Abstract
Background
Analgesics, sedatives, and neuromuscular blockers are commonly used medications for mechanically ventilated air medical transport patients. Prior research in the emergency department (ED) and intensive care unit (ICU) has demonstrated that depth of sedation is associated with increased mechanical ventilation duration, delirium, increased hospital length-of-stay (LOS), and decreased survival. The objectives of this study were to evaluate current sedation practices in the prehospital setting and to determine the impact on clinical outcomes.
Methods
A retrospective cohort study of mechanically ventilated patients transferred by air ambulance to a single 812-bed Midwestern academic medical center from July 2013 to May 2018 was conducted. Prehospital sedation medications and depth of sedation [Richmond Agitation-Sedation Scale score (RASS)] were measured. Primary outcome was hospital LOS. Secondary outcomes were delirium, length of mechanical ventilation, in-hospital mortality, and need for neurosurgical procedures. Univariate analyses were used to measure the association between sedatives, sedation depth, and clinical outcomes. Multivariable models adjusted for potentially confounding covariates to measure the impact of predictors on clinical outcomes.
Results
Three hundred twenty-seven patients were included. Among those patients, 79.2% of patients received sedatives, with 41% of these patients achieving deep sedation (RASS = −4). Among patients receiving sedation, 58.3% received at least one dose of benzodiazepines. Moderate and deep sedation was associated with an increase in LOS of 59% (aRR: 1.59; 95%CI: 1.40–1.81)and 24% (aRR: 1.24; 95% CI: 1.10–1.40), respectively. Benzodiazepines were associated with a mean increase of 2.9 days in the hospital (95% CI, 0.7–5.1). No association existed between either specific medications or depth of sedation and the development of delirium.
Conclusions
Prehospital moderate and deep sedation, as well as benzodiazepine administration, is associated with increased hospital LOS. Our findings point toward sedation being a modifiable risk factor and suggest an important need for further research of sedation practices in the pre-hospital setting.
Keywords: Air ambulances, intubation, deep sedation, emergency medical services (EMS), delirium
Introduction
The effect of sedation on clinical outcomes in mechanically ventilated patients has been studied in both the intensive care unit (ICU) and in the emergency department (ED) [1–4]. For example, depth of sedation has been shown to be associated with delirium, increased mechanical ventilation duration, increased length-of-stay (LOS), and decreased survival [2]. In a recent study of mechanically ventilated patients in the ED, deep sedation was provided to nearly 2/3 of ventilated patients, of which 71% eventually developed delirium [1].Delirium has particular significance on outcomes for critically ill patients, as patients with delirium have an increased risk of prolonged hospital stays, mortality, and long-term cognitive impairment [5, 6]. There is also increased recognition that sedation during the early course of mechanical ventilation is especially impactful. Sedation during the first 48 hours of mechanical ventilation in the ICU has been shown to influence mortality and mechanical ventilation duration [7]. In addition, certain sedatives, such as dexmedetomidine, have been shown to be effective agents contributing to better outcomes in both neurological and non-neurological ICU patients [3, 8, 9]. In the ED, deep sedation is common and associated with worse outcomes [1, 10].
The provision of some combination of analgesics, sedatives, and neuromuscular blockers is a near-ubiquitous intervention for the more than 550,000 critically ill patients requiring air medical transport annually [11]. Despite this, there is no data examining the potential impact of sedation in the prehospital environment on mechanically ventilated patients. As early sedation practices have been shown to not only impact patient-oriented outcomes, but also subsequent sedation practices in the ICU [12], quantifying the impact of prehospital sedation could have important clinical implications. The objectives of this study were to evaluate current sedation practices in the prehospital setting and to determine the impact on clinical outcomes. We hypothesized that deep sedation in the prehospital environment would be associated with worse clinical outcomes, specifically increased LOS, increased time on mechanical ventilation, increased hospital mortality risk, development of delirium, and need for neurosurgical interventions.
Methods
Study Design, Setting, and Population
This study was a retrospective cohort study of all (i.e. neonatal, pediatric, adult) mechanically ventilated patients transferred by air ambulance to a Midwestern university hospital with a 60,000-visit ED between July 2013 and May 2018. Prehospital arrivals from three air ambulance services were abstracted from electronic medical records. Records with incomplete or insufficient documentation, and patients who did not require mechanical ventilation during air transport were excluded. If a patient was transported multiple times during the study period, only the first encounter was used. As there were three ambulance services that serve the institution, we used a stratified random sample to obtain a representative sample from each service. This was done for efficiency and time required to extract data from all charts, and to account for representation from all three services. The local Institutional Review Board approved this study under waiver of informed consent, and the study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [13].
Measurements of Primary Exposures and Covariates
Data were collected from air transport logs and manual extraction of hospital medical records by a single trained research assistant, using a standardized data collection form in Research Electric Data Capture (REDCap) software. Minimum and maximum values of each continuous variable were set in the software to limit errors in data abstraction, and intermittent, informal audits of random records were performed by the research assistant to promote accuracy. The primary exposure was sedative and paralytic medications during air medical transport. Medications of interest included benzodiazepines, ketamine, opioids, propofol, other hypnotic sedatives (e.g. etomidate), and neuromuscular blockers (e.g. succinylcholine, rocuronium, vecuronium). Combinations of medications administered also included (1) opioids and benzodiazepines, (2) opioids and propofol, and (3) opioids, benzodiazepines, and propofol. These medications were characterized in two ways: 1) cumulative dose and 2) dichotomously (administered vs. not) among those who received any sedation. Medication data were gathered only from the air transport record in order to limit error and uncertainty from outside health records. Doses of medications were summarized as follows: bolus doses were recorded at the time of administration; continuous infusions were recorded as constant infusions rates at a dose that was used for the majority of the flight.
We extracted baseline demographics (age, gender, race/ethnicity, insurance), comorbid conditions, and indication for mechanical ventilation (neurological vs. non-neurological). Comorbid conditions included: diabetes, chronic obstructive pulmonary disease (COPD), congestive heart failure, stroke, coronary artery disease, dementia, pneumonia, traumatic brain injury, and other psychiatric disorders (schizophrenia, bipolar disorder, etc). Ventilator settings (mode, tidal volume, FiO2, and positive end-expiratory pressure), vital signs (e.g. heart rate, blood pressure), and Glasgow Coma Scale (GCS) were collected from the accepting hospital ED admission note. If no GCS value was recorded in the receiving hospital ED, the GCS value was obtained from the air ambulance record upon arrival at the receiving hospital. Air ambulance transport departure/arrival times were recorded to calculate medication infusion duration.
Exposures
The principle exposures tested in this study were: 1) drug selection; 2) depth of sedation; and 3) dosing. To measure sedation depth during transport and in the ED, the Richmond Agitation-Sedation Scale (RASS) was used [14]. This scale ranges from +4 (combative) to −5 (unarousable) [9]. When explicit scores were not provided, subjective descriptions during the initial patient assessments by the EMS crew and ED staff were used to record the appropriate RASS value. To measure sedative dosing, two residency-trained emergency medicine (EM) clinical pharmacists, blinded to all study outcomes, categorized each patient dose as “low,” “intermediate,” and “high” based on the cumulative dose of sedative and analgesic medication the patient received during the flight. This was done was because no universal equivalent dosing between different sedative agents are available, and because the impact of low dose-deep sedation and high dose-mild sedation on clinical outcomes was hypothesized to differ. Examples of each of the dose classifications which represent the cumulative dose received during flight are as follows: mild sedation: midazolam < 5 mg, lorazepam ≤ 4mg, benzodiazepine plus opioid combination: fentanyl < 100 mcg plus midazolam < 5 mg; moderate sedation: benzodiazepine plus opioid combination: fentanyl ≥100 mcg plus midazolam ≥ 5 mg, propofol continuous infusion ≤ 20 mcg/kg/minute, ketamine 0.31–0.99 mg/kg; deep sedation: propofol continuous infusion > 20 mcg/kg/minute, propofol continuous infusion plus opioid (e.g., fentanyl) administration, ≥ 3 sedative medications administered. Discrepancies were resolved by review of a third blinded EM clinical pharmacist. Both depth of sedation and drug dosing adjudication was performed blinded to clinical outcomes.
Primary Outcomes
The primary outcome was hospital LOS.
Secondary Outcomes
Secondary outcomes were delirium and 28-day ventilator-free days. Delirium was measured as either a diagnosis of delirium using ICD-10 codes in the medical record or symptoms consistent with delirium, specifically acute onset of agitation, confusion, aggressive behavior, or insomnia. Clinical symptoms described in patients’ charts were used due to inconsistent use of a standardized screening tool, such as the CAM-ICU [15]. Also, 28-day ventilator-free days were used to account for right-censored data in a population with high mortality, such that this measure accounted the number of days alive and free from mechanical ventilation and was not biased by the number of deaths. Other secondary outcomes included the receipt of neurosurgery and intracranial pressure (ICP) monitoring (among those with a neurological indication for intubation), and hospital mortality. The reason for measuring neurosurgery and ICP monitoring was to determine if sedatives and/or sedation depth confound the physical exam and consequently lead to more neurological interventions.
Statistical Data Analysis
Evaluation of Covariates
We measured differences in demographics, comorbidities, clinical presentation (pre-hospital and ED RASS, vital signs), and ventilator settings across each outcome. Bivariate comparisons between each outcome and covariate were conducted using Wilcoxon rank-sum tests for continuous variables and Fisher exact or Pearson’s chi-squared tests for categorical variables.
Evaluation of Primary and Secondary Outcomes
Binary outcomes, such as our secondary outcomes of delirium, need for neurosurgery and/or intracranial pressure monitoring, and in-hospital mortality, were evaluated through logistic regression. In order to account for potential interaction between depth of sedation and neurological indication for intubation on the outcome of in-hospital mortality, we stratified the relationship by status of neurological indication. Continuous outcomes, such as our primary outcome of hospital LOS, as well as the time on mechanical ventilation were evaluated descriptively (i.e. mean and standard deviation). As each LOS and time on ventilation was a count of days that were right-skewed, we modeled the association between sedation and level of dosing for each continuous outcome through generalized estimating equations, using an identity link and Poisson distribution.
Final Multivariable Models
The final models developed were built by purposeful manual backward selection of covariates that were associated with each outcome. For example, we removed demographic and clinical covariates or variables which were not independently associated with the outcome in the full model, did not significantly impact measures of sedation, and did not improve the overall model fit (evaluated by Akaike Information Criterion values). Final adjusted measures were evaluated as relative risks (RR, 95%CI) for continuous outcomes and odds ratios (OR, 95%CI) for binary outcomes. For in-hospital mortality, we tested for effect modification by indication for neurosurgery by fitting an interaction between sedation dosing and neurosurgery indication. All tests were considered significant at alpha < 0.05 using 2-tailed tests. Analyses were completed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Power analyses
We estimated that to detect a 15% increase in hospital LOS in patients deeply sedated vs. those with light sedation, 276 patients would be needed to have 80% power at an alpha of 0.05. After inclusion and exclusion criteria, there were 327 patients available for study inclusion, providing us with assurance that we could test the hypothesis with adequate power.
Results
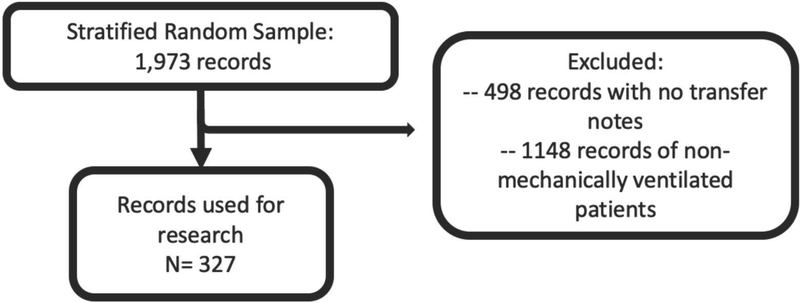
There were 3,664 patients who were transported via air ambulance, of which 1,973 patients were selected in the stratified random sample. After screening for eligibility criteria, 327 patients were included in the study (Figure 1).
Figure 1.
Patient Inclusion Flow Diagram
Characteristics of Population
The mean patient age was 63 years (SD: 20.3), of which 4.6% were <18 years (n=15) and 2.8% were ≤1 year (1.8%). Males represented 58.1% of the sample. Baseline characteristics are in Table 1.
Table 1.
Demographic and Clinical Characteristics of Patient Population on Mechanical Ventilation during Air Transport1
| Characteristic | Overall (n=327) | Delirium (n=84) | p-value2 | Neurosur gery3 (n=64) | p-value2 | ICP Monitor3 (n=35) | p-value2 | Death (n=121) | p-value2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age - Median (IQR) | 63 | (47–74) | 61 | (44–74) | 0.499 | 59 | (49–69) | 0.001 | 54 | (45–66) | 0.001 | 66 | (59–79) | <0.001 |
| Sex - n (%) | ||||||||||||||
| Male | 190 | (58.1) | 49 | (58.3) | 0.961 | 33 | (51.6) | 0.354 | 20 | (57.1) | 0.902 | 70 | (57.9) | 0.934 |
| Female | 137 | (41.9) | 35 | (41.7) | 31 | (48.4) | 15 | (42.9) | 51 | (42.1) | ||||
| Race - n (%) | ||||||||||||||
| Caucasian/White | 282 | (86.2) | 77 | (91.7) | 0.230 | 51 | (79.7) | 0.337 | 28 | (80.0) | 0.686 | 103 | (85.1) | 0.273 |
| African American/Black | 16 | (4.9) | 3 | (3.6) | 6 | (9.4) | 2 | (5.7) | 4 | (3.3) | ||||
| Other | 29 | (8.9) | 4 | (4.8) | 7 | (10.9) | 5 | (14.3) | 14 | (11.6) | ||||
| Insurance - n (%) | ||||||||||||||
| Medicaid | 63 | (19.3) | 24 | (28.6) | 0.139 | 10 | (15.6) | 0.021 | 8 | (22.9) | 0.022 | 13 | (10.7) | <0.001 |
| Medicare | 164 | (50.2) | 40 | (47.6) | 27 | (42.2) | 13 | (37.1) | 82 | (67.8) | ||||
| Self-pay | 20 | (6.1) | 4 | (4.8) | 4 | (6.3) | 2 | (5.7) | 7 | (5.8) | ||||
| Private | 66 | (20.2) | 13 | (15.5) | 20 | (31.3) | 12 | (34.3) | 15 | (12.4) | ||||
| Other | 14 | (4.3) | 3 | (3.6) | 3 | (4.7) | 0 | (0.0) | 4 | (3.3) | ||||
| Helicopter Service | ||||||||||||||
| Aircare | 145 | (44.3) | 38 | (45.2) | 0.736 | 27 | (42.2) | 0.394 | 14 | (40.0) | 0.283 | 54 | (44.6) | 0.455 |
| Lifeguard | 76 | (23.2) | 17 | (20.2) | 19 | (29.7) | 12 | (34.3) | 32 | (26.4) | ||||
| Medforce | 106 | (32.4) | 29 | (34.5) | 18 | (28.1) | 9 | (25.7) | 35 | (28.9) | ||||
| Co-Morbidities - n (%) | ||||||||||||||
| Coronary Artery Disease | 69 | (21.1) | 8 | (9.5) | 0.003 | 13 | (20.3) | 0.493 | 6 | (17.1) | 0.343 | 34 | (28.1) | 0.018 |
| Congestive Heart Failure | 29 | (8.9) | 5 | (6.0) | 0.276 | 3 | (4.7) | 0.069 | 1 | (2.9) | 0.109 | 15 | (12.4) | 0.085 |
| Diabetes | 78 | (23.9) | 17 | (20.2) | 0.367 | 13 | (20.3) | 0.298 | 5 | (14.3) | 0.108 | 30 | (24.8) | 0.789 |
| Stroke | 42 | (12.8) | 6 | (7.1) | 0.070 | 9 | (14.1) | 0.329 | 6 | (17.1) | 0.905 | 24 | (19.8) | 0.004 |
| Dementia | 11 | (3.4) | 1 | (1.2) | 0.301 | 1 | (1.6) | 0.179 | 1 | (2.9) | 0.636 | 8 | (6.6) | 0.022 |
| TBI | 5 | (1.5) | 2 | (2.4) | 0.273 | 0 | (0.0) | *** | 0 | (0.0) | *** | 1 | (0.8) | 0.655 |
| Pneumonia | 15 | (4.6) | 3 | (3.6) | 0.768 | 0 | (0.0) | *** | 0 | (0.0) | *** | 4 | (3.3) | 0.585 |
| COPD | 53 | (16.2) | 17 | (20.2) | 0.245 | 3 | (4.7) | 0.038 | 0 | (0.0) | *** | 22 | (18.2) | 0.458 |
| Other Psychiatric Disorder | 73 | (22.3) | 29 | (34.5) | 0.002 | 12 | (18.8) | 0.859 | 4 | (11.4) | 0.183 | 14 | (11.6) | <0.001 |
| Neurosurgery | 70 | (21.4) | 19 | (22.6) | 0.753 | *** | *** | *** | 35 | (100.0) | <0.001 | 18 | (14.9) | 0.027 |
| ICP Monitoring | 39 | (11.9) | 11 | (13.1) | 0.702 | 35 | (54.7) | <0.001 | *** | *** | *** | 10 | (8.3) | 0.012 |
| RASS Score, Prehospital3 | ||||||||||||||
| Awake | 40 | (12.2) | 10 | (11.9) | 0.835 | 4 | (6.3) | 0.099 | 2 | (5.7) | 0.282 | 6 | (5.0) | 0.004 |
| Light/Moderately Sedated | 19 | (5.8) | 6 | (7.1) | 53 | (82.8) | 4 | (11.4) | 5 | (4.1) | ||||
| Heavily Sedated | 267 | (81.7) | 68 | (81.0) | 7 | (10.9) | 29 | (82.9) | 109 | (90.1) | ||||
| RASS Score, ED3 | (0.0) | |||||||||||||
| Awake | 18 | (5.5) | 5 | (6.0) | 0.867 | 2 | (3.1) | 0.662 | 1 | (2.9) | 0.917 | 2 | (1.7) | 0.003 |
| Light/Moderately Sedated | 32 | (9.8) | 7 | (8.3) | 6 | (9.4) | 3 | (8.6) | 6 | (5.0) | ||||
| Heavily Sedated | 275 | (84.1) | 71 | (84.5) | 56 | (87.5) | 31 | (88.6) | 112 | (92.6) | ||||
| Vital Signs - Median (IQR) | ||||||||||||||
| Diastolic BP (mmHg) | 73 | (62–89) | 70 | (61–90) | 0.517 | 83 | (69–97) | 0.011 | 83 | (76–96) | 0.020 | 74 | (60–90) | 0.581 |
| Systolic BP (mmHg) | 127 | (107–148) | 125 | (103–143) | 0.313 | 134 | (118–153) | 0.844 | 134 | (118–152) | 0.740 | 132 | (107–154) | 0.334 |
| GCS | 3 | (3–6) | 3 | (3–6) | 0.255 | 3 | (3–6) | 0.252 | 3 | (3–7) | 0.112 | 3 | (3–4) | 0.013 |
| Heart Rate (bpm) | 85 | (71–100) | 82 | (73–98) | 0.861 | 78 | (64–95) | 0.151 | 77 | (61–94) | 0.076 | 85 | (70–100) | 0.541 |
| Respiratory Rate | 18 | (16–20) | 18 | (16–20) | 0.093 | 18 | (16–20) | 0.680 | 18 | (16–20) | 0.955 | 18 | (16–21) | 0.989 |
| SpO2 (%) | 98 | (96–100) | 98 | (96–100) | 0.882 | 99 | (96–100) | 0.759 | 99 | (95–100) | 0.611 | 98 | (96–100) | 0.257 |
| Ventilator Settings - Median (IQR) | ||||||||||||||
| Fi02 | 60 | (40–100) | 50 | (40–98) | 0.367 | 50 | (40–78) | 0.167 | 50 | (40–100) | 0.674 | 60 | (50–80) | 0.695 |
| Mode | 1 | (1–2) | 1 | (1–2) | 0.924 | 1 | (1–2) | 0.306 | 1 | (1–2) | 0.109 | 1 | (1–1) | 0.014 |
| PEEP | 5.0 | (5–6) | 5 | (5–6) | 0.493 | 5 | (5–6) | 0.117 | 5 | (5–6) | 0.414 | 5 | (5–6) | 0.823 |
| Tidal volume | 500 | (440–500) | 500 | (440–500) | 0.570 | 500 | (450–550) | 0.411 | 500 | (450–550) | 0.645 | 500 | (425–500) | 0.419 |
Abbreviations. Unadjusted risk ratio (uRR); Standard deviation (SD); Traumatic Brain Injury (TBI); Chronic Obstructive Pulmonary Disease (COPD); Intracranial Pressure (ICP) 2
P value comparisons comparing each demographic characteristic by presence or absence of outcome. Wilcoxon rank sum test continuous variables, and Fisher exact (cells <5) or Χ2 (cells ≥5) for categorical variables
Among those with a neurological indication for intubation only
Heavily sedated (RASS values −5, −4), light/moderately sedated (−3, −1), and awake (−1,0, 1, 2, 3, 4).
Primary Outcomes: Hospital LOS
The overall mean hospital LOS was 8.1 days (SD 10.3), and appeared to increase with higher levels of depth of prehospital sedation (Table 2). Compared to those receiving no sedation, moderate and deep sedation was associated with an increase in LOS of 78% and 51%, respectively. Of patients who received sedatives, 58.3% were given at least one dose of benzodiazepines, and patients who were exposed to benzodiazepines had an increase in hospital LOS by 2.6 days (Table 2)
Table 2.
Association between Medications Administered in Flight and Hospital Length of Stay and Ventilator-Free Days1
| Medication Characteristic | Hospital Length-of-Stay | 28-Day Ventilator-Free Days | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | uRR | 95%CI | Mean | (SD) | uRR | 95%CI | |||||
| Deptd of Sedation | ||||||||||||
| None | 5.8 | (7.7) | Ref | 9.7 | (12.5) | Ref | ||||||
| Mild | 6.6 | (7.3) | 1.14 | 0.99–1.31 | 17.8 | (12.1) | 1.83 | 1.66–2.02 | ||||
| Moderate | 10. 4 | (16.0) | 1.78 | 1.57–2.01 | 16.4 | (12.3) | 1.69 | 1.53–1.86 | ||||
| Deep | 8.8 | (8.4) | 1.51 | 1.34–1.69 | 17.9 | (11.9) | 1.85 | 1.69–2.01 | ||||
| Medication2 | Exposed | Unexposed | Exposed | Unexposed | ||||||||
| Mean | (SD) | Mean | (SD) | uRR | 95%CI | Mean | (SD) | Mean | (SD) | uRR | 95%CI | |
| Benzodiazepines | 9.8 | (12. 9) | 7.2 | (6.9) | 1.3 6 | 18. 2 | (11. 6) | 16. 5 | (12. 6) | 1.1 1 | 1.04–1.17 | |
| Ketamine | 10. 7 | (10. 1) | 8.5 | (10. 9) | 1.2 5 | 1.10–1.42 | 20. 4 | (10. 7) | 17. 2 | (12. 1) | 1.1 9 | 1.08–1.30 |
| Opioids | 8.6 | (9.9) | 8.9 | (12. 1) | 0.9 7 | 0.89–1.05 | 17. 8 | (11. 9) | 17. 1 | (12. 2) | 1.0 4 | 0.98–1.10 |
| Propofol | 8.6 | (7.9) | 8.8 | (11. 9) | 0.9 8 | 0.90–1.08 | 18. 1 | (11. 9) | 17. 2 | (12. 1) | 1.0 5 | 0.98–1.12 |
| Sedative Hypnotic | 8.3 | (8.4) | 8.9 | (11. 3) | 0.9 3 | 0.83–1.04 | 17. 1 | (12. 5) | 15. 6 | (11. 9) | 0.9 7 | 0.90–1.05 |
| Paralytics | ||||||||||||
| Short Acting | 10. 1 | (12. 2) | 8.5 | (10. 6) | 1.1 8 | 1.05–1.32 | 18. 5 | (11. 8) | 17. 3 | (12. 1) | 1.0 7 | 0.99–1.16 |
| Intermediate Acting | 9.3 | (14. 0) | 8.5 | (8.9) | 1.1 0 | 1.01–1.20 | 17. 8 | (11. 8) | 17. 3 | (12. 1) | 1.0 3 | 0.97–1.10 |
| Combinations | ||||||||||||
| Opioids, Benzodiazepine s | 9.8 | (11. 3) | 8.2 | (10. 6) | 1.1 9 | 1.09–1.29 | 18. 4 | (11. 7) | 17. 0 | (12. 2) | 1.0 8 | 1.01–1.15 |
| Opioids, Propofol | 9.3 | (8.5) | 8.7 | (11. 2) | 1.0 7 | 0.95–1.20 | 19. 9 | (10. 9) | 17. 0 | (12. 2) | 1.1 7 | 1.08–1.26 |
| Opioids, Benzodiazepines Propofol | 8.5 | (6.2) | 8.8 | (11. 0) | 0.9 8 | 0.79–1.20 | 23. 8 | (8.2) | 17. 2 | (12. 1) | 1.3 9 | 1.22–1.57 |
Abbreviations. Unadjusted risk ratio (uRR); Standard deviation (SD);
Numbers and percentages presented are for those who received any sedation (mild, moderate, or deep) only.
In the final adjusted model evaluating those receiving any sedation, an increased hospital LOS was still observed with moderate (aRR: 1.59; 95%CI: 1.40–1.81) and deep sedation (aRR: 1.24; 95%CI: 1.10–1.40) (Table 3).
Table 3.
Final Adjusted Multivariable Models for Delirium, Death, and Hospital Length of Stay1
| Clinical Characteristic and Presentation | Delirium2 | Death | Hospital Length of Stay2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intubation Indication -Neuro Only2 | Intubation Indication -Non-Neuro Only2 | |||||||||
| aOR3 | 95%CI | aOR4 | 95%CI | aOR4 | 95%CI | aRR5 | 95%CI | |||
| Pre-hospital RASS (Ref = Awake/Alert) | ||||||||||
| Moderately Sedated | 1.7 7 | 0.48–6.53 | 1.5 5 | 0.23–10.41 | 0.8 9 | 0.07–12.17 | 1.3 5 | 1.12–1.62 | ||
| Heavily Sedated | 1.3 9 | 0.60–3.24 | 2.4 0 | 0.59–9.82 | 1.4 9 | 0.33–6.62 | 0.9 1 | 0.81–1.02 | ||
| Sedation (Ref = Mild) | ||||||||||
| Moderate | 1.6 3 | 0.67–3.96 | 1.6 5 | 0.60–4.58 | 0.3 3 | 0.05–2.07 | 1.5 9 | 1.40–1.81 | ||
| Deep | 1.7 2 | 0.78–3.78 | 1.1 7 | 0.46–2.99 | 0.5 9 | 0.16–2.16 | 1.2 4 | 1.10–1.40 | ||
Abbreviations: aOR = adjusted odds ratio; aRR = adjusted relative risk
Among those receiving any sedation (mild, moderate, or deep) only.
Adjusted for pre-hospital RASS, level of sedation, neurological indication for intubation, coronary artery disease, and other psychiatric co-morbidities. N= 259 observations in model.
Adjusted for pre-hospital RASS, level of sedation, age, and other psychiatric co-morbidities. N=160 observations in model with neurological indicator for intubation, N=99 in model with non-neurological indicator for intubation.
Adjusted for pre-hospital RASS, level of sedation, age, insurance, and history of coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, other psychiatric comorbidities, stroke, and traumatic brain injury. N=259 observations in model.
Secondary Outcome: 28-Day Ventilator Free Days
Sedation was associated with an increase in ventilator-free days. A combination of opioids, benzodiazepines, and propofol was associated with a 39% (uRR: 1.22–1.57) increase in ventilator-free days (Table 2).
Secondary Outcome: Delirium
A quarter of patients (25.7%) developed delirium during their hospital admission, of which 81% had received some level of sedation (Table 4). In the final model adjusted for potential confounders for those receiving any sedation, there was no difference in the delirium outcome among those receiving moderate sedation (aOR: 1.63; 95%CI: 0.67–3.96) or deep sedation (aOR: 1.72; 95%CI: 0.78–3.78) compared to those who were mildly sedated (Table 3).
Table 4.
Association between Medications Administered in Flight and Delirium, Performing Neurosurgery, ICP Monitoring, and Death
| Depth of Sedation and Medications | Delirium | Neurosurgery1 | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % | uOR | 95%CI | n | % | uOR | 95%CI | |
| Depth of Sedation | ||||||||
| None | 16 | (19.0) | Ref | 5 | (7.8) | Ref | ||
| Mild | 11 | (13.1) | 0.80 | 0.34–1.89 | 13 | (20.3) | 2.48 | 0.75–8.22 |
| Moderate | 19 | (22.6) | 1.24 | 0.57–2.67 | 14 | (21.9) | 1.75 | 0.55–5.61 |
| Deep | 38 | (45.2) | 1.29 | 0.66–2.53 | 32 | (50.0) | 2.67 | 0.91–7.83 |
| Medications2 | ||||||||
| Benzodiazepines | 43 | (63.2) | 1.32 | 0.75–2.34 | 32 | (54.2) | 0.69 | 0.34–1.32 |
| Ketamine | 8 | (11.8) | 1.37 | 0.56–3.32 | 6 | (10.2) | 2.75 | 0.74–10.16 |
| Opioids | 39 | (57.4) | 0.95 | 0.54–1.66 | 35 | (59.3) | 1.08 | 0.56–2.08 |
| Propofol | 22 | (32.4) | 1.21 | 0.67–2.21 | 23 | (39.0) | 1.59 | 0.81–3.12 |
| Sedative Hypnotic | 9 | (13.2) | 0.60 | 0.27–1.30 | 9 | (15.3) | 0.65 | 0.28–1.52 |
| Paralytics | ||||||||
| Short Acting | 8 | (11.8) | 0.75 | 0.32–1.72 | 6 | (10.2) | 0.49 | 0.18–1.30 |
| Intermediate Acting | 19 | (27.9) | 0.72 | 0.39–1.32 | 15 | (25.4) | 0.59 | 0.29–1.20 |
| Combinations | ||||||||
| Opioids + Benzodiazepines | 27 | (39.7) | 1.40 | 0.79–2.49 | 18 | (30.5) | 0.64 | 0.33–1.27 |
| Opioids + Propofol | 8 | (11.8) | 0.75 | 0.32–1.72 | 14 | (23.7) | 2.55 | 1.07–6.06 |
| Opioids + Benzodiazepines + Propofol | 2 | (2.9) | 0.61 | 0.13–2.91 | 4 | (6.8) | 2.38 | 0.51–11.0 |
| Depth of Sedation and Medications | ICP Monitoring1 | Death | ||||||
| n | % | uOR | 95%CI | n | % | uOR | 95%CI | |
| Depth of Sedation | ||||||||
| None | 4 | (11.4) | Ref | 41 | (33.9) | Ref | ||
| Mild | 7 | (20.0) | 1.36 | 0.35–5.27 | 17 | (14.0) | 0.29 | 0.14–0.61 |
| Moderate | 5 | (14.3) | 0.64 | 0.16–2.64 | 24 | (19.8) | 0.35 | 0.18–0.70 |
| Deep | 19 | (54.3) | 1.64 | 0.50–5.36 | 39 | (32.2) | 0.27 | 0.15–0.50 |
| Medications2 | ||||||||
| Benzodiazepines | 16 | (51.6) | 0.65 | 0.30–1.44 | 41 | (51.3) | 0.66 | 0.38–1.12 |
| Ketamine | 5 | (16.1) | 4.77 | 1.29–17.67 | 5 | (6.3) | 0.53 | 0.19–1.47 |
| Opioids | 22 | (71.0) | 2.00 | 0.85–4.67 | 45 | (56.3) | 0.89 | 0.52–1.51 |
| Propofol | 14 | (45.2) | 1.97 | 0.88–4.40 | 22 | (27.5) | 0.88 | 0.49–1.58 |
| Sedative Hypnotic | 4 | (12.9) | 0.56 | 0.18–1.74 | 16 | (20.0) | 1.15 | 0.59–2.24 |
| Paralytics | ||||||||
| Short Acting | 2 | (6.5) | 0.32 | 0.07–1.43 | 10 | (12.5) | 0.80 | 0.70–1.75 |
| Intermediate Acting | 9 | (29.0) | 0.82 | 0.35–1.93 | 25 | (31.3) | 0.88 | 0.50–1.55 |
| Combinations | ||||||||
| Opioids + Benzodiazepines | 10 | (32.3) | 0.79 | 0.34–1.79 | 8 | (10.0) | 0.77 | 0.44–1.36 |
| Opioids + Propofol | 10 | (32.3) | 3.62 | 1.43–9.13 | 24 | (30.0) | 0.58 | 0.25–1.32 |
| Opioids + Benzodiazepines + Propofol | 2 | (6.5) | 1.71 | 0.32–9.26 | 1 | (1.3) | 0.21 | 0.03–1.70 |
Among those with a neurological indication for intubation only
Numbers and percentages presented are for those who received any sedation (mild, moderate, or deep) only.
Secondary Outcomes: Neurosurgery and ICP monitoring
Among those with a neurological indication for intubation, 35% of patients had neurosurgery performed (Table 1). Nearly all (92.2%) of these patients had some level of sedation, though the odds of neurosurgery did not appear to be different between those who were given sedatives compared to those who were not. Among those receiving any medications, the odds of neurosurgery were 2.55 (95%CI: 1.07–6.06) times greater among those receiving an opioid-propofol combination compared to those who did not receive this combination (Table 4). There was no significant difference in placement of ICP monitors associated with depth of sedation (Table 4).
Secondary Outcome: In-Hospital Mortality
In-hospital mortality was 37%, and approximately two-thirds of patients who died had received sedatives. Specific medications were not significantly associated with in-hospital mortality. In evaluating in-hospital mortality among those receiving any sedation in the final adjusted model, there was no association between moderate (aOR: 1.10; 95%CI: 0.47–2.58) and deep (aOR: 0.92; 95CI: 0.43–1.97) sedation compared to those receiving mild sedation (Table 3). There was evidence of effect modification by the level of neurological indication for intubation between depth of sedation and in-hospital mortality (p=0.032) and the relationship between pre-hospital sedation and in-hospital mortality are presented stratified by neurological indication.. Within each stratum, however, there was no significant difference between level of sedation and in-hospital mortality (Table 3).
Discussion
During air medical transport of critically ill patients, sedation and paralysis are important interventions in providing comfort and safety for the patient, as well as safety for the air ambulance crew [16]. Sedation in the air medical environment presents unique challenges in comparison to the ED and ICU settings. Loud noise and vibration, frequent transfer of the patient (i.e. from transferring hospital to helicopter to receiving hospital), and limited space to exam the patient are just a few of the difficulties when evaluating and managing sedation depth of the patient. Data, though, are increasingly finding that sedation influences long-term outcomes. A recent multicenter, prospective cohort study by Fuller et. al demonstrated association between sedation practices and long-term clinical outcomes [10]. Our objective for this study was to test the hypothesis that deep sedation in the prehospital setting is associated with poor outcomes.
Our most significant finding may be the association between depth of sedation and our primary outcome of LOS. When compared to patients who had no sedation, those receiving mild, moderate, and deep sedation experienced an increase in hospital LOS of 14%, 78%, and 51%, respectively. Additionally, among all patients who received at least one sedative medication, moderate sedation (RASS score = −3) and deep sedation (RASS score = −4) were associated with an increase in LOS of 59% and 24%, respectively. While this finding could be related to severity of illness, it could also be related to the lingering effect of prehospital medications.
In addition to the effect of depth of sedation on hospital LOS, benzodiazepines specifically were associated with an increased hospital LOS of 2.6 days. This finding was significant, albeit not surprising, as the toxicity and risks of benzodiazepines are well documented [4, 17, 18]. While benzodiazepines are certainly warranted in certain emergency situations, a 36% increase of time in the hospital points toward a need for caution when considering this class of drugs, especially considering the frequency of administration seen in our study. The high frequency of benzodiazepine usage (and the high variability of sedatives used in general) may be related to lack of sedation protocols in air medical programs.
While previous studies have shown an association between depth of sedation and delirium in the ICU and ED setting, our data showed no such association in the pre-hospital setting.
Additionally, no specific sedative or paralytic medications were associated with increased risk of delirium. These findings could indicate that medication selection and depth of sedation during the short time period of air medical transport plays less of a role in the development of delirium as compared to patients who stay much longer periods in the ED or ICU setting.
Interestingly, patients receiving no sedative medications had an increased risk of in-hospital death compared to those who were sedated to any depth. This is likely due to certain patients with severe neurological conditions not requiring sedation, rather than a true cause-and-effect. Furthermore, even when patients were stratified by indication for intubation (neurological vs non-neurological), there was no association between mortality and depth of sedation within each stratum. The other secondary outcomes of neurosurgery and ICP monitoring were not associated with depth of sedation or medications.
While our study focused primarily on the consequences of deep sedation, the risk of undersedation should not be ignored. Patients who are not sedated adequately are at risk for extreme discomfort and distress due to increased awareness of external and internal stimuli. This adverse reaction could lead to agitation and increased blood pressure, worsening their medical condition, especially in the situations of a severe head injury or hemorrhagic stroke. Additionally, agitation caused by undersedation places the patient at higher risk of self-extubation and removal of IVs/tubes/lines, which can be precarious in the tight quarters of air ambulances. While our study suggests risks of deep sedation, a measured approach should be taken in reducing the amount of sedatives administered.
Limitations
Our study has several limitations. Medications given at the receiving hospital (i.e. in the ED or ICU) were not factored into the final analyses due to limited resources and time, though this should be considered in future studies due to its possibility as a significant confounder. As a retrospective cohort study, our study is limited by documentation in the medical chart. We selected objective exposures (drug dosing) and outcomes (mortality, LOS) to limit the effect of ascertainment bias, but this was difficult in the assessment of delirium. Notation of delirium in patients’ charts was sporadic, which is consistent with prior reports [5]. RASS scores were not consistently noted, so our subjective assessment from the prehospital or inpatient record was the most appropriate substitute. This assessment could have under-reported delirium, however. This inconsistent RASS notation also made it challenging to account for sedation depth in the setting of neuromuscular blockade, creating the possibility of categorizing medically paralyzed patients as deeply sedated, when in fact they were not. Additionally, the three ambulance services in our study all used RASS scores exclusively to measure sedation, so there was no opportunity to use a different sedation scale (such as the RAMSAY scale). Finally, while an APACHE-II score was calculated for all patients, it was not feasible to incorporate these data into the results. Future studies would ideally further account for severity of illness [19].
Conclusions
In this study, prehospital moderate and deep sedation is associated with increased hospital LOS. Sedation was not associated with increased in-hospital mortality or incidence of delirium. Prehospital benzodiazepine administration is associated with longer hospital stays by more than 2 days. These findings point toward the risks of moderate to deep sedation, as well as benzodiazepine administration, leading to increased LOS. Our findings suggest that there is important need for further validation in other systems of sedation practices in the pre-hospital setting, especially because data from ED and inpatient cohorts may not apply to prehospital environment.
Acknowledgements
Each of the authors meets criteria for authorship and claim responsibility for the research. All authors participated in the concept and design, analysis and interpretation, drafting and revising the manuscript, and approve the submitted manuscript. No authors have conflicts of interest to report.
Prior Presentations: Oral presentation at the 2018 Great Plains Society for Academic Emergency Medicine meeting (St. Louis, Missouri); Poster presentation at the 2019 National Association of EMS Physicians meeting (Austin, Texas).
Funding Sources/Disclosures: This research was funded by the T35 HL007485/HL/NHLBI NIH HHS/United States (Medical Student Research Funding).
REFERENCES
- 1.Stephens RJ, Ablordeppey E, Drewry AM, Palmer C, Wessman BT, Mohr NM, Roberts BW, Liang SY, Kollef MH, and Fuller BM, Analgosedation Practices and the Impact of Sedation Depth on Clinical Outcomes Among Patients Requiring Mechanical Ventilation in the ED: A Cohort Study. Chest, 2017. 152(5): p. 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephens RJ, Dettmer MR, Roberts BW, Ablordeppey E, Fowler SA, Kollef MH, and Fuller BM, Practice Patterns and Outcomes Associated With Early Sedation Depth in Mechanically Ventilated Patients: A Systematic Review and Meta-Analysis. Crit Care Med, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, and Ely EW, Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. Jama, 2007. 298(22): p. 2644–53. [DOI] [PubMed] [Google Scholar]
- 4.Skrupky LP, Drewry AM, Wessman B, Field RR, Fagley RE, Varghese L, Lieu A, Olatunde J, Micek ST, Kollef MH, and Boyle WA, Clinical effectiveness of a sedation protocol minimizing benzodiazepine infusions and favoring early dexmedetomidine: a before-after study. Crit Care, 2015. 19: p. 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr., Inouye SK, Bernard GR, and Dittus RS, Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. Jama, 2004. 291(14): p. 1753–62. [DOI] [PubMed] [Google Scholar]
- 6.Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, Fang YF, Shieh MH, and Kuo HP, The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med, 2004. 32(11): p. 2254–9. [DOI] [PubMed] [Google Scholar]
- 7.Shehabi Y, Howe BD, Bellomo R, Arabi YM, Bailey M, Bass FE, Bin Kadiman S, McArthur CJ, Murray L, Reade MC, Seppelt IM, Takala J, Wise MP, and Webb SA, Early Sedation with Dexmedetomidine in Critically Ill Patients. N Engl J Med, 2019. 380(26): p. 2506–2517. [DOI] [PubMed] [Google Scholar]
- 8.Humble SS, Wilson LD, Leath TC, Marshall MD, Sun DZ, Pandharipande PP, and Patel MB, ICU sedation with dexmedetomidine after severe traumatic brain injury. Brain Inj, 2016. 30(10): p. 1266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, and Rocha MG, Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. Jama, 2009. 301(5): p. 489–99. [DOI] [PubMed] [Google Scholar]
- 10.Fuller BM, Roberts BW, Mohr NM, Knight W.A.t., Adeoye O, Pappal RD, Marshall S, Alunday R, Dettmer M, Goyal M, Gibson C, Levine BJ, Gardner-Gray JM, Mosier J, Dargin J, Mackay F, Johnson NJ, Lokhandwala S, Hough CL, Tonna JE, Tsolinas R, Lin F, Qasim ZA, Harvey CE, Bassin B, Stephens RJ, Yan Y, Carpenter CR, Kollef MH, and Avidan MS, The ED-SED Study: A Multicenter, Prospective Cohort Study of Practice Patterns and Clinical Outcomes Associated With Emergency Department SEDation for Mechanically Ventilated Patients. Crit Care Med, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Association of Air Medical Services. Fact Sheet and FAQs [cited 2019; Available from: https://aams.org/member-services/fact-sheet-faqs/.
- 12.Stoltze AJ, Wong TS, Harland KK, Ahmed A, Fuller BM, and Mohr NM, Prehospital tidal volume influences hospital tidal volume: A cohort study. J Crit Care, 2015. 30(3): p. 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, and Vandenbroucke JP, The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. International Journal of Surgery, 2014. 12(12): p. 1495–1499.25046131 [Google Scholar]
- 14.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, and Elswick RK, The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med, 2002. 166(10): p. 1338–44. [DOI] [PubMed] [Google Scholar]
- 15.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, and Inouye SK, Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med, 2001. 29(7): p. 1370–9. [DOI] [PubMed] [Google Scholar]
- 16.Chevron V, Menard JF, Richard JC, Girault C, Leroy J, and Bonmarchand G, Unplanned extubation: risk factors of development and predictive criteria for reintubation. Crit Care Med, 1998. 26(6): p. 1049–53. [DOI] [PubMed] [Google Scholar]
- 17.Olfson M, King M, and Schoenbaum M, Benzodiazepine use in the United States. JAMA Psychiatry, 2015. 72(2): p. 136–42. [DOI] [PubMed] [Google Scholar]
- 18.Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, and Takala J, Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. Jama, 2012. 307(11): p. 1151–60. [DOI] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, and Zimmerman JE, APACHE II: a severity of disease classification system. Crit Care Med, 1985. 13(10): p. 818–29. [PubMed] [Google Scholar]