an improved tool for risk-adjusted analysis in comparative surgical audit. Br J Surg. 2002;89:763–8. doi: 10.1046/j.1365-2168.2002.02080.x. [DOI] [PubMed] [Google Scholar]
- 15.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–60. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. WHO/NMH/NHD/MNM/11.1. [Accessed on 22/11/2019]. http://www.who.int/vmnis/indicators/haemoglobin.pdf .
- 17.Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32:88–105. doi: 10.1097/EJA.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 18.Demiral HS, Unal D, Sayın MM. Blood transfusion in major artroplasties: frequency, causes and investigation of transfusion effect on outcome. Anestezi Dergisi. 2018;26:148–58. [Google Scholar]
- 19.Council of Europe. Guide to the preparation, use and quality assurance of blood components. 19th Edition. [Accessed on 03/09/2019]. Available from: https://www.edqm.eu/en/blood-guide.
- 20.Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia. 2017;72:233–47. doi: 10.1111/anae.13773. [DOI] [PubMed] [Google Scholar]
- 21.Klein AA, Collier TJ, Brar MS, et al. Association of Cardiothoracic Anaesthetists (ACTA) The incidence and importance of anaemia in patients undergoing cardiac surgery in the UK - the first Association of Cardiothoracic Anaesthetists national audit. Anaesthesia. 2016;71:627–35. doi: 10.1111/anae.13423. [DOI] [PubMed] [Google Scholar]
- 22.Peters F, Ellermann I, Steinbicker AU. Intravenous iron for treatment of anemia in the 3 perisurgical phases: a review and analysis of the current literature. Anesth Analg. 2018;126:1268–82. doi: 10.1213/ANE.0000000000002591. [DOI] [PubMed] [Google Scholar]
- 23.Qian F, Osler TM, Eaton MP, et al. Variation of blood transfusion in patients undergoing major noncardiac surgery. Ann Surg. 2013;257:266–78. doi: 10.1097/SLA.0b013e31825ffc37. [DOI] [PubMed] [Google Scholar]
- 24.Madjdpour C, Spahn DR, Weiskopf RB. Anemia and perioperative red blood celltransfusion: a matter of tolerance. Crit Care Med. 2006;34:S102–8. doi: 10.1097/01.CCM.0000214317.26717.73. [DOI] [PubMed] [Google Scholar]
- 25.Lindquist DE, Stewart DW, Brewster A, et al. Comparison of postoperative bleeding in total hip and knee arthroplasty patients receiving rivaroxaban, enoxaparin, or aspirin for thromboprophylaxis. Clin Appl Thromb Hemost. 2018;24:1315–21. doi: 10.1177/1076029618772337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet. 2013;381:1855–65. doi: 10.1016/S0140-6736(13)60808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isbister JP, Shander A, Spahn DR, et al. Adverse blood transfusion outcomes: establishing causation. Transfus Med Rev. 2011;25:89–101. doi: 10.1016/j.tmrv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 29.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–62. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gombotz H, Rehak PH, Shander A, et al. The second Austrian benchmark study for blood use in elective surgery: results and practice change. Transfusion. 2014;54:2646–57. doi: 10.1111/trf.12687. [DOI] [PubMed] [Google Scholar]
- 31.Meier J, Filipescu D, Kozek-Langenecker S, et al. Intraoperative transfusion practices in Europe. Br J Anaesth. 2016;116:255–61. doi: 10.1093/bja/aev456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barr PJ, Donnelly M, Morris K, Parker M, Cardwell C, Bailie KE. The epidemiology of red cell transfusion. Vox Sang. 2010;99:239–50. doi: 10.1111/j.1423-0410.2010.01349.x. [DOI] [PubMed] [Google Scholar]
- 33.Meybohm P, Froessler B, Goodnough LT, et al. Simplified international recommendations for the implementation of patient blood management (SIR4PBM) Perioper Med. 2017;6:5. doi: 10.1186/s13741-017-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franchini M, Marano G, Veropalumbo E, et al. Patient Blood Management: a revolutionary approach to transfusion medicine. Blood Transfus. 2019;17:191–5. doi: 10.2450/2019.0109-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meybohm P, Choorapoikayil S, Wessels A, et al. Washed cell salvage in surgical patients: a review and meta-analysis of prospective randomized trials under PRISMA. Medicine. 2016;95:e4490. doi: 10.1097/MD.0000000000004490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;1886;19:CD00. [Google Scholar]
- 37.Corredor C, Wasowicz M, Karkouti K, et al. The role of point-of-care platelet function testing in predicting postoperative bleeding following cardiac surgery: a systematic review and meta-analysis. Anaesthesia. 2015;70:715–31. doi: 10.1111/anae.13083. [DOI] [PubMed] [Google Scholar]
- 38.Fahrendorff M, Oliveri RS, Johansson PI. The use of viscoelastic haemostatic assays in goal-directing treatment with allogeneic blood products - a systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med. 2017;25:39. doi: 10.1186/s13049-017-0378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frietsch T, Shander A, Faraoni D, Hardy JF. Patient Blood Management is not about blood transfusion: it is about patients’ outcomes. Blood Transfus. 2019;17:331–333. doi: 10.2450/2019.0126-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.