Abstract
Background
Postpartum haemorrhage (PPH) is still a leading cause of maternal morbidity in the US. We aimed to reassess national trends in severe and non-severe PPH using recent data.
Material and methods
We performed a cross-sectional study using the 2001–2012 Nationwide Inpatient Sample of the Healthcare Cost and Utilization Project. Delivery-related hospitalisations with PPH were identified using the International Classification of Diseases (9th revision). Rates were calculated per 1,000 delivery hospitalisations. All statistical analyses accounted for the complex sampling design of the data source.
Results
Rates of non-severe PPH did not change significantly from 2001–2002 to 2011–2012 (25.5 and 24.2 per 1,000; p=0.058). The rates of PPH requiring blood transfusions for caesarean deliveries more than doubled in this time period from 2.0 to 4.8 (p<0.001). The overall rate of PPH with a procedure other than blood transfusion has risen from 0.9 to 1.9. Specifically, rates of hysterectomy (0.5 to 0.7; p<0.001), embolisation (0.3 to 0.5; p<0.001), and uterine tamponade use (0.09 to 0.69; p<0.001) increased over the time period.
Discussion
Population-based surveillance data show an increasing rate of only severe PPH in the US. Rates of medical/surgical intervention including hysterectomy, embolisation and uterine tamponade use are also rising.
Keywords: postpartum haemorrhage, blood transfusion, nationwide inpatient sample, maternal mortality, maternal morbidity
INTRODUCTION
Postpartum haemorrhage (PPH) is the leading cause of maternal mortality worldwide1. In the US, PPH increased by 26% from 1994 to 20062. Prior national reports showed increasing transfusion rates in 2004 among delivery hospitalisations3,4.
Postpartum haemorrhage places women at increased risk for significant morbidity and mortality by a direct effect on the heart, kidneys and liver, plus the indirect consequences from related interventions. Blood transfusion has associated risks such as anaphylactic reaction, lung injury, antibody formation for future pregnancy, and risk of infection5. Other interventions, such as uterotonic agents, uterine tamponade devices, uterine artery embolisation, antifibrinolytic therapy or peripartum hysterectomy, attempt to address the most common categories of aetiology for PPH: uterine atony and retained placenta or morbidly adherent placenta, lacerations/incisional extensions or coagulopathy/medical complications6,7. Uterine artery embolisation and peripartum hysterectomy complications increase the risk for thromboembolism or infection. While some risk factors that place women at increased risk for PPH during delivery can be identified, severe PPH can often occur in the absence of identifiable risk factors8.
Ultimately, our study sought to make available current trends for this important peripartum outcome so the impact of national/state efforts to reduce maternal morbidity and mortality could be assessed over time. Our primary objective was to characterise recent national trends for PPH from 2000 to 2012 and to determine if the trend previously seen in published data has continued, plateaued or decreased. Secondary objectives included distinguishing the trends separately for severe and non-severe PPH, as well as understanding rates of intervention to treat severe PPH.
MATERIALS AND METHODS
Our study was a cross-sectional study design using the Nationwide Inpatient Sample (NIS) database. The Healthcare Cost and Utilization Project created NIS as a database tool to help keep track of national estimates of inpatient hospital data, by compiling state data from a 20% stratified sample of hospital discharges across US community hospitals. Hospital stratification is based on five key factors: ownership, bed size, teaching status, urban-rural location, and region. As the nation’s largest all-payer inpatient care database, it includes data from nearly 8 million hospital discharges and 1,000 hospitals. Our study used NIS data from 1994 to 2012, but focused primarily on the years 2000 to 2012.
This study was deemed exempt by the Duke University Health System Institutional Review Board because these data are publicly available and there are no personal identifiers included. NIS data contain safeguards to protect the privacy of individual patients, physicians, and hospitals. For the time period we specified, discharge diagnoses and procedure codes were based on the International Classification of Diseases, 9th Revision, Clinical Modifications (ICD-9-CM). All delivery-related discharge records were defined by inclusion of a delivery code. Delivery hospitalisations were also identified by diagnosis-related group codes. There are no linking codes between hospitalisations so individuals could not be followed for multiple admissions (Table I).
Table I.
| • Caesarean delivery 74.X, except 74.91 |
| • General delivery V27, 72.X, 73.X and 650–659 |
| • 2000–2007 diagnosis-related group codes 370, 371, 372, 373, 374 and 375 |
| • 2008–2012, diagnosis-related group codes 765 or 766 and 767, 768, 774 and 775 |
ICD-9-CM: International Classification of Diseases, 9th revision, Clinical Modifications.
Delivery hospitalisations with PPH were identified by ICD-9-CM codes 666.XX. Severe PPH was defined as cases of PPH that required interventions such as blood transfusion, hysterectomy, repair of obstetric laceration, uterine artery ligation/embolisation, and tamponade of the uterus or vagina with the respective ICD-9-CM codes of 99.0X, (683, 683.1, 683.9, 684, 684.1, 684.9, 685, 685.1, 686, 686.1, 686.9, 687, 687.1, 687.9, 689), (75.50, 75.52), (397.9, 992.9, 682.5, 682.4), and 758. Non-severe PPH was by default all PPH not categorised as severe PPH. Atonic PPH was defined using ICD-9-CM code 666.1X and non-atonic PPH using codes 666.0X, 666.2X, 666.3X. Induction of labor was defined using ICD-9-CM code 73.4.
We calculated the rates per 1,000 delivery hospitalisations for severe and non-severe PPH from 2000 to 2012, and specifically examined the trend of PPH requiring blood transfusions. We plotted the rates per 1,000 delivery hospitalisation (by combined overall deliveries, then by caesarean/vaginal deliveries) for interventions to manage severe PPH including hysterectomy, repair of obstetric laceration, and uterine artery ligation/embolisation. A patient may have had multiple interventions and so was counted in each group for which they had an intervention. This grouping was kept consistent in the analysis over the time periods. We assessed the significance of trends in rates using previously published methods2. Given that uterine atony is one of the leading causes of PPH, we explored the trend of PPH due to atony over this time period, and also individually focused on this outcome by delivery type. For the tables, we focused on the time period that was most relevant from 2000 to 2012; however, for the figures, we wanted to study a longer time period (1994–2012) so that we could more effectively illustrate the trends.
Estimates in our study are generalisable to the US population as all counts and proportions were weighted with the use of the weighting variables in the NIS that account for the complex sampling design. All analyses were performed using SAS software (version 9.1; SAS Institute Inc., Cary, NC, USA) and SUDAAN (version 9.0; RTI International, Research Triangle, NC, USA).
RESULTS
The total number of deliveries per year was consistent over the time period studied, with 7,878,870 delivery hospitalisations from 2001–2002 and 7,437,199 delivery hospitalisations from 2011–2012. The percentage of deliveries via caesarean increased from 25.8 to 33.2% over the 12-year time period. Demographic data are presented in Table II. As with prior studies2, women in the later time period were older, had government insurance, multiple gestations, and more comorbidities such as hypertension or diabetes mellitus.
Table II.
Demographic data*
| Percentage ± SE | ||
|---|---|---|
| 2001–2002 (n=7,878,870) | 2011–2012 (n=7,437,199) | |
| Age, years | ||
| 12–18 | 6.3 (0.1) | 5.6 (0.1) |
| 19–25 | 38.9 (0.5) | 33.5 (0.5) |
| 26–35 | 48.7 (0.4) | 49.1 (0.4) |
| 36–45 | 11.0 (0.2) | 11.5 (0.3) |
| 46–55 | 0.07 (0.004) | 0.09 (0.005) |
| Payer | ||
| Medicaid/Medicare | 37.1 (1.0) | 44.4 (0.8) |
| Private insurance | 56.6 (1.2) | 50.0 (0.8) |
| Self | 6.1 (0.5) | 5.4 (0.2) |
| Mode of delivery | ||
| Vaginal | 71.6 | 65.1 |
| Vaginal birth after caesarean | 2.7 | 1.7 |
| Repeat caesarean | 10.3 | 15.3 |
| Primary caesarean | 15.5 | 17.9 |
| Multiple birth | 1.6 (0.04) | 1.8 (0.03) |
| Hypertensive disorder in pregnancy (any) | 6.9 (0.1) | 8.5 (0.1) |
| Pre-gestational and gestational diabetes mellitus | 0.7 (0.03) | 1.0 (0.02) |
All demographic variables p<0.001 between two time periods.
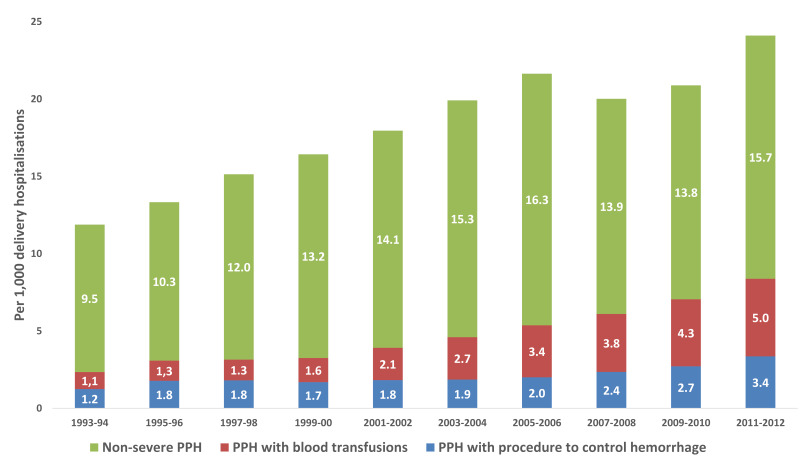
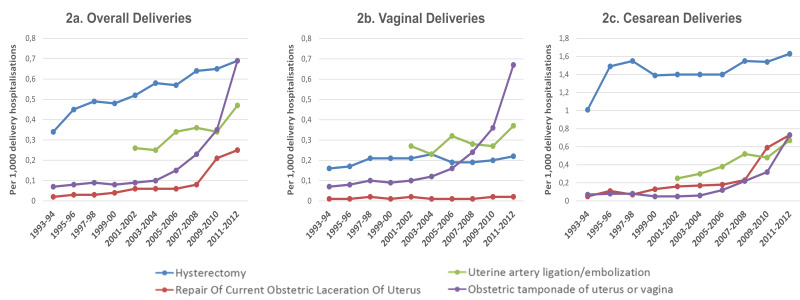
The rates of PPH requiring blood transfusions for overall delivery hospitalisations increased in this time period from 1.6 in 2001–2002 to 3.8 in 2011–2012 (p<0.001). Specifically, for caesarean deliveries, this rate more than doubled from 2.0 in 2001–2002 to 4.8 in 2011–2012 (p<0.001) (Figure 1). The overall rate of PPH with a procedure other than blood transfusion has risen from 0.9 to 1.9 (p<0.001). Rates of hysterectomy (0.5 to 0.7; p<0.001), embolisation (0.3 to 0.5; p<0.001), and uterine tamponade use (0.09 to 0.69; p<0.001) increased over the time period (Figure 2a). The trends were similar when individually assessed by examining data according to vaginal and caesarean delivery (Figure 2b and c). In contrast to severe PPH, there was no significant difference in the rate of non-severe PPH for all deliveries between the time period 2001–2002 and the time period 2011–2012 (25.5 and 24.2 per 1,000; p=0.058 (Online_Supplementary_Content, Figure S1a for overall deliveries and Online_Supplementary_Content, Figure S1b for vaginal deliveries only).
Figure 1.
Prevalence of postpartum haemorrhage (PPH) per 1,000 caesarean delivery hospitalisations requiring blood transfusion or procedure for intervention
Grouped into non-severe PPH and severe PPH (requiring either transfusion or additional surgical intervention).
Figure 2.
Prevalence of procedures to control postpartum haemorrhage (PPH) per 1,000 delivery hospitalisations, the Nationwide Inpatient Sample, USA
Separated into graphs for overall (2a), vaginal (2b) and caesarean deliveries (2c).
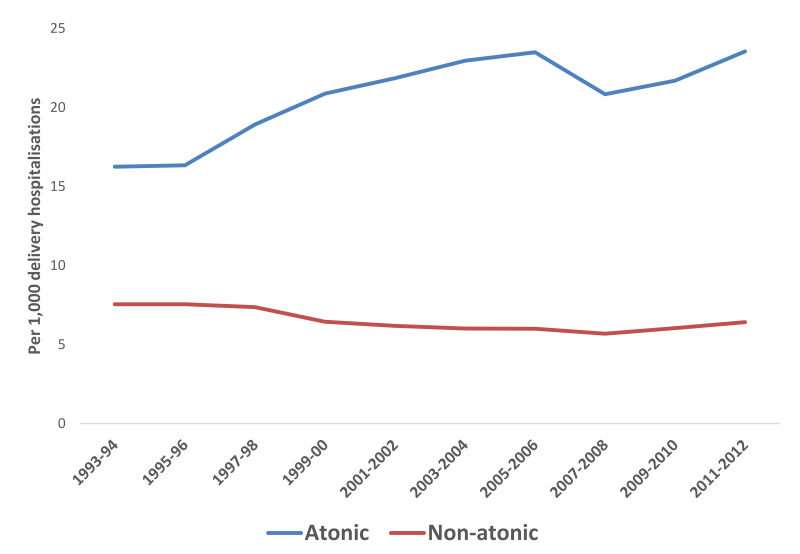
Since uterine atony is one of the most common causes of severe PPH, we assessed specific trends for PPH due to atony over this time period. Overall, the trend continued to increase for rates of PPH due to uterine atony (Figure 3). The rates of PPH caused by uterine atony increased 14.1% from the time period 2001–2002 to the time period 2011–2012 in vaginal deliveries that were induced, but decreased 18.3% in vaginal deliveries that were not induced (Table III). The cases of PPH caused by uterine atony increased among all caesarean deliveries by over 60%.
Figure 3.
Prevalence of PPH per 1,000 overall delivery hospitalisations by atonic versus non-atonic cause of haemorrhage
Table III.
Cases of postpartum haemorrhage by delivery type and uterine atony
| Type of delivery | Delivery distribution, % (95%CI) | Cases of postpartum haemorrhage caused by uterine atony*, n (95%CI) | ||||
|---|---|---|---|---|---|---|
| 2001–2002 | 2011–2012 | 2001–2002 | 2011–2012 | Change in cases, n | Change in cases, % | |
| Overall | 100 | 100 | 172,196 (167,399–176,153) | 175,149 (171,249–179,536) | 2,953 | 1.7% |
| Vaginal | 74.2 (74.6–73.7) | 66.7 (67.1–66.3) | 143,045 (139,369–146,378) | 127,885 (124,457–131,057) | −15,160 | −10.6% |
| Induced | 13.5 (12.9–14.2) | 16.4 (16.0–16.8) | 33,880 (32,864–35,022) | 38,678 (37,510–39,969) | 4,8 | 14.2% |
| Not induced | 60.6 (59.9–61.4) | 50.3 (49.8–50.8) | 109,165 (106,793–111,788) | 89,207 (87,165–91,421) | −19,958 | −18.3% |
| Caesarean | 25.8 (26.3–25.4) | 33.3 (33.7–32.9) | 29,151 (28,699–29,536) | 47,264 (46,253–48,067) | 18,113 | 61.1% |
| Induced | 2.6 (2.5–2.8) | 4.0 (3.9–4.1) | 5,475 (5,344–5,634) | 8,936 (8,712–9,252) | 3,46 | 63.2% |
| Not induced | 23.2 (22.7–23.7) | 29.3 (28.9–29.6) | 23,676 (23,392–24,007) | 38,328 (37,720–39,077) | 14,652 | 61.9% |
All comparisons between two time periods p<0.001 with exception of overall cases of postpartum haemorrhage caused by uterine atony p>0.05.
DISCUSSION
Our findings show that, over the past 10–15 years, the rates of PPH at the time of delivery across the US have continued to rise. Specifically, the rates of severe PPH, as defined by needing transfusion or other surgical intervention, have been increasing, while non-severe PPH trends appeared stable. Over the same time period, the rate of caesarean delivery has increased significantly from 5.5% in 1997 to 32.7% in 201316,17. With increased caesarean delivery rates comes increased obstetric interventions and increased risk for intra-abdominal scar tissue and abnormal placentation18. Abnormal placentation risk can be as high as 40% when a woman has had 3 prior caesarean deliveries and a placenta previa.
Rates of PPH requiring blood transfusion have also gone up in Canada and Ireland as evaluated by similar assessment methods using ICD-9-CM codes19,20. One of the leading risk factors for severe PPH or morbidity with delivery includes abnormal placentation8,21. The ability to diagnose potential abnormal placentation has improved with ultrasonography and magnetic resonance imaging techniques22,23, but these women still incur increased morbidity in terms of transfusion and other interventions. Other international data suggest a trend towards increasing PPH rates and rates of abnormal placentation20. Bateman et al.8, examining PPH risk factors directly related to uterine atony from the NIS database, found antepartum haemorrhage, retained placenta, and multiple gestations to be common risk factors. More broadly, these risk factors overlap with other studies looking at risk factors for transfusion in the obstetric population24,25. The ability to accurately predict risk for severe PPH has been suboptimal, in part due to the relative rarity of the outcome26. Improved prediction models that consider both pre-delivery and intrapartum risk factors are needed.
The importance of prediction for severe PPH is that it helps with patient counseling, ensures the preparedness of the clinical teams involved (obstetrics, anaesthesiology, transfusion medicine), and has the potential to foresee the need for decision-making about transfer of care prior to onset of severe PPH to a tertiary care centre with more equipped staff and blood management services, including massive transfusion policies. Our study points to increasing rates of interventions such as uterine tamponade and uterine artery embolisation. It is unclear if employing these strategies either helps mitigate blood transfusion and further morbidity or delays definitive care and increases risk for blood transfusion.
Given the new reVITALize recommendation27 to change the definition of PPH from an estimation of blood loss >500 mL with vaginal delivery and > 1,000 mL with caesarean delivery to just 1,000 mL with all deliveries, this will likely affect the trend in PPH we have seen documented by hospitals and in charts. There will likely be a time lag until this is widely adopted and used consistently; however, we should not interpret a decline in rates of PPH as misclassification. It is also important to note that clinical providers routinely underestimate clinical blood loss volume.
Further studies should identify if national trends in blood utilisation among all inpatients explain part or all of the trend seen in the obstetric patients with our data. In attempts to reverse these alarming trends, newer haemostatic agents and technologies commonly used in trauma or other surgical fields, such as antifibrinolytic therapy and viscoelastic testing, are being explored for their potential role in blood management in severe PPH27,28. Given the findings of the WOMAN Trial in 2017, and the consideration made by the American College of Obstetricians and Gynecologists to use tranexamic acid in haemorrhage refractory to first-line interventions27,29, additional clinical and translational work using antifibrinolytic therapy are needed to optimise mode of administration, dosing, and specific populations who should or should not receive the therapy. Reliable and accurate methods of monitoring haemorrhage severity are desperately needed in obstetrics to improve quality of research for future epidemiological and clinical studies.
The main strength of our study is the use of a large robust dataset to reflect national trends in rates and risk factors related to PPH at delivery. We explored the aetiology of the increased rates of severe and non-severe PPH, and did not limit our study to the diagnosis of uterine atony. Focusing on the related interventions pregnant mothers undergo related to PPH is important to highlight the changes in management practices. For example, the trend over the past 10 years has been one of an increasing use of uterine tamponade devices and uterine artery ligation/embolisation.
Our study has limitations. The NIS dataset is limited by the reliance on billing coding and most often these are under-reported by providers and staff. Therefore, the rates of true PPH are likely underestimated in our study. Misclassification is possible given not all hospitals in the dataset use standard definitions for PPH and attributable risk factors are not always reported. Diagnosis of abnormal placentation is an important clinical variable, but we were unable to isolate this within the dataset. Finally, the pre-delivery risk factors such as suspected abnormal placentation by imaging, antenatal suspected abruption vs intrapartum abruption, degree of anaemia, duration of labor, duration of oxytocin use, or estimated foetal weight by ultrasound measurements are not incorporated as variables in the NIS.
CONCLUSIONS
Ultimately, the overall increasing trend of PPH will draw the attention of clinicians, hospitals and industry to the importance of better understanding this phenomenon. Specifically, we need to analyse the related risk factors further at system levels with more granular data to better identify women at risk for severe haemorrhage. Local, state and national education efforts, simulations, toolkits, and quality improvement projects may halt, and ideally reverse, this trend. Multidisciplinary approaches will help recognise, aggressively treat, and ideally prevent these clinical outcomes.
Supplementary Information
ACKNOWLEDGEMENTS
The Authors would like to acknowledge Dr. Elena Kuklina and Dr. William M. Callaghan at the Division of Reproductive Health, Centers for Disease Control and Prevention for their contribution in data analysis. This paper was presented in part at the 36th Annual Society for Maternal Fetal Medicine Conference, February 1–6, 2016, Atlanta, GA, USA.
Footnotes
FUNDING AND RESOURCES
Children’s National CTSI grant (KL2TR001877). This publication was supported by Award Numbers UL1TR001876 and KL2TR001877 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
AUTHORSHIP CONTRIBUTIONS
AJ and CG helped with the study design and review of the manuscript. HA helped with the study concept, study design and writing of the manuscript. EK and BC helped with the analysis, and their contributions are acknowledged.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Say L, Chou D, Gemmill A, Tuncalp O, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–33. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 2.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol. 2010;202:353.e1–6. doi: 10.1016/j.ajog.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. Am J Obstet Gynecol. 2008;199:133.e1–8. doi: 10.1016/j.ajog.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Kuklina EV, Meikle SF, Jamieson DJ, et al. Severe obstetric morbidity in the United States: 1998–2005. Obstet Gynecol. 2009;113:293–9. doi: 10.1097/AOG.0b013e3181954e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ISBT Working Party on Hemovigilance. Proposed standard definitions for surveillance of non infectious adverse transfusion reactions. [Accessed on 10/05/2019]. Available at: https://www.isbtweb.org/fileadmin/user_upload/Proposed_definitions_2011_surveillance_non_infectious_adverse_reactions_haemovigilance_incl_TRALI_correction_2013.pdf.
- 6.James AH, McLintock C, Lockhart E. Postpartum hemorrhage: when uterotonics and sutures fail. Am J Hematol. 2012;87(Suppl 1):S16–22. doi: 10.1002/ajh.23156. [DOI] [PubMed] [Google Scholar]
- 7.James AH, Grotegut C, Ahmadzia H, et al. Management of Coagulopathy in Postpartum Hemorrhage. Semin Thromb Hemost. 2016;42:724–31. doi: 10.1055/s-0036-1593417. [DOI] [PubMed] [Google Scholar]
- 8.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110:1368–73. doi: 10.1213/ANE.0b013e3181d74898. [DOI] [PubMed] [Google Scholar]
- 9.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–36. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 10.Grotegut CA, Kuklina EV, Anstrom KJ, et al. Factors associated with the change in prevalence of cardiomyopathy at delivery in the period 2000–2009: a population-based prevalence study. BJOG. 2014;121:1386–94. doi: 10.1111/1471-0528.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James AH, Jamison MG, Biswas MS, et al. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113:1564–71. doi: 10.1161/CIRCULATIONAHA.105.576751. [DOI] [PubMed] [Google Scholar]
- 12.Kuklina E, Callaghan W. Chronic heart disease and severe obstetric morbidity among hospitalisations for pregnancy in the USA: 1995–2006. BJOG. 2011;118:345–52. doi: 10.1111/j.1471-0528.2010.02743.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuklina EV, Whiteman MK, Hillis SD, et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J. 2008;12:469–77. doi: 10.1007/s10995-007-0256-6. [DOI] [PubMed] [Google Scholar]
- 14.Podulka J, Stranges E, Steiner C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet] Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Feb–2011 Apr. Hospitalizations related to childbirth, 2008: Statistical Brief #110. [PubMed] [Google Scholar]
- 15.Grotegut CA, Chisholm CA, Johnson LN, et al. Medical and obstetric complications among pregnant women aged 45 and older. PLoS One. 2014;9:e96237. doi: 10.1371/journal.pone.0096237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottoms SF, Rosen MG, Sokol RJ. The increase in the caesarean birth rate. N Engl J Med. 1980;302:559–63. doi: 10.1056/NEJM198003063021006. [DOI] [PubMed] [Google Scholar]
- 17.Osterman MJ, Martin JA. Trends in low-risk caesarean delivery in the United States, 1990–2013. Natl Vital Stat Rep. 2014;63:1–16. [PubMed] [Google Scholar]
- 18.Silver RM, Landon MB, Rouse DJ, et al. National Institute of Child H, Human Development Maternal-Fetal Medicine Units N. Maternal morbidity associated with multiple repeat caesarean deliveries. Obstet Gynecol. 2006;107:1226–32. doi: 10.1097/01.AOG.0000219750.79480.84. [DOI] [PubMed] [Google Scholar]
- 19.Joseph KS, Rouleau J, Kramer MS, et al. Maternal Health Study Group of the Canadian Perinatal Surveillance S. Investigation of an increase in postpartum haemorrhage in Canada. BJOG. 2007;114:751–9. doi: 10.1111/j.1471-0528.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 20.Lutomski JE, Byrne BM, Devane D, Greene RA. Increasing trends in atonic postpartum haemorrhage in Ireland: an 11-year population-based cohort study. BJOG. 2012;119:306–14. doi: 10.1111/j.1471-0528.2011.03198.x. [DOI] [PubMed] [Google Scholar]
- 21.Grobman WA, Bailit JL, Rice MM, et al. Eunice Kennedy Shriver National Institute of Child H, Human Development Maternal-Fetal Medicine Units N. Frequency of and factors associated with severe maternal morbidity. Obstet Gynecol. 2014;123:804–10. doi: 10.1097/AOG.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abuhamad A. Morbidly adherent placenta. Semin Perinatol. 2013;37:359–64. doi: 10.1053/j.semperi.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Familiari A, Liberati M, Lim P, et al. Diagnostic accuracy of magnetic resonance imaging in detecting the severity of abnormal invasive placenta: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97:507–20. doi: 10.1111/aogs.13258. [DOI] [PubMed] [Google Scholar]
- 24.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265–72. doi: 10.1111/j.1471-0528.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 25.Rouse DJ, Leindecker S, Landon M, et al. National Institute of Child H, Human Development Maternal-Fetal Medicine Units N. The MFMU caesarean Registry: uterine atony after primary caesarean delivery. Am J Obstet Gynecol. 2005;193:1056–60. doi: 10.1016/j.ajog.2005.07.077. [DOI] [PubMed] [Google Scholar]
- 26.Dilla AJ, Waters JH, Yazer MH. Clinical validation of risk stratification criteria for peripartum hemorrhage. Obstet Gynecol. 2013;122:120–6. doi: 10.1097/AOG.0b013e3182941c78. [DOI] [PubMed] [Google Scholar]
- 27.Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130:e168–e86. doi: 10.1097/AOG.0000000000002351. [DOI] [PubMed] [Google Scholar]
- 28.Snegovskikh D, Souza D, Walton Z, et al. Point-of-care viscoelastic testing improves the outcome of pregnancies complicated by severe postpartum hemorrhage. J Clin Anesth. 2018;44:50–6. doi: 10.1016/j.jclinane.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 29.WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–16. doi: 10.1016/S0140-6736(17)30638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.