Abstract
Purpose:
Prescribing guideline-recommended anti-emetics is an effective strategy to prevent CINV. However, the rate of guideline-concordant care is not well-understood. The purpose of this study was to describe the proportion of pediatric, adolescent, and young adult patients receiving HEC or MEC who received guideline-concordant antiemetic prophylaxis for acute CINV, and to identify potential predictors of guideline-concordant antiemetic prophylaxis.
Methods:
Using electronic health record data from 2016 through 2018, a retrospective single-institution cohort study was conducted to investigate how often patients less than 26 years of age receiving moderately or highly emetogenic chemotherapy receive guideline-concordant prophylaxis for acute CINV. Guideline-concordant care was defined according to guidelines from the Pediatric Oncology Group of Ontario for patients < 18 years and the American Society of Clinical Oncology for those ≥ 18 years. Independent variables included: sex, age, insurance status, race, ethnicity, cancer type, chemotherapy regimen, clinical setting, chemotherapy emetogenicity, and patient location. Predictors of receiving guideline-concordant care were determined using multiple logistic regression.
Results:
Of 180 eligible patients, 65 (36.1%) received guideline-concordant care. In multivariable analysis, being treated in adult oncology setting (aOR: 14.3, CI95: 5.3 – 38.6), with a cisplatin-based regimen (aOR: 3.5, CI95:1.4 – 9.0), solid tumor diagnosis (aOR: 2.2, CI95: 1.0 – 4.8), and commercial insurance (aOR: 2.4, CI95: 1.1 – 5.2) were associated with significantly higher likelihood of receiving guideline-concordant care.
Conclusions:
Multi-level factors were associated with receiving guideline concordant care for prevention of CINV in children, adolescents, and young adults receiving emetogenic chemotherapy. These findings can inform current efforts to optimize implementation strategies for supportive care guidelines
Keywords: Cancer, guideline-concordant care, implementation science, pediatric, AYA
Background
Symptom management for children, adolescents and young adults with cancer reduces adverse effects of therapy, keeps treatment on schedule by reducing delays, and improves overall patient outcomes.1 Chemotherapy, a common treatment modality for cancer, often causes nausea and vomiting, both of which significantly impact quality of life.2–4 Nausea and vomiting are also some of the most feared adverse effects of chemotherapy.2,5,6 It is therefore important to prevent and manage chemotherapy-induced nausea and vomiting (CINV) which can be achieved through administering anti-emetics according to rigorously-developed evidence-based clinical practice guidelines.
Provision of guideline-concordant care for prevention of CINV requires knowledge about treatment-specific factors. Chemotherapeutic agents, alone or in combination, have been classified based on their emetogenic potential as high, moderate, low or minimal. 7,8 Guideline recommendations based on these classifications are available for both pediatric and adult cancer patients.8–11 Although some patients receiving prophylaxis still experience CINV, studies show that administering prophylactic regimens concordant with published guidelines significantly reduces and controls symptoms for patients receiving moderately emetogenic or highly emetogenic chemotherapy (MEC; HEC).12–14
Despite rigorously-developed guidelines and availability of effective medications to prevent and treat CINV in pediatric cancer patients, the prescription of antiemetic medications in this population varies widely. One study found that 78% of sites self-reported a standardized approach to prophylaxis. However, only 41% reported that the approach was consistent with. published guidelines.15 Some reasons for not providing guideline concordant care include lack of awareness of the guideline, concerns about drug interactions (specifically neurokinin-1 receptor blockers), and contraindications to dexamethasone use due to concomitant medication concerns or adverse effects of steroids.16–18 It is also hypothesized that guidelines may be viewed as less robust in children because the data for guideline development are often extrapolated from adult data.19,20
The negative effects of inconsistent care are important, as children and adolescents with cancer may experience CINV unnecessarily or may receive medication that conveys no benefit. Indeed, for children and adolescents, the reported prevalence of both vomiting and nausea symptoms are frequently higher than that observed in adult patients. For example, a recent pooled synthesis of patient-reported symptoms in adults receiving active cancer treatment observed a prevalence of 40% for nausea and 27% for vomiting.21 In children and adolescents receiving active therapy, vomiting has been self-reported in 10 – 48% and nausea in 18 – 81%.4,22 In studies examining complete protection of CINV symptoms, in children and adolescents receiving emetogenic chemotherapy, fewer than 50% achieved complete protection from CINV symptoms.23,24
Symptom management, specifically prevention of CINV, is imperative to maximize patient outcomes in cancer treatment. Though guidelines are available for children, adolescents, and young adults with cancer, it is not currently known how often they receive guideline-concordant care and what factors are associated with receiving this care. The purpose of this study was to describe the proportion of pediatric, adolescent, and young adult patients receiving HEC or MEC who received guideline-concordant antiemetic prophylaxis for acute CINV, and to identify potential predictors of guideline-concordant antiemetic prophylaxis.
Methods
Data Source
A retrospective cohort study using the electronic health record (EHR) data of a large, urban hospital complex that includes a stand-alone children’s hospital, an adult hospital, and both pediatric and adult outpatient cancer clinics all within an NCI-designated Comprehensive Cancer Center was conducted. This study was approved by the Institutional Review Board at Columbia University; a waiver of HIPAA authorization was granted (IRB-AAAR9461)
Sample
Subjects < 26 years of age who received either MEC or HEC between January 1, 2016 and December 31, 2018 for an oncologic diagnosis were included. Emetogenicity was classified according to the 2011 Pediatric Oncology Group of Ontario (POGO) guideline7 for patients ≤ 18 years and to the American Society of Clinical Oncology (ASCO) guideline11 for patients > 18 years. For patients receiving multiple eligible regimens, only the first regimen within the specified time window was included. Patients with disease recurrence including patients undergoing stem cell transplantation were included if the episode was the first chemotherapy administration for treatment of the recurrence. Patients who received prior chemotherapy at another institution were excluded, unless this was the first treatment for a new disease recurrence.
Antiemetics
Anti-emetic administration was identified both from an EHR-generated list, and by confirmatory, manual EHR review. In the EHR, all medication administration records were assessed to ensure every drug administration was captured, even if the patient’s location within the hospital varied within the hospital. For example, if a patient received ondansetron in the outpatient setting and was admitted for chemotherapy to the inpatient setting, this was captured during EHR review and abstraction procedures.
Each patient encounter for chemotherapy administration was assessed to identify if the patient received the following classes of antiemetic: neurokinin-1 receptor blockers (NK1RAs), 5HT3 serotonin receptor antagonists (5HT3-blockers), and dexamethasone. To be considered guideline-concordant, antiemetic prophylaxis needed to: be prescribed and administered prior to the administration of chemotherapy; and include the individual agents recommended based on the patient’s age and chemotherapy regimen. For dexamethasone, any administration prior to chemotherapy was considered guideline-concordant including treatment-related regimens as well as antiemetic regimen. Antiemetic dose was not considered when adjudicating guideline- concordance. NKIRAs included fosaprepitant and aprepitant, which were the NKIRAs on the hospital formulary, and 5-HT3 serotonin receptor antagonists included ondansetron, granisetron, and palonosetron. To capture other medications prescribed for anti-emetic prophylaxis, a list was developed from a review of CINV guidelines both for prevention of acute CINV, treatment of refractory CINV, and management of anticipatory CINV.11,25,26
Clinical and demographic characteristics
Independent variables (e.g. clinical, system-level, and sociodemographic) were abstracted from the EHR as potential factors associated with receiving guideline-recommended CINV prophylaxis.4,6,22,27 Clinical factors included: primary oncologic diagnosis (i.e. leukemia, lymphoma, solid tumor, central nervous system tumor), cancer recurrence, chemotherapy regimen, emetogenicity of chemotherapy regimen, and co-morbidities at the time of chemotherapy initiation (e.g. prolonged QTc, other organ toxicity). System-level factors included: location of chemotherapy administration (inpatient or outpatient), and clinical setting (pediatric or adult oncology). Sociodemographic characteristics included: age at the time of chemotherapy administration, categorized as < 12 years or 12- < 26 years. Race and ethnicity were also collected, along with primary insurance status, which was categorized as commercial/private insurance, governmental (i.e., Medicaid/Medicare), or uninsured/self-pay. Sociodemographic characteristics were abstracted from the EHR, including a patient-completed intake form completed at the first outpatient oncology clinic visit.
Primary Outcome
The primary outcome was receipt of guideline-concordant care for the prevention of acute CINV for patients receiving HEC or MEC (binary outcome). For patients < 18 years, guideline-concordant care was defined using the POGO guideline,9 which recommends provision of a 5HT3-blocker, dexamethasone and, for patients 12 – 18 years, addition of an NK1RA in those receiving HEC. For patients receiving MEC, the guideline recommends administration of a 5HT3-blocker and dexamethasone (Figure 1).
Figure 1:
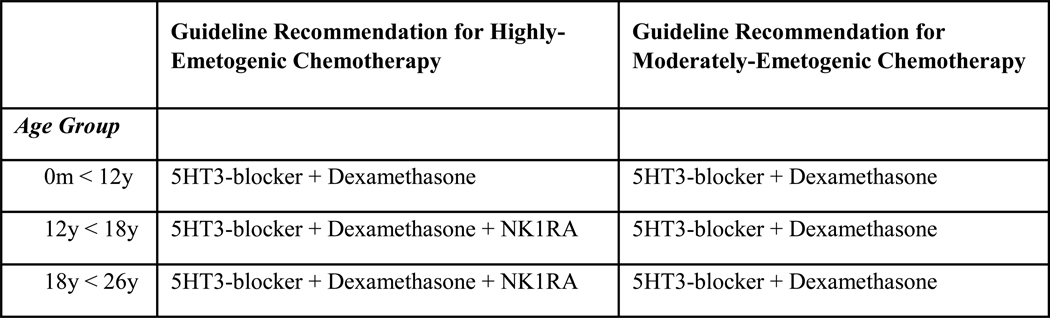
Definition of Guideline-Concordant Care by Age and Emetogenicity
For young adults 18 to < 26 years, guideline-concordant care was defined using the ASCO guideline, which recommends a 5HT3 blocker, dexamethasone and an NK1RAs for those receiving HEC.11 For patients receiving MEC, this guideline recommends administration of a 5HT3-blocker and dexamethasone (Figure 1).
Although both the pediatric and adult guidelines were updated in 2017, 8,28, we used the preceding publications (e.g. 2013 for pediatric, and 2011 for adults) given the time frame of study evaluation (2016 to 2018) and the typical lag between guideline publication and implementation.29
Secondary Outcomes
Recognizing that administration of dexamethasone and NK1RAs are associated with unique issues such as toxicities and drug interactions, we describe receipt of dexamethasone (binary outcome) and NK1RAs (binary outcome) for patients where these drugs were recommended without obvious contraindication. We also reported if patients received other medications for antiemetic prophylaxis.
Statistical Analysis
Descriptive statistics were computed to assess for the frequency of clinical, system-level, and sociodemographic characteristics as well as for the primary and secondary outcomes. Certain variables were collapsed into binary outcomes, specifically race (white, non-white), ethnicity (Hispanic, non-Hispanic), age (< 12 years, ≥ 12 years), cancer type (solid tumor, all other cancers), and chemotherapy regimen (cisplatin-based, non-cisplatin based) for bivariate and multivariable analyses. Associations between the predictors and the primary outcome, guideline- recommended anti-emetic regimen were calculated using Chi-square tests and logistic regression. Variables with a p-value < 0.1 in the bivariate analysis were included in the multivariable logistic regression model and independent variables with a p-value of < 0.05 were included in the final model. Odds ratios and 95% Wald confidence intervals were reported.
Chi-square tests were used to assess for correlation between age and provider setting, and p-values were reported. Testing for interaction was conducted using likelihood ratios (LR) test between provider setting and insurance status. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Initially, 295 patients were identified. After chart review, 115 were excluded due to not having cancer (n= 47), chemotherapy dosing not classified as HEC or MEC (n= 18), receipt of prior chemotherapy (n= 25), and duplicate patient records (n=20). Baseline characteristics of the 180 patients included in the final analyses are presented in Table 1. A slight majority (54%) were male, 41% had commercial insurance at the time of treatment, 61% were white and 63% were non-Hispanic. Most were seen by pediatric oncology providers (73%) in the inpatient setting (73%). Of those treated in the adult oncology setting, the youngest patient was 20 years at the time of treatment; of those treated in the pediatric oncology setting, the oldest patient was 24 years at the time of treatment. The most common cancers were solid tumor (41%) and leukemia (24%), and most received HEC (71%) with 19% of the sample receiving cisplatin-based regimens, 14% receiving dactinomycin and 14% receiving combination chemotherapy classified as HEC when administered concurrently (e.g. cyclophosphamide and doxorubicin). Seventy-four percent (n=133) did not have any co-morbid conditions at the time of chemotherapy administration. Of those who did, asthma (n=8), post-organ transplantation (n=8), and congenital syndromes (n=10) were the most common conditions.
Table 1:
Summary of Sample Characteristics (n=180)
| Independent Variable | N (%) |
|---|---|
| Sex | |
| Male | 98 (54.4%) |
| Female | 82 (45.6%) |
| Insurance (primary) | |
| Medicaid/Medicare | 103 (57.2%) |
| Commercial | 73 (40.6%) |
| Self-pay/non-insured | 4 (2.2%) |
| Age group | |
| 0–5M | 5 (2.8%) |
| 6M – 11Y | 67 (37.2%) |
| 12Y – 17Y | 36 (20%) |
| 18Y < 26Y | 72 (40%) |
| Race | |
| White | 110 (61.1%) |
| Black | 32 (17.8%) |
| Asian | 13 (7.2%) |
| Unknown | 25 (13.9%) |
| Ethnicity | |
| Non-Hispanic | 114 (63.3%) |
| Hispanic | 64 (35.6%) |
| Unknown | 2 (1.1%) |
| Location | |
| Inpatient | 132 (73.3%) |
| Outpatient | 48 (26.7%) |
| Provider setting | |
| Pediatric Oncology | 132 (73.3%) |
| Adult Oncology | 48 (26.7%) |
Of the 180 patients, 36% received guideline-concordant antiemetics prior to the administration of chemotherapy. In the bivariate analysis (table 2) five independent variables were significantly associated with receiving guideline-concordant care. Race was not included due to the large number of patients with “unknown” status (14%). In the multivariable model, four variables: provider type (adult oncology), primary insurance (commercial), tumor type (solid), and chemotherapy regimen (cisplatin-based), were significantly associated with receiving guideline concordant care at the 5% level (table 3). Age group was not included in the multivariable analysis as it was highly correlated with provider type (p<.0001).
Table 2:
Bivariate Analysis of Independent Variables and Outcome: Guideline Concordant Care Received
| GCC Received (n) | Not Received (n) | p-value | |
|---|---|---|---|
| Total | 65 | 115 | |
| Sex | .90 | ||
| Male | 35 | 63 | |
| Female | 30 | 52 | |
| Insurance (primary) | .02* | ||
| Non-commercial | 31 | 76 | |
| Commercial | 34 | 39 | |
| Age group: young vs. old >12y | .03* | ||
| 0–11Y | 19 | 53 | |
| 12Y < 26Y | 46 | 62 | |
| Race | .01** | ||
| White | 31 | 79 | |
| Non-White | 34 | 36 | |
| Ethnicity | .50 | ||
| Non-Hispanic | 39 | 75 | |
| Hispanic | 26 | 40 | |
| Location | .20 | ||
| Inpatient | 44 | 88 | |
| Outpatient | 21 | 27 | |
| Provider setting | <.0001* | ||
| Pediatric Oncology | 29 | 103 | |
| Adult Oncology | 36 | 12 | |
| Emetogenicity | .33 | ||
| Highly-emetogenic (HEC) | 43 | 84 | |
| Moderately-emetogenic (MEC) | 22 | 31 | |
| Chemotherapy type | <.001* | ||
| Cisplatin based therapy | 21 | 13 | |
| Non-Cisplatin based therapy | 44 | 102 | |
| Cancer type: solid vs. not | .007* | ||
| Solid tumor | 35 | 38 | |
| All others | 30 | 77 | |
| Cancer status | .91 | ||
| First occurrence | 10 | 17 | |
| Relapse | 55 | 98 |
Significant at the 5% level, included in multivariable logistic regression
Excluded from multivariable regression due to 14% “unknown” status
Table 3:
Results of Multivariable Logistic Regression
| Independent variable | Reduced Model* |
|---|---|
| Provider setting (ref: pediatric oncology) | aOR 10.9 (4.8 – 25.0, p<.0001) |
| Primary Insurance (ref: Medicaid/Medicare) | aOR 2.3 (1.1 – 4.9, p=.03) |
| Tumor type (ref: non-solid tumor) | aOR: 2.3 (1.1 – 5.0, p=.03) |
| Chemotherapy regimen (ref: non-Cisplatin-based) | aOR: 3.6 (1.4 – 9.3, p<.01) |
aOR > 1 favors guideline-concordant care
Sensitivity Analysis and Interactions
A sensitivity analysis was conducted to explore the relationship between emetogenicity and other independent variables. Bivariate analysis between emetogenicity and all independent variables revealed no significant associations with provider setting and age at the (p < .05) level. Pediatric oncology patients were not significantly more likely to receive HEC than adult oncology patients (p=.07), and younger patients < 18 years were not more likely to receive HEC than patients ≥ 18 years (p = .08). Lastly, we assessed for interaction between provider setting and insurance status using the LR test and an interaction between the two variables was not observed (p > .05).
Secondary Outcomes: Receipt of Antiemetic by Drug Class
Forty-four percent (n=80) of patients received dexamethasone, and 26% (n=46) received NK1RA. In those with solid tumors, 62% (45/73) received dexamethasone; of those with non-solid tumors, 33% (35/107) received dexamethasone. In the latter patients without a solid tumor diagnosis, 13% (14/107) received one of three recommended alternative antiemetic medications.
Receipt of NK1RA for patients receiving HEC was also examined by age group and interacting chemotherapy (i.e. cyclophosphamide, ifosfamide, anthracycline). By age, none of the patients 0 – 6-months old and 10% of patients 7-months – 11 years old received NK1RA. In patients older than 12 years and receiving HEC, 26% of patients 12 – 17 years old, and 64% of ≥ 18 years received NK1RAs. Overall, 49% (35/71) of patients ≥ 12 years received NK1RA. In patients ≥ 12 years who received HEC regimens not known to interact with NK1RAs, 63% (24/38) received NK1RA. In the 115 patients who did not receive guideline-concordant prophylaxis, 19% (n=22) received another medication for antiemetic prophylaxis (e.g. diphenhydramine, lorazepam).
Discussion
Our study found that in a cohort of patients less than 26 years of age receiving MEC or HEC, only 36% received guideline concordant anti-emetic prophylaxis. Factors associated with receiving guideline-concordant antiemetic prophylaxis included treatment in an adult oncology clinic, having a solid tumor, receiving cisplatin-based chemotherapy, and having commercial insurance.
Our observation that rates of guideline-concordant antiemetic prophylaxis are low is consistent with the limited literature. Prior studies of adult cancer patients report that 10 – 41% of patients receive guideline-recommended prophylaxis.30,31 In pediatric oncology, a multi-institutional survey conducted through the Children’s Oncology Group (COG) found that less than half of 36 participating sites had institution-specific guidelines for antiemetic prophylaxis. Of these, 28% reported that local practice was adapted from guidelines endorsed by the COG.15 Our study found that patients treated by adult oncologists were significantly more likely to receive guideline-concordant care.
Commonly-cited provider-level barriers to guideline implementation and adherence include lack of awareness, mistrust of guidelines, and barriers related to changing previous practice.32 Specifically for providers prescribing antiemetics, lack of awareness is possible both for the guideline recommendations for antiemetic prophylaxis as well as the classification of emetic potential of chemotherapy. Pediatric providers may also have a mistrust of pediatric-specific guidelines since they are often based, at least in part, on indirect evidence from studies in adults or in rigidly-defined populations.19 For example, in our study, patients with solid tumors may have received appropriate antiemetic prophylaxis more frequently due to specific issues with prescribing dexamethasone in leukemia and brain tumor patients, the most common pediatric tumors. Use of dexamethasone for CINV in these populations is limited as a result of concomitant dosing of other corticosteroids, such as prednisone which is commonly part of the anti-tumor regimen in leukemia, or to the side effect profile of corticosteroids, including behavioral, infectious, and bone-related toxicities.33–35 The 2013 pediatric guideline recommends that patients who cannot receive dexamethasone or an NK1RA36 should receive secondary medications as prophylaxis. In contrast, the adult guideline does not provide guideline for patients who are not eligible to receive dexamethasone, suggesting that concerns about concomitant medications are increased in pediatric setting. We found that a negligible number of patients in this study who may not have been able to receive NK1RAs or dexamethasone received one of the three pediatric guideline-recommended medications and less than 20% received a non-guideline-recommended medication for antiemetic prophylaxis. It is possible that providers are cautious with these medications because of potential side effects such as extrapyramidal effects and sedation.9 This supports the need for a more robust evidence-base to support the development of pediatric-specific guidelines, including rigorous testing of antiemetics specifically in pediatric patients. 19,37 Further research is needed to identify alternative antiemetic regimens when certain drug classes, specifically NK1RAs and dexamethasone cannot be used.
We also identified expected patient-level clinical factors associated with receiving guideline concordant care, specifically receiving a cisplatin-based regimen and having a solid tumor diagnosis. In CINV studies, much of the efficacy data for antiemetics comes from patients receiving cisplatin-based therapy.11 We found that patients receiving cisplatin-based regimens were significantly more likely to receive guideline concordant care, suggesting that awareness about the need for optimized prophylaxis is heightened in this population. In contrast, only 14% of patients receiving dactinomycin and 14% of patients receiving combination chemotherapy, both classified as HEC, received guideline concordant care. Future interventions that increase awareness of the classification of chemotherapy emetogenicity are therefore warranted.
Finally, we identified disparities in the receipt of guideline-concordant care based on insurance. In our cohort, primary commercial insurance was significantly associated with likelihood of receiving guideline concordant care for CINV (p=.02). This disparity in CINV management should be further explored across multiple institutions to better understand what may be driving the consideration of insurance status during antiemetic selection. A previous study of adult breast cancer patients described disparities in receipt of guideline recommended antiemetics related to race, reporting that black (vs. white) women were 11% less likely to be prescribed NK1RAs.27 It is notable that over 70% of patients in our study received chemotherapy in the inpatient setting where medications are not charged individually, but rather are bundled. It is possible, therefore, that the difference in CINV care based on insurance is a proxy for other sociodemographic disparities not identified in this single-institution study. Larger studies in multiple settings will be necessary to further explore disparities in delivering guideline-concordant care for CINV prevention and management.
Strengths and Limitations
This study has some strengths and limitations worth discussing. First, this is a single-institution study, thus limiting the generalizability of the findings. However, because of patient-level information from the EHR, the reliability of these data is strong, which is a common challenge in larger studies using administrative databases. Another limitation was the large number of patients with “unknown” race, which made it difficult to further examine the disparities observed by insurance. Capturing patient-level sociodemographic data is an ongoing challenge for many healthcare systems, and the Institute of Medicine and other governmental agencies continue to emphasize the importance of these data in healthcare disparities research, and quality measures and improvement initiatives.38,39 These and local initiatives should be encouraged to improve the accuracy of data reporting. Another limitation of this study is related to the definition of guideline-concordant care for patients who may not be eligible to receive certain classes of anti-emetics. Further research is clearly needed in this area to provide guidance on antiemetic prophylaxis in patients receiving highly- and moderately-emetogenic regimens that may preclude them from receiving certain anti-emetics to ensure optimal symptom management.
Finally, due to our limited sample size, we may have been under-powered to identify relationships between other provider, systems, or patient factors and guideline concordant care. This work would be strengthened by broadening the study to include multiple institutions. Despite these limitations, this study adds to the very limited literature and provides information on guideline concordant CINV management in children, adolescents and young adults using individual, patient-level data from a large, academic medical center.
Future implications
Multi-level implementation strategies have been cited in the literature as effective mechanisms to address patient-, provider-, and system-level barriers to guideline implementation. Current initiatives, such as an ongoing trial through the COG (NCT02847130), aim to elucidate the barriers and facilitators to implementing supportive care guidelines in pediatric cancer settings, and to improve understanding of these guidelines at the provider-level. Our study supports the need for ongoing research in this area to increase guideline uptake in pediatric and adolescent oncology. Additionally, strategies such as clinical decision support integrated into the EHR, audit and feedback, changing organizational climate, and collaborations may be useful to improve cancer care delivery across multiple settings.40,41 These approaches can help to identify patient-level clinical and sociodemographic factors and provide guideline-based decision support. Other strategies, such as enhanced education and efforts to increase awareness of quality improvement strategies that clinicians feel comfortable adopting should also be explored further.42 Certainly, a multi-level approach to increase the provision of guideline-based care is needed to address cancer symptom management.
Policy implications are also important to acknowledge; children and adolescents are frequently underrepresented in drug safety data. The U.S. Food and Drug Association acknowledges this limitation and now incentivizes pharmaceutical companies to include a pediatric-specific component of new drug applications.43,44 At present, however, there remains a paucity of generalizable pediatric-specific data.45 Federal and pharmaceutical funding should support the development of medications specifically for pediatric populations. Echoing prior literature about the importance of guideline development in pediatric oncology,19 increasing the evidence base upon which these guidelines can be developed will be critical going forward.
Conclusion
In summary, in this retrospective cohort study of children, adolescent, and young adults with cancer who received MEC or HEC, we report an overall low rate of guideline-concordant care with prophylaxis and management of CINV. Factors associated with receiving guideline-concordant care include provider specialty, chemotherapy with cisplatin-based regimen, commercial insurance, and solid tumor. Future implementation strategies should be aimed at addressing barriers to guideline-concordant care in pediatric oncology, and to examining reasons for inconsistent practices across tumor types and sociodemographic populations.
Table 3.1:
Summary of Sample Characteristics (n=180)
| Emetogenicity | |
| Highly-emetogenic (HEC) | 127 (70.6%) |
| Moderately-emetogenic (MEC) | 53 (29.4%) |
| Chemotherapy type | |
| Carboplatin | 12 (6.7%) |
| Cisplatin | 34 (18.9%) |
| Dacarbazine | 12 (6.7%) |
| Dactinomycin | 21 (11.7%) |
| Other HEC | 48 (26.7%) |
| MEC | 53 (29.4%) |
| Cancer type | |
| Solid tumor | 73 (40.6%) |
| Lymphoma | 48 (26.7%) |
| CNS | 16 (8.9%) |
| Leukemia | 43 (23.9%) |
| Cancer status | |
| First occurrence | 153 (85%) |
| Relapse | 27 (15%) |
Acknowledgments
Funding Sources
This research was made possible by the Reducing Health Disparities through Informatics (RHeaDI) training grant (T32 NR007969) funded by NINR (PI: Bakken) as well as a Doctoral Degree Scholarship in Cancer Nursing, DSCN-18–068-01, from the American Cancer Society. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or the American Cancer Society.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
None of the authors have a conflict of interest.
Dr. Beauchemin has primary control over all of the data. Due to the sensitive nature of the data (e.g. it is not deidentified), the data cannot be shared at this time. If requested, a data use agreement could be explored.
References
- 1.Multinational Association of Supportive Care in Cancer. https://www.mascc.org/about-mascc. https://www.mascc.org/about-mascc. Accessed January, 2019.
- 2.Lorusso D, Bria E, Costantini A, Di Maio M, Rosti G, Mancuso A. Patients’ perception of chemotherapy side effects: Expectations, doctor-patient communication and impact on quality of life - An Italian survey. European Journal of Cancer Care. 2017;26(2):n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 3.Baggott CR. Children’s perspectives on symptoms and health related quality of life during cancer chemotherapy, University of California, San Francisco; 2009. [Google Scholar]
- 4.Rodgers C, Kollar D, Taylor O, et al. Nausea and vomiting perspectives among children receiving moderate to highly emetogenic chemotherapy treatment. Cancer Nursing. 2012;35(3):203–210. [DOI] [PubMed] [Google Scholar]
- 5.Sun CC, Bodurka DC, Weaver CB, et al. Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2005;13(4):219–227. [DOI] [PubMed] [Google Scholar]
- 6.Baggott C, Dodd M, Kennedy C, et al. Changes in children’s reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses. 2010;27(6):307–315. [DOI] [PubMed] [Google Scholar]
- 7.Dupuis LL, Boodhan S, Sung L, et al. Guideline for the classification of the acute emetogenic potential of antineoplastic medication in pediatric cancer patients. Pediatric blood & cancer. 2011;57(2):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017:Jco2017744789. [Google Scholar]
- 9.Dupuis LL, Boodhan S, Holdsworth M, et al. Guideline for the prevention of acute nausea and vomiting due to antineoplastic medication in pediatric cancer patients. Pediatric blood & cancer. 2013;60(7):1073–1082. [DOI] [PubMed] [Google Scholar]
- 10.Hesketh PJ, Bohlke K, Lyman GH, et al. Antiemetics: American Society of Clinical Oncology Focused Guideline Update. Journal of Clinical Oncology. 2016;34(4):381–386. [DOI] [PubMed] [Google Scholar]
- 11.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(31):4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flank J, Dupuis LL. Comparative effectiveness research in antineoplastic-induced nausea and vomiting control in children. Journal of comparative effectiveness research. 2014;3(2):185–196. [DOI] [PubMed] [Google Scholar]
- 13.Gilmore JW, Peacock NW, Gu A, et al. Antiemetic Guideline Consistency and Incidence of Chemotherapy-Induced Nausea and Vomiting in US Community Oncology Practice: INSPIRE Study. 2014;10(1):68–74. [DOI] [PubMed] [Google Scholar]
- 14.Aapro M, Molassiotis A, Dicato M, et al. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Annals of oncology : official journal of the European Society for Medical Oncology. 2012;23(8):1986–1992. [DOI] [PubMed] [Google Scholar]
- 15.Patel P, Robinson PD, Orsey A, et al. Chemotherapy-Induced Nausea and Vomiting Prophylaxis: Practice Within the Children’s Oncology Group. Pediatric blood & cancer. 2016;63(5):887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stacey D, Carley M, Ballantyne B, Skrutkowski M, Whynot A. Perceived factors influencing nurses’ use of evidence-informed protocols for remote cancer treatment-related symptom management: A mixed methods study. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2015;19(3):268–277. [DOI] [PubMed] [Google Scholar]
- 17.Jun J, Kovner CT, Stimpfel AW. Barriers and facilitators of nurses’ use of clinical practice guidelines: An integrative review. International journal of nursing studies. 2016;60:54–68. [DOI] [PubMed] [Google Scholar]
- 18.Van Laar ES, Desai JM, Jatoi A. Professional educational needs for chemotherapy-induced nausea and vomiting (CINV): multinational survey results from 2388 health care providers. Supportive Care in Cancer. 2015;23(1):151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeffen EA, Kremer LC, Mulder RL, et al. The importance of evidence-based supportive care practice guidelines in childhood cancer-a plea for their development and implementation. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2017;25(4):1121–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institute of Medicine. Clinical Practice Guidelines We Can Trust. 2011. [Google Scholar]
- 21.Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013;21(6):1525–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hockenberry MJ, Hooke MC, Gregurich M, McCarthy K, Sambuco G, Krull K. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncology nursing forum. 2010;37(1):E16–27. [DOI] [PubMed] [Google Scholar]
- 23.Holdsworth MT, Raisch DW, Frost J. Acute and delayed nausea and emesis control in pediatric oncology patients. Cancer. 2006;106(4):931–940. [DOI] [PubMed] [Google Scholar]
- .Flank J, Nadeem K, Moledina S, et al. Nausea and vomiting in children and adolescents receiving intrathecal methotrexate: A prospective, observational study. Pediatric blood & cancer. 2017;64(10). [DOI] [PubMed] [Google Scholar]
- 25.Ettinger DS, Armstrong DK, Barbour S, et al. Antiemesis. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10(4):456–485. [DOI] [PubMed] [Google Scholar]
- 26.Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. Journal of Clinical Oncology. 2006;24(18):2932–2947. [DOI] [PubMed] [Google Scholar]
- 27.Check D, Reeder-Hayes K, Zullig L, et al. Examining racial variation in antiemetic use and post-chemotherapy health care utilization for nausea and vomiting among breast cancer patients. Supportive Care in Cancer. 2016;24(12):4839–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel P, Robinson PD, Thackray J, et al. Guideline for the prevention of acute chemotherapy-induced nausea and vomiting in pediatric cancer patients: A focused update. Pediatric blood & cancer. 2017. [DOI] [PubMed] [Google Scholar]
- 29.Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. Journal of the Royal Society of Medicine. 2011;104(12):510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez DR, Liao KP, Giordano S, Nguyen H, Smith BD, Elting LS. Adherence to national guidelines for antiemesis prophylaxis in patients undergoing chemotherapy for lung cancer: a population- based study. Cancer. 2013;119(7):1428–1436. [DOI] [PubMed] [Google Scholar]
- 31.Check DK, Reeder-Hayes KE, Basch EM, Zullig LL, Weinberger M, Dusetzina SB. Investigating racial disparities in use of NK1 receptor antagonists to prevent chemotherapy-induced nausea and vomiting among women with breast cancer. Breast cancer research and treatment. 2016;156(2):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. Jama. 1999;282(15):1458–1465. [DOI] [PubMed] [Google Scholar]
- 33.Pound CM, Clark C, Ni A, Athale U, Lewis V, Halton JM. Corticosteroids, behavior, and quality of life in children treated for acute lymphoblastic leukemia; a multicentered trial. Journal of pediatric hematology/oncology. 2012;34(7):517–523 [DOI] [PubMed] [Google Scholar]
- 34.Roth P, Happold C, Weller M. Corticosteroid use in neuro-oncology: an update. Neuro-oncology practice. 2015;2(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupuis LL, Nathan PC. Optimizing emetic control in children receiving antineoplastic therapy. Pediatric Drugs. 2010;12(1):51–61. [DOI] [PubMed] [Google Scholar]
- 36.Patel P, Leeder JS, Piquette-Miller M, Dupuis LL. Aprepitant and fosaprepitant drug interactions: a systematic review. British journal of clinical pharmacology. 2017;83(10):2148–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruggiero A, Rizzo D, Catalano M, Coccia P, Triarico S, Attiná G. Acute chemotherapy-induced nausea and vomiting in children with cancer: Still waiting for a common consensus on treatment. The Journal of international medical research. 2018;46(6):2149–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agency for Healthcare Research and Quality. Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. https://www.ahrq.gov/research/findings/final-reports/iomracereport/reldata1.html. Published April 2018. Accessed April, 2019.
- 39.Crossing the quality chasm: A new health system for the 21st Century. [press release]. Washington, DC: The National Academies Press; 2001. [PubMed] [Google Scholar]
- 40.Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implementation science : IS. 2015;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell SA, Chambers DA. Leveraging Implementation Science to Improve Cancer Care Delivery and Patient Outcomes. Journal of oncology practice. 2017:Jop2017024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood M, Hall L, Hockenberry M, Borinstein S. Improving Adherence to Evidence-Based Guidelines for Chemotherapy-Induced Nausea and Vomiting. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses. 2015;32(4):195–200. [DOI] [PubMed] [Google Scholar]
- 43.Barone A, Casey D, McKee AE, Reaman G. Cancer drugs approved for use in children: Impact of legislative initiatives and future opportunities. Pediatric blood & cancer. 2019:e27809. [DOI] [PubMed] [Google Scholar]
- 44.Avant D, Wharton GT, Murphy D. Characteristics and Changes of Pediatric Therapeutic Trials under the Best Pharmaceuticals for Children Act. The Journal of pediatrics. 2018;192:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMahon AW, Dal Pan G. Assessing Drug Safety in Children - The Role of Real-World Data. The New England journal of medicine. 2018;378(23):2155–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]


