Abstract
Background
Pathogen reduction technology (PRT) may damage platelet (PLT) components. To study this, metabolic activity and haemostatic function of buffy coat (BC) PLT concentrates, with or without riboflavin and UV light PRT treatment, were compared.
Material and methods
Twenty-four BC PLT concentrates, leukoreduced and diluted in additive solution, were grouped into 12 type-matched pairs, which were pooled and divided into 12 non-PRT-treated BC PLT concentrates (control units) and 12 riboflavin and UV PRT-treated BC PLT concentrates (test units). Haemostatic function and metabolic parameters were monitored by thrombelastography at days 1, 3, 7 and 14 post collection in both PLT groups.
Results
Loss of PLT discoid shape, glucose consumption, lactate production, and decrease in pH were greater in the PRT-treated PLTs than in control PLTs over time (p<0.001). PLT haemostatic function evaluated by clot strength was also significantly weaker in PRT-treated PLTs compared with the excellent clot quality of control PLTs at day 7 (maximum amplitude: 41.27 vs 64.27; p<0.001), and even at day 14 (21.16 vs 60.39; p<0.001) of storage.
Discussion
Pathogen reduction technology treatment accelerates and increases platelet storage lesion, resulting in glucose depletion, lactate accumulation, PLT acidification, and discoid shape loss. The clots produced by control PLTs at day 14 were still remarkably strong, whereas at day 7 PRT-treated PLTs produced weaker clots compared to the control group. Clinical trials investigating the efficacy of PRT-treated PLTs transfused at the end of the storage period (day 7), when the in vitro clot strength is weaker, are needed.
Keywords: blood component preparations, blood centre operations
INTRODUCTION
In spite of advances in laboratory testing and donor screening, a small risk of viral1,2, bacterial3,4, and parasite5–9 contamination of platelet (PLT) concentrates persists. There is also a permanent threat from newly emerging or re-emerging blood transfusion-transmitted pathogens10–12. Pathogen reduction technology (PRT) for blood and blood components has the potential to prevent pathogen proliferation and increase blood safety.
The different PRT methods for plasma, PLTs, red blood cells and whole blood (WB) are classified according to the photosensitising substance and the wavelength of UV irradiation. This study is based on PLTs treated by Mirasol® PRT (Terumo BCT Europe N.V., Zaventem, Belgium), which uses ribof lavin (vitamin B2), a photosensitising reactive, together with UV light in the range of 265–370 nm. The ribof lavin is added to the PLT component and activated by UV light illumination to produce free oxygen radicals that permanently damage the nucleic acid of the infectious agent. As a result, pathogen proliferation in the blood component is blocked13.
The use of PRT has other advantages. These include: the replacement of gamma irradiation by the inactivation of white blood cells (WBCs), thus eliminating the risk of transfusion-transmitted graft-versus-host disease14; prevention of alloimmunisation (which still needs to be confirmed by evidence from clinical practice15,16); fewer PLT transfusion reactions17,18; and, in some settings, PLT supply is improved by storage time being increased from five to seven days19. Although PRT treatment of PLTs is generally recognised to have significant benefits, there is justifiable concern regarding its impairment of haemostatic function. Comprehensive in vitro testing and standard quality control assays have been carried out to assess the effects of PRT treatment on PLT metabolic and biological variables20,21. However, the differences in haemostatic function of PRT-treated PLTs in vitro compared to that observed after transfusion in vivo is not completely understood.
With the aim of shedding new light on the PLT functional impairment associated with PRT treatment, a study was designed to evaluate the effect of ribof lavin and UV light PRT on the haemostatic function of PLTs diluted in additive solution and stored at room temperature for 14 days. PLT function was monitored by thrombelastography (TEG), a technique that provides a global evaluation of haemostasis by measuring blood coagulation as a dynamic process under low-shear conditions22,23. Metabolic parameters were also investigated, since lactate production and pH have been correlated with in vivo recovery and survival time in human subjects21.
On the other hand, PLT concentrates stored at room temperature undergo progressive damage and loss of quality in vitro, which is known as platelet storage lesion (PSL)24. Since PSL impairs the function of conventional non-PRT-treated PLTs stored at room temperature, a parallel control study was carried out on the PSL of conventional PLTs diluted in additive solution, stored in the same conditions as PRT-treated PLTs for 14 days, beyond the conventional PLT storage time of five days.
Thus, 24 buffy coat (BC) PLT concentrates were grouped into 12 type-matched pairs, which were pooled and divided into 12 non-PRT-treated BC PLT concentrates (control units) and 12 PRT-treated BC PLT concentrates (test units), both diluted in platelet additive solution and leukoreduced. The PSL development over time was studied by measuring PLT metabolic activity and haemostatic function by TEG in the 24 BC PLT concentrates at days 1, 3, 7 and 14 post-collection.
MATERIALS AND METHODS
Study design and preparation of whole blood-derived buffy coat platelets
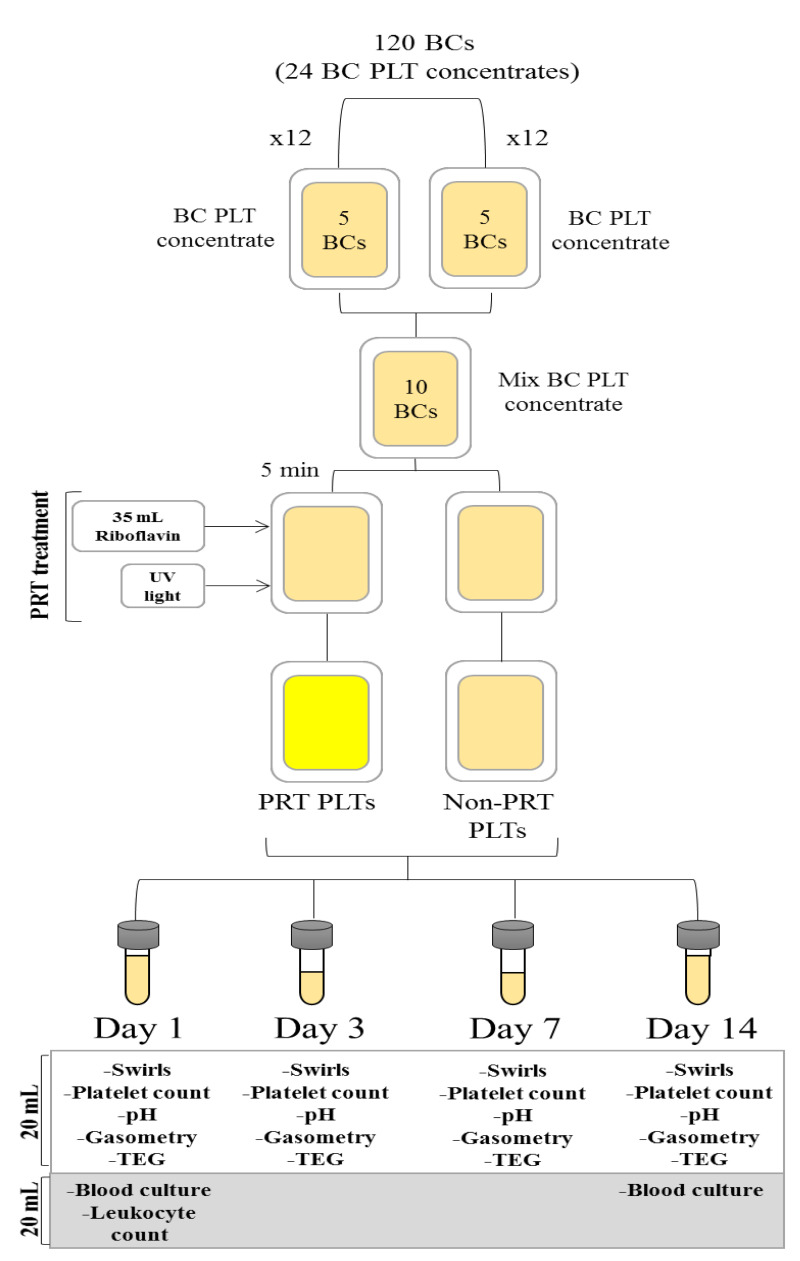
Whole blood units were obtained from healthy voluntary blood donors who gave informed consent that the donated blood not utilised for transfusion could be used for research purposes. In the study, 120 WB units (450±45mL) were collected (day 0) into top-and-bottom bags containing 63 mL CPD (Fresenius Kabi, Bad Homburg, Germany) to generate 12 paired BC PLT concentrates, each one containing five BCs.
After donation, the WB units were immediately placed on butane-1,4-diol plates (Compocool®, Fresenius Kabi) and kept overnight at room temperature. BCs were obtained the day after collection (day 1) by hard spin (3,530 × rpm, 14 min, 22 °C, acceleration 7, deceleration 5, HeraeusTM CryofugeTM 6000i, Thermo Scientific, MA, USA) and separated using an automated blood component extractor (COMPOMAT G5®, Fresenius Kabi).
In order to elaborate the WB-derived BC PLTs, five ABO Rh isogroup BCs were pooled in 300 mL of PAS-E (Terumo-Platelet Additive Solution + [T-PAS+], Shibuya, Tokio, Japan) and centrifuged using an automated centrifuge separator (1,337 rhpm, 22 ºC, integral 45000; TACSI System; TERUMO BCT, Somerset, NJ, USA) including a leukoreduction filter, obtaining a final proportion of 35% plasma and 65% PAS-E.
To prepare the BC PLT concentrates, two ABO Rh isogroup WB-derived BC PLT concentrates were pooled in one bag. After being homogeneously mixed, the content was divided into two equal PLT concentrates: one was PRT-treated (test unit) using ribof lavin and UV light (Mirasol PRT® system; TerumoBCT, Lakewood, CO, USA) and the other was not PRT-treated (control unit).
Riboflavin and UV light treatment
The WB-derived BC PLT concentrates of each pair were randomly assigned to the test or control group. The test units were treated with ribof lavin and UV light-PRT within 8 hours (h) of PLT concentrate preparation and 32 h of collection, according to the manufacturer’s instructions. After transferring the PLT test unit into an illumination/storage bag, the ribof lavin solution was added (35±5 mL). The PLT unit was then placed in the illuminator and exposed to UV light in the range of 265–370 nm for approximately 5 minutes. Both PRT-treated and non-treated PLTs were stored for 14 days post collection at room temperature in continuous agitation, protected from light in an enclosed PLT storage cabinet. (Platelet Incubator I-192 and Platelet Agitator H-192, Grifols, Barcelona, Spain). The storage temperature (20–24 ºC) was monitored and recorded in each daily shift over the storage time.
Sampling of platelet concentrates
After the WB-derived BC PLT concentrate had been gently mixed, a mean 20 mL of PLT from each unit was taken using a sterile connector device (Terumo Sterile Connecting Device, TSCD-II®, Shibuya, Tokio, Japan). The following laboratory testing was performed at days 1, 3, 7 and 14 post collection: a PLT count was made by a haematology analyser (Cell-Dyn Ruby, Abbot Park, IL, USA), the pH at 22 ºC was determined by a pHmeter (XS instruments: Laboratory Instruments, Carpi, MO, Italy), swirling was assessed by visual inspection using a scale from 0 (swirl absence) to 3 (abundant swirls), TEG analysis (TEG® 5000 and Version 4.2, Haemoscope Corp., Niles, IL, USA), and analysis of metabolic substances including glucose (mmol/L), bicarbonate (mmol/L), lactate (mmol/L), pCO2 (mmHg), and pO2 (mmHg) by a gasometer (GEM Premier® 4000, Instrumentation Laboratory, Bedford, MA, USA). Leukoreduction testing was performed in WB-derived non-PRT-treated BC PLTs at day 1 by flow cytometry (Guava® EasyCyteTM Single Sample Flow Cytometer, Merck Millipore, Darmstadt, Germany). An additional sample of 20 mL was taken under sterile conditions from both control and test WB-derived BC PLTs at days 1 and 14 for bacterial contamination testing using the BACT/ALERT® system (bioMérieux, Marcy-l’Étoile, France) (Figure 1).
Figure 1.
Experimental design
Haemostatic function evaluated by thrombelastography
Platelet haemostatic function was evaluated by TEG analysis using TEG haemostasis system software (TEG® 5000 and Version 4.2, Haemoscope Corp., Niles, IL, USA). All samples were analysed less than 1 h after sample collection. Concentration of platelets was adjusted to 200×109 platelets/L and an aliquot of AB plasma was added if necessary. For the citrate-kaolin (CK) test, one milliliter of the dilution of 200×109 PLTs/L was introduced into the kaolin-loaded tube (Haemoscope Corp., Braintree, MA, USA) and mixed by inversion 3–5 times. A volume of 340 μL of the mix from the CK tube was introduced into a 37 ºC cup pre-loaded with 20 μL of 0.2 M CaCl2 for the re-calcification. The variables recorded from the TEG system were reaction time R (min), which represents clotting time (i.e., the time between sample loading and detection of initial fibrin formation ref lecting plasma clotting factors); α angle (degree), which indicates the kinetics of the clot formation (i.e., ref lecting PLT function, fibrinogen and plasma components residing on the PLT surface), and the maximum amplitude (MA) (mm), which evaluates the maximum strength of the clot depending on the interaction between fibrin and PLTs. The recommended values of TEG parameters in WB are 3–8 min for R, 55–78 degrees for α angle, and 51–69 mm for MA25.
Platelet metabolic activity
To study the PLT metabolic activity, 2 mL samples of each WB-derived BC PLT concentrate were sterilely transferred into heparinised syringes (Syringes PICO50 with TIPCAP stoppers, Radiometer Iberica, Madrid, Spain). The heparinised syringes were transported refrigerated (2–4 ºC) to the Balearic Islands University Hospital (Hospital Universitari Son Espases). Glucose (mmol/L), bicarbonate (mmol/L), lactate (mmol/L), pCO2 (mmHg), and pO2 (mmHg) were analysed using a gasometer (GEM Premier 4000, Instrumentation Laboratory) within 4 h of sample collection.
Statistical analysis
Baseline variables in the PRT PLTs and non-PRT PLTs were compared by paired two-sided t-tests. Significant group effects were analysed using the Bonferroni correction. Repeated-measure analyses were performed using a mixed model (SPSS mixed linear models algorithm, MIXED), assuming a compound symmetry co-variance structure among the repeated measurements, after analysing the information criteria for other co-variance matrix options (unstructured, autoregressive, and autoregressive heterogenous variances). The model included group (PRT PLT, non-PRT PLT), time (days), and group × time effects. Data are presented as means±standard deviation (SD) and p<0.05 was considered significant. Statistical calculations were performed using computer software (IBM SPSS Statistics v. 25).
RESULTS
Volume and platelet concentration
The PLT volume unit decreased in both treated and non-treated groups over the 14 days due to sample collection for the determination of PLT variables. At days 1 and 14, 40 mL were collected, and 20 mL were collected at days 3 and 7. Overall, 120 mL were extracted for PLT variable analysis (Figure 1). It is worth highlighting that the PLT loss due to sample collection throughout the storage did not influence TEG performance, since the PLT concentration of all TEG-studied samples was adjusted to 200×109 platelets/L by adding AB plasma if necessary.
Swirling and pH values
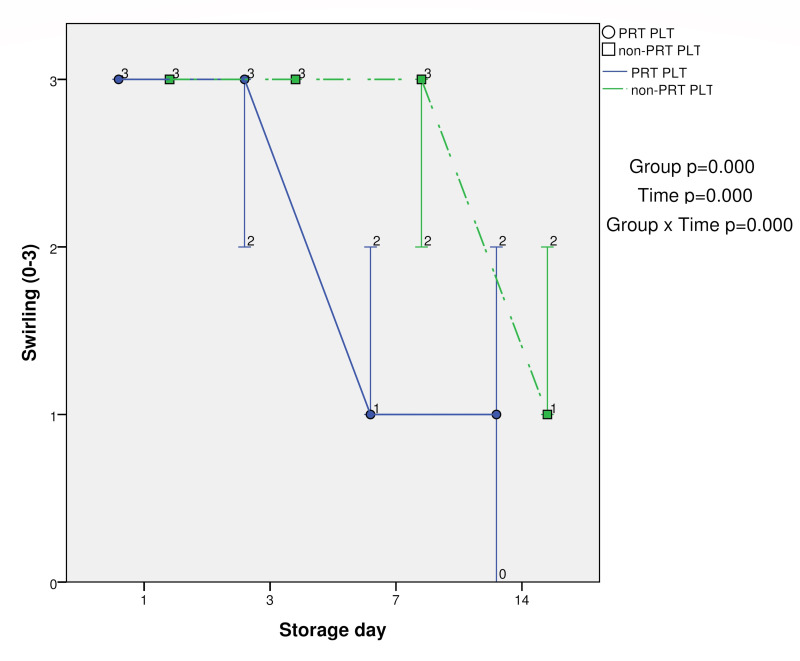
Swirling was significantly lower at days 7 and 14 in PRT-treated PLTs compared with control PLTs (p<0.05). The group (PRT PLT vs non-PRT PLT), time, and group × time effects were also statistically significant (p<0.001) (Table I and Figure 2).
Table I.
Results (mean±standard deviation) of the metabolic and haemostatic tests at days 1, 3, 7 and 14 in pathogen reduction technology (PRT) platelets (PLT) and non-PRT PLT groups
| Variables | PLT Group | Day 1 (mean±SD) | Day 3 (mean±SD) | Day 7 (mean±SD) | Day 14 (mean±SD) | †Group p value | †Time p value | †Group × Time p value |
|---|---|---|---|---|---|---|---|---|
| Swirling (0–3) | PRT PLT | 3±0.00 | 2.83±0.39 | 1.33±0.49* | 0.75±0.62* | 0.000 | 0.000 | 0.000 |
| non-PRT PLT | 3±0.00 | 3±0.00 | 2.58±0.51 | 1.42±0.51 | ||||
| pH | PRT PLT | 7.11±0.05 | 6.98±0.09* | 6.8±0.05* | 6.82±0.08* | 0.000 | 0.000 | 0.000 |
| non-PRT PLT | 7.11±0.05 | 7.21±0.06 | 7.17±0.05 | 7.13±0.1 | ||||
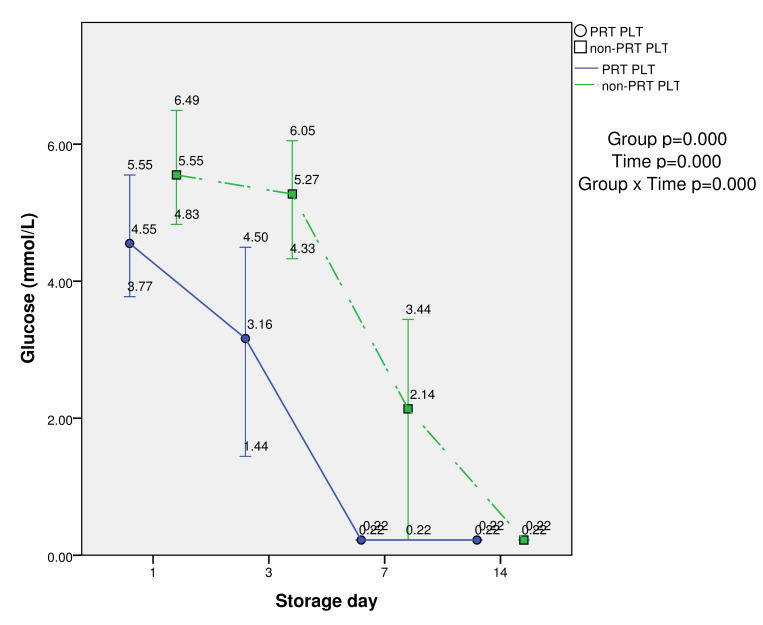
| Glucose (mmol/L) | PRT PLT | 4.57±0.65* | 3.05±0.99* | 0.22±0* | 0.22±0 | 0.000 | 0.000 | 0.000 |
| non-PRT PLT | 5.56±0.6 | 5.15±0.55 | 2.29±0.92 | 0.22±0 | ||||
| Bicarbonate (mmol/L) | PRT PLT | 5.26±0.6* | 3.88±1.38* | 0.34±0.63* | 0±0* | 0.000 | 0.000 | 0.000 |
| non-PRT PLT | 6.11±0.49 | 5.39±0.43 | 3.89±0.67 | 0.94±1.34 | ||||
| Lactate (mmol/L) | PRT PLT | 6.96±0.99 | 9.69±1.58* | 16.84±1.65* | 17.04±1.63* | 0.004 | 0.000 | 0.000 |
| non-PRT PLT | 6.12±1.03 | 7.24±1.05 | 13.11±1.87 | 18.7±1.47 | ||||
| PCO2 (mmHg) | PRT PLT | 20.17±1.8* | 19.33±1.61* | 8.5±2.15* | 6±0.00 | 0.024 | 0.000 | 0.000 |
| non-PRT PLT | 17.83±1.95 | 14±1.48 | 11.17±1.47 | 6.25±0.62 | ||||
| pO2 (mmHg) | PRT PLT | 149.25±21.15 | 148.83±26.74 | 190.67±15.01* | 198.75±16.16 | 0.884 | 0.000 | 0.077 |
| non-PRT PLT | 156.42±25.79 | 158.58±21.54 | 174.5±14.37 | 195±19.06 | ||||
| R (min) | PRT PLT | 8.82±3.28 | 6.74±1.19 | 6.37±1.19 | 7.02±1.73 | 0.239 | 0.002 | 0.346 |
| non-PRT PLT | 7.4±1.74 | 6.78±0.69 | 6.28±0.92 | 6.25±0.89 | ||||
| Angle (degrees) | PRT PLT | 75.31±5.62 | 78.79±1.62 | 67.89±11.06* | 65.38±15.52* | 0.026 | 0.022 | 0.001 |
| non-PRT PLT | 78.52±0.94 | 74.39±8.6 | 79.1±1.35 | 76.72±1.73 | ||||
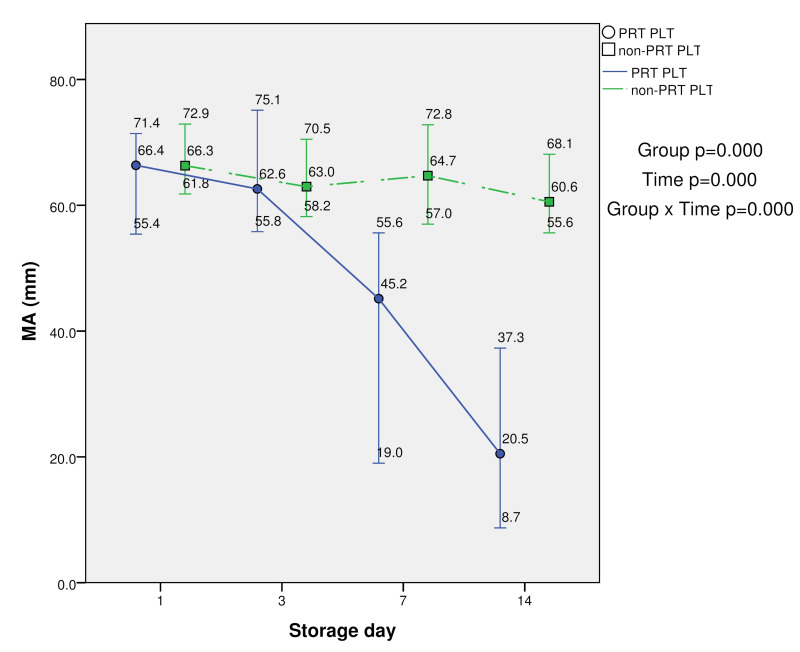
| MA (mm) | PRT PLT | 64.94±5.54 | 63.6±5.73 | 41.27±13.48* | 21.16±7.52* | 0.000 | 0.000 | 0.000 |
| non-PRT PLT | 66.7±4 | 63.61±4.44 | 64.27±4.98 | 60.39±3.79 |
Significant group effects were analyzed using the Bonferroni correction, p<0.05
Effect of group (PRT PLT, non-PRT PLT), time (days), and group × time was analysed by a mixed model (p-values)
Figure 2.
Swirling in PRT PLT and non-PRT PLT groups over 14-day storage period (median, minimum and maximum values)
The mean pH values remained over 7.0 over 14 days in non-PRT PLT group. However, the pH values in PRT-treated PLT units were significant lower at days 3, 7 and 14 compared with the control (p<0.05), but never less than 6.4. The group (PRT PLT vs non-PRT PLT), time, and group × time effects were also statistically significant (p<0.001) (Table I ).
Platelet metabolic activity
Promptly after PRT treatment, the glucose and bicarbonate values decreased significantly, and at day 1 the levels of lactate and PCO2 increased significantly (p<0.05). Ribof lavin and UV light PRT seems to significantly enhance PLT metabolic activity immediately after treatment. The evolution of these metabolic values over time was different for each variable.
Glucose values were significantly lower at days 1, 3 and 7 in PRT-treated PLTs compared with the control (p<0.05). The group (PRT PLT vs non-PRT PLT), time, and group × time effects were also statistically significant (p<0.001). The values were extremely low in both PLT groups at day 14 (mean 0.2 mmol/L) (Table I and Figure 3).
Figure 3.
Metabolic parameter (glucose) in PRT PLT and non-PRT PLT groups over 14-day storage period (median, minimum and maximum values)
Bicarbonate values were also significantly lower in PRT-treated PLTs at days 1, 3, 7 and 14 compared with the control (p<0.05). The group (PRT PLT vs non-PRT PLT), time, and group × time effects were also statistically significant (p<0.001). The reduction began earlier in PRT-treated PLTs, i.e., at day 3, with a gradual decline at day 7 and total absence at day 14 (Table I).
Lactate values were significantly higher at days 3, 7 and 14 in PRT-treated PLT compared with the control (p<0.05). The group (PRT PLT vs non-PRT PLT; p=0.004), time, and group × time effects were also statistically significant (p<0.001). Also, the increase began earlier, i.e., at day 3, in PRT-treated PLTs (Table I).
PCO2 values were significantly higher at days 1 and 3 in PRT-treated PLTs compared with the control (p<0.05). The group (PRT PLT vs non-PRT PLT; p=0.024), time, and group × time effects were also statistically significant (p<0.001) (Table I).
pO2 had significantly increased at day 7 in PRT-treated PLTs compared with the control. However, only the time effect was statistically significant (p<0.001), but not the group (PRT PLT vs non-PRT PLT) nor group × time effects (Table I).
Leukoreduction testing
The leukocyte content was measured at day 1 by flow cytometry before the PRT treatment. All PLTs presented leukocyte levels lower than 1×106 per unit, in accordance with the Council of Europe guidelines26.
Bacterial contamination screening
All bacterial contamination screening tests were negative after five days of culture for both test and control PLT groups at days 1 and 14.
Haemostatic function evaluated by thrombelastography
The evolution of TEG parameters over time was different for each variable. The R (range: 3–8 min) values were statistically significant in the time analysis (p=0.001), but neither group (PRT PLT vs non-PRT PLT) nor group × time effects were statistically significant.
The α angle (range: 55–78 degrees) was significantly lower in PRT-treated PLTs at days 7 and 14 compared with the control (p<0.05), some α angles being <55 degrees at day 14. The group (PRT PLT vs non-PRT PLT), time, and group × time effects were also statistically significant (p<0.05).
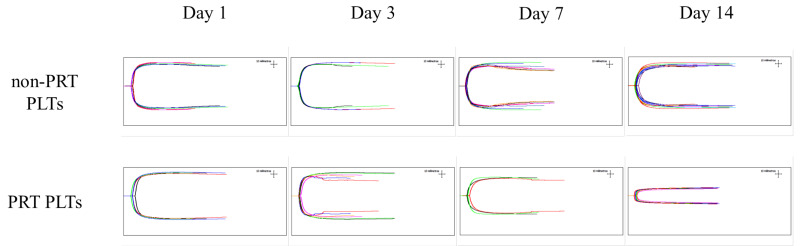
Maximum amplitude (MA) (51–69 mm) values were significantly lower in PRT-treated PLTs at days 7 and 14 compared with the control (p<0.05). The group (PRT PLT vs non-PRT PLT), time, and group × time effects were also statistically significant (p<0.001). In fact, the mean MA values at days 7 and 14 were below 51 mm in PRT-treated PLTs. In contrast, in non-PRT-treated PLTs the mean MA values at days 7 and 14 were higher than 51 and the decline was very slight during storage, indicating very good clot strength, even at day 14. However, the clot strength in PRT-treated PLTs was lower than expected at day 7, and even more so at day 14 (Table I, and Figures 4 and 5).
Figure 4.
TEG parameter (maximum amplitude, MA) in PRT PLT and non-PRT PLT groups over 14-day storage period (median, minimum and maximum values)
Figure 5.
TEG indicating in vitro clot strength (maximum amplitude, MA) in PRT PLT and non-PRT PLT groups over 14-day storage period
DISCUSSION
Pathogen reduction technology treatment had an immediate impact on PLT metabolic activity at day 1, resulting in increased glucose and bicarbonate consumption. In addition, PLT haemostatic functional impairment developed at different rates in the PRT-treated PLT group compared with the control over the 14-day storage period.
In the PRT-treated PLTs the increase in metabolic activity was greater and took place earlier compared to the control group, with a significantly higher bicarbonate and glucose consumption at days 1, 3 and 7. Although the swirling rate declined over the storage period in both groups, the reduction was significantly greater in PRT-treated PLTs at days 7 and 14. Consequently, PLT discoid shape loss was faster and more pronounced in PRT-treated PLTs over time. The mean pH values remained >7.0 over 14 days in the non-PRT PLT group, being lower in the PRT-treated PLT group, although always above the limit accepted by the Council of Europe guidelines for PLT component release (>6.4)27. Similar findings regarding in vitro PLT viability have been described by other authors, although the PLT storage time studied was less than 14 days27,28.
In this study, an average loss of 1.8 % volume and 3.4% PLTs was observed in the PLT test unit at day 1, after transfer to an illumination/storage bag for UV light PRT treatment before adding the ribof lavin solution. The loss of volume and PLTs associated with PRT was less than that described by other authors, who have reported a 5% PLT loss due to the ribof lavin and UV light PRT procedure29. A possible explanation is that the fine research work of a single operator may differ from routine work involving several operators.
Clot strength, measured by MA, was of excellent quality in the control group throughout the 14-day storage period at room temperature, but was below the levels considered optimal in the PRT-treated PLTs at day 7, and more so at day 14, in the same storage conditions. It would appear that PRT treatment accelerates and increases the PSL that occur naturally during PLT storage at room temperature by increasing glycolysis, which results in glucose depletion, lactate accumulation and PLT component acidification. PLT availability and safety are compromised not only by the risk of bacterial contamination but also the development of PSL, which are defined as a series of structural and functional modifications that occur when PLTs are exposed to artificial surfaces and high centrifugation forces during PLT concentrate preparation24.
Interestingly, it has been reported that lactate production and pH are correlated with PLT recovery and survival time in human subjects, i.e., the higher the lactate production and pH decrease, the lower the PLT survival and recovery in vivo21. Also, PLT concentrates exhibiting swirling as an indicator of discoid shape retention in vitro are expected to be functional in vivo at the time of transfusion30.
According to our results, PLT discoid shape loss, glucose consumption, lactate production and decreasing pH over time were more accentuated and occurred more rapidly in PRT-treated PLTs compared with the control, which could reflect an acceleration of PSL induced by PRT treatment. It may, therefore, be inferred that the survival and recovery of PRT-treated PLTs in vivo would be much lower than that of conventional PLTs. In addition, PLT haemostatic function (i.e., clot strength in vitro) was also significantly weaker in PRT-treated PLTs at day 7, and especially at day 14, compared with the good clot strength in the control group throughout the storage period.
While most trials investigating transfusion efficacy of PRT-treated PLTs have reported that all transfusion increment parameters were significantly lower than in conventional PLTs, no increased risk of bleeding has been described31–37. In fact, the mean corrected count increment (CCI) values for both 1 h and 24 h post transfusion were lower for PRT-treated PLTs than non-treated PLTs in a non-inferiority randomised control trial aiming to assess the efficacy and safety of ribof lavin and UV light treatment for PLTs performed by the Mirasol Clinical Evaluation Study Group31,32. Another study investigating the safety and efficacy of ribof lavin and UV light-treated PLTs compared with conventional PLTs found that, although the transfusion dose was similar for both, the post-transfusion PLT count increments at 1 h and 24 h were significantly lower in patients receiving PRT-treated PLTs compared to the control group. Nevertheless, the PRT-treated PLTs showed a high safety and efficacy profile, as there was no difference in the frequency and type of adverse transfusion reactions and the risk of bleeding between PRT-treated and control PLTs33. The same finding has been reported in a systematic review of PRT-treated PLTs for the prevention of bleeding in patients with oncological or haematologic diseases. Although PRT-treated PLTs result in an increased number of PLT transfusions, there is no evidence that they enhance the risk of death, bleeding, or serious adverse events34. The Pathogen Reduction Evaluation and Predictive Analytical Rating Score (PREPAReS) also showed that, despite a roughly equal PLT content in PRT-treated and conventional PLT products, all transfusion increment parameters were significantly lower for the former. However, there were no differences in the occurrence of grade 2 or higher bleeding between the PLT groups35.
A long-standing question has been whether the reduced cell viability associated with PRT is only due to cellular losses in a proportion of PRT-treated PLTs27,38 or to the functional impairment of all treated PLTs as well39–40. It is worth noting that ribof lavin and UV light treatment leads to hyper-reactive PLTs, probably due to a continuous basal PLT degranulation during storage41, and this could accelerate the PSL. The PRT damage (affecting either a proportion of PLTs or all of them) can result in an enhanced cellular metabolism, reduced clot strength, and lower PLT increments post transfusion compared with non-treated PLTs. However, as far as we know, it does not result in more bleeding events in patients transfused with PRT-treated PLTs31–37.
If only a proportion of PRT-treated PLTs are damaged, these hyper-activated PLTs can be removed from circulation faster42 than untreated PLTs, which would account for the lower post-transfusion increments. However, they should not produce more bleeding complications in patients transfused with PRT-treated PLTs, since a PLT dose equal to half the standard dose does not increase bleeding events43. On the other hand, if all the PLTs treated with ribof lavin and UV light are impaired, they could experience some functional recovery, since after transfusion the PLTs again have access to glucose from the blood stream, the bioactive substances released are diluted into the patient’s total intravascular volume, and although the post-transfusion increments will be lower, the patients receiving PRT-treated PLTs will not bleed more31–37.
With respect to the clot strength, it has been reported that ribof lavin and UV light PRT can influence PLT haemostatic function evaluated by TEG. Ostrowski et al. report that ribof lavin and UV light PRT of BC PLTs in additive solution results in lower clot strength measured by MA compared with non-PRT-treated BC PLTs in additive solution at day 8 of storage44. In addition, a pilot study comparing the TEG haemostatic function of PLTs treated with ribof lavin and UV light and conventional PLTs in patients with thrombocytopenia found that, while the latter provided better MA values than PRT-treated PLTs at 1 h post transfusion, no differences between groups were observed at 24 h post transfusion45.
Surprisingly, in the present study, non-PRT-treated PLTs created strong clots in vitro after storage at room temperature for 14 days. Conversely, the clot strength in PRT-treated PLTs at day 7, and particularly at day 14, was weaker than in the control group. Whether or not the ability to form clots and other PLT variables altered over the storage time recover once the PRT-treated PLTs are transfused should be investigated in clinical studies.
CONCLUSIONS
Haemostasis is a very complex process involving positive and negative feedback mechanisms in which PLTs, coagulation factors and the endothelium closely interact46. The efficacy of PLT transfusion is undoubtedly more effectively investigated in vivo. The clinical relevance of the impact of PRT treatment on PLT metabolic activity and haemostatic function should be further investigated in clinical trials. Studies on bleeding events and/or other clinical parameters in patients receiving PRT-treated PLTs at the end of the storage period (day 7), when the clot strength is apparently weaker, could verify whether or not the results of the present in vitro study correlate with clinical practice.
ACKNOWLEDGEMENTS
We would like to acknowledge donors from the Balearic Islands. We would also like to thank the laboratory technician team for their contribution to the implementation of pathogen reduction technology in our blood bank. The authors also wish to thank Lucy Brzoska for her invaluable advice on the revision of the manuscript.
Footnotes
FUNDING AND RESOURCES
This research study was supported by the Comissió de Docència i Investigació de la Fundació Banc de Sang i Teixits de les Illes Balears.
AUTHORSHIP CONTRIBUTIONS
CB-S and TJ-M contributed equally to this work. CB-S and TJ-M designed the experiment and wrote the manuscript. CB-S, DM-G and MQ-O performed the experimental assays. All authors contributed to editing the manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Weusten J, van Drimmelen H, Vermeulen M, Lelie N. A mathematical model for estimating residual transmission risk of occult hepatitis B virus infection with different blood safety scenarios. Transfusion. 2017;57:841–9. doi: 10.1111/trf.14050. [DOI] [PubMed] [Google Scholar]
- 2.Tedder RS, Ijaz S, Kitchen A, et al. Hepatitis E risks: pigs or blood-that is the question. Transfusion. 2017;57:267–72. doi: 10.1111/trf.13976. [DOI] [PubMed] [Google Scholar]
- 3.Abela MA, Fenning S, Maguire KA, Morris KG. Bacterial contamination of platelet components not detected by BacT/ALERT. Transfus Med. 2018;28:65–70. doi: 10.1111/tme.12458. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez-Arcos S, DiFranco C, McIntyre T, Goldman M. Residual risk of bacterial contamination of platelets: six years of experience with sterility testing. Transfusion. 2017;57:2174–81. doi: 10.1111/trf.14202. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez-Marco T, Riera C, Fisa R, et al. The utility of pathogen inactivation technology: a real-life example of Leishmania infantum inactivation in platelets from a donor with an asymptomatic infection. Blood Transfus. 2012;10:536–41. doi: 10.2450/2012.0178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez-Marco T, Fisa R, Girona-Llobera E, et al. Transfusion-transmitted leishmaniasis: a practical review. Transfusion. 2016;56:S45–51. doi: 10.1111/trf.13344. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez-Marco T, Riera C, Girona-Llobera E, et al. Strategies for reducing the risk of transfusion-transmitted leishmaniasis in an area endemic for Leishmania infantum: a patient- and donor-targeted approach. Blood Transfus. 2018;16:130–6. doi: 10.2450/2017.0201-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin RJ, Stramer SL, Leiby DA, et al. Trypanosoma cruzi infection in North America and Spain: evidence in support of transfusion transmission. Transfusion. 2012;52:1913–21. doi: 10.1111/j.1537-2995.2011.03554.x. [DOI] [PubMed] [Google Scholar]
- 9.Cancino-Faure B, Fisa R, Riera C, et al. Evidence of meaningful levels of Trypanosoma cruzi in platelet concentrates from seropositive blood donors. Transfusion. 2015;55:1249–55. doi: 10.1111/trf.12989. [DOI] [PubMed] [Google Scholar]
- 10.Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: following the path of dengue and chikungunya? Lancet. 2015;386:243–4. doi: 10.1016/S0140-6736(15)61273-9. [DOI] [PubMed] [Google Scholar]
- 11.Vanlandingham DL, Keil SD, Horne KM, et al. Photochemical inactivation of chikungunya virus in plasma and platelets using the Mirasol pathogen reduction technology system. Transfusion. 2013;53:284–90. doi: 10.1111/j.1537-2995.2012.03717.x. [DOI] [PubMed] [Google Scholar]
- 12.Cap AP, Pidcoke HF, Keil SD, et al. Treatment of blood with a pathogen reduction technology using ultraviolet light and riboflavin inactivates Ebola virus in vitro. Transfusion. 2016;56:S6–15. doi: 10.1111/trf.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drew VJ, Barro L, Seghatchian J, Burnouf T. Towards pathogen inactivation of red blood cells and whole blood targeting viral DNA/RNA: design, technologies, and future prospects for developing countries. Blood Transfus. 2017;15:512–21. doi: 10.2450/2017.0344-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corash L, Lin L. Novel processes for inactivation of leukocytes to prevent transfusion-associated graft-versus-host disease. Bone Marrow Transplant. 2004;33:1–7. doi: 10.1038/sj.bmt.1704284. [DOI] [PubMed] [Google Scholar]
- 15.Muench MO, Heitman JW, Inglis H, et al. Reduced alloimmunization in mice following repeated transfusion with pathogen-reduced platelets. Transfusion. 2016;56:1419–29. doi: 10.1111/trf.13579. [DOI] [PubMed] [Google Scholar]
- 16.Norris PJ, Kaidarova Z, Maiorana E, et al. Ultraviolet light based pathogen inactivation and alloimmunization after platelet transfusion: results from a randomized trial. Transfusion. 2018;58:1210–7. doi: 10.1111/trf.14534. [DOI] [PubMed] [Google Scholar]
- 17.Łetowska M, Przybylska Z, Piotrowski D, et al. Hemovigilance survey of pathogen-reduced blood components in the Warsaw Region in the 2009 to 2013 period. Transfusion. 2016;56:S39–44. doi: 10.1111/trf.13330. [DOI] [PubMed] [Google Scholar]
- 18.Piotrowski D, Przybylska-Baluta Z, Jimenez-Marco T, et al. Passive haemovigilance of blood components treated with a riboflavin-based pathogen reduction technology. Blood Transfus. 2017;23:1–4. doi: 10.2450/2017.0268-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girona-Llobera E, Jimenez-Marco T, Galmes-Trueba A, et al. Reducing the financial impact of pathogen inactivation technology for platelet components: our experience. Transfusion. 2014;54:158–68. doi: 10.1111/trf.12232. [DOI] [PubMed] [Google Scholar]
- 20.Li J, de Korte D, Woolum MD, et al. Pathogen reduction of buffy coat platelet concentrates using riboflavin and light: comparisons with pathogen-reduction technology-treated apheresis platelet products. Vox Sang. 2004;87:82–90. doi: 10.1111/j.1423-0410.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- 21.Goodrich RP, Li J, Pieters H, et al. Correlation of in vitro platelet quality measurements with in vivo platelet viability in human subjects. Vox Sang. 2006;90:279–85. doi: 10.1111/j.1423-0410.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 22.Bontekoe IJ, van der Meer PF, Korte D. Thromboelastography as a tool to evaluate blood of healthy volunteers and blood component quality: a review. Vox Sang. 2019;114:643–57. doi: 10.1111/vox.12823. [DOI] [PubMed] [Google Scholar]
- 23.Curry NS, Davenport R, Pavord S, et al. The use of viscoelastic haemostatic assays in the management of major bleeding: s British Society for Haematology Guideline. Br J Haematol. 2018;182:789–806. doi: 10.1111/bjh.15524. [DOI] [PubMed] [Google Scholar]
- 24.Ng MSY, Tung JP, Fraser JF. Platelet storage lesions: what more do we know now? Transfus Med Rev. 2018;32:144–54. doi: 10.1016/j.tmrv.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Scarpelini S, Rhind SG, Nascimento B, et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res. 2009;42:1210–7. doi: 10.1590/s0100-879x2009001200015. [DOI] [PubMed] [Google Scholar]
- 26.European Directorate for the Quality of Medicines & HealthCare. Guide for the preparation, use and quality assurance of blood components. 19th Ed. Council of Europe; 2017. [Accessed on: 2/12/2019]. Available at: https://www.edqm.eu/en/blood-transfusion-guides-1608.html. [Google Scholar]
- 27.Picker SM, Schneider V, Oustianskaia L, Gathof BS. Cell viability during platelet storage in correlation to cellular metabolism after different pathogen reduction technologies. Transfusion. 2009;49:2311–8. doi: 10.1111/j.1537-2995.2009.02316.x. [DOI] [PubMed] [Google Scholar]
- 28.Castrillo A, Cardoso M, Rouse L. Treatment of buffy coat platelets in platelet additive solution with the Mirasol pathogen reduction technology system. Transfus Med Hemother. 2013;40:44–48. doi: 10.1159/000345679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Meer PF, Couture C, Hervig T, et al. Experience with semi-routine production of riboflavin and UV-B pathogen-inactivated platelet concentrates in three blood centres. Vox Sang. 2017;112:9–17. doi: 10.1111/vox.12465. [DOI] [PubMed] [Google Scholar]
- 30.Mathai J, Resmi KR, Sulochana PV, et al. Suitability of measurement of swirling as a marker of platelet shape change in concentrates stored for transfusion. Platelets. 2006;17:393–6. doi: 10.1080/09537100600757695. [DOI] [PubMed] [Google Scholar]
- 31.Cazenave JP, Follea G, Bardiaux L, et al. A randomized controlled clinical trial evaluating the performance and safety of platelets treated with MIRASOL pathogen reduction technology. Transfusion. 2010;50:2362–75. doi: 10.1111/j.1537-2995.2010.02694.x. [DOI] [PubMed] [Google Scholar]
- 32.Cook RJ, Heddle NM. Clinical trials evaluating pathogen-reduced platelet products: methodologic issues and recommendations. Transfusion. 2013;53:1843–55. doi: 10.1111/j.1537-2995.2012.03951.x. [DOI] [PubMed] [Google Scholar]
- 33.Rebulla P, Vaglio S, Beccaria F, et al. Clinical effectiveness of platelets in additive solution treated with two commercial pathogen-reduction technologies. Transfusion. 2017;57:1171–83. doi: 10.1111/trf.14042. [DOI] [PubMed] [Google Scholar]
- 34.Estcourt LJ, Malouf R, Hopewell S, et al. Pathogen-reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev. 2017;7:CD009072. doi: 10.1002/14651858.CD009072.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Meer PF, Ypma PF, van Geloven N, et al. Hemostatic efficacy of pathogen-inactivated-versus untreated-platelets: a randomized controlled trial. Blood. 2018;132:223–31. doi: 10.1182/blood-2018-02-831289. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez-Marco T, Garcia-Recio M, Girona-Llobera E. Our experience in riboflavin and ultraviolet light pathogen reduction technology for platelets: from platelet production to patient care. Transfusion. 2018;58:1881–9. doi: 10.1111/trf.14797. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez-Marco T, Garcia-Recio M, Girona-Llobera E. Use and safety of riboflavin and UV light-treated platelet transfusions in children over a five-year period: focusing on neonates. Transfusion. 2019;59:3580–8. doi: 10.1111/trf.15538. [DOI] [PubMed] [Google Scholar]
- 38.Murphy S, Snyder E, Cable R, et al. SPRINT Study Group. Platelet dose consistency and its effect on the number of platelet transfusions for support of thrombocytopenia: an analysis of the SPRINT trial of platelets photochemically treated with amotosalen HCl and ultraviolet A light. Transfusion. 2006;46:24–33. doi: 10.1111/j.1537-2995.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 39.Apelseth TO, Bruserud O, Wentzel-Larsen T, et al. In-vitro evaluation of metabolic changes and residual platelet responsiveness in photochemically-treated and gamma-irradiated single donor platelet concentrates during long-term storage. Transfusion. 2007;47:657–65. doi: 10.1111/j.1537-2995.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 40.Keuren JF, Cawenberghs S, Heeremans J, et al. Platelet ADP response deteriorates in synthetic storage media. Transfusion. 2006;46:204–12. doi: 10.1111/j.1537-2995.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 41.Zeddies S, De Cuyper IM, van der Meer PF, et al. Pathogen reduction treatment using riboflavin and ultraviolet light impairs platelet reactivity toward specific agonists in vitro. Transfusion. 2014;54:2292–300. doi: 10.1111/trf.12636. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser-Guignard J, Canellini G, Lion N, et al. The clinical and biological impact of new pathogen inactivation technologies on platelet concentrates. Blood Rev. 2014;28:235–41. doi: 10.1016/j.blre.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Slichter SJ, Kaufman RM, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362:600–13. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostrowski SR, Bochsen L, Windeløv NA, et al. Hemostatic function of buffy coat platelets in additive solution treated with pathogen reduction technology. Transfusion. 2011;51:344–56. doi: 10.1111/j.1537-2995.2010.02821.x. [DOI] [PubMed] [Google Scholar]
- 45.Johansson PI, Simonsen AC, Brown PN, et al. A pilot study to assess the hemostatic function of pathogen-reduced platelets in patients with thrombocytopenia. Transfusion. 2013;53:2043–52. doi: 10.1111/trf.12055. [DOI] [PubMed] [Google Scholar]
- 46.Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22:1381–9. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]