Abstract
This chapter discusses the mechanisms of action of hormonal male contraception, which suppresses the hypothalamic-pituitary-testis axis. When the intratesticular concentration of testosterone is subsequently suppressed to adequately low concentrations, spermatogenesis is arrested. Androgens are a necessary hormonal male contraceptive component because they not only suppress the hypothalamic-pituitary-testis axis, but also provide the male hormone necessary to maintain peripheral androgen functions. Past studies using testosterone alone and testosterone combined with progestins demonstrated contraceptive efficacy in the female partner at rates similar to combined hormonal female methods. Newer hormonal male contraceptive formulations and the alternative routes of administration are discussed, along with potential barriers, challenges, and opportunities for hormonal male contraceptive development. Novel methods that are safe, effective, reversible, user-friendly, and coitus-independent are intrinsic to equitably meet the various needs and limitations of an increasingly diverse population.
Keywords: Androgens, Progestins, Spermatogenesis suppression, Acceptability, Male contraception emerging market
Introduction
Hormonal contraceptive pills for women have undergone numerous advances since their initial FDA approval in 1960 [1]. The original female combined hormonal contraceptive has been replaced with an array of synthetic contraceptive steroid hormone combinations with innovative routes of administration aimed at improving contraceptive compliance and efficacy [2]. However, this expansion of methods has been limited to female methods, as hormonal male contraception has been confined to clinical trials for nearly half a century. The absence of hormonal male contraceptives from the market is notable given that nearly a quarter of all contraceptive use is currently male-mediated, inclusive of condoms, vasectomy, and withdrawal (coitus interruptus) [3]. While vasectomy is one of the safest and most effective methods of contraception, it is considered permanent and data suggest its decreasing uptake [4]. Vasectomy reversal is complex, cost-prohibitive, and fertility is not guaranteed [5]. Condoms and withdrawal, while commonly used, are among the least effective methods of pregnancy prevention, with typical risk of failure within the first 12 months of use estimated at 13% and 20%, respectively, based on United States (US) population data [6]. Even among experienced users, global data show 12-month typical-use failure rates of 5% among condom users and 13% among withdrawal users [7]. A reversible hormonal male contraceptive would thus fill a gap in men’s options for family planning. Furthermore, men are increasingly interested in becoming involved—US family planning clinics noted nearly double the uptake (5%–9%) of services among men from 2003 to 2014 [8]. Consequently, men may comprise a ready market for novel, hormonal male contraceptives.
How do hormonal male contraceptives work?
Hormonal female contraceptives, comprised of progestin-only and combined estrogen and progestin, prevent pregnancy through multiple distinct pathways, dependent upon their dosing. Combined hormonal methods suppress ovulation through negative feedback onto the hypothalamus and pituitary gland. Other mechanisms of action associated with progestin activity include the thickening of cervical mucus to inhibit sperm entry into the cervix, impeding ciliary transport of the ovum, and theoretically modifying the endometrium to become less receptive to implantation. Analogously, hormonal male contraceptives suppress spermatogenesis through negative feedback by the combined contraceptive steroid hormones on the hypothalamic-pituitary axis.
Androgens and progestins
Hormonal male contraceptive-mediated sperm suppression is dependent upon exogenous steroid hormones to provide negative feedback inhibition on the hypothalamus and pituitary. This results in the suppression of the secretion of gonadotropin-releasing hormone (GnRH) and the gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) (Fig. 1). LH suppression inhibits Leydig cell function, decreasing the concentration of intratesticular testosterone beyond the critical level needed for spermatogenesis. High intratesticular testosterone concentration is required for the initiation and maintenance of spermatogenesis. The testosterone concentration achieved by exogenous testosterone for hormonal male contraceptive regimens is adequate to maintain peripheral androgen functions but is less than the high intratesticular testosterone concentrations required to stimulate spermatogenesis [9]. Testosterone binds to the androgen receptor in the Sertoli cells of the testes to stimulate spermatogenesis. The importance of intratesticular testosterone in spermatogenesis was proven in mouse models where knockouts of the androgen receptor in different cell types of the testis resulted in spermatogenic arrest [10]. In addition, the suppression of FSH results in defective Sertoli function, impairing spermatogenesis. Because decreasing intratesticular testosterone is so important to hormonal male contraceptive action, men can become symptomatically hypogonadal if circulating testosterone levels are low. Thus, current hormonal male contraceptive methods in clinical trials generally include testosterone or another androgen to provide the androgenic activity necessary for maintaining sexual function, secondary sex characteristics, and other nonreproductive organ systems including the bone, muscle, adipose tissue, and brain.
Fig. 1.
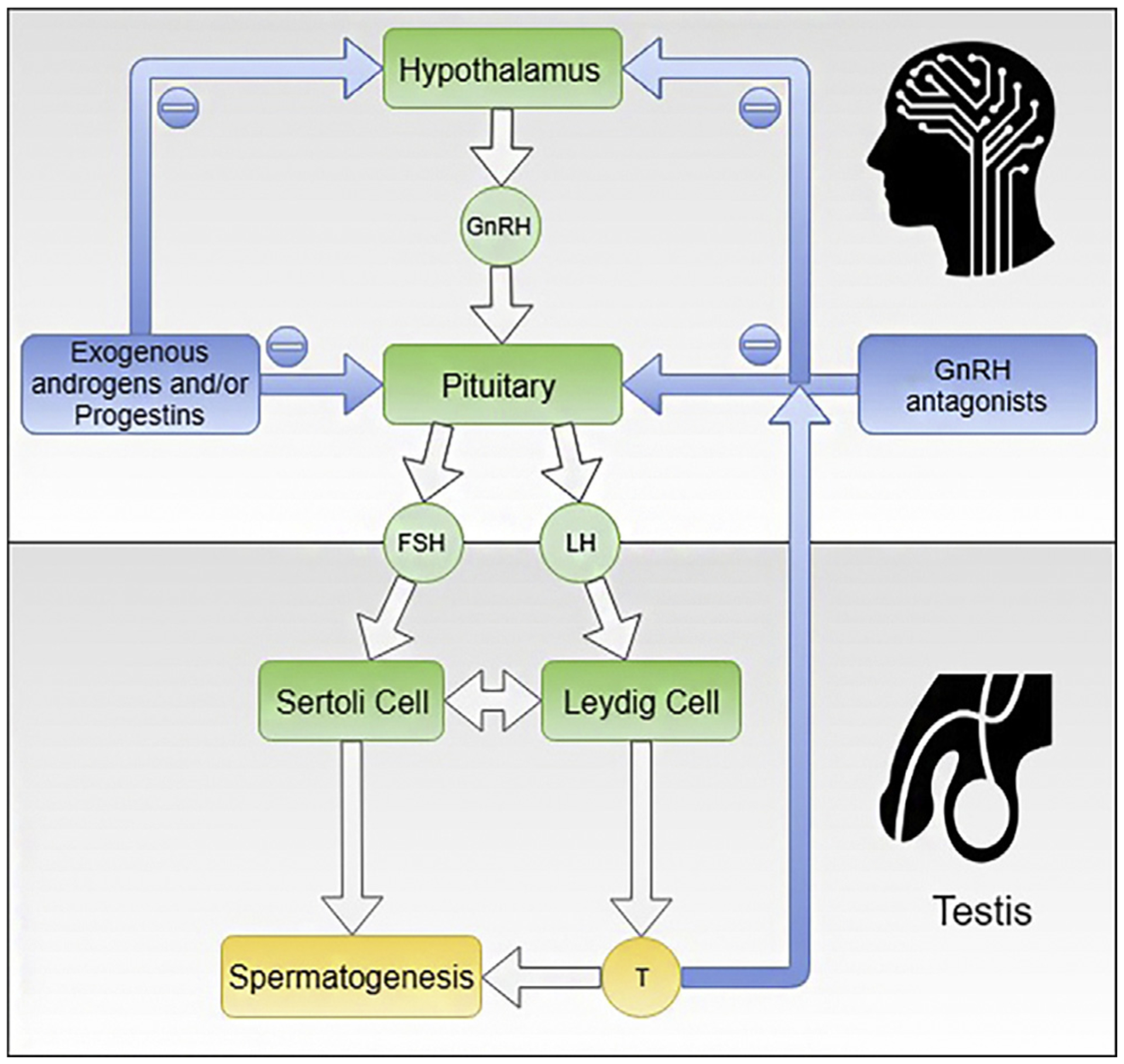
This figure shows the hypothalamic-pituitary-testis axis. Spermatogenesis is dependent on the high testosterone concentration within the testis and FSH acting on the Sertoli cells to allow the completion of qualitatively and quantitatively normal spermatogenesis. Exogenous androgens and/or progestins or Gonadotropin-Releasing Hormone (GnRH) antagonists suppress the production of LH and FSH leading to low intratesticular testosterone and low Sertoli function resulting in the suppression of spermatogenesis. Androgen action in the men is sustained by the exogenously administered testosterone or another androgen. This is the basis of hormonal male contraception.
GnRH analogs
GnRH agonists cause an initial release of gonadotropins, followed by downregulation and the suppression of LH and FSH, which results in decreased intratesticular testosterone and spermatogenesis suppression. However, trials adding GnRH agonists to paradoxically suppress testosterone release from the Leydig cells were not reliably effective in suppressing spermatogenesis to adequate levels for contraception [11–13]. In contrast, GnRH antagonists rapidly inhibit the production and secretion of LH and FSH, and are effective in suppressing sperm output. Unfortunately, GnRH antagonists are short acting and have to be administered daily or every 2 weeks subcutaneously; they can cause a local skin reaction, and are expensive to produce [14–18]. Longer acting or orally bioavailable GNRH antagonists have not been further developed for hormonal male contraception.
What sperm concentration threshold will prevent pregnancy in couples? Will this threshold be reached in men?
The threshold concentration of sperm whereby the risk of pregnancy is considered equivalent to that of couples using female hormonal contraceptives was determined in early hormonal male contraceptive efficacy trials sponsored by the World Health Organization. In these contraceptive efficacy clinical trials, couples were recruited and male partners received weekly injections of a synthetic testosterone ester (testosterone enanthate, TE). Male partners were followed with serial semen analyses until they demonstrated azoospermia or oligozoospermia, defined as a sperm concentration < 5 million/mL. Couples then relied on the experimental hormonal male contraceptive exclusively for pregnancy prevention. The contraceptive threshold for sperm concentration was lowered to 3 million/mL during the study to further decrease the risk of pregnancy. After nearly 50 person-years, four pregnancies resulted among couples where the male partner had oligozoospermia (0.1–3.0 million/mL). No pregnancies occurred among couples where the male partner was consistently azoospermic in 230 person-years of exposure to the risk of pregnancy. For oligozoospermic men, the extrapolated pregnancy rate was 1.4 per 100 person-years (95% CI: 0.4 to 3.7) [19,20]. At the 10th Summit meeting on hormonal male contraception in 2006, the threshold was lowered further to ≤1 million/mL [21], giving a pregnancy rate of 0.7 per 100 person-years [20], which is equivalent to the most effective methods of reversible female hormonal contraception [2,22]. This threshold (<1 million/mL) has since been used as a surrogate of contraceptive efficacy and for entry into the efficacy phase of hormonal male contraceptive studies with acceptable contractive failure rates of about 2% [23–26].
While hormonal female contraceptives are reliable within a week of starting them during any day of a woman’s menstrual cycle, suppressing spermatogenesis can take approximately 70–75 days in men [27]. Thus, the assessment of sperm output in male contraceptive trials have a minimum of three months but preferably six months of exposure to the medications to allow for two complete spermatogenic cycles. It is not anticipated that many participants would demonstrate the suppression of spermatogenesis to very low levels within 4 weeks. In other hormonal male contraceptive clinical trials using a combination of testosterone and a progestin, a small proportion of men showed early sperm output suppression by 28 days [23,26,28,29]. But the majority of men reached severe oligozoospermia by 12 weeks of treatment. Thus, for a male contraceptive to be fully effective, it is anticipated there will be a delay of about 12 weeks. This waiting period of about 8–16 weeks is not different from the waiting period of about the same duration after vas occlusion [30].
Is there an emerging market for hormonal male contraceptives?
The barriers to a marketable hormonal male contraceptive method are both socioeconomic and biological/physiological. Neither are insurmountable. From a social standpoint, the early development of contraceptives was aimed at female users, given its expected impact on the enormous physical and social burdens of unintended pregnancy. Early successes and perceived demand prompted an explosion of research into understanding reproductive endocrinology. With research and product development biased favoring females, gynecology became a well-recognized, influential field, with andrology only later becoming a distinct subspecialty for urologists and endocrinologists. This sex-based imbalance in research aimed at understanding and manipulating the reproductive system, created a greater barrier for male contraceptive research with the perception that hormonal male contraceptive might be more challenging to develop [31]. In reality, hormonal compounds with the ability to reversibly suppress spermatogenesis had been described in 1939 [32], well before the FDA’s approval of the first oral hormonal contraceptive pill for women in 1960. However, the disproportionate efforts to develop and distribute contraceptive methods for women rather than men could be justified by their direct impact on women’s rates of unintended pregnancy and their inherent morbidities. With hormonal female contraceptive, the health-related benefits from the avoidance of unintended pregnancy, well-outweighed their off-target risks (e.g., thromboembolic disease). As a hormonal male contraceptive would only pose physical risk to male users without any obvious health benefits, their development over methods for women was not prioritized by the industry such that in the early 2000s, all major pharmaceutical companies (e.g., Schering AG, Wyeth, and Organon) that had been actively involved in male contraceptive research and development discontinued their programs owing partly to merging with larger pharmaceutical companies with other priorities [33]. Pharmaceutical support for male contraceptive development declined in spite of population surveys indicating men’s willingness to use novel male contraceptives [34]. Nevertheless, acceptability surveys of the male contraceptive market show that men across the globe would be willing to use male contraception if available [34–36] and that women would trust male partners to use them [37]. Ongoing support for male contraceptive research and development is supported by the National Institute for Child Health and Human Development (NICHD) in the US and nonprofit, nongovernmental organizations, such as the Male Contraceptive Initiative [38]. Furthermore, as gender equity is becoming a global priority, nonprofits and nongovernment organizations are setting expectations for the inclusion of men and male partners such that the demand for male contraception is expected to rise [39]. For example, when Planned Parenthood surveyed its clients in 2016 about what the organization should consider prioritizing in its research portfolio, male contraception was one of the highest areas of reported interest1. Additionally, online campaigns where male hormonal contraception researchers dialogued with an online audience using the popular platform, Reddit, were one of the most well-attended and engaged events of the site in 2018 and 20192. Should interest and demonstrated efficacy and safety continue to rise, male hormonal contraception may not be as elusive as previously purported.
Can lessons learned from past hormonal male contraceptive trials guide the development of new approaches?
Androgens
In the 1970s, the National Institutes of Health (NIH) supported trials of weekly intramuscular TE [40–42] that reversibly suppressed gonadotropins were well tolerated in men. Many others followed that confirmed androgens reversibly suppressed sperm production [43,44] (Table 1). In 1990, two studies undertaken by the World Health Organization showed that this suppression of spermatogenesis by exogenous weekly TE injections was uniformly reversible, and that for men who suppressed sperm concentrations to <3 million/mL including many men with azoospermia, pregnancy rates were comparable to that of female oral contraceptives [19,20]. The administration of testosterone alone efficiently suppresses spermatogenesis in men to sufficiently low-sperm concentration in over 95% of men with more Asian men attaining azoospermia than non-Asian men in these studies. This was followed by late phase 2 and 3 studies in China, where testosterone undecanoate was administered once every month confirming the contraceptive efficacy of androgen alone regimens [24,25]. Because serum testosterone was in the supra-physiological range, adverse events referable to testosterone (acne, weight gain, increase in hematocrit, and decrease in HDL-cholesterol) were reported [45]. Moreover, the average time of attaining the suppression of sperm concentration to <1 million/ml (a threshold compatible with the prevention of pregnancy in the partner) was about 11–12 weeks and there may be sperm rebound [25]. Lessons learned from testosterone alone clinical trials for male hormonal concentration include: (1) Reducing the testosterone dose may decrease adverse events, (2) Adding another gonadotropin-suppressing agent may reduce the time for the suppression of sperm output sufficient to confer efficacy and prevent sperm rebound, (3) Oral testosterone preparations currently available do not last for 24 h, and (4) Long-acting intramuscular injection may have less adverse events than shorter acting injection.
Table 1.
Androgens and progestins tested in clinical trials.
| Androgens | Progestins |
|---|---|
| Oral — testosterone undecanoate, dimethandrolone undecanoatea, 11beta-Methyl-19-Nortestosterone-17beta-Dodecylcarbonatea | Oral — levonorgestrel, desogestrel, cyproterone acetate |
| Transdermal- testosterone gela, patch, cream | Transdermal — nestorone gela |
| Injection — testosterone enanthate, testosterone undecanoate, testosterone decanoate, 19-nortestosterone-hexoxyphenylpropionate, dimethandrolone undecanoatea | Injection — depot medroxyprogesterone acetate, norethisterone enanthate |
| Implant — testosterone, 7 alpha methyl-19 nor-testosterone (MENT) | Implant — levonorgestrel, etonogestrel |
Currently in clinical trials.
Androgens/progestin combinations
Multiple testosterone-progestin combinations (Table 1) have been shown to be safe and effective in suppressing spermatogenesis in shorter term studies. Analyses of data generated from over 1700 men participating in hormonal male contraceptive trials showed that the addition of a progestin to the androgens enhanced the suppression of the hypothalamic-pituitary-testis action resulting in faster and more profound suppression of spermatogenesis in men [46] and may reduce the dose of androgen required. However, the combined use of androgens plus progestins for male hormonal contraception increases the complexity as the pharmacokinetic and pharmacodynamic actions of the progestin may not match that of the androgen, and progestins may have additional potential adverse effects [43,47,48].
A small contraceptive efficacy study utilized testosterone implants with depot medroxyprogesterone acetate injections in men. There was no pregnancy in the female partner after an exposure to pregnancy risks of 35.5 person years [23]. The discontinuation rate was high partly related to symptomatic androgen insufficiency and sperm rebound that required a protocol change to reduce the interval between the insertions of testosterone implants from every 6 to 4 months. In a Phase II multicenter study of intramuscular testosterone undecanoate and norethisterone enanthate injections every 8 weeks (an androgenic progestin), 320 participants entered the suppression phase [49] and 95.9% of continuing users suppressed their sperm concentration to under 1 million/mL by 24 weeks. In all, 266 participants entered the efficacy phase, 4 pregnancies occurred during the efficacy phase, with a pregnancy rate of 1.57 per 100 continuing users and the calculated Pearl index was 2.18 pregnancies per 100 person-years. All pregnancies occurred before week 16 of the efficacy phase. This study was terminated early because of high adverse event rates and frequent complaints of mood disorders. Though most complaints were mild, there were reports of moderate and severe symptoms at several sites including one suicide, one suicide attempt, and a third serious adverse event of depression (Behre et al., 2016). Other side effects occurring in over 10% of participants included acne, injection site pain, increased libido, and myalgia. It is important to note that depression was not specifically tracked on enrollment into the study, and not prospectively assessed. Despite these concerns and despite the premature trial termination, approximately 80% of male participants and their female partners reported satisfaction with the method and would consider using it in the future. In a previously reported doubleblind, placebo-controlled clinical trial using combinations of testosterone undecanoate and etonogestrel implants with sperm output as the primary endpoint, weight gain, mood changes, acne, sweating, and libido change were reported more frequently in the active treatment than the placebo group [50]. Because in contraceptive efficacy trials pregnancy in the partner is the endpoint, a placebo arm cannot be ethically justified. The association of hormonal male contraceptives with any new-onset or worsening mood-related and sexual disorders will require prospective defined monitoring and assessment during the trial. These studies demonstrate that the inclusion of a placebo group in earlier phase studies will help to delineate drug-related adverse events and that prospective monitoring before, during, and after the trial is essential to determine causality of adverse events, such as mood disorders and sexual dysfunction. Furthermore, the balance between adequate androgen and progestin concentration should be optimized by dose-finding studies during hormonal male contraceptive development.
Can biological barriers to male contraception be overcome?
Achieve adequate suppression of spermatogenesis
Biological barriers to the release of a marketable male contraceptive are dependent on several characteristics: the consistent suppression of spermatogenesis providing contraception, contraceptive latency, reversibility, and off-target effects and/or potential risks. The doses of androgen required for suppressing spermatogenesis adequately were also supraphysiological, which may increase the risk of adverse events [45]. These limitations led to the recognition that male hormonal contraception with androgens alone may need to include another agent with gonadotropin suppressive activities such as a progestin to more consistently and completely suppress gonadotropins. In more recent trials of combined androgen and progestin methods [26,29,50], complete suppression of the hypothalamicpituitary-testicular axis and spermatogenesis still required an average of 2–3 months of continuing use. In men, spermatogenesis takes approximately 72 days for an immature spermatogonia to develop into the mature spermatozoa [27]. Spermatozoa then require an additional 10–14 days to be released into the reproductive tract and appear in the ejaculate. Current research includes the investigation into post-testicular methods that will shorten the latency periods to be comparable to those seen in women’s methods—women who initiate hormonal methods need only wait 7 days for contraceptive protection. As this latency period is a biological constant, novel compounds that have actions on spermiogenesis, spermiation, or in the epididymis may lead to shorter waiting time. It should be noted that vasectomy requires a postsurgery waiting period of about 8–16 weeks to guarantee azoospermia. While hormonal methods for men are expected to be completely reversible, recovery from complete sperm suppression is not immediate and can require at least three months for more than half of users, based on an integrated analysis of past male hormonal contraception clinical trials [51]. In clinical trials, semen analyses are a prerequisite for monitoring sperm suppression, “dipstick” [52] and “computerized imaging” [53] are being developed as home self-monitoring kits. These new methods have to be modified and validated to be able to detect accurately the sperm concentration near 1 million/ml for use in hormonal male contraception.
Lower the potential adverse effects of androgens and progestins in men
Androgens are known to stimulate erythropoiesis and increase hemoglobin and hematocrit in men. Adjusting the dose of androgens to result in little or no change of the red cell indices are the goals of more recent hormonal male contraceptive clinical trials [29,54]. In some male hormonal contraception clinical trials weight gain has been reported, but it has not been determined if this is related to the androgen or the progestin or both, and it is not known whether the increase in weight is due to increases in lean mass, fat mass, or water retention. Decreases in HDL cholesterol are anticipated, as it is a known effect of androgens. Oral androgens may lead to more changes in the lipoproteins that may affect HDL or LDL-cholesterol concentrations because of the first pass effect in the liver of about 20% [55,56]. Further studies are required to determine what effects nonaromatizable androgens will have on changes in HDL- and LDL cholesterol [57], and the impact of different classes of progestins on lipid levels. For hormonal combinations that incorporate more androgenic progestins, such as oral levonorgestrel, dose-dependent decreases in HDL cholesterol and weight gain have been observed, without significant changes in LDL-cholesterol levels [58]. In another study, when a transdermal testosterone gel was combined with a potent progestin gel (Nestorone), a smaller decrease in HDL-cholesterol levels were noted compared to a comparable dose of testosterone alone [29]. In a different study paradigm, men who were given various progestins alone for two weeks exhibited decreases in their HDL-cholesterol levels, which were ameliorated with the addition of testosterone (Zitzmann 2017). Thus, while progestins may improve the efficacy of these male hormonal contraception formulations, they may also impact user lipid profiles varying with the delivery methods: a pill, gel, injection, or implant. The clinical significance of these metabolic changes associated with male hormonal contraception use should be studied in long-term clinical trials with targeted, prospective assessments of changes from baseline during use and reversion to baseline when men discontinue using the study drug. The impact of lipid changes on possible cardiovascular disease risks cannot be assessed until we have a hormonal male contraceptive on the market and large scale longer term epidemiological studies become possible.
Monitor sexual function and mood changes prospectively
In some of these studies, men reported libido changes (increase/decrease), mood disorders and mood swings [23,26]. These self-reported adverse events were more frequent in the active treatment group compared to placebo in one study [50]. In most studies, the mood was not quantified and not tracked. These outcomes should be prospectively monitored at baseline and during treatment and recovery periods using a validated questionnaire of sexual function and mood. The results of the questionnaire can then be evaluated against self-reports from the participant to better understand their clinical relevance. The dosing and pharmacokinetics of combined androgen and progestin male hormonal contraception formulations need to be optimized to maintain effective spermatogenesis suppression and adequate androgen replacement, while minimizing sexual, mood, and other off-target effects. Fortunately, long-term clinical efficacy trials are underway.
What are the next steps?
To ensure that men will use hormonal male contraceptives, formulations need to be convenient, effective, reversible, and affordable. Consequently, trials of novel androgens and combinations of androgens/progestins are ongoing, supported by NICHD, NIH funding.
Transdermal testosterone and nestorone
Testosterone and nestorone are absorbed and formed a reservoir under the skin that releases the steroid hormones gradually into the circulation resulting in a steady concentration of the hormones in the blood. Unlike oral drugs, gels are not affected by first-pass metabolism by the liver, and thus they may have a different effect on proteins and lipoproteins made by the liver.
The development of the testosterone/nestorone combined gel included: (1) A dose finding, first-in-man study of nestorone in men [59], (2) A six-month study showing that the selected nestorone dose with a standard dose of testosterone used for testosterone replacement in hypogonadal men effectively suppressed spermatogenesis [29], (3) A pharmacokinetics study of the combined gel [60], and (4) A study to determine transfer of the hormones to the female partner under an experimental paradigm [61]. Following these studies, a Phase IIb trial of a self-applied, nestorone/testosterone combination transdermal gel started at the end of 2018 (NCT03452111). The primary endpoint is the cumulative pregnancy rate in 420 couples. This study is currently recruiting couples in centers in North and South America, Europe and Africa.
Novel androgens
Researchers are working on creating a formulation that can be taken orally. Oral testosterone is rapidly metabolized by the liver (first-pass effect). Preparations of testosterone such as methyl testosterone carry concern for hepatotoxicity. Oral testosterone undecanoate is in use in other countries and a new formulation has just been approved for use in the US as of 2019. However, its usability may be limited for male contraception by a twice-daily dosing schedule.
Novel androgens with progestational activity have been found to be safe and well tolerated in Phase I trials [62,63]. These compounds allow the development of compounds with activities on both the androgen and progesterone receptors as a single agent hormonal male contraceptive. 7 α -methyl-19-nortestosterone (MENT) implants have been examined in men [64]. Dimethandrolone undecanoate (7α, 11β-dimethyl-19-nortestosterone) and 11β-methyl-19-nortestosterone (11β-MNTDC), both derivatives of 19-nortestosterone with androgenic and progestational activity that are bioavailable and active after oral administration, have been studied in animals for their effects on spermatogenesis suppression and to examine their safety profile [65–67]. Phase I clinical studies of these oral androgens have been completed showing that serum gonadotropins and testosterone are rapidly and markedly decreased, while the androgen effects are maintained [62,63,68,69]. Adverse events seen in short-term studies with these oral androgens with progestational activity include dose-dependent weight gain, decrease in HDL cholesterol, and changes in mood. Longer studies are ongoing to demonstrate that these novel compounds suppress spermatogenesis as effectively as the combination of separate androgens and progestins.
An injectable formulation for intramuscular injections is also being tested in Phase I studies of these novel androgens with progestin activity.
Summary
Hormonal male contraceptives have a sound mechanism of action and have been proven in clinical trials to effectively prevent pregnancy. Currently, hormonal male contraceptive development efforts aim at combining androgen and progestins to suppress spermatogenesis reversibly. The adverse events are usually mild and reversible upon the discontinuation of medications; further dose optimization of these steroids is necessary before conducting large clinical trials.
Surveys conducted in men and women indicate that at least 50% of the men would try a new male contraceptive method and that their female partner would trust them in using the method consistently. Continued cultural shift that engages men in family planning and reproductive responsibility seems likely. Industries should recognize that innovation in male contraception will supplement or synergize with women’s use of female contraceptives; they would not be expected to be less likely to use female-controlled methods. As men and women focus on their education and career-related pathways and delay childbearing, both groups will need contraceptive methods and will have independent reasons to use them. Continued empowerment and equitable treatment of women may create greater expectations for men to participate in family planning, further expanding the market for male contraception. Developing new therapeutic compounds is also critical. The pharmacokinetic and pharmacodynamic effects of contraceptive steroid hormone is challenging to study. The ratio of activity of the administered hormones and their conversion to effector hormones need to be considered in new drug development. The development of a wide range of safe and effective medications allow for the selection of compounds that minimize off-target effects while providing the end goal of reliable, safe, and effective contraception. The lessons learned from the development of male hormonal methods may guide the pathway for nonhormonal compounds with specific targets allowing for a wider range of safe, reversible, and effective fertility regulation in men.
Practice points.
Current male contraceptive methods include withdrawal and condoms, which are dependent on the time of sexual intercourse or vasectomy, which is considered irreversible
There is a demand for a safe, efficacious, reversible, affordable contraceptive for men
The male hormonal methods are in late phase clinical trials and closest to reaching the market
Research agenda.
Optimization of dose of androgen/progestin combination to maximize effective suppression of spermatogenesis and to minimize adverse effects.
Determination of the adequacy of androgen action when serum testosterone levels are suppressed as with the novel androgens.
Assess the landscape, prepare and test the market for male hormonal contraception in the form of a pill, gel, injection or implant.
Development of androgen and progesterone receptor modulators with specific actions specifically on the production and secretion of gonadotropins
Declaration of Competing Interest
The authors received support for clinical trials under the Contraceptive Clinical Trials Network, Eunice Shriver National Institute of Child Health and Human Development (HHSN275201300024I), National Institutes of Health, Bethesda, USA. Dr. Yuen is a recipient of a F32HD097932 fellowship award from Eunice Shriver National Institute of Child Health and Human Development.
Footnotes
References
- [1].De Leo V, Musacchio MC, Cappelli V, Piomboni P, Morgante G. Hormonal contraceptives: pharmacology tailored to women’s health. Hum Reprod Update 2016;22(5):634–46. [DOI] [PubMed] [Google Scholar]
- *[2].Trussell J Contraceptive failure in the United States. Contraception 2011;83(5):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ross J, Hardee K. Use of male methods of contraception worldwide. J Biosoc Sci 2017;49(5):648–63. [DOI] [PubMed] [Google Scholar]
- [4].Ostrowski KA, Holt SK, Haynes B, Davies BJ, Fuchs EF, Walsh TJ. Evaluation of vasectomy trends in the United States. Urology 2018;118:76–9. [DOI] [PubMed] [Google Scholar]
- [5].Herrel LA, Goodman M, Goldstein M, Hsiao W. Outcomes of microsurgical vasovasostomy for vasectomy reversal: a meta-analysis and systematic review. Urology 2015;85(4):819–25. [DOI] [PubMed] [Google Scholar]
- [6].Sundaram A, Vaughan B, Kost K, Bankole A, Finer L, Singh S, et al. Contraceptive Failure in the United States: estimates from the 2006–2010 national survey of family growth. Perspect Sex Reprod Health 2017;49(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Polis CB, Bradley SE, Bankole A, Onda T, Croft T, Singh S. Typical-use contraceptive failure rates in 43 countries with Demographic and Health Survey data: summary of a detailed report. Contraception 2016;94(1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Besera G, Moskosky S, Pazol K, Fowler C, Warner L, Johnson DM, et al. Male attendance at title X family planning clinics - United States, 2003–2014. MMWR Morb Mortal Wkly Rep 2016;65(23):602–5. [DOI] [PubMed] [Google Scholar]
- [9].Coviello AD, Bremner WJ, Matsumoto AM, Herbst KL, Amory JK, Anawalt BD, et al. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J Androl 2004;25(6):931–8. [DOI] [PubMed] [Google Scholar]
- [10].Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev 2009;30(2):119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bhasin S, Heber D, Steiner B, Peterson M, Blaisch B, Campfield LA, et al. Hormonal effects of GnRH agonist in the human male: II. Testosterone enhances gonadotrophin suppression induced by GnRH agonist. Clin Endocrinol 1984;20(2): 119–28. [DOI] [PubMed] [Google Scholar]
- [12].Bhasin S, Heber D, Steiner BS, Handelsman DJ, Swerdloff RS. Hormonal effects of gonadotropin-releasing hormone (GnRH) agonist in the human male. III. Effects of long term combined treatment with GnRH agonist and androgen. J Clin Endocrinol Metab 1985;60(5):998–1003. [DOI] [PubMed] [Google Scholar]
- [13].Behre HM, Nashan D, Hubert W, Nieschlag E. Depot gonadotropin-releasing hormone agonist blunts the androgen-induced suppression of spermatogenesis in a clinical trial of male contraception. J Clin Endocrinol Metab 1992;74(1): 84–90. [DOI] [PubMed] [Google Scholar]
- [14].Bagatell CJ, Matsumoto AM, Christensen RB, Rivier JE, Bremner WJ. Comparison of a gonadotropin releasing-hormone antagonist plus testosterone (T) versus T alone as potential male contraceptive regimens. J Clin Endocrinol Metab 1993;77(2):427–32. [DOI] [PubMed] [Google Scholar]
- [15].Bagatell CJ, Rivier JE, Bremner WJ. Dose effects of the gonadotropin-releasing hormone antagonist, Nal-Glu, combined with testosterone enanthate on gonadotropin levels in normal men. Fertil Steril 1995;64(1):139–45. [PubMed] [Google Scholar]
- [16].Tom L, Bhasin S, Salameh W, Steiner B, Peterson M, Sokol RZ, et al. Induction of azoospermia in normal men with combined Nal-Glu gonadotropin-releasing hormone antagonist and testosterone enanthate. J Clin Endocrinol Metab 1992;75(2):476–83. [DOI] [PubMed] [Google Scholar]
- [17].Behre HM, Klein B, Steinmeyer E, McGregor GP, Voigt K, Nieschlag E. Effective suppression of luteinizing hormone and testosterone by single doses of the new gonadotropin-releasing hormone antagonist cetrorelix (SB-75) in normal men. J Clin Endocrinol Metab 1992;75(2):393–8. [DOI] [PubMed] [Google Scholar]
- [18].Behre HM, Kliesch S, Puhse G, Reissmann T, Nieschlag E. High loading and low maintenance doses of a gonadotropin-releasing hormone antagonist effectively suppress serum luteinizing hormone, follicle-stimulating hormone, and testosterone in normal men. J Clin Endocrinol Metab 1997;82(5):1403–8. [DOI] [PubMed] [Google Scholar]
- [19].World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility. Lancet 1990;336(8721):955–9. [PubMed] [Google Scholar]
- *[20].World Health Organization Task Force on the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril 1996;65(4):821–9. [PubMed] [Google Scholar]
- [21].Aaltonen P, Amory JK, Anderson RA, Behre HM, Bialy G, Blithe D, et al. 10th Summit Meeting consensus: recommendations for regulatory approval for hormonal male contraception. J Androl 2007;28(3):362–3. [DOI] [PubMed] [Google Scholar]
- [22].World Health Organization, Johns Hopkins School of Public Health. Family planning: a global handbook for providers. https://wwwfphandbookorg/; 2018. [Google Scholar]
- [23].Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI, et al. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab 2003;88(10):4659–67. [DOI] [PubMed] [Google Scholar]
- [24].Gu YQ, Wang XH, Xu D, Peng L, Cheng LF, Huang MK, et al. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab 2003;88(2):562–8. [DOI] [PubMed] [Google Scholar]
- *[25].Gu Y, Liang X, Wu W, Liu M, Song S, Cheng L, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab 2009;94(6):1910–5. [DOI] [PubMed] [Google Scholar]
- [26].Behre HM, Zitzmann M, Anderson RA, Handelsman DJ, Lestari SW, McLachlan RI, et al. Efficacy and safety of an injectable combination hormonal contraceptive for men. J Clin Endocrinol Metab 2016:jc20162141. [DOI] [PubMed] [Google Scholar]
- [27].Clermont Y Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 1972;52(1):198–236. [DOI] [PubMed] [Google Scholar]
- [28].Gu YQ, Tong JS, Ma DZ, Wang XH, Yuan D, Tang WH, et al. Male hormonal contraception: effects of injections of testosterone undecanoate and depot medroxyprogesterone acetate at eight-week intervals in Chinese men. J Clin Endocrinol Metab 2004;89(5):2254–62. [DOI] [PubMed] [Google Scholar]
- *[29].Ilani N, Roth MY, Amory JK, Swerdloff RS, Dart C, Page ST, et al. A new combination of testosterone and nestorone transdermal gels for male hormonal contraception. J Clin Endocrinol Metab 2012;97(10):3476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sharlip IM, Belker AM, Honig S, Labrecque M, Marmar JL, Ross LS, et al. AUA clinical guideline: vasectomy. 2012. https://wwwauanetorg/guidelines/vasectomy-(2012-reviewed-for-currency-2015). [DOI] [PubMed]
- [31].Oudshoorn N The Male Pill: a biography of a technology in the making. Durham, North Carolina: Duke University Press; 2003. [Google Scholar]
- [32].Heckel NJ. Production of Oligospermia in a man by the use of testosterone propionate. Proc Soc Exp Biol Med 1939;40(4): 658–9. [Google Scholar]
- *[33].Dorman E, Bishai D. Demand for male contraception. Expert Rev Pharmacoecon Outcomes Res 2012;12(5):605–13. [DOI] [PubMed] [Google Scholar]
- *[34].Heinemann K, Saad F, Wiesemes M, White S, Heinemann L. Attitudes toward male fertility control: results of a multinational survey on four continents. Hum Reprod 2005;20(2):549–56. [DOI] [PubMed] [Google Scholar]
- [35].Heinemann K, Saad F, Wiesemes M, Heinemann LA. Expectations toward a novel male fertility control method and potential user types: results of a multinational survey. J Androl 2005;26(2):155–62. [DOI] [PubMed] [Google Scholar]
- [36].Glasier A Acceptability of contraception for men: a review. Contraception 2010;82(5):453–6. [DOI] [PubMed] [Google Scholar]
- *[37].Glasier AF, Anakwe R, Everington D, Martin CW, van der Spuy Z, Cheng L, et al. Would women trust their partners to use a male pill? Hum Reprod 2000;15(3):646–9. [DOI] [PubMed] [Google Scholar]
- [38].Male contraceptive initiative. https://www.malecontraceptive.org/; Feb 18, 2019.
- [39].Wang C, Sitruk-Ware R, Serfaty D. It is time for new male contraceptives! Andrology 2016;4(5):773–5. [DOI] [PubMed] [Google Scholar]
- [40].Cunningham GR, Silverman VE, Thornby J, Kohler PO. The potential for an androgen male contraceptive. J Clin Endocrinol Metab 1979;49(4):520–6. [DOI] [PubMed] [Google Scholar]
- [41].Steinberger E, Smith KD, Rodriguez-Rigau LJ. Suppression and recovery of sperm production in men treated with testosterone enanthate for one year. A study of a possible reversible male contraceptive. In: Endocrine approach to male contraception Copenhagen. Scriptor; 1978. p. 748–60. [Google Scholar]
- [42].Swerdloff RS, Palacios A, McClure RD, Campfield LA, Brosman SA. Male contraception: clinical assessment of chronic administration of testosterone enanthate. In: Endocrine approach to male contraception Copenhagen. Scriptor; 1978. p. 731–47. [Google Scholar]
- [43].Wang C, Swerdloff RS. Hormonal approaches to male contraception. Curr Opin Urol 2010;20(6):520–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chao J, Page ST, Anderson RA. Male contraception. Best Pract Res Clin Obstet Gynaecol 2014;28(6):845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wu FC, Farley TM, Peregoudov A, Waites GM. Effects of testosterone enanthate in normal men: experience from a multicenter contraceptive efficacy study. World Health Organization Task Force on Methods for the regulation of male fertility. Fertil Steril 1996;65(3):626–36. [PubMed] [Google Scholar]
- [46].Liu PY, Swerdloff RS, Anawalt BD, Anderson RA, Bremner WJ, Elliesen J, et al. Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: an integrated analysis. J Clin Endocrinol Metab 2008; 93(5):1774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Meriggiola MC, Farley TM, Mbizvo MT. A review of androgen-progestin regimens for male contraception. J Androl 2003; 24(4):466–83. [DOI] [PubMed] [Google Scholar]
- [48].Gava G, Meriggiola MC. Update on male hormonal contraception. Therapeutic advances in endocrinology and metabolism 2019;10. 2042018819834846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[49].Behre HM, Zitzmann M, Anderson RA, Handelsman DJ, Lestari SW, McLachlan RI, et al. Efficacy and Safety of an Injectable combination hormonal contraceptive for men. J Clin Endocrinol Metab 2016;101(12):4779–88. [DOI] [PubMed] [Google Scholar]
- [50].Mommers E, Kersemaekers WM, Elliesen J, Kepers M, Apter D, Behre HM, et al. Male hormonal contraception: a double-blind, placebo-controlled study. J Clin Endocrinol Metab 2008;93(7):2572–80. [DOI] [PubMed] [Google Scholar]
- *[51].Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet 2006;367(9520):1412–20. [DOI] [PubMed] [Google Scholar]
- [52].Coppola MA, Klotz KL, Kim KA, Cho HY, Kang J, Shetty J, et al. SpermCheck Fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum Reprod 2010;25(4):853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Daloglu MU, Ozcan A. Computational imaging of sperm locomotion. Biol Reprod 2017;97(2):182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ilani N, Swerdloff RS, Wang C. Male hormonal contraception: potential risks and benefits. Rev Endocr Metab Disord 2; 12(2):107–17. [DOI] [PubMed] [Google Scholar]
- [55].Yin AY, Htun M, Swerdloff RS, Diaz-Arjonilla M, Dudley RE, Faulkner S, et al. Reexamination of pharmacokinetics of oral testosterone undecanoate in hypogonadal men with a new self-emulsifying formulation. J Androl 2012;33(2): 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jockenhovel F, Bullmann C, Schubert M, Vogel E, Reinhardt W, Reinwein D, et al. Influence of various modes of androgen substitution on serum lipids and lipoproteins in hypogonadal men. Metabolism 1999;48(5):590–6. [DOI] [PubMed] [Google Scholar]
- [57].Friedl KE, Hannan CJ Jr, Jones RE, Plymate SR. High-density lipoprotein cholesterol is not decreased if an aromatizable androgen is administered. Metabolism 1990;39(1):69–74. [DOI] [PubMed] [Google Scholar]
- [58].Anawalt BD, Bebb RA, Bremner WJ, Matsumoto AM. A lower dosage levonorgestrel and testosterone combination effectively suppresses spermatogenesis and circulating gonadotropin levels with fewer metabolic effects than higher dosage combinations. J Androl 1999;20(3):407–14. [PubMed] [Google Scholar]
- [59].Mahabadi V, Amory JK, Swerdloff RS, Bremner WJ, Page ST, Sitruk-Ware R, et al. Combined transdermal testosterone gel and the progestin nestorone suppresses serum gonadotropins in men. J Clin Endocrinol Metab 2009;94(7):2313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Anawalt BD, Roth MY, Ceponis J, Surampudi V, Amory JK, Swerdloff RS, et al. Combined nestorone-testosterone gel suppresses serum gonadotropins to concentrations associated with effective hormonal contraception in men. Andrology 2019;7(6):878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yuen F, Wu S, Thirumalai A, Swerdloff RS, Page ST, Liu PY, et al. Preventing secondary exposure to women from men applying a novel nestorone/testosterone contraceptive gel. Andrology 2019;7(2):235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[62].Wu S, Yuen F, Swerdloff RS, Pak Y, Thirumalai A, Liu PY, et al. Safety and pharmacokinetics of single-dose novel oral androgen 11beta-methyl-19-nortestosterone-17beta-dodecylcarbonate in men. J Clin Endocrinol Metab 2019;104(3):629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Thirumalai A, Ceponis J, Amory JK, Swerdloff R, Surampudi V, Liu PY, et al. Effects of 28 Days of oral dimethandrolone undecanoate in healthy men: a prototype male pill. J Clin Endocrinol Metab 2019;104(2):423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].von Eckardstein S, Noe G, Brache V, Nieschlag E, Croxatto H, Alvarez F, et al. A clinical trial of 7 alpha-methyl-19-nortestosterone implants for possible use as a long-acting contraceptive for men. J Clin Endocrinol Metab 2003;88(11): 5232–9. [DOI] [PubMed] [Google Scholar]
- [65].Attardi BJ, Engbring JA, Gropp D, Hild SA. Development of dimethandrolone 17beta-undecanoate (DMAU) as an oral male hormonal contraceptive: induction of infertility and recovery of fertility in adult male rabbits. J Androl 2011;32(5):530–40. [DOI] [PubMed] [Google Scholar]
- [66].Attardi BJ, Marck BT, Matsumoto AM, Koduri S, Hild SA. Long-term effects of dimethandrolone 17beta-undecanoate and 11beta-methyl-19-nortestosterone 17beta-dodecylcarbonate on body composition, bone mineral density, serum gonadotropins, and androgenic/anabolic activity in castrated male rats. J Androl 2011;32(2):183–92. [DOI] [PubMed] [Google Scholar]
- [67].Hild SA, Attardi BJ, Koduri S, Till BA, Reel JR. Effects of synthetic androgens on liver function using the rabbit as a model. J Androl 2010;31(5):472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Surampudi P, Page ST, Swerdloff RS, Nya-Ngatchou JJ, Liu PY, Amory JK, et al. Single, escalating dose pharmacokinetics, safety and food effects of a new oral androgen dimethandrolone undecanoate in man: a prototype oral male hormonal contraceptive. Andrology 2014;2(4):579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ayoub R, Page ST, Swerdloff RS, Liu PY, Amory JK, Leung A, et al. Comparison of the single dose pharmacokinetics, pharmacodynamics, and safety of two novel oral formulations of dimethandrolone undecanoate (DMAU): a potential oral, male contraceptive. Andrology 2017;5(2):278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


