Janos Selye - a half-Hungarian, half-Austrian - endocrinologist exposed rats to a diverse range of acute non-specific nocuous agents in his laboratory at the Department of Biochemistry at the McGill University, Montreal, Canada, and published his first results in a very brief paper in “Nature” on the 4th of July in 1936 [1]. He discovered that exposure to cold, tissue injury, spinal shock, excessive muscular exercise, etc., resulted in the same typical syndrome,
independent of the nature of the damaging agent and represented a response to damage. Later he named this “typical syndrome” as stress, for which he was nominated for the first time for a Noble prize in 1949 in Physiology or Medicine. Sixteen more nominations followed, however he never received the reward. Nevertheless, we owe a lot to his discoveries that are not only important in the field of physiology but also provide an important conceptual way of thinking about the pathophysiology of critical illness in general.
More than a hundred years ago Sir William Osler observed that most of his patients, didn't seem to die from the infection but rather from the body's response to that infection [2]. A very similar finding to that of Selye's, emphasizing the importance of the “response to damage”, known today as the host response. In 1989 Roger Bone's group established the term “sepsis syndrome” characterized by a handful of simple systemic responses to infection [3]. This concept was expanded and structured by a consensus conference in 1991 where the term “systemic inflammatory response syndrome” (SIRS) was introduced [4]. However, SIRS overlooked the fact that these systemic responses are necessary to help us through a certain disease or injury, and these symptoms simply indicate that our immune system has increased its activity in order to conquer the injury, the “acute non-specific nocuous agents” that have attacked our homeostasis. Therefore, it is not the systemic response per se that is the problem but rather when it goes out of control. In other words, when the immune response becomes dysregulated the two antagonistic forces of the immune system - the pro-, and anti-inflammation -, lose the ability to act in harmony and the balance between the two becomes imbalanced [5]. This concept was acknowledged by Singer et al. in the Sepsis 3.0 definition, and sepsis is now seen as a life-threatening organ dysfunction caused by a dysregulated host response to infection [6].
However, a dysregulated immune response is not a privilege of sepsis but can also occur in other infectious or non-infectious insults. Major surgery, multiple trauma, burns, and ischemia-reperfusion injuries can provoke a similar host response as seen in infections [7]. Today it is also well acknowledged that not only bacterial but also viral and fungal infections may cause a dysregulated immune response [8,9].
The terminology has now been adjusted to accommodate this concept: “hyperinflammation”, “cytokine release syndrome (CRS)” or “cytokine storm” are becoming routine parts of our vocabulary both at the bedside and in scientific papers. This is similar to the term “vasoplegic syndrome or shock” that is more and more frequently replacing and equivalent to the term “septic shock” in scenarios when there is no pathogen in its etiology [10].
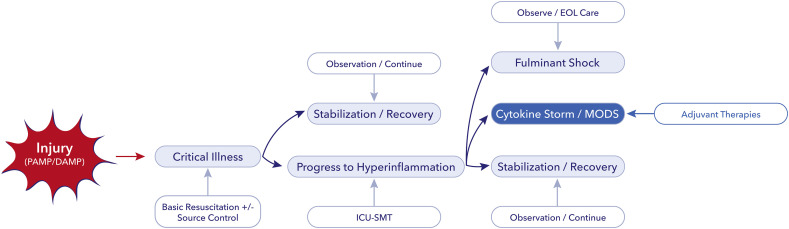
Cytokine storm resulting in vasoplegic shock and multiple organ failure is a life threatening condition, which renders the need for full intensive care support. The process can start by any injury and may evolve to multiple organ dysfunction or fulminant shock in case of dysfunctional immune response is depicted in Fig. 1 . Fortunately, most patients will respond to standard medical therapy including resuscitation, source control (if needed) and organ support. However, there is a considerable group of patients who do not show signs of improvement despite all efforts. This is the population in whom adjunctive therapy - such as blood purification with plasma exchange, hemadsorption, convalescent plasma, or other types of immune-modulation, could be considered in order to regain control over the disease and the dysfunctional immune response [11].
Fig. 1.
Treatment escalation according to clinical and inflammatory status.
DAMP, damage associated molecular pattern; PAMP, pathogen associated molecular pattern; ICU, intensive care unit; SMT, standard medical therapy; EOL, end of life; MODS, multiple organ dysfunction syndrome. Blue boxes indicate the progress, white boxes the possible interventions. For further explanation please see text. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the past several adjuvant therapies have been tested, one of them being blood purification. There are a number of modalities such as: hemofiltration, hemoperfusion, intermittent or continuous high-volume hemofiltration, therapeutic plasma exchange (TPE) and hemadsorption. The rationale for blood purification is to eliminate the excessive amounts of pro- and also anti-inflammatory mediators that are released in large quantities due to the dysregulated response, to regain balance in order to re-establish “immune homeostasis”. Despite the theoretical promise, these therapies have failed to demonstrate a clear survival benefit in large randomized controlled studies [11].
There is increasing evidence that in a subset of COVID-19 patients a very similar cytokine storm or CRS to that seen in septic shock, or in hemophagocytic lymph histiocytosis, occurs [8,9,12,13,14]. This cytokine storm plays a pivotal role in the widespread inflammation that can cause multi-organ damage in these patients [9]. As part of this process acute respiratory distress syndrome (ARDS) can also develop, which is the main culprit for the high mortality seen in COVID-19 patients who require mechanical ventilation [14]. Pathology studies have demonstrated that in several cases histological changes are very similar to that of seen in typical ARDS [15]. Several authors have proposed that blood purification therapies could thus beneficial effects in the treatment of critically ill COVID-19 patients [9,15,16].
In the current issue of Journal of Critical Care, Faqihi et al. reported a prospective case series of 10 COVID-19 patients treated with TPE for severe ARDS who were also in multiple organ failure due to CRS [17]. The authors observed that after 5–7 TPE sessions the most important outcome parameters, such as the sequential organ function assessment score (SOFA), the PaO2/FiO2 ratio, serum lactate, ferritin, D-dimer and interleukin-6 (IL-6) levels significantly improved. The median duration of mechanical ventilation was 9 (IQR: 7–12) days, ICU length of stay was 15 (13−20) days. No adverse events were reported, and 9 out of 10 patients survived until day 28. The authors concluded that TPE may have a potential survival benefit in life threatening COVID-19 conditions.
There are obvious limitations in this study, as with any small sample size case series, however, there several issues worth discussing.
First element is our attitude to collecting and sharing data and experiences during a pandemic, which caught us more-or-less off guard. There are major challenges in situations like this from obtaining consent to actually executing any study. Therefore, prospective randomized trials – the most important source of evidence generation - are extremely difficult to do for several reasons. Therefore, we were all learning as we went along. This process meant that the number of publications related to COVID-19 was, on some days, around 250, passing 15,000 on PubMed (www.pubmed.ncpi.nlm.nih.gov) within less than 2 months that is an unprecedented event in medical history – with only very few prospective randomized trials. As Derek Angus depicted in a recent viewpoint: “In a rapidly changing pandemic, perfection will be the enemy of the good” [18]. Indeed, proper design of a clinical trial on the role of any treatment in COVID-19 could easily take months and result in the complete loss of information as it never had the chance to take off, which is in fact the case with several trials at present.
Unfortunately, from the work by Faqihi et al. we are not sure whether the observed improvement over the 5–7 days was due to TPE or whether these patients would have improved anyway, as there was no control group. One would expect a more rapid response for such an intensive treatment as blood purification. Indeed, two recent studies on extracorporeal cytokine adsorption in septic shock and ARDS reported significant improvement in both clinical and inflammatory markers after just one single treatment [19,20]. Nevertheless, despite all limitations Faqihi and coworkers taking the time and effort to collect data on a specific treatment in a prospective fashion during these difficult times of the pandemic should be congratulated.
The other issue is that of pioneering something that has not yet been proven. Potentially, there are two ways one can approach the problem: waiting for the evidence to be generated, or generating it yourself. This is especially true for adjunctive therapies in critically ill patients were blood purification is a perfect example of this. Without any clear evidence, blood purification technologies, like extracorporeal cytokine adsorption, have been accepted by several authorities [21], and already more than 800 COVID-19 patients worldwide have been treated with the CytoSorb device.
Given the devastating consequences of COVID-19 efforts to increase our understanding of the disease and unfold the possibility of alternative therapeutic interventions remains important and should be encouraged. The results presented by Faqihi et al. [17] underline the potentially catastrophic role of unrestricted inflammation in these patients and reports on their positive experience and treatment safety. In accordance with this, some authors believe that controlling/attenuating the inflammatory response may be as important as targeting the virus itself [9]. A similar opinion was articulated by Brouwer and coworkers recently [19]. They suggested that as septic shock originates primarily from the dysregulated host response and not the pathogen per se, these patients in refractory septic shock may benefit more from cytokine adsorption therapy than antibiotics alone. Therefore, robust randomized controlled trials are needed. Fortunately, there are many clinical trials in the blood purification domain registered on ClinicalTrials.gov, hence answers are about to come, hopefully sooner rather than later.
Declaration of competing interest
ZM is a medical director at CytoSorbents Europe.
References
- 1.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 2.Osler W. Kessinger Publishing; 1904. The evolution of modern medicine. Reprint 2004. [Google Scholar]
- 3.Bone R.C., Fisher C.J., Clemmer T.P., Slotman G.J., Metz C.A., Balk R.A. Sepsis syndrome: a valid clinical entity. Crit Care Med. 1989;17(5):389–393. [PubMed] [Google Scholar]
- 4.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 5.Hchkiss R.S., Monneret G., Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birndt S., Schenk T., Heinevetter B., Brunkhorst F.M., Maschmeyer G. Hemophagocytic lymphohistiocytosis in adults: collaborative analysis of 137 cases of a nationwide German registry. J Cancer Res Clin Oncol. 2020;146(4):1065–1077. doi: 10.1007/s00432-020-03139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busse L.W., Barker N., Petersen C. Vasoplegic syndrome following cardiothoracic surgery – review of pathophysiology and update of treatment options. Crit Care. 2020;24 doi: 10.1186/s13054-020-2743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laszlo I., Trasy D., Molnar Z., Fazakas J. Sepsis: from pathophysiology to individualized patient care. J Immunol Res. 2015 doi: 10.1155/2015/510436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honore P.M., Hoste E., Molnar Z., Joannes-Boyau O., Malbrain M.L.N.G., Forni L.G. Cytokine removal in human septic shock: where are we and where are we going? Ann Intensive Care. 2019;9 doi: 10.1186/s13613-019-0530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W.J., Ni Y.Z., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical charecteristics of of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J., Xia P., Zhou Y., Liu Z., Zhou X., Wang J. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol. 2020;2020:214. doi: 10.1016/j.clim.2020.108408. 108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronco C., Navalesi P., Vincent J.L. Coronavirus epidemic: preparing for extracorporeal organ support in intensive care. Lancet Respir Med. 2020;8(3):240–241. doi: 10.1016/S2213-2600(20)30060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reference for Faqihi et al, for Elsevier to fill in. 2020. [Google Scholar]
- 18.Angus D.C. Optimizing the trade-off between learning and doing in a pandemic. JAMA. 2020;323(19):1895–1896. doi: 10.1001/jama.2020.4984. [DOI] [PubMed] [Google Scholar]
- 19.Hawchar F., Laszlo I., Oveges N., Trasy D., Ondrik Z., Molnar Z. Extracorporeal cytokine adsorption in septic shock: a proof of concept randomized, controlled pilot study. I Crit Care. 2019;49:172–178. doi: 10.1016/j.jcrc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Kogelmann K., Scheller M., Drüner M., Jarczak D. Use of hemoadsorption in sepsis-associated ECMO-dependent severe ARDS: a case series. J Intensive Care Soc. 2020;21(2):183–190. doi: 10.1177/1751143718818992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.https://www.mdmag.com/medical-news/fda-emergency-use-authorization-cytosorb-in-covid19-patients