Abstract
Diminished response to name, a potential early marker of autism spectrum disorder (ASD), may also indicate risk for other disorders characterized by attention problems, including attention-deficit/hyperactivity disorder (ADHD). Using a familial risk design, we examined whether response to name ability at 6, 12, 18, 24, and 36 months of age differed between three 36-month outcome groups: ASD, ADHD Concerns, or a Comparison group. Persistent differences between the ASD and Comparison groups were evident beginning at 12 months; differences between the ADHD Concerns and Comparison groups were evident between 12–18 months only. Results suggest that response to name may be a general marker for ASD and ADHD risk in infancy but a specific indicator of ASD by 24-months.
Keywords: Autism spectrum disorder, attention-deficit/hyperactivity disorder, infancy, social communication, early detection
The ability to respond to one’s own name is a central skill for social communication development. Indeed, measurement of this ability is included within assessment instruments for autism spectrum disorder (ASD) risk in infants and toddlers (Bryson, Zwaigenbaum, McDermott, Rombough, & Brian, 2008) as well as diagnostic tools for ASD (Lord, Rutter, DiLavore, Gotham, & Bishop, 2012). The ability to recognize and respond to one’s name emerges within the first year of development (Mandel, Jusczyk, & Pisoni, 1995; Parise, Friederici, & Striano, 2010), and individual differences in this behavior—as early as 9 to 12 months of age—can serve as a behavioral marker for ASD (Campbell et al., 2019; Miller et al., 2017; Nadig et al., 2007).
Although the ability to respond to one’s name is considered a social communication ability, it also involves other skills including the disengagement, shifting, and re-engagement of attention (Bryson et al., 2008; Landry & Bryson, 2004; Ljungberg, Parmentier, Jones, Marsja, & Neely, 2014; Parise et al., 2010). One implication of this is that diminished response to name amongst children with ASD may in part reflect attention regulation difficulties that are also a feature of ASD (Baranek et al., 2013; Bryson et al., 2018; Jones, Gliga, Bedford, Charman, & Johnson, 2014; Landry & Bryson, 2004; Tye et al., 2014). This leaves open the possibility that failure to orient to name may be a behavioral marker not just for ASD, but also for other neurodevelopmental disorders characterized by attention difficulties.
One prevalent neurodevelopmental disorder characterized by difficulties with attention regulation is attention-deficit/hyperactivity disorder (ADHD: (Mahone, Mostofsky, Lasker, Zee, & Denckla, 2009; Pauli-Pott & Becker, 2011). Although ADHD is typically diagnosed in middle childhood, the core symptoms often emerge earlier (Bufferd, Dougherty, Carlson, Rose, & Klein, 2012; Curchack-Lichtin, Chacko, & Halperin, 2014). For instance, it is estimated that 2.4% of children from the general population meet criteria for ADHD by age 3 (Bufferd et al., 2012). In addition, children as young as 3 who display elevated symptoms of ADHD are more likely to meet diagnostic criteria for ADHD when transitioning to school (Curchack-Lichtin et al., 2014). Although most studies of ADHD focus on children preschool aged or older, there is some evidence that individual differences associated with ADHD may be apparent in infancy and toddlerhood. On parent-rated temperament measures, children at high risk for ADHD exhibit lower levels of effortful control and higher levels of negative affect and activity than low-risk children as early as 12 months of age (Auerbach et al., 2008). Moreover, there is evidence that, relative to typically developing peers, 8- to 11-year-olds with ADHD (but who have a family history of ASD) display atypical sustained attention development from 3- to 24-months of age (Miller, Iosif, Young, Hill, & Ozonoff, 2018).
Taken together, based on evidence that impaired attention regulation is a feature of both ASD (Baranek et al., 2013; Bryson et al., 2018; Jones et al., 2014; Landry & Bryson, 2004; Tye et al., 2014) and ADHD (Mahone et al., 2009; Pauli-Pott & Becker, 2011), and that attention regulation may be involved in response to name (Landry & Bryson, 2004; Ljungberg et al., 2014; Parise et al., 2010), it is plausible that deficits responding to name during infancy may serve as an early marker for both ASD and ADHD. To date, although no study has examined this directly, some have examined whether diminished likelihood of responding to name is present for other clinical samples, including children with non-ASD developmental delays (Clifford, Young, & Williamson, 2007), intellectual disability (Osterling, Dawson, & Munson, 2002), or language disorders (Gabrielsen et al., 2015). While some of these studies provide evidence that the ability to respond to name does not differ between typically developing children and those with developmental conditions other than ASD (Gabrielsen et al., 2015; Osterling et al., 2002) such findings are not consistent (Zhang et al., 2018). Notably, some data suggests that diminished response to name for some clinical conditions is apparent at a level in-between that observed among non-clinical samples and those with ASD. For instance, studies that retrospectively coded response to name from video taken at 9–12 months (Baranek, 1999) or 12–24 months of age (Clifford, Young, & Williamson, 2007) found that children with non-ASD developmental delay had greater difficulty responding to name than typically developing children, although they were still more responsive than children with ASD.
In summary, although there is evidence that response to name is an early marker for later ASD, there is the potential that diminished response to name may also be indicative of risk for other disorders characterized by attention problems, including ADHD. Studies comparing response to name ability among children with ASD and other clinical groups are important to help determine if deficiencies in this behavior early in life are specific to ASD or more general to other disorders, with implications for screening, early identification, and early differential diagnosis. The primary aim of the current study was to address this gap in research. In particular, orienting to name responses were examined in a sample enriched for outcomes of ASD and elevated ADHD symptoms (infants at risk for ASD, infants at risk for ADHD, and infants at low risk for both conditions) in order to evaluate whether diminished response to name is a specific early marker for ASD or whether it may serve as a marker of generalized atypical neurodevelopment. This sample is independent from other samples with previously published data from this task (Miller et al., 2017; Nadig et al., 2007). Consistent with the prior literature, we hypothesized that infants who developed ASD by 36 months of age would be more likely to fail to respond to name by 12 months than infants with non-clinical outcomes, with differences persisting through 36 months of age. Additionally, we hypothesized that infants with concerns for ADHD at 36 months of age (i.e., elevated symptoms based on clinical observation, parent, and—when available—teacher report) would demonstrate intermediate performance between the ASD and non-clinical comparison groups from 12–36 months of age. An additional objective was to determine prognostic values of failure to orient to name for ASD relative to a sample including children with ADHD concerns.
METHOD
Overview
This study uses data collected from a sample of infants participating in an ongoing longitudinal study of infants with a family history of ASD (ASD-risk group; n = 90), a family history of ADHD (ADHD-risk group; n = 38), or no family history of either condition (low-risk group; n =38). The primary measure of interest, the “orients to name” task, was administered as part of a larger assessment battery at visits occurring at 6, 12, 18, 24, and 36 months of age. This study was approved by the University’s Institutional Review Board and informed consent was obtained from parents before assessments. All evaluations were conducted by Masters or Ph.D.-level examiners who were unaware of the child’s familial risk group membership and without access to details of participant performance from previous visits.
Participants
The primary inclusion criterion for the ASD-risk group was status as a younger sibling of a child with ASD, with older sibling (proband) diagnosis confirmed using the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2012) and the Social Communication Questionnaire (Rutter, Bailey, & Lord, 2003). ASD-risk group exclusion criteria included birth before 32 weeks of gestation and a known genetic disorder in the proband. The primary inclusion criterion for the ADHD-risk group was status as a first-degree relative of someone with ADHD (i.e., older sibling or parent with ADHD). Proband ADHD diagnoses were confirmed with an intake screener (Diagnostic and Statistical Manual, 5th Edition checklist for ADHD), clinician diagnostic reports, or clinician documentation of treatment for ADHD. If medical records were unavailable for sibling probands, the study team conducted a diagnostic evaluation (including parent- and teacher-completed ADHD symptom rating scales and behavioral observation during cognitive testing). When records were not available for parent probands, eligibility was based on self-report of prior ADHD diagnosis and a T-score ≥ 65 on the ADHD Index from the Conners Adult ADHD Rating Scale (CAARS; Conners, Erhardt, & Sparrow, 1999), rated by partner/spouse. ADHD-risk group exclusion criteria included birth before 32-weeks’ gestation; ASD in first-, second-, or third-degree relatives; or a known genetic disorder in the proband. The primary inclusion criterion for the low-risk group was status as a younger sibling of a child with typical development. Exclusion criteria for the low-risk group were birth before 36 weeks of gestation; developmental, learning, or medical conditions in any older sibling; and ASD or ADHD in any first-, second-, or third-degree relative.
Outcome classification at 36-months.
At 36 months of age, participants were classified into one of three outcome groups: ASD, ADHD Concerns, or a Comparison group. Those classified with ASD met DSM-5 diagnostic criteria for ASD and had ADOS-2 calibrated severity scores at or above 4 (Lord et al., 2012). Classification of the ADHD Concerns outcome group was designed to capture children who exhibited clinically-relevant levels of ADHD symptoms that may reflect increased propensity for developing the full phenotype over time. In particular, those classified with ADHD Concerns had: (a) a clinical best estimate outcome of “ADHD Concerns” based on examiner observation; and (b) at least 4 DSM-5 ADHD symptoms within any one symptom category (i.e., inattention or hyperactivity-impulsivity) or at least 5 DSM-5 symptoms across symptom categories (i.e., inattentive and hyperactive-impulsive combined) across raters (examiner, parent, teacher); and (c) at least 1 symptom endorsed by a parent or teacher on the ADHD Rating Scale, Preschool Version (McGoey, DuPaul, Haley, & Shelton, 2007). The Comparison group included all participants with ADOS and ADHD symptom data at the 36-month visit who did not meet criteria for ASD or ADHD Concerns, but did not exclude children with other non-diagnosable concerns (e.g., speech-language delays). Diagnosis of ASD and completion of the DSM-5 ADHD checklist was conducted by, or under supervision of, a licensed psychologist.
The final analyzed sample included 157 participants: 26 with ASD outcomes (n = 25 ASD-risk, n = 1 ADHD-risk), 18 with ADHD Concerns outcomes (n = 8 ASD-risk, n = 9 ADHD-risk, n = 1 low-risk), and 113 with Comparison group outcomes (n = 52 ASD-risk, n = 26 ADHD-risk, n = 35 low-risk). Group characteristics, including ADHD and ASD symptom severity at 36-months, are presented in Table 1.
Table 1.
Sample characteristics.
| ASD group n = 26 | ADHD Concerns group n = 18 | Comparison group n = 113 | p-value | |
|---|---|---|---|---|
| Male sex (n, %) | 16 (62%) | 12 (67%) | 59 (52%) | ns |
| Household income ( ≤ $80 000)1 | 12 (46%)a,b | 12 (67%)b | 35 (31%)a | .03 |
| Familial risk | <.001 | |||
| ADHD risk | 1 (4%) | 9 (50%) | 26 (23%) | |
| ASD risk | 25 (96%) | 8 (44%) | 52 (46%) | |
| Low risk | 0 (0%) | 1 (6%) | 35 (31%) | |
| Mullen Scales of Early Learning T-scores2, 36 months (mean, SD) | ||||
| Visual reception | 37.6 (14.7)a | 46.3 (14.6)a | 58.8 (11.7)b | <.001 |
| Fine motor | 30.5 (11.1)a | 39.1 (12.2)a | 49.1 (11.5)b | <.001 |
| Receptive language | 34.5 (12.5)a | 44.9 (1.1)b | 50.8 (8.4)c | <.001 |
| Expressive language | 36.9 (14.2)a | 47.3 (11.3)b | 54.6 (7.5)c | <.001 |
| Early learning composite | 73.0 (20.8)a | 89.4 (17.9)b | 106.7 (15.7)c | <.001 |
| ADHD Rating Scale Total Score3, 36 months (mean, SD) | 19.7 (9.8)a | 18.3 (8.1)a | 8.9 (5.7)b | <.001 |
| ADOS calibrated total severity score (mean, SD) | 6.5 (2.1)a | 1.7(1.1)b | 1.5 (0.8)b | <.001 |
| First Visit (n, %) | .01 | |||
| 6 months | 22 (85%)a | 8 (44%)b | 68 (60%)b | |
| 12 months | 4 (15%) | 7 (39%) | 43 (38%) | |
| 18 months | 0 (0%) | 3 (17%) | 2 (2%) | |
| Missing visits (median, IQR) | 0 (1) | 1 (1) | 0 (1) | ns |
Abbreviations: ASD = autism spectrum disorder; ADHD = attention-deficit/hyperactivity disorder; SD = Standard deviation; IQR = Interquartile range.
Missing data for ADHD Concerns (n = 1), ASD (n = 2), and Comparison (n = 14) groups
Missing data for Comparison group (n = 1).
Missing data for ADHD Concerns (n = 1), ASD (n = 2), and Comparison (n = 4) groups.
p-value are for overall group differences assessed using logistic regression for sex, ethnicity, and household income (excluding decline to state/missing values), 1-way ANOVA for Mullen Scales of Early Learning, ADHD Rating Scale, and ADOS calibrated total severity scores, non-parametric Kruskal-Wallis ANOVA for first visit, and Poisson regression model for number of missing visits. Overall p-values of < .05 were followed by post-hoc pairwise group comparisons; groups with different superscript letters differ significantly after Tukey-Kramer adjustment for multiple comparisons. Fisher’s exact test was used to examine overall group differences for familial risk.
Measures
Orients to Name.
This task, adapted from the Autism Observation Scale for Infants (Bryson et al., 2008), was administered at 6, 12,18, 24, and 36 months of age. It involves an examiner calling the infant’s name in a clear voice at a normal volume on 2 occasions (a “press”) while the infant is engaged with toys and seated in the parent’s lap, at least 2 feet from (and not facing) the examiner. Each press consists of up to 2 trials. Participant behavior was scored as follows, consistent with the Autism Observation Scale for Infants scoring guidelines (Bryson et al., 2008): 0 = orients to name with eye contact on both presses, at least one of which is on the first trial; 1 = orients with eye contact at least once; or 2 = does not orient on any trial. Consistent with previous research (Miller et al., 2017), for this study we consider a score of 0 or 1 to be “passing,” and a 2 to constitute a “failure,” resulting in a dichotomous measure of the orienting response.
Measures of sample characteristics
Autism Diagnostic Observation Schedule, 2nd Ed. (ADOS-2).
The ADOS-2 is a semi-structured interaction and observation that measures symptoms of ASD. It provides a calibrated severity score with a value of 4 and above indicative of autism spectrum (Gotham, Pickles, & Lord, 2009). The ADOS-2 (Lord et al., 2012) was used for diagnostic classification purposes in probands with ASD (to verify inclusion criteria) and the participant (to determine outcome at 36 months of age).
Child & Adolescent Symptom Inventory, 5th Ed. (CASI-5; Gadow & Sprafkin, 2013).
This is a caregiver-completed checklist for symptoms of common childhood disorders defined in the DSM-5. Parents completed the ADHD section of the CASI-5 on sibling probands as part of confirming risk group classification.
Attention-Deficit/Hyperactivity Disorder Rating Scale, Preschool Version (ADHD-RS).
This parent report version of the ADHD Rating Scale has been modified for use with preschool children. It was administered at 36 months of age to determine outcome classification. McGoey et al. (2007) provide normative and reliability/validity data in preschoolers. Similar adaptations have been used in 24-month-old children (Gimpel & Kuhn, 2000). Whenever possible, an observer familiar with the child (e.g., daycare provider) also completed this rating scale.
Mullen Scales of Early Learning (Mullen, 1995).
The Mullen Scales of Early Learning is a standardized developmental test for children from birth to 68 months of age and was used to evaluate functioning with respect to four domains: visual reception, fine motor, receptive language, and expressive language. These subscales have excellent levels of internal consistency (median 0.91) and test-retest reliability (median 0.84). An overall score, the Early Learning Composite, can also be obtained.
Missing Data.
Missing response to name data were due to variation in first enrollment visit, missed visits, and skipped or incomplete administrations. All 157 infants had data available for at least 2 visits; 51% had data for all 5 visits, 42% for 4 visits, 6% for 3 visits, and 1% for 2 visits. Number of visits with missing response to name data did not significantly differ by 36-month outcome group (p = 0.12)
Statistical Analysis Plan
Longitudinal differences between outcome groups.
A generalized linear mixed effects model (McCulloch, Searle, & Neuhaus, 2008), with binomial response distribution and logit link function, was used to analyze the likelihood of response to name failure as predicted by child age at each visit and outcome group at 36 months. The model included fixed effects for outcome group (ASD, ADHD Concerns, or Comparison group) and child age in months (centered at 6 months), including linear, quadratic, and cubic effects for child age, as well as age by outcome group interactions. Following significant overall tests for the interaction between outcome group and age, we examined pairwise differences between outcome groups at each visit age. The model included a random effect for intercept to account for the within-person correlation. Hypothesis tests were 2-sided with p values of < .05 considered statistically significant.
Prognostic value.
To further illustrate the utility of failing the response to name task as an early behavioral marker for ASD or ADHD Concerns, as well as to evaluate whether failure to respond to name demonstrates predictive specificity to ASD, we calculated prognostic value estimates. Specifically, we examined estimates for sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Three sets of analyses were conducted, one for ASD vs ADHD Concerns group and Comparison group collapsed together, one for ADHD Concerns vs ASD and Comparison groups collapsed together, and one for ASD and ADHD Concerns groups collapsed together (reflecting generalized neurodevelopmental risk) vs Comparison group. Since sample size varied by visit, multiple imputations for incomplete longitudinal binary data (Yamaguchi, Misumi, & Maruo, 2018) were used for missing values, so that predictive values would be comparable across ages. We generated 100 data sets using multilevel multiple imputations with fully conditional specification with the logistic regression. Simulation studies have shown that this approach yields unbiased estimates with coverage probabilities for the confidence intervals close to the nominal level (Yamaguchi et al., 2018). Estimates were combined according to Rubin’s rules (Rubin, 1987) and 95% confidence intervals were calculated using Wilson’s method for proportions adapted for multiple imputations (Lott & Reiter, 2018). Along with calculating prognostic values for each visit separately, we also calculated prognostic values for two different patterns of failing response to name across multiple visits. In particular we examined prognostic values of (1) failing for at least one visit at 12, 18, or 24 months, and (2) failing at multiple visits prior to 36 months (i.e., failing at two or more of the visits at 6, 12, 18, and 24 months).
The generalized linear mixed effects model was implemented using the GLIMMIX Procedure in SAS 9.4 (SAS Institute, Cary, North Carolina). Multiple imputations were implemented in SAS 9.4 using the MI and MIANALYZE procedures, while predictive value estimates and 95% confidence intervals were calculated on imputed data and then pooled in R 3.6.0 (R Core Team, 2019) using the mice 3.5.0 package (van Buuren & Groothuis-Oudshoorn, 2011).
RESULTS
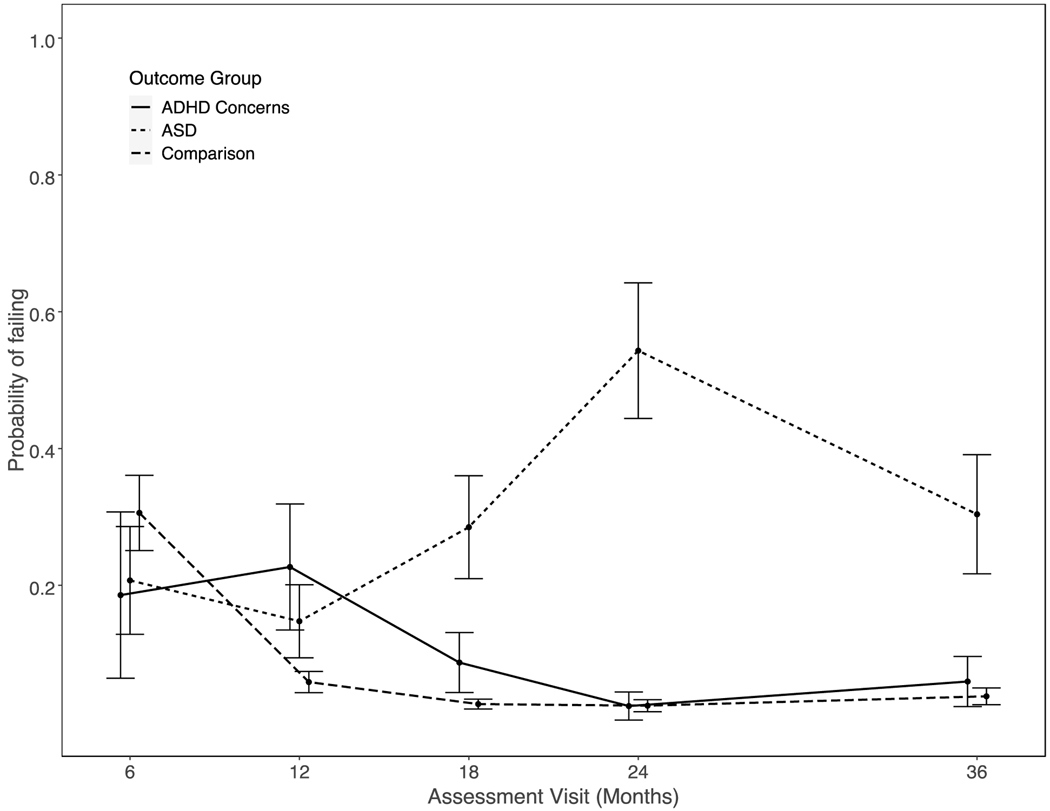
Results for the longitudinal analysis of failing to respond to name are summarized in Tables 2 and 3. The interactions of outcome group with linear age effect, χ2(2) = 3.35, p = 0.04, quadratic age effect, χ2(2) = 3.39, p = 0.03, and cubic age effect, χ2(2) = 4.16, p = 0.02, were all significant, indicating the three groups had different trajectories of failure to orient. At 6 months of age, the three groups did not differ significantly from one another. As illustrated in Figure 1, the likelihood of failing to respond to name for the ADHD Concerns and Comparison groups declined across subsequent visits. In contrast, the likelihood of failing to respond to name for the ASD group increased through to the 24-month visit. Significant differences between the ASD and Comparison groups emerged at 12 months of age (Odds Ratio [OR] = 2.78, 95% Confidence Interval [CI] = 1.02 – 7.56) and increased in magnitude through 24 months of age (OR = 48.24, 95% CI = 15.48 – 141.23), remaining different at 36 months of age (OR = 11.13, 95% CI = 3.91 – 31.68). Relative to the ASD group, children with ADHD Concerns outcomes were significantly less likely to fail the response to name task beginning at 18 months of age (OR = 0.24, 95% CI = 0.07 – 0.87) and persisting through 36 months of age (OR = 0.15, 95% CI = 0.03 – 0.66). The odds of failing to respond in the ADHD Concerns group were significantly greater than in the Comparison group at both 12 (OR = 4.71, 95% CI = 1.46 – 15.21) and 18 (OR = 3.52, 95% CI = 1.04 – 11.88) months of age, but differences between these groups were non-significant at all other ages (i.e., 6, 24, 36).
Table 2.
Results (parameter estimates and SE) of the longitudinal analyses for failing response to name.
| Model Parameter | Estimate (SE)a |
|---|---|
| Intercept | −0.8195 (0.2587)** |
| Outcome Group | |
| ADHD Concerns | −0.6590 (0.8444) |
| ASD | −0.5223 (0.5451) |
| Age (months) | −0.4417 (0.1009)*** |
| Age (months) × Outcome Group | |
| Age (months)× ADHD Concerns | 0.6605 (0.2645)* |
| Age (months) × ASD | 0.2139 (0.1767) |
| Age (months)2 | 0.0210 (0.0094)* |
| Age (months)2 × Outcome Group | |
| Age (months)2 × ADHD Concerns | −0.055 (0.0242)* |
| Age (months)2 × ASD | 0.0100 (0.0157) |
| Age (months)3 | −0.0003 (0.0002) |
| Age (months)3 × Outcome Group | |
| Age (months)3 × ADHD Concerns | 0.0012 (0.0005)* |
| Age3 (months) × ASD | −0.0005 (0.0003) |
Abbreviations: ASD = autism spectrum disorder; ADHD = attention-deficit/hyperactivity disorder; SE = Standard error.
Fixed effect estimates and standard errors on logit scale from a generalized linear mixed effects model for binary data that includes a random effect for intercept to account for within-subject clustering. Age2= Quadratic effect for age; Age3= Cubic effect for age
p < .05
p < .01
p < .001
Table 3.
Odds Ratios (95% Confidence Intervals) for 36-month outcome group contrasts from generalized linear mixed effects model of failing response to name.
| Visit Age | ADHD Concern vs ASD | ADHD Concern vs Comparison | ASD vs Comparison |
|---|---|---|---|
| 6 Months | 0.87 (0.14 – 5.49) | 0.52 (0.10 – 2.72) | 0.59 (0.20 – 1.73) |
| 12 Months | 1.70 (0.45 – 6.41) | 4.71 (1.46 – 15.21)** | 2.78 (1.02 – 7.56 )* |
| 18 Months | 0.24 (0.07 – 0.89) | 3.52 (1.04 –11.88)* | 14.71 (5.92 – 36.55)*** |
| 24 Months | 0.02 (0.00 – 0.14)*** | 0.98 (0.15 – 6.59) | 48.24 (16.48 –141.23)*** |
| 36 Months | 0.15 (0.03 – 0.66)* | 1.61 (0.38 – 6.84) | 11.13 (3.91 – 31.68)*** |
Abbreviations: ASD = autism spectrum disorder; ADHD = attention-deficit/hyperactivity disorder.
Outcome group contrasts are based on a generalized linear mixed effects model for binary data that includes a random effect for intercept to account for within-subject clustering.
p < .05
p < .01
p < .001
Figure 1.
Outcome group probability (and SE) of failing response to name task by visit age.
Prognostic values
Prognostic values are presented in Table 4, including prognostic values for each visit, as well as the prognostic values for patterns of failing over multiple visits. Overall, the sensitivity and positive predictive values, illustrate that failing response to name at 24 months was a better predictor of ASD (Sensitivity = 0.58, 95% CI: 0.36 – 0.72; PPV = 0.71, 95% CI = 0.68 – 0.75) than failing at other visits or patterns of failing across multiple visits. For the ADHD Concerns group, sensitivity and positive predictive values tended to be comparable to the ASD group at the 6- and 18-month visits, but considerably lower at the 24-month visit (Sensitivity = 0.05, 95% CI = 0.02 – 0.13; PPV =0 .00, 95% CI = 0.00 – 0.01). Thus, failing response to name at 12–18 months was predictive of both ASD and ADHD Concerns, while failing at 24 months was predictive of ASD. Specificity values (the proportion of individuals within the Comparison group who ‘passed’ the response to name task) ranged from 0.67 at 6 months to above 0.90 at all other visits. Negative predictive values fell between 0.80 and 0.91 when examined for the ASD and ADHD Concerns groups separately, and between 0.70 and 0.82 when these groups were collapsed. Sensitivity and positive predictive values tended to be lower than specificity and negative predictive values, indicating that passing the response to name task is a better marker for not having an outcome of ASD or ADHD Concerns than failing response to name is for having an outcome of ASD or ADHD Concerns.
Table 4.
Prognostic values (estimate and 95% confidence intervals) of failing response to name task for identifying 36-month outcome group.
| Visit age or pattern | ASD Sensitivity | ADHD Sensitivity | Combined ASD & ADHD Sensitivity | Comparison Specificity | ASD vs. All Others PPV | ADHD vs. All Others PPV | Combined ASD & ADHD PPV | ASD vs. All Others NPV | ADHD vs. All NPV | Combined ASD & ADHD NPV |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 months | .24 (.14 - .36) |
.27 (.11 - .49) |
.25 (.15 - .35) |
.67 (.63 - .74) |
.13 (.11 - .16) |
.10 (.07 - .13) |
.23 (.20 - .27) |
.80 (.78 - .84) |
.86 (.84 - .90) |
.70 (.67 - .74) |
| 12 months | .26 (.16 - .38) |
.30 (.14 - .46) |
.28 (.18 - .37) |
.93 (.92 - .96) |
.34 (.31 - .38) |
.25 (.23 - .30) |
.60 (.57 - .64) |
.85 (.83 - .89) |
.90 (.88 - .93) |
.77 (.74 - .81) |
| 18 months | .22 (.11 - .33) |
.13 (.05 - .24) |
.18 (.11 - .26) |
.98 (.97 - 1.00) |
.53 (.49 - .57) |
.23 (.19 - .25) |
.74 (.72 - .79) |
.85 (.82 - .88) |
.88 (.86 - .91) |
.75 (.73 - .75) |
| 24 months | .58 (.36 - .72) |
.05 (.02 - .13) |
.34 (.23 - .43) |
.95 (.94 - .97) |
.71 (.68 - .75) |
.00 (.00 - .01) |
.71 (.68 - .75) |
.91 (.89 - .94) |
.87 (.85 - .90) |
.78 (.76 - .83) |
| Multiple visits at 6–24 months | .46 (.28 - .61) |
.16 (.07 - .30) |
.33 (.22 - .43) |
.95 (.94 - .99) |
.60 (.56 - .65) |
.14 (.11 - .17) |
.73 (.71 - .78) |
.89 (.87 - .92) |
.89 (.87 - .92) |
.79 (.76 - .83) |
| At least one visit at 12–24 months | .61 (.37 - .76) |
.37 (.19 - .55) |
.51 (.61 - .35) |
.86 (.84 - .90) |
.41 (.38 - .46) |
.17 (.14 - .21) |
.58 (.55 - .63) |
.91 (.90 - .94) |
.91 (.89 - .94) |
.82 (.80 - .86) |
Abbreviations: ASD = autism spectrum disorder; ADHD = attention-deficit/hyperactivity disorder.
Estimates and confidence intervals are derived from 100 complete datasets derived using multiple imputation. Sensitivity = true positives/(true positives + false negatives); Specificity = true negatives/(true negatives + false positives); positive predictive value (PPV) = true positives/(true positives + false positives); negative predictive value (NPV) = true negatives/(true negatives + false negatives).
DISCUSSION
This study prospectively measured response to name ability of infants at elevated familial risk for ASD, elevated familial risk for ADHD, or low familial risk for both diagnoses, to examine whether longitudinal differences in this ability differed between those with 36-month outcomes of ASD, ADHD Concerns, or a Comparison group. Although familial risk designs have previously been used to examine the predictive value of infant response to name ability to later ASD in other samples (Miller et al., 2017; Nadig et al., 2007), no such prospective design has yet examined if differences in response to name ability are related to risk for ADHD. Comparing this ability among children with an outcome of ASD or ADHD concerns is important given that it involves regulation of attention (Ljungberg et al., 2014; Parise et al., 2010) which is often impacted in both ASD (Bryson et al., 2018; Jones, Gliga, Bedford, Charman, & Johnson, 2014; Tye et al., 2014) and ADHD (Mahone et al., 2009; Pauli-Pott & Becker, 2011).
Results highlight the importance of a developmental perspective when examining shared versus distinct early markers of neurodevelopmental disorders, given that outcome group differences in response to name depended upon age. Consistent with our hypotheses, differences between the ASD and Comparison groups were apparent at 12 months of age and persisted through to 36 months of age. Our hypothesis that the ADHD Concerns group would demonstrate intermediate performance between the ASD and Comparison groups was generally supported. Specifically, at 12 months of age, the likelihood of failing to respond to name in the ADHD Concerns group was greater than in the Comparison group and did not differ from the ASD group; at 18 months of age, the likelihood of failing for the ADHD Concerns group was still greater than the Comparison group but less than the ASD group; and at 24 and 36 months of age, the ADHD Concerns and Comparison groups did not differ.
There are several factors that might account for the pattern of findings for longitudinal group differences observed in this study. One possibility is that differences in the ability to respond to name might reflect differences in developmental trajectories of underlying neurocognitive functions. At birth, attention is reflexive with infants attracted to looking at basic but salient features. Over the ensuing months, typically developing infants become capable of more complex abilities: by 4 months they begin to show eye movements indicative of voluntary attention (Johnson, 1994; Richards, 2001); and from 6–12 months they begin to sustain attention on complex stimuli such as faces (Reynolds & Romano, 2016). These early developing attentional abilities are integrated with development of social cognition, (Mundy & Newell, 2007; Perra & Gattis, 2010). Within this developmental context, differences in response to name in the infant and toddler periods may reflect different underlying processes during different developmental periods. For example, one explanation of our findings might be that, at 12 months of age, response to name may serve more as a marker for shared attention problems that are characteristic of both ASD and ADHD, whereas by 24 months of age, response to name may serve more as a marker for social communication problems. Testing this hypothesis, along with alternative mechanistic explanations for differences in trajectories of response to name across infancy, will require further research beyond outcome group comparisons.
To further examine the clinical utility of failing response to name as a behavioral marker for ASD or ADHD Concerns, we also calculated prognostic values. As expected based on the results of the longitudinal analysis, the prognostic utility depended on both age and the outcome group of interest. At 12–18 months, response to name functioned better as a non-specific marker for ASD or ADHD Concerns than a specific marker, while at 24 months it was a marker specific to ASD. Although little research has examined infant screening tools for ADHD, numerous studies have examined methods of screening for ASD and have been the subject of a recent meta-analysis (Sánchez-García, Galindo-Villardón, Nieto-Librero, Martín-Rodero, & Robins, 2019). While the highest ASD sensitivity value of 0.61 (95% CI = 0.37 – 0.76) found in the current study is below the pooled meta-analysis value of 0.72 (95% CI = 0.61 – 0.81), both this study and the meta-analysis found that sensitivity values tend to be lower than specificity values. Thus, like other screening measures, response to name has somewhat greater value predicting individuals who do not have ASD than for predicting those who do.
As with all research, this study is not without limitations. The sample sizes for the clinical groups are relatively small, potentially reducing our ability to detect group differences more subtle than those identified. Larger studies should permit examining group differences in more nuanced response to name behavior (e.g., consistent response vs. inconsistent response vs. no response). Another limitation is that outcome groups, particularly the ADHD Concerns group, included children either at familial risk for ADHD or familial risk for ASD. Although approximately half of this outcome group was comprised of children at familial risk for ADHD (and not ASD), it is not yet known whether concern for ADHD in the context of familial risk for ASD is similar to concern for ADHD in the absence of such risk. Future, larger studies powered to examine the intersection of familial risk and outcome will be needed to address this question. Since PPVs and NPVs are sensitive to comparison group characteristics, some caution should be taken in generalizing the application of these prognostic values to other settings. Although the comparison group in this study did not exclude those with other non-diagnosable concerns, calculating PPVs and NPVs within a larger, representative sample would be a valuable addition. In addition, while the ADHD Concerns group was defined by elevated ADHD symptoms, which has been shown to be predictive of a later diagnosis of ADHD in previous samples (Curchack-Lichtin et al., 2014), this remains to be verified in this sample with continued longitudinal observation. Finally, consistent with previous studies (Miller et al., 2017), the present investigation scored response to name behavior as eye contact within a specific time limit, although it is plausible that alternative approaches that quantify behavior at a more nuanced level (e.g., latency to respond; Campbell et al., 2019) or incorporate measurement of psychophysiology (e.g., electroencephalogram; (Arslan et al., 2020), may allow greater detection of individual variation on response to name ability. Despite these limitations, this study is novel in including infants at familial risk for both ASD and ADHD, resulting in a sample enriched for both ASD and ADHD outcomes.
In conclusion, this study (1) uses an independent sample to replicate findings from previous studies examining response to name as an early marker for ASD (e.g., Miller et al., 2017; Nadig et al., 2007), and (2) expands upon this work by including an outcome group with ADHD concerns. By including an ADHD concerns outcome group, the study provides unique insight into early identification, demonstrating that, within a clinical context and alongside validated parent-report screeners, response to name might serve as a brief, initial observational screening measure for ASD and ADHD risk at 12 months but a more specific marker for ASD at 18 months and later. Response to name is an appealing screening measure given it can be administered relatively quickly and easily. This research illustrates the importance of testing the utility of tools for early screening of ASD multiple times across early development using clinically diverse samples characterized by potentially partially shared underlying mechanisms. To refine the utility of response to name as a screening measure, further prospective studies with representative samples might examine alternative scoring methods aimed at improving sensitivity for ASD (e.g., response latency).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- Arslan M, Warreyn P, Dewaele N, Wiersema JR, Demurie E, & Roeyers H (2020). Development of neural responses to hearing their own name in infants at low and high risk for autism spectrum disorder. Developmental Cognitive Neuroscience, 41, 100739. 10.1016/j.dcn.2019.100739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach JG, Berger A, Atzaba-Poria N, Arbelle S, Cypin N, Friedman A, & Landau R (2008). Temperament at 7, 12, and 25 months in children at familial risk for ADHD. Infant and Child Development, 17(4), 321–338. 10.1002/icd.579 [DOI] [Google Scholar]
- Baranek GT (1999). Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders, 29(3), 213–224. [DOI] [PubMed] [Google Scholar]
- Baranek Grace T., Watson LR, Boyd BA, Poe MD, David FJ, & McGuire L (2013). Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Development and Psychopathology, 25(2), 307–320. 10.1017/S0954579412001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson SE, Garon N, McMullen T, Brian J, Zwaigenbaum L, Armstrong V, … Szatmari P (2018). Impaired disengagement of attention and its relationship to emotional distress in infants at high-risk for autism spectrum disorder. Journal of Clinical and Experimental Neuropsychology, 40(5), 487–501. 10.1080/13803395.2017.1372368 [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, & Brian J (2008). The Autism Observation Scale for Infants: Scale development and reliability data. Journal of Autism and Developmental Disorders, 38(4), 731–738. 10.1007/s10803-007-0440-y [DOI] [PubMed] [Google Scholar]
- Bufferd SJ, Dougherty LR, Carlson GA, Rose S, & Klein DN (2012). Psychiatric disorders in preschoolers: Continuity from ages 3 to 6. American Journal of Psychiatry, 169(11), 1157–1164. 10.1176/appi.ajp.2012.12020268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K, Carpenter KL, Hashemi J, Espinosa S, Marsan S, Borg JS, … Dawson G (2019). Computer vision analysis captures atypical attention in toddlers with autism. Autism, 23(3), 619–628. 10.1177/1362361318766247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S, Young R, & Williamson P (2007). Assessing the early characteristics of autistic disorder using video analysis. Journal of Autism and Developmental Disorders, 37(2), 301–313. 10.1007/s10803-006-0160-8 [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, & Sparrow E (1999). CAARS. Adult ADHD Rating Scales. Technical manual. Toronto, Ontario, Canada: Multi-Health Systems. [Google Scholar]
- Curchack-Lichtin JT, Chacko A, & Halperin JM (2014). Changes in ADHD symptom endorsement: Preschool to school age. Journal of Abnormal Child Psychology, 42(6), 993–1004. 10.1007/s10802-013-9834-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen TP, Farley M, Speer L, Villalobos M, Baker CN, & Miller J (2015). Identifying autism in a brief observation. PEDIATRICS, 135(2), e330–e338. 10.1542/peds.2014-1428 [DOI] [PubMed] [Google Scholar]
- Gadow KD, & Sprafkin J (2013). Child & Adolescent Symptom Inventory-5. Stony Brook, NY: Checkmate Plus. [Google Scholar]
- Gimpel GA, & Kuhn BR (2000). Maternal report of attention deficit hyperactivity disorder symptoms in preschool children. Child: Care, Health and Development, 26(3), 163–176. 10.1046/j.1365-2214.2000.00126.x [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(5), 693–705. 10.1007/s10803-008-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH (1994). Visual attention and the control of eye movements in early infancy. Attention and Performance XV: Conscious and Nonconscious Information Processing, 291–310. [Google Scholar]
- Jones EJH, Gliga T, Bedford R, Charman T, & Johnson MH (2014). Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience & Biobehavioral Reviews, 39, 1–33. 10.1016/j.neubiorev.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry R, & Bryson SE (2004). Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry, 45(6), 1115–1122. 10.1111/j.1469-7610.2004.00304.x [DOI] [PubMed] [Google Scholar]
- Ljungberg JK, Parmentier FBR, Jones DM, Marsja E, & Neely G (2014). ‘What’s in a name?’ ‘No more than when it’s mine own’. Evidence from auditory oddball distraction. Acta Psychologica, 150, 161–166. 10.1016/j.actpsy.2014.05.009 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule (2nd ed.). Torrance, CA: western Psychological Services. [Google Scholar]
- Lott A, & Reiter JP (2018). Wilson confidence intervals for binomial proportions with multiple imputation for missing data. The American Statistician, 1–7. 10.1080/00031305.2018.1473796 [DOI] [Google Scholar]
- Mahone EM, Mostofsky SH, Lasker AG, Zee D, & Denckla MB (2009). Oculomotor anomalies in attention-deficit/hyperactivity disorder: evidence for deficits in response preparation and inhibition. Journal of the American Academy of Child & Adolescent Psychiatry, 48(7), 749–756. 10.1097/CHI.0b013e3181a565f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel DR, Jusczyk PW, & Pisoni DB (1995). Infants’ recognition of the sound patterns of their own names. Psychological Science, 6(5), 314–317. 10.1111/j.1467-9280.1995.tb00517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch C, Searle S, & Neuhaus J (2008). Generalized Linear, and Mixed Models. Hoboken, NJ: Wiley. [Google Scholar]
- McGoey KE, DuPaul GJ, Haley E, & Shelton TL (2007). Parent and teacher ratings of attention-deficit/hyperactivity disorder in preschool: The ADHD rating scale-IV preschool version. Journal of Psychopathology and Behavioral Assessment, 29(4), 269–276. 10.1007/s10862-007-9048-y [DOI] [Google Scholar]
- Miller M, Iosif A-M, Hill M, Young GS, Schwichtenberg AJ, & Ozonoff S (2017). Response to name in infants developing autism spectrum disorder: A prospective study. The Journal of Pediatrics, 183, 141–146.e1. 10.1016/j.jpeds.2016.12.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Iosif A-M, Young GS, Hill MM, & Ozonoff S (2018). Early detection of ADHD: Insights from infant siblings of children with autism. Journal of Clinical Child & Adolescent Psychology, 47(5), 737–744. 10.1080/15374416.2016.1220314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen Scales of Early Learning, AGS Edition: Manual and Item Administrative Books. American Guidance Services, Inc. [Google Scholar]
- Mundy P, & Newell L (2007). Attention, joint attention, and social cognition. Current Directions in Psychological Science, 16(5), 269–274. 10.1111/j.1467-8721.2007.00518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadig AS, Ozonoff S, Young GS, Rozga A, Sigman M, & Rogers SJ (2007). A Prospective study of response to name in infants at risk for autism. Archives of Pediatrics & Adolescent Medicine, 161(4), 378. 10.1001/archpedi.161.4.378 [DOI] [PubMed] [Google Scholar]
- Osterling JA, Dawson G, & Munson JA (2002). Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology, 14(2), 239–251. 10.1017/S0954579402002031 [DOI] [PubMed] [Google Scholar]
- Parise E, Friederici AD, & Striano T (2010). “Did You Call Me?” 5-Month-Old Infants Own Name Guides Their Attention. PLoS ONE, 5(12), e14208. 10.1371/journal.pone.0014208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli-Pott U, & Becker K (2011). Neuropsychological basic deficits in preschoolers at risk for ADHD: A meta-analysis. Clinical Psychology Review, 31, 626–637. (Peer Reviewed Journal: 2011–05663-001). [DOI] [PubMed] [Google Scholar]
- Perra Oliver., & Gattis Merideth (2010). The control of social attention from 1 to 4 months. British Journal of Developmental Psychology, 28(4), 891–908. 10.1348/026151010X487014 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2019). R: A language and environment for statistical computing. Retrieved from http://www.R-project.org/
- Reynolds GD, & Romano AC (2016). The development of attention systems and working memory in infancy. Frontiers in Systems Neuroscience, 10. 10.3389/fnsys.2016.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE (2001). Cortical indexes of saccade planning in infants. Infancy, 2(2), 123–133. 10.1207/S15327078IN0202_1 [DOI] [Google Scholar]
- Rubin DB. (Ed.). (1987). Multiple Imputation for Nonresponse in Surveys. 10.1002/9780470316696 [DOI] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). The Social Communication Questionnaire. Los Angeles: Western Psychological Services. [Google Scholar]
- Sánchez-García AB, Galindo-Villardón P, Nieto-Librero AB, Martín-Rodero H, & Robins DL (2019). Toddler screening for autism spectrum disorder: A meta-analysis of diagnostic accuracy. Journal of Autism and Developmental Disorders, 49(5), 1837–1852. 10.1007/s10803-018-03865-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye C, Asherson P, Ashwood KL, Azadi B, Bolton P, & McLoughlin G (2014). Attention and inhibition in children with ASD, ADHD and co-morbid ASD + ADHD: An event-related potential study. Psychological Medicine, 44(5), 1101–1116. 10.1017/S0033291713001049 [DOI] [PubMed] [Google Scholar]
- van Buuren S, & Groothuis-Oudshoorn K (2011). mice: Multivariate imputation by chained equations in R. Journal of Statistical Software, 45(3). 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- Yamaguchi Y, Misumi T, & Maruo K (2018). A comparison of multiple imputation methods for incomplete longitudinal binary data. Journal of Biopharmaceutical Statistics, 28(4), 645–667. 10.1080/10543406.2017.1372772 [DOI] [PubMed] [Google Scholar]
- Zhang D, Roche L, Bartl-Pokorny KD, Krieber M, McLay L, Bölte S, … Marschik PB (2018). Response to name and its value for the early detection of developmental disorders: Insights from autism spectrum disorder, Rett syndrome, and fragile X syndrome. A perspectives paper. Research in Developmental Disabilities, 82, 95–108. 10.1016/j.ridd.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]