Abstract
OBJECTIVE:
Many sepsis patients receive initial care from prehospital Emergency Medical Services (EMS). While earlier sepsis care improves outcomes, the characteristics, care and outcomes of those traded by EMS versus those arriving directly to an emergency department (ED) are currently not detailed. We sought to determine differences in hospital presentation, course and outcomes between EMS and non-EMS patients enrolled in the Protocolized Care of Early Septic Shock (ProCESS) trial.
METHODS:
We performed a secondary analysis of ProCESS, which studied ED patients with septic shock. EMS care was the primary exposure. We determined differences in demographics, clinical features, interventions and hospital course between EMS and non-EMS patients. Using mixed models, we determined the association between EMS care and 60-day mortality.
RESULTS:
Among 1,341 patients, 826 (61.6%) received initial EMS care. EMS patients were older, more likely to be black (OR 1.49, 95% CI 1.14-1.95) or nursing home residents (5.57, 3.61-8.60), and more likely to have chronic respiratory disease (1.36, 1.04-1.78), cerebral vascular disease (1.56; 1.04-2.33), peripheral vascular disease (2.02; 1.29-3.16), and dementia (3.53; 2.04-6.10). EMS patients were more likely to present with coma (4.48; 2.53-7.96) or elevated lactate (1.30; 1.04-1.63), and to receive mechanical ventilation in the ED (7.16; 4.34-11.79). There were no differences in infection source or total intravenous fluids. Initial differences in vasopressor use (1.66; 1.22-2.26) resolved at 6 hours (1.18; 0.94-1.47). Initial differences in APACHE II (EMS 21.8 vs. non-EMS 19.0) narrowed by 48 hours (17.9 vs. 16.3, [EMS X time] interaction p=0.003). Although EMS patients exhibited higher 60-day mortality, after adjustment for confounders, this association was not significant (1.09, 95% CI: 0.78-1.55).
CONCLUSIONS:
While EMS sepsis patients presented with worse chronic, non-modifiable characteristics and higher acuity than non-EMS patients, differences in acuity narrowed after initial hospital care. Despite having higher illness burden, EMS patients did not have worse adjusted short-term mortality.
Keywords: EMS, Prehospital, Sepsis, Septic Shock
INTRODUCTION
Sepsis, the syndrome of infection and concurrent organ dysfunction, results in almost 850,000 emergency department (ED) visits, over 600,000 hospitalizations and 90,000 in hospital deaths in the US each year. (1) Sepsis is a time-sensitive condition, with early recognition, infection therapy and resuscitation optimizing patient outcomes. Current national guidelines and quality measures specify performance benchmarks for lactate measurement, antibiotic administration, intravenous fluid resuscitation, and use of vasopressors in the initial hospital care of sepsis. (2)
Emergency medical services (EMS) systems are an important component of the national health care delivery system, providing critically ill patients with early life-saving care and coordinated access prior to hospital arrival. (3, 4) Of all patients transported by EMS, around 3.3% are subsequently hospitalized with severe sepsis, more than the estimated EMS incidence rate of acute myocardial infarction or stroke, with an in-hospital mortality rate of almost 20%. (5) Among higher acuity sepsis patients, more than 40% present to the ED by EMS. (5) Given the time-critical nature of sepsis, many EMS agencies target elements of early sepsis care, including notification to receiving facilities to expedite ED sepsis care. (6-9)
An important question is whether EMS care might be associated with differences in initial hospital sepsis course and outcomes. To better understand this potential relationship, we examined data from Protocolized Care of Early Septic Shock (ProCESS), a multicenter randomized trial evaluating strategies of ED sepsis resuscitation. (10) We describe and compare the presentation, hospital course and outcomes between ProCESS trial patients who did and did not receive initial EMS care.
METHODS
Study design
We conducted a secondary analysis of data from the ProCESS trial. The Institutional Review Boards of the University of Alabama at Birmingham and the University of Texas Health Science Center at Houston approved our design.
Study Setting and Population – the ProCESS Trial
The ProCESS clinical trial occurred at 31 academic hospitals, each with at least 40,000 annual emergency department visits. (10)The purpose of that study was to evaluate clinical outcomes of different resuscitation protocols in a broad cohort of patients with septic shock, specifically whether protocols involving central hemodynamic monitoring were more or less effective than those without it. The trial randomly assigned patients to three treatment strategies: 1) Early Goal Directed Therapy as described by Rivers et al with resuscitation guides by use of invasive central oxygenation catheter monitoring, 2) Protocolized Standard Care of fluids and vasopressors guided by the bedside evaluation but not invasive physiologic data, and 3) “wild type” resuscitation following clinician discretion. All three arms had other aspects of care per usual recommendations, including early antibiotics. The trial found no difference in 30-day survival between the three resuscitation strategies.(11)
ProCESS included adults presenting to the ED with septic shock, defined as [infection (suspected infection source + ≥2 systemic inflammatory response syndrome (SIRS) criteria) + hypotension (SBP< 90 mmHg despite 1 liter of intravenous fluid, need for vasopressors or a lactate ≥4 mg/dL)]. The trial excluded patients who were pregnant, required immediate surgery, had a known CD4 count < 50/mm2, had an advance directive that would impede protocol implementation, had a contraindication for central venous catheterization, had a high likelihood of refusing blood transfusion, had a treating physician who determined that resuscitation was futile, were participating in a different concomitant and active interventional study, or transferred from another inpatient setting.
Exposure
In the current analysis, the primary exposure was transportation to the ED by EMS. EMS transport was indicated by study personnel during the trial enrollment process. We did not conduct independent validation of this variable. With the exception of prehospital intravenous fluids, ProCESS did not collect other information regarding other prehospital-specific characteristics or course(s) of care.
Covariates and Outcomes
Covariates in the analysis included patient characteristics, age, sex, race, ethnicity, comorbidities, nursing home residence, pre-trial intervention characteristics (prehospital intravenous fluids, and characteristics on ED arrival (mental status, lactate level, hypotension and mechanical ventilation), source of infection, and pre-enrollment interventions). We identified resuscitation care from baseline to 72 hours (time to randomization, trial arm, intravenous fluids, vasopressors, blood transfusion, mechanical ventilation, APACHE II score, other care), and patient outcomes (organ failure, 60-day, 90-day and 1-year death, discharge destination). We characterized organ failure using the Sequential Organ Failure Assessment (SOFA). We also examined mortality at 60 days, 90 days and 1 year.
Data Analysis
We initially analyzed the data using descriptive techniques. We stratified the cohort by exposure to EMS care. We determined the proportion of EMS cases for each clinical site. Using univariable odds ratios, we determined differences in patient characteristics, pre- and post-randomization care, and outcomes between EMS and non-EMS patients. Cardiac, respiratory and renal failure determination used the respective categories of each patient’s sequential organ failure assessment (SOFA) scores through the first week. We compared baseline, 24-, 48- and 72-hours APACHE II scores between EMS and non-EMS patients using linear mixed models, modeling APACHE II as the outcome, EMS, time and [EMS X time] as independent variables. Additionally, we account for survival bias by censoring individuals lost to follow-up at 24, 48 and 72 hours, comparing APACHE II scores the censored and non-censored sub-populations.
To assess any potential association between EMS exposure and patient outcomes, we used multivariable logistic regression, adjusting for age, sex, race, ethnicity, nursing home, initial lactate levels, initial systolic blood pressure, baseline APACHE II. We repeated the analysis using mixed models fitting study site as a random (clustering) effect to eliminate any potential confounding effect from both the heterogeneous distribution of enrollments and inconsistent incidence of EMS care between sites. We again addressed survival bias by accounting for patients lost to follow up with the implementation of a Fine and Gray’s competing risk analysis, considering those patients as a competing risk factor for each outcome. We censored individuals at lost to follow-up at 24, 48 and 72 hours for competing events. Other event censoring depended on the outcome and end of the follow up period (one year).We conducted all analyses using Stata version 14.0 (Stata, Inc., College Station, Texas).
RESULTS
Characteristics of Study Subjects
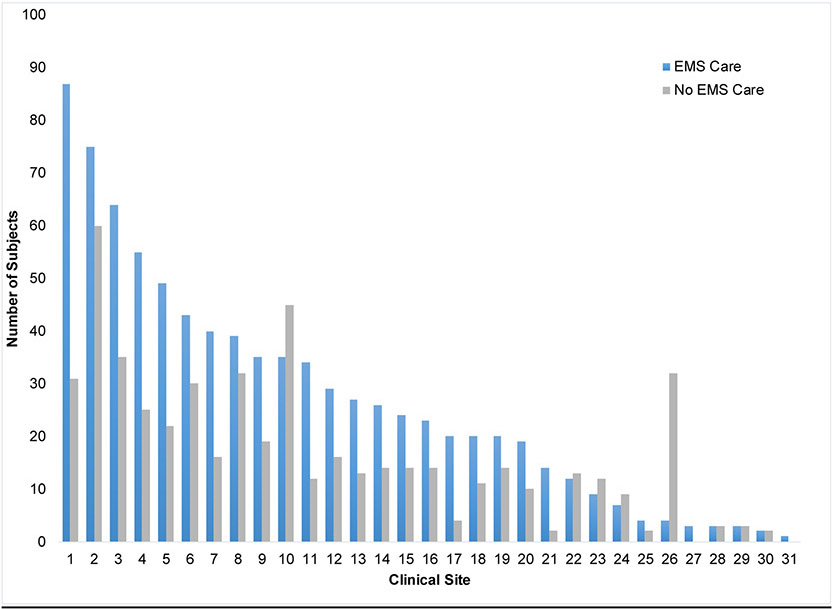
Of 1,341 subjects enrolled in the ProCESS Trial, 826 (61.6%) received initial EMS care. The proportion of EMS patients among enrolled subjects varied across sites (median 64.5%; IQR 52.5-68.9%; min 11.1%, max 100.0%). (Table 1) EMS patients were older (63.6 vs 57.2) and more likely to be Black and non-Hispanic than non-EMS patients. (Table 1) EMS patients were more likely to have a history of chronic respiratory disease, cerebral vascular disease, peripheral vascular disease, chronic dementia and peptic ulcer disease. Charlson comorbidity scores did not differ between EMS and non-EMS patients (p = 0.13). EMS patients were notably more likely to reside in a nursing home.
TABLE 1.
Characteristics of patients enrolled in the ProCESS trial, stratified by receipt of initial Emergency Medical Services (EMS) care.
| Characteristic | EMS Care (n=826) |
No EMS Care (n=515) |
Odds Ratio (95% CI) or P-values |
|---|---|---|---|
| Age (years - SD) | 63.6 (15.6) | 57.2 (16.0) | P <0.001 |
| Sex | |||
| Male | 459 (55.6) | 289 (56.1) | Ref |
| Female | 367 (44.4) | 226 (43.9) | 1.02 (0.82-1.28) |
| Race | P=0.003 | ||
| White | 549 (66.5) | 367 (71.3) | Ref |
| Black | 230 (27.8) | 103 (20.0) | 1.49 (1.14-1.95) |
| Asian | 15 (1.8) | 11 (2.1) | 0.91 (0.41-2.01) |
| Other | 32 (3.9) | 34 (6.6) | 0.63 (0.38-1.4) |
| Ethnicity | |||
| Non-Hispanic | 768 (93.0) | 428 (83.4) | Ref |
| Hispanic | 58 (7.0) | 85 (16.6) | 0.38 (0.27-0.54) |
| Comorbidities | |||
| Hypertension | 497 (60.2) | 292 (56.7) | 1.15 (0.92-1.44) |
| Diabetes Mellitus | 285 (34.5) | 173 (33.7) | 1.04 (0.82-1.31) |
| Chronic Respiratory Disease | 200 (24.2) | 98 (19.0) | 1.36 (1.04-1.78) |
| Cancer | 141 (17.1) | 93 (18.1) | 0.93 (0.70-1.25) |
| Renal Impairment | 120 (14.6) | 93 (18.1) | 0.77 (0.57-1.04) |
| Congestive Heart Failure | 107 (13.0) | 54 (10.5) | 1.27 (0.90-1.80) |
| Prior Myocardial Infection | 97 (11.7) | 46 (8.9) | 1.36 (0.94-1.96) |
| Cerebral Vascular Disease | 89 (10.8) | 37 (7.2) | 1.56 (1.04-2.33) |
| Peripheral Vascular Disease | 83 (10.1) | 27 (5.2) | 2.02 (1.29-3.16) |
| Chronic Dementia | 84 (10.2) | 16 (3.1) | 3.53 (2.04-6.10) |
| Hepatic Cirrhosis | 52 (6.3) | 35 (6.8) | 0.92 (0.59-1.44) |
| Peptic Ulcer Disease | 52 (6.3) | 20 (3.9) | 1.66 (0.98-2.82) |
| AIDS and Related Syndromes | 20 (2.4) | 18 (3.5) | 0.68 (0.36-1.31) |
| Charlson Score - Median (IQR) | 2 (1-4) | 2 (0-4) | P=0.13 |
| Nursing Home Residence | |||
| Yes | 184 (22.3) | 25 (4.9) | 5.57 (3.61-8.60) |
| No | 642 (77.7) | 486 (95.1) | Ref |
Main Results
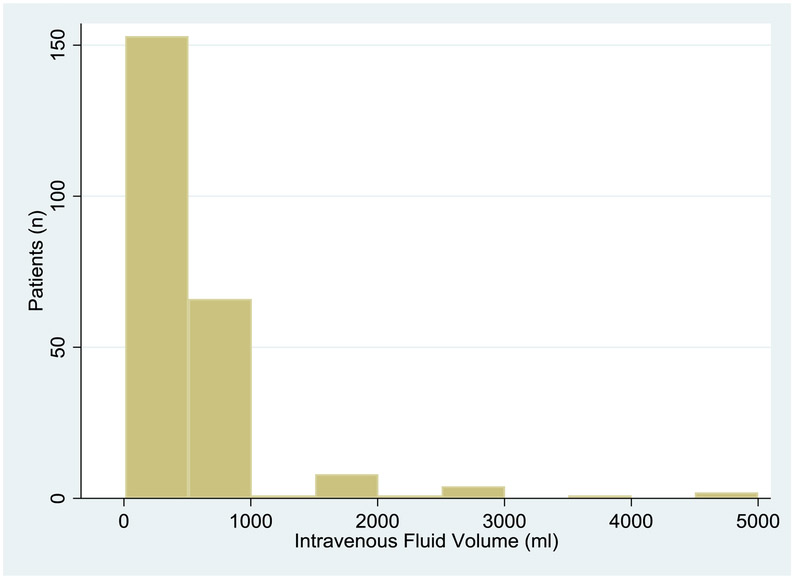
Among EMS patients, 236 received intravenous fluids. Among EMS patients receiving intravenous fluids, the median volume was 500 ml (IQR 225-1000, min 10, max 5,000) . (Table 2, Figure 1) EMS performed intubation on 43 (5.2%) subjects; 13 EMS patients had a prior history of chronic mechanical ventilation. EMS patients were over 4 times more likely to arrive at the ED in a comatose or near-comatose state. Elevated lactate was more common among EMS patients, but refractory hypotension was less common. Infection sources were similar between EMS and non-EMS patients. Pre-randomization intravenous fluids were similar between EMS and non-EMS patients. Pre-randomization intravenous antibiotic administration was slightly lower for EMS than non-EMS patients. Pre-randomization vasopressor use and mechanical ventilation were more common in EMS patients.
TABLE 2.
ProCESS pre-intervention characteristics, stratified by receipt of initial EMS care.
| Characteristic | EMS Care (n=826) |
No EMS Care (n=515) |
Odds Ratio (95% CI) and P-values |
|---|---|---|---|
| Estimated prehospital intravenous fluids (ml) – median (IQR), min, max† | 500 (225-1000), 10, 5000 | -* | - |
| Emergency Department Presentation | |||
| Mechanical ventilation on ED arrival | 56 (6.8) | 9 (1.7)** | 4.09 (2.00-8.34) |
| Prior history of chronic invasive mechanical ventilation (n=61) | 13 (25.5) | 2 (20.0) | 1.37 (0.26-7.29) |
| Mental status comatose or near-comatose | 92 (11.1) | 14 (2.7) | 4.48 (2.53-7.96) |
| Pre-intervention normal or minimally impaired mental | 548 (66.3) | 368 (71.5) | 0.79 (0.62-1.00) |
| Pre-intervention lactate ≥4 mg/dL | 513 (62.1) | 287 (55.7) | 1.30 (1.04-1.63) |
| Pre-intervention lactate level (mg/dL) | 5.1 (3.3) | 4.6 (3.2) | P=0.01 |
| Refractory hypotension (SBP≤90 mm Hg) | 430 (52.1) | 297 (57.7) | 0.80 (0.64-0.99) |
| Pre-intervention systolic blood pressure - Mean (SD) | 102.5 (29.7) | 97.9 (27.0) | P=0.005 |
| Source of Infection | |||
| Pneumonia | 286 (34.6) | 157 (30.5) | 1.31 (0.63-2.76) |
| Intra-abdominal | 108 (13.1) | 69 (13.4) | 1.13 (0.52-2.45) |
| Urinary tract infection | 174 (21.1) | 110 (21.4) | 1.14 (0.54-2.42) |
| Skin or soft tissue | 52 (6.3) | 44 (8.5) | 0.85 (0.38-1.93) |
| Central nervous system | 8 (1.0) | 2 (0.4) | 2.89 (0.52-15.90) |
| Endocarditis | 5 (0.6) | 2 (0.4) | 1.81 (0.30-10.79) |
| Catheter related | 19 (2.3) | 19 (3.7) | 0.72 (0.28-1.88) |
| Unknown Source | 107 (13.0) | 63 (12.2) | 1.23 (0.56-2.67) |
| Other | 49 (5.9) | 36 (7.0) | 0.98 (0.43-2.26) |
| No infection | 286 (34.6) | 157 (30.5) | Ref |
| Pre-randomization | |||
| Intravenous fluids (ml) | 2,223±51*** | 2,127±59 | P=0.30 |
| Fluids per body weight (ml/kg) | 29.7±22.0 | 27.9±19.1 | P=0.11 |
| Vasopressor use | 162 (19.6) | 66 (12.8) | 1.66 (1.22-2.26) |
| Dobutamine Use | 0 (0) | 0 (0) | -- |
| Blood Transfusion | 15 (1.8) | 6 (1.2) | 1.60 (0.60-4.07) |
| Mechanical Ventilation | 170 (20.6) | 18 (3.5) | 7.16 (4.34-11.79) |
| Intravenous Antibiotics | 611 (74.0) | 411 (79.8) | 0.72 (0.55-0.94) |
| Corticosteroids | 82 (9.9) | 39 (7.6) | 1.34 (0.90-2.00) |
| Activated Protein C | 0 (0) | 0 (0) | -- |
Four (4) participants in the non-EMS group received intravenous fluids prior to ED arrival.
Includes subjects transferred from another hospital facility.
Includes prehospital intravenous fluids
Includes only n=236 EMS patients who received intravenous fluids.
FIGURE 1.
Prehospital intravenous fluids. Includes only EMS patients who received intravenous fluids (n=236).
Time from ED arrival to trial randomization was almost 30 minutes shorter for EMS patients (P < 0.001). (Table 3) During the first 6 hours after randomization, vasopressor use and mechanical ventilation were more common in EMS patients. The intravenous fluid volume given between randomization-6 hours did not differ between EMS and non-EMS patients. In study hours 6-72, mechanical ventilation remained more common in EMS patients. EMS and non-EMS patients had similar intravenous fluid use and pressor use. Central venous catheterization was more common in EMS patients.
TABLE 3.
Resuscitation care from baseline to 72 hours, stratified by receipt of initial EMS care.
| Characteristic | EMS Care (n=826) |
No EMS Care (n=515) |
Odds Ratio (95% CI) and P-values |
|---|---|---|---|
| Trial Randomization | |||
| Time from arrival to randomization (mins) | 177.1 (98.7) | 204.3 (121.4) | P<0.001 |
| Time eligibility to randomization (mins) | 68.3 (37.5) | 69.2 (76.7) | P=0.77 |
| Trial Intervention Arm | |||
| Early Goal Directed Therapy | 266 (32.2) | 173 (33.6) | 1.03 (0.79-1.35) |
| Protocolized Standard Care | 287 (34.7) | 159 (30.9) | 1.21 (0.92-1.58) |
| Usual Care | 273 (33.1) | 183 (35.5) | Ref |
| Randomization to Hour 6 | |||
| Intravenous fluids (ml) | 2,827±1,872 | 2,719±1,960 | P=0.31 |
| Vasopressor use | 444 (53.8) | 231 (44.8) | 1.43 (1.14-1.78) |
| Dobutamine Use | 28 (3.4) | 16 (3.1) | 1.09 (0.59-2.04) |
| Blood Transfusion | 89 (10.8) | 45 (8.7) | 1.26 (0.86-1.84) |
| Mechanical Ventilation | 263 (31.8) | 62 (12.0) | 3.41 (2.52-4.62) |
| Hours 6-72 | |||
| Intravenous fluids (ml) | 4,628±4,096 | 4,491±3,930 | P=0.54 |
| Vasopressor use | 391 (47.3) | 223 (43.3) | 1.18 (0.94-1.47) |
| Dobutamine Use | 25 (3.0) | 13 (2.5) | 1.20 (0.61-2.38) |
| Blood Transfusion | 172 (20.8) | 90 (17.5) | 1.24 (0.94-1.65) |
| Mechanical Ventilation | 305 (36.9) | 110 (21.4) | 2.16 (1.67-2.78) |
| Ancillary Care | |||
| Central Venous Catheterization | 591 (71.6) | 336 (65.2) | 1.34 (1.06-1.70) |
| Central Venous Oximeter Catheterization | 266 (32.2) | 177 (34.4) | 0.91 (0.72-1.14) |
| Intravenous Antibiotics | 803 (97.2) | 500 (97.1) | 1.05 (0.54-2.03) |
| Corticosteroids | 96 (11.6) | 43 (8.3) | 1.44 (0.99-2.11) |
| Activated Protein C | 1 (0.1) | 1 (0.2) | 0.62 (0.04-9.98) |
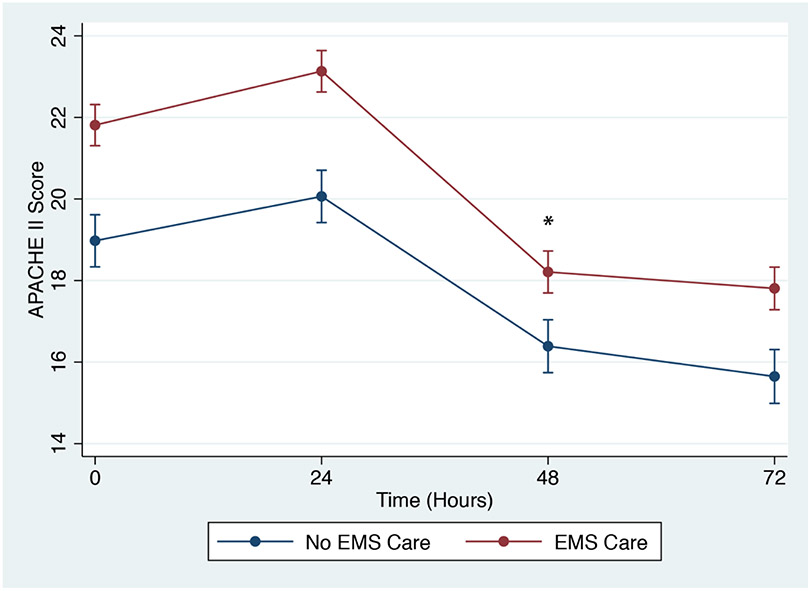
Cardiovascular and respiratory organ failure were higher and renal failure were lower in EMS than non-EMS patients in the first week. Only the association with respiratory failure persisted after adjusting for clinical confounders. APACHE II scores were higher for EMS than non-EMS patients at all time points. The gap in APACHE II slightly narrowed at 48 hours (EMS X 48-hour time interaction p=0.003), but scores from both cohorts trended down over time. (Figure 2) APACHE II scores remained higher for EMS than non-EMS patients after censoring patients who died early or withdrew from the study (Appendix 3). Death (60-day, 90-day and 1-year) was more common in EMS patients. (Table 4) However, initial EMS care was not associated with sepsis mortality after adjustment for clinical confounders. On competing risks regression, EMS care was still not associated with sepsis mortality.EMS patients were more likely to be discharged to nursing home or long-term acute facility or transferred to another hospital.
FIGURE 2.
Association of APACHE II scores with EMS care. *[EMS X time] multiplicative interaction significant at 48 hours (p=0.003). (Full mixed model in Appendix 2)
TABLE 4.
Patient outcomes, stratified by receipt of initial EMS care.
| Outcome | EMS Care (n=826) |
No EMS Care (n=515) |
Odds Ratio (95% CI) |
Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
|---|---|---|---|---|---|
| Organ Failure (in first week) | |||||
| Cardiovascular | 522 (63.2) | 287 (55.7) | 1.36 (1.09-1.71) | 1.26 (0.97-1.64) | 1.15 (0.88-1.51) |
| Respiratory | 348/813 (42.8) |
124/513 (24.2) |
2.34 (1.84-3.00) | 1.79 (1.33-2.41) | 1.75 (1.30-2.37) |
| Renal | 28/732 (3.8) | 19/446 (4.3) | 0.89 (0.49-1.62) | 0.64 (0.32-1.28) | 0.64 (0.32-1.28) |
| Death | |||||
| 60-day death | 187 (22.6) | 72 (14.0) | 1.80 (1.34-2.42) | 1.11 (0.79-1.56) | 1.09 (0.78-1.55) |
| 90-day death | 279/762 (36.6) | 117/470 (24.9) | 1.74 (1.35-2.25) | 1.11 (0.82-1.49) | 1.05 (0.82-1.49) |
| 1-year death | 374/825 (45.3) | 159/514 (30.9) | 1.85 (1.47-1.33) | 1.16 (0.88-1.52) | 1.15 (0.87-1.52) |
| Discharge Status at 60 days | |||||
| Discharged home | 352 (55.1) | 346 (78.1) | Ref | Ref | Ref |
| Discharged to nursing home | 185 (29.0) | 67 (15.1) | 2.71 (1.98-3.72) | 1.37 (0.95-1.98) | 1.44 (0.97-2.15) |
| Long term acute facility | 47 (7.4) | 13 (2.9) | 3.55 (1.89-6.68) | 2.69 (1.32-5.49) | 2.86 (1.35-6.07) |
| Other | 34 (5.3) | 10 (2.3) | 3.34 (1.62-6.87) | 2.86 (1.31-6.20) | 3.01 (1.36-6.68) |
| Not Discharged | 7 (1.1) | 6 (1.4) | 1.15 (0.38-3.45) | 0.75 (0.22-2.54) | 0.64 (0.17-2.37) |
| Transferred to another care hospital | 14 (2.2) | 1 (0.2) | 13.76 (1.80-105.22) | 9.33 (1.19-73.27) | 8.73 (1.07-71.55) |
Model 1 - logistic regression model adjusted for age, sex, race, ethnicity, nursing home, initial lactate levels, initial systolic blood pressure, baseline APACHE II.
Model 2 - mixed effects logistic regression model, with site as a random intercept
DISCUSSION
We found that over half of the patients enrolled in the ProCESS trial were transported to the hospital by EMS. Compared with non-EMS cases, EMS septic shock patients presented with worse chronic, non-modifiable characteristics and higher acuity, but differences in acuity largely resolved over the initial hospital course. Although EMS patients exhibited higher unadjusted mortality, these associations were not significant after adjustment for confounders.
Current literature supports that EMS patients present with higher acuity and receive more timely sepsis resuscitation care, but with varying effects on outcomes. In a study of over 4,500 patients admitted for infection, Wang et al. found that EMS transported over one third of the patients, cared for higher acuity patients and was associated with higher overall adjusted mortality rates. (12) Studnek, et al. found that EMS cared for more than half of 300 ED severe sepsis patients; EMS patients presented with higher rates of organ failure but experienced shorter times to antibiotic and protocol initiation. (13) In a series of 361 patients, Peltan, et al. found that those receiving EMS advanced life support care experience shorter door-to-antibiotic time than those receiving EMS basic life support care or no EMS care.(14) Femling et al showed that of 400 patients with severe sepsis or septic shock, mortality was not associated with EMS care. (15) Band et al. confirmed that among 1,000 severe sepsis patients, prehospital care did not impact mortality, but did shorten ED treatment times for key sepsis bundle care elements. (16) Our observations extend upon these findings, offering details illustrating the association between EMS care and clinical benefit in the first 72 hours of hospital care.
Across the 31 enrolling sites of the trial, we observed variations in the proportion of patients arriving by EMS (Appendix 1). We cannot know why this exists however when adjusted for in our mortality models, it does continue to shift our outcomes towards no difference between EMS and non-EMS care. Though this shift is minimal and we are unable to determine significance from it, its effects are consistent and may indicate variability in outcomes between sites, unrelated to acuity. We also saw that EMS patients presented with higher acuity than those accessing care otherwise; this finding is an important reminder that EMS transport may act as a marker for higher illness severity. Finally, differences in higher initial acuity narrowed during the ensuing 48-72 hours of hospital care. There was an associated increase in early use of vasopressors and mechanical ventilation, suggesting the possibility that EMS patients received more proactive initial resuscitation care. However, the exact reasons for the narrowing of this gap are unknown. The narrowing in difference in acuity may potentially explain the absence of adjusted mortality differences, but the results of the analysis cannot be used to prove causality.
Our findings might be construed as evidence that EMS care does not influence later sepsis care and outcomes. However, this evidence reflects the impacts of EMS care prior to the implementation of organized sepsis detection or care strategies. Seymour et al suggested the need for more organized prehospital care in severe sepsis patients, showing that over 13,000 severe sepsis patients received an average of 45 minutes of prehospital care, but that less than one third of the patients arrived to the ED with even peripheral intravenous access. (5) While not addressed by our study, we may have observed larger treatment and outcome differences with the presence of organized prehospital sepsis care. Several efforts have already alluded towards a benefit from the implementation of more organized prehospital sepsis care protocols. For example, in two separate studies, Borrelli et al and Hunter et al demonstrated that earlier recognition of sepsis in the prehospital setting resulted in better sepsis bundle-care compliance and faster execution of each sepsis care element. (8, 17) Incorporating a venous lactate meter to prehospital care was shown to benefit patient survival when used to initiate sepsis alerts. (7) Prehospital placement of intravenous access and subsequent administration of prehospital fluids have also both been linked with improved hospital outcomes. (18) Walchok et al showed that a comprehensive sepsis core measure bundle, including the collection of blood cultures and venous blood for lactate as well as the administration of intravenous fluids and antibiotics following an initiation of their sepsis alert, could be feasible. (9) An important consideration is that common screening tools such as the quick Sequential Organ Failure Assessment (qSOFA) and Systemic Inflammatory Response Syndrome (SIRS) criteria have poor sensitivity in the prehospital setting. (19, 20) While many of these studies look at the effects of implementing new protocols, we hope that with our findings, additional studies will scrutinize specific elements of prehospital care and EMS agency data to identify the precise EMS characteristics and measures currently taken that directly benefit the hospital course and outcomes of these sick septic patients.
LIMITATIONS
The ProCESS trial was not designed to study EMS care. Our data came from the convenience sample population enrolled in the trial, which likely omitted many other patients with sepsis not enrolled in the trial. We have only limited data on the course of prehospital care provided to EMS patients. We do not know the specific EMS agencies or their organizational or operational characteristics. We also could not assess regional variations. We observed variations in the number and proportion of EMS sepsis patients at each enrolling center; these differences may have been due to trial screening or enrollment practices and may not reflect epidemiologic differences across sites. Our observations reflect associations between EMS care and sepsis course and outcomes, not causality.
CONCLUSION
In our data of ED patients with septic shock, EMS transported sepsis patients had worse chronic, non-modifiable characteristics and higher acuity than non-EMS patients, differences in acuity narrowed after initial hospital care. Despite having higher illness burden, EMS patients did not have worse adjusted short-term mortality.
Acknowledgments
FUNDING SOURCES/DISCLOSURES
Supported by award P50-GM076659 from NIH/NIGMS.
Appendix
APPENDIX 1.
Distribution of EMS care subjects across ProCESS clinical sites.
APPENDIX 2. Mixed model of association of APACHE II score with EMS care and time.
| Variable | β (95% CI) | P-value |
|---|---|---|
| EMS Care | 2.84 (2.02,3.65) | <0.001 |
| Time | ||
| 24 hours | 1.09 (0.56-1.61) | <0.001 |
| 48 hours | −.58 (−3.12, −2.05) | <0.001 |
| 72 hours | −3.33 (−3,87, −2.78) | <0.001 |
| [EMS X Time] Interaction | ||
| EMS * 24 hours | 0.23 (−0.43, 0.90) | 0.49 |
| EMS * 48 hours | −1.02 (−1.69, −0.34) | 0.003 |
| EMS * 72 hours | −0.68 (−1.37, 0.02) | 0.06 |
APPENDIX 3. Distribution of APACHE II mean scores among censored and non-censored patients. Censored cases were patients who died early or withdrew from the study.
| Characteristic | Time Point (in hours) | |||
|---|---|---|---|---|
| 0 | 24 | 48 | 72 | |
| Total, n | 1,341 | 1,341 | 1,312 | 1,248 |
| Censored,n | ||||
| EMS care | - | 21 (2.5%) | 41 (5.1%) | 51 (6.7%) |
| Non-EMS care | - | 8 (1.6%) | 23 (4.5%) | 37 (7.6%) |
| Baseline APACHE II Scores among censored patients (mean[SD]) | ||||
| EMS care | - | 26.9 (10.3) | 24.7 (9.3) | 20.2 (9.0) |
| Non-EMS care | - | 15.1 (10.6) | 20.8 (8.1) | 16.7 (7.1) |
| Baseline APACHE II scores among non-censored patients (mean[SD]) | ||||
| EMS care | 21.8 (7.9) | 21.7 (7.7) | 21.5 (7.6) | 21.6 (7.5) |
| Non-EMS care | 19.0 (6.8) | 19.0 (6.8) | 18.9 (6.7) | 19.1 (6.6) |
| APACHE II Scores (mean[SD]) | ||||
| EMS care | 21.8 (7.9) | 23.0 (8.5) | 17.9 (6.6) | 17.6 (6.6) |
| Non-EMS care | 19.0 (6.8) | 20.1 (8.1) | 16.3 (6.7) | 15.6 (5.9) |
APPENDIX 4. Competing risk analysis showing association between EMS care and risk of sepsis mortality accounting for site-related clustering. Participants lost during the initial 72 hours were censored as the competing factor for this analysis.
| Outcome | Crude OR (95% CI) |
Adjusted* OR (95% CI) |
|---|---|---|
| 60-day mortality | 1.70 (1.30-2.24) | 1.26 (0.87-1.80) |
| 90-day mortality | 1.60 (1.32-1.94) | 1.23 (0.95-1.58) |
| 1- year mortality | 1.56 (1.32-1.84) | 1.19 (0.96-1.47) |
Cox Proportional Hazard model adjusted for age, sex, race, ethnicity, nursing home, initial lactate levels, initial systolic blood pressure, baseline APACHE II score at multiple time points.
Footnotes
PRIOR PRESENTATIONS
Presented at the Society for Academic Emergency Medicine Annual Meeting, May 16, 2019, Las Vegas, Nevada.
Drs. Liu, Wang, Chaudhary, Huang, and Yealy report no conflicts of interest.
REFERENCES
- 1.Wang HE, Jones AR, Donnelly JP. Revised National Estimates of Emergency Department Visits for Sepsis in the United States. Crit Care Med. 2017;45(9): 1443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–77. [DOI] [PubMed] [Google Scholar]
- 3.Larribau R, Deham H, Niquille M, Sarasin FP. Improvement of out-of-hospital cardiac arrest survival rate after implementation of the 2010 resuscitation guidelines. PLoS One. 2018;13(9):e0204169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Council NEA. EMS Makes a Difference: Improved clinical outcomes and downstream healthcare savings. A position statement of the National EMS Advisory Council. Annals of Emergency Medicine. 2011;57(2):169.21251525 [Google Scholar]
- 5.Seymour CW, Rea TD, Kahn JM, Walkey AJ, Yealy DM, Angus DC. Severe sepsis in pre-hospital emergency care: analysis of incidence, care, and outcome. Am J Respir Crit Care Med. 2012; 186(12): 1264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane D, Ichelson RI, Drennan IR, Scales DC. Prehospital management and identification of sepsis by emergency medical services: a systematic review. Emerg Med J. 2016;33(6):408–13. [DOI] [PubMed] [Google Scholar]
- 7.Guerra WF, Mayfield TR, Meyers MS, Clouatre AE, Riccio JC. Early detection and treatment of patients with severe sepsis by prehospital personnel. J Emerg Med. 2013;44(6): 1116–25. [DOI] [PubMed] [Google Scholar]
- 8.Hunter CL, Silvestri S, Stone A, Shaughnessy A, Miller S, Rodriguez A, et al. Prehospital sepsis alert notification decreases time to initiation of CMS sepsis core measures. Am J Emerg Med. 2019;37(1): 114–7. [DOI] [PubMed] [Google Scholar]
- 9.Walchok JG, Pirrallo RG, Furmanek D, Lutz M, Shope C, Giles B, et al. Paramedic-Initiated CMS Sepsis Core Measure Bundle Prior to Hospital Arrival: A Stepwise Approach. Prehosp Emerg Care. 2017;21(3):291–300. [DOI] [PubMed] [Google Scholar]
- 10.ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. The New England journal of medicine. 2014;370(18): 1683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England journal of medicine. 2001;345(19):1368–77. [DOI] [PubMed] [Google Scholar]
- 12.Wang HE, Weaver MD, Shapiro NI, Yealy DM. Opportunities for Emergency Medical Services care of sepsis. Resuscitation. 2010;81(2): 193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Studnek JR, Artho MR, Garner CL Jr, Jones AE. The impact of emergency medical services on the ED care of severe sepsis. Am J Emerg Med. 2012;30(1):51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peltan ID, Mitchell KH, Rudd KE, Mann BA, Carlbom DJ, Rea TD, et al. Prehospital Care and Emergency Department Door-to-Antibiotic Time in Sepsis. Annals of the American Thoracic Society. 2018;15(12): 1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Femling J, Weiss S, Hauswald E, Tarby D. EMS patients and walk-in patients presenting with severe sepsis: differences in management and outcome. South Med J. 2014;107(12):751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Band RA, Gaieski DF, Hylton JH, Shofer FS, Goyal M, Meisel ZF. Arriving by emergency medical services improves time to treatment endpoints for patients with severe sepsis or septic shock. Acad Emerg Med. 2011;18(9):934–40. [DOI] [PubMed] [Google Scholar]
- 17.Borrelli G, Koch E, Sterk E, Lovett S, Rech MA. Early recognition of sepsis through emergency medical services pre-hospital screening. Am J Emerg Med. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Seymour CW, Cooke CR, Heckbert SR, Spertus JA, Callaway CW, Martin-Gill C, et al. Prehospital intravenous access and fluid resuscitation in severe sepsis: an observational cohort study. Crit Care. 2014;18(5):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tusgul S, Carron PN, Yersin B, Calandra T, Dami F. Low sensitivity of qSOFA, SIRS criteria and sepsis definition to identify infected patients at risk of complication in the prehospital setting and at the emergency department triage. Scand J Trauma Resusc Emerg Med. 2017;25(1): 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorsett M, Kroll M, Smith CS, Asaro P, Liang SY, Moy HP. qSOFA Has Poor Sensitivity for Prehospital Identification of Severe Sepsis and Septic Shock. Prehosp Emerg Care. 2017;21(4):489–97. [DOI] [PubMed] [Google Scholar]