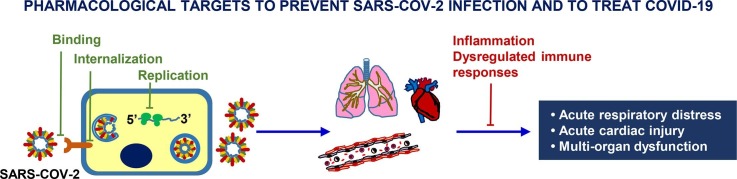
Graphical abstract
Abbreviations: 2019-nCoV, 2019 novel coronavirus; 3CLpro, chymotrypsin‐like protease; AAK1, AP2-associated kinase 1; ADE, antibody-dependent enhancement, ALI, acute lung injury; ARDS, acute respiratory distress syndrome; CAR, chimeric antigen receptors; CAS, Chemical Abstracts Service; CoV, coronavirus; COVID-19, Corona Virus Disease 2019; CT, computed tomography; DAMPs, danger-associated molecular patterns; E, envelope; ERGIC, endoplasmic reticulum-Golgi apparatus intermediate compartment; GAK, cyclin G–associated kinase; G-CSF, granulocyte-colony stimulating factor; HBV, hepatitis B virus; HCV, hepatitis C virus; HScore, hemophagocytosis score; ICU, intensive care unit; IL, interleukin; IP-10, interferon-γ-inducible protein 10; IRF3, interferon regulatory factor 3; JAK, Janus kinases; M, membrane; mAb, monoclonal antibody; MCP, monocyte chemoattractant protein; MERS-CoV, Middle East respiratory syndrome coronavirus; MIP1α, macrophage inflammatory protein; Mpro, main protease; MRSA, methicillin-resistant Staphylococcus Aureus; MSCs, mesenchymal stem cells; LMWH, low molecular weight heparin; N, nucleocapsid; NF-kB, nuclear factor-kB; nsps, nonstructural proteins; ORF, open reading frames; PDE-5, phosphodiesterase-5; PLpro, papain‐like protease; PRRs, pattern recognition receptors; RBD, receptor-binding domain; S, spike; ACE2, angiotensin-converting enzyme 2; S1P, sphingosine 1-phosphate; SARS, severe acute respiratory syndrome; sHLH, secondary hemophagocytic lymphohistiocytosis; STAT, signal transducer and activator of transcription; TLR, Toll-like receptor; TMPRSS2, transmembrane protease serine 2 protease; TNFα, tumor necrosis factor α; tPa, tissue-type plasminogen activator; UFH, unfractionated heparin; VEGF-A, vascular endothelial growth factor-A; VIP, vasoactive intestinal polypeptide
Keywords: COVID-19, Coronavirus, SARS-CoV-2, Cytokine storm, Antiviral agents, Drug repositioning
Abstract
On March 11, 2020, the World Health Organization (WHO) declared the severe acute respiratory syndrome caused by coronavirus 2 (SARS-CoV-2) a global pandemic. As of July 2020, SARS-CoV-2 has infected more than 14 million people and provoked more than 590,000 deaths, worldwide. From the beginning, a variety of pharmacological treatments has been empirically used to cope with the life-threatening complications associated with Corona Virus Disease 2019 (COVID-19). Thus far, only a couple of them and not consistently across reports have been shown to further decrease mortality, respect to what can be achieved with supportive care. In most cases, and due to the urgency imposed by the number and severity of the patients’ clinical conditions, the choice of treatment has been limited to repurposed drugs, approved for other indications, or investigational agents used for other viral infections often rendered available on a compassionate-use basis. The rationale for drug selection was mainly, though not exclusively, based either i) on the activity against other coronaviruses or RNA viruses in order to potentially hamper viral entry and replication in the epithelial cells of the airways, and/or ii) on the ability to modulate the excessive inflammatory reaction deriving from dysregulated host immune responses against the SARS-CoV-2. In several months, an exceptionally large number of clinical trials have been designed to evaluate the safety and efficacy of anti-COVID-19 therapies in different clinical settings (treatment or pre- and post-exposure prophylaxis) and levels of disease severity, but only few of them have been completed so far. This review focuses on the molecular mechanisms of action that have provided the scientific rationale for the empirical use and evaluation in clinical trials of structurally different and often functionally unrelated drugs during the SARS-CoV-2 pandemic.
1. Introduction
On January 30, 2020, the World Health Organization (WHO) declared the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; initially named 2019 novel coronavirus or 2019-nCoV) a public health emergency of international concern, highlighting the need for a coordinated international intervention to limit virus spreading. Few weeks later, on March 11, 2020, because of the rapid diffusion of the infection, the WHO announced that SARS-CoV-2 infection was a global pandemic. The first cases of respiratory disease caused by 2019-CoV-2, thereafter officially named COVID-19 (Corona Virus Disease 2019), likely occurred from a zoonotic transmission in China in December 2019 and since then infection has spread across 213 countries and territories. As of July 2020, SARS-CoV-2 has infected more than 14,000,000 people and caused more than 590,000 deaths (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ accessed July 19, 2020).
Coronaviridae define a family of hundreds of enveloped, positive-sense, single-stranded RNA viruses that are known to cause diseases in animals. Sometimes these viruses become able to overcome the species barriers (spillover event) and, so far, 7 coronaviruses are known to cause human diseases. Among these, four human coronaviruses (i.e., HCoV-229E, HCoV-NL63, HCoV-OC43 and HKU1) typically affect the upper respiratory tract and cause relatively minor symptoms. However, the other three coronaviruses [severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV-2] are able to replicate in the lower respiratory tract and are responsible for severe forms of pneumonia that can be fatal [1]. Phylogenetic analysis indicates that SARS-CoV-2 has high similarity (88–89%) with two coronaviruses circulating in Rhinolophus (horseshoe bats) [2], but it is less closely related to the SARS-CoV (~79% similarity) and MERS-CoV (~50% similarity). Based on the sequence analysis of the 29.8 kb viral genome and on the presence of bats and live animals in the seafood wholesale market in Wuhan (Hubei province, China), where SARS-CoV-2 was detected for the first time, this virus might have arisen from bats or materials contaminated by bat droppings in the Chinese seafood market areas and transmitted to humans either directly or through an intermediate host [3].
Similar to the other respiratory coronaviruses, SARS-CoV-2 is transmitted primarily via the respiratory route in the form of droplets, with a possible, though yet unproven, fecal-oral transmission route [4], [5]. The virus is stable for several hours to days in aerosols and on various types of surfaces, suggesting that transmission may occur by person-to-person droplets as well as by contact with fomites in the proximity of infected patients [6]. Although many individuals remain asymptomatic, 97.5% of diseased patients display clinical symptoms within 11.5 days [7]. Patients with COVID-19 may exhibit mild to moderate symptoms, most commonly fever, fatigue, dry cough, anosmia/dysgeusia, or severe pneumonia with dyspnea, tachypnea, and hypoxemia. Actually, dyspnea is predictive of severe COVID-19 and intensive care unit (ICU) admission [8]. Other symptoms less frequently reported include muscle and joint pain, headache, diarrhea, nausea or vomiting, hemoptysis [9]. Severe COVID-19 is associated to acute lung injury (ALI) and/or acute respiratory distress syndrome (ARDS) that generally occur 8–9 days after symptom onset. As with SARS-CoV infection, an aggressive inflammatory reaction is responsible for the damage to the lung, indicating that the disease severity also depends on dysregulation of the host immune responses. Respiratory failure is the most common cause of death (>70%) of fatal COVID-19 cases. Furthermore, the massive release of cytokines by the immune system can result in cytokine storm and septic shock and/or multiple organs dysfunction syndromes in 28% of fatal cases [10], [11]. Other causes of death are cardiac failure, coagulopathy and renal failure [11]. SARS-CoV-2 appears also to target the central nervous system with anosmia and dysgeusia as early symptoms and convulsions that may develop later on [12].
Currently, the standard of care in patients showing ARDS includes oxygen therapy together with the administration of parenteral fluids. Furthermore, many patients with severe respiratory distress, hypoxemia and ARDS require invasive mechanical ventilation, and, if the situation deteriorates, extracorporeal membrane oxygenation support [13]. Therapeutic interventions including administration of drugs may vary from country to country and it is extremely difficult to harmonize the different protocols due also to the different disease stages of the patients (asymptomatic, pre-symptomatic, mild, severe, under mechanical ventilation). The scarce knowledge of the SARS-CoV-2 biology and of the host-pathogen interactions leading to COVID-19 has markedly hampered the prompt identification of suitable targets for the development of new therapies.
A large number of exploratory clinical trials and pivotal studies are being carried out worldwide. Among them, the international “Solidarity trial” launched by the WHO on March 2020 with the aim to find an effective treatment for COVID-19 patients by comparing four different treatments (i.e., lopinavir/ritonavir, lopinavir/ritonavir plus interferon-β, chloroquine/hydroxychloroquine or remdesivir) against standard of care (see also Sections 2 and 3). Presently, regulatory authorities all over the world underline the need of common and rigorous approaches to clinical trials in order to generate more robust evidence on the safety/efficacy of the different anti-SARS-CoV-2 treatments or vaccines that are being tested.
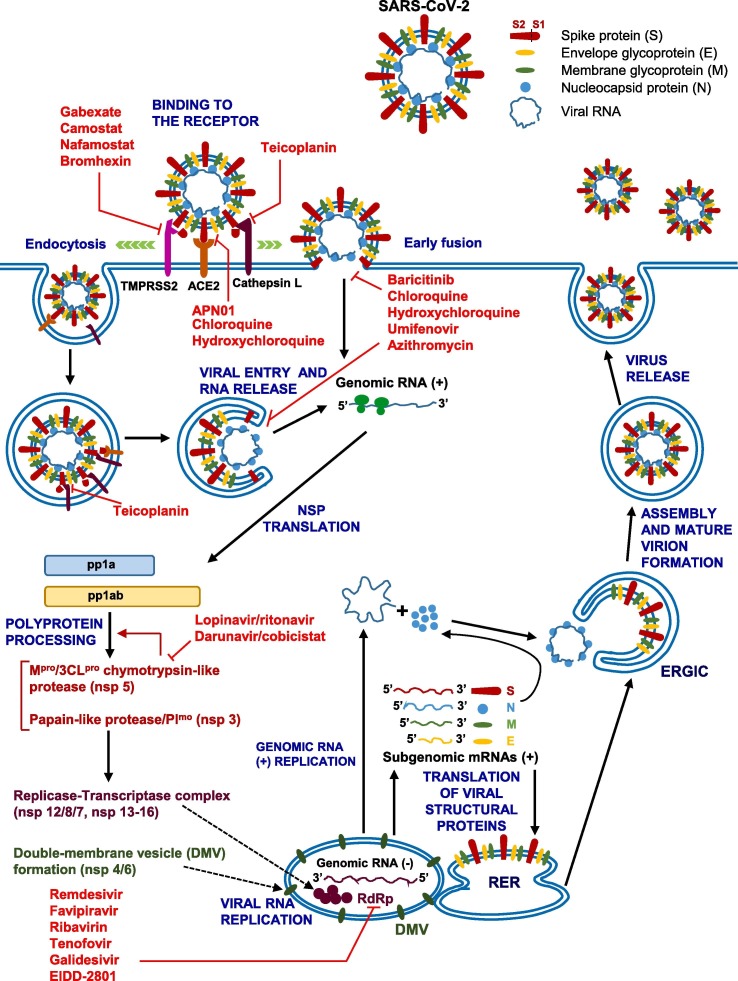
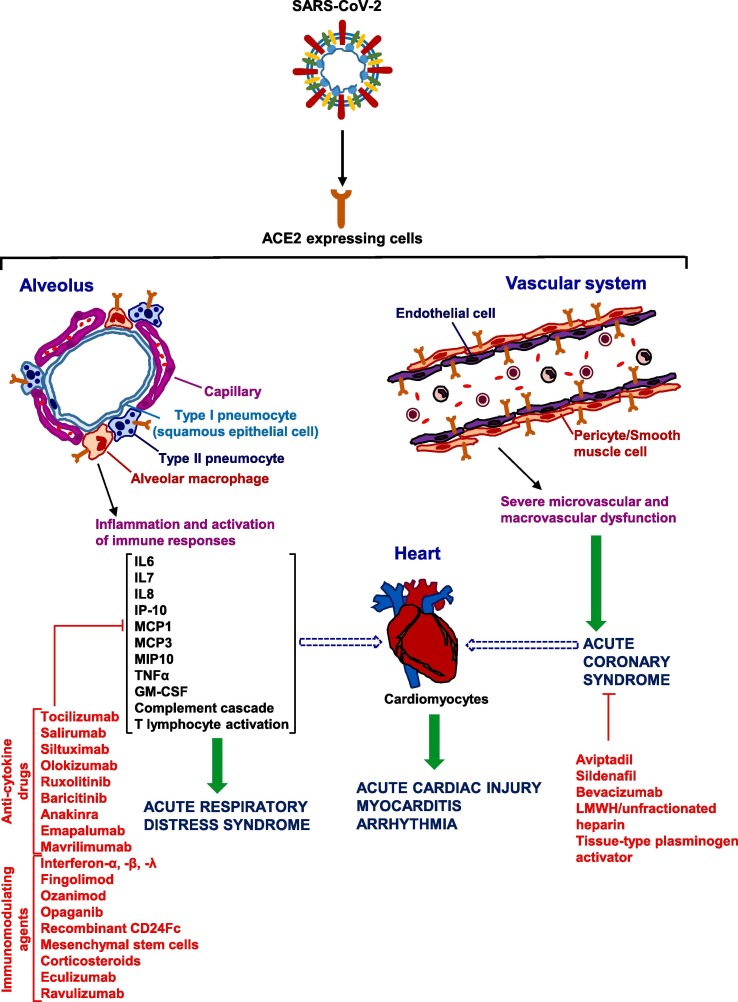
Here, we review the recently published literature on the pharmacological treatments used so far and/or undergoing evaluation in clinical trials, with focus on the molecular mechanisms of action of repurposed or investigational drugs, classified as agents directly targeting the virus (Fig. 1 and Table 1 ) and those used to treat the respiratory distress, inflammation associated with the cytokine release syndrome and cardiovascular complications (Fig. 2 and Table 2 ). In addition, we summarize the main clinical trials completed or still ongoing in SARS-CoV-2 infected patients.
Fig. 1.
Schematic diagram of SARS-CoV-2 replication cycle in human cells and potential viral targets of repurposed drugs that have been empirically used and tested in clinical trials for COVID-19 treatment. During the viral replication cycle, SARS-CoV-2 spike (S) protein binds to ACE2 in host cells and after the attachment step, the entry process requires the S protein priming by cellular proteases (i.e., TMPRSS2, cathepsin L, furin). Fusion of the virus and cell membranes likely occurs both at the plasma membrane (early fusion) and endosomal level (endocytosis) after which the release of the nucleocapsid into the cytoplasm takes place. Most of viral genome sequence is directly translated to produce the polyproteins pp1a and pp1ab, which are processed by viral proteases (3CLpro/Mpro, PLpro) into 16 nonstructural proteins (nsps), including RNA-dependent RNA polymerase (RdRp) and other proteins that form the replication-transcription complex, which is anchored to double-membrane vesicles (DMV) integrated into a reticulovesicular network of modified endoplasmic reticulum membranes. The viral RdRp synthesizes a full-length complementary negative-strand RNA as template for the production of positive-strand genome of the virus progeny and a set of subgenomic mRNAs deriving from negative-sense RNA intermediates (not shown). Subgenomic mRNAs are translated into structural proteins in the rough endoplasmic reticulum (RER) [spike (S), membrane (M), envelope (E) proteins], or in the cytosol [nucleocapsid (N) protein]. The S, E and M move along the intermediate compartment of the endoplasmic reticulum-Golgi (ERGIC). The viral genomic RNA is encapsulated by the nucleocapsid N protein and, thereafter, buds into the ERGIC and acquires a membrane containing the S, E and M structural proteins. Finally, the virus is released by exocytosis. Blunt red arrows indicate the potential targets of the listed drugs. See the text for further details. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Drugs potentially targeting SARS-CoV-2: mechanism of action, approved indications, and clinical studies on their use for COVID-19.
| Drugs | Mechanism of action against SARS-CoV-2 | Approved use | Trial code (NCT)a and/or reference with clinical study results |
|---|---|---|---|
| Drugs that interfere with SARS-CoV-2 entry | |||
| Umifenovir | Inhibition of virus attachment and internalization | Prophylaxis and treatment of influenza A and B (Russia and China) |
[38], [39], [40], [41], [42], [43], [44] NCT04260594 NCT04350684 NCT04273763 |
| Baricitinib | Inhibition of endocytosis | Rheumatoid arthritis | [46], [47] (see Table 2) |
| Chloroquine Hydroxychloroquine ± azythromycin |
Inhibition of endocytosisb | Malariac Systemic lupus erythematosusc Rheumatoid arthritisc |
[54–58/NCT04323527, 62,63] NCT04364815 NCT04340544 NCT04334382 NCT04342221 NCT04315896 NCT04261517 NCT04420247 NCT04325893 NCT04365231 NCT04410562 NCT04346329 NCT04331834 NCT04328467 NCT04372017 NCT04397328 NCT04330144 NCT04408456 NCT04318015 NCT04349228 NCT04328285 NCT04352933 NCT04341441 NCT04363450 NCT04403100 |
| Camostat | Inhibition of TMPRSS2 | Pancreatitis (Japan) |
NCT04321096 NCT04353284 NCT04355052 NCT04338906 |
| Nafamostat | Inhibition of TMPRSS2 | Pancreatitis Disseminated intravascular coagulation and anticoagulation in extracorporeal circulation (Japan) |
[75] NCT04352400 NCT04418128 |
| Gabexate | Inhibition of TMPRSS2 | Pancreatitis (Italy and Japan) |
– |
| APN01 (recombinant human ACE2) | Inhibition of ACE2-mediated virus entry | NCT04335136 | |
| Teicoplanin | Inhibition of cathepsin L | Treatment of resistant Gram-positive bacterial infections | [84] |
| Inhibitors of translation, processing and replication of SARS-CoV-2 | |||
| Lopinavir/ritonavir | Inhibition of 3CLpro/Mpro protease | HIV |
[95], [97] NCT04307693 NCT04343768 NCT04276688 NCT04365582 NCT04372628 NCT04328285 NCT04386070 NCT04321174 |
| Darunavir/cobiscistat | Inhibition of 3CLpro/Mpro protease | HIV |
NCT04252274 NCT04425382 |
| Remdesivir | Inhibition of RdRpd | COVID-19 (Japan, EMA) |
[114], [115], [116], [117], [118] NCT04280705 NCT04292899 NCT04257656 NCT04315948 NCT04292730 NCT04321616 NCT04409262 NCT04401579 |
| Favipiravir | Inhibition of RdRp | Treatment of influenza A and B (Japan) COVID-19 (Russia) |
[127] NCT04346628 NCT04336904 NCT04425460 NCT04358549 NCT04349241 NCT04402203 NCT04387760 NCT04373733 NCT04359615 NCT04411433 NCT04392973 NCT04310228 |
| Ribavirin | Inhibition of RdRp | HCV | [133/NCT04276688] NCT04392427 NCT04356677 |
| Tenofovir | Inhibition of RdRp | HIV and HBV chronic infection |
NCT04405271 NCT04334928 |
| Galidesivir | Inhibition of RdRp | – | NCT03891420 |
| EIDD-2801 | Inhibition of RdRp | – |
NCT04405739 NCT04405570 |
NCT: ClinicalTrials.gov identifier; data from ClinicalTrials.gov accessed on June 2020. Due to the rapidly evolving situation and the increasing number of clinical trials, the reported list of clinical trials does not mean to be exhaustive.
These agents might also have additional mechanisms contributing to the antiviral activity against SARS-CoV-2.
These indications apply to chloroquine and hydroxychloroquine. Azithromycin is a macrolide antibiotic used for a number of bacterial infections.
RdRp, RNA-dependent RNA polymerase.
Fig. 2.
Main targets of repurposed drugs that have been empirically used and tested in clinical trials for the COVID-19 respiratory distress and cardiovascular complications associated with the cytokine release syndrome. SARS-CoV-2 entry into type II pneumocytes, endothelial cells and cardiomyocytes results in inflammation with acute respiratory distress, acute cardiac injury and multi-organ dysfunction (not depicted in the drawing). Infection of cells in the respiratory tract, particularly of type II pneumocytes, by SARS-CoV-2 may result in an excessive inflammatory reaction and immune cell overactivation, with high levels of cytokines such as IL6, IL7, IL8, TNFα, IP-10, MCP1, MCP3, MIP1α, etc. (cytokine storm). Cardiac complications can be due to: direct damage upon virus entry through ACE2 in coronary endothelial cells and cardiomyocytes, massive cytokine release with hyperinflammation and dysregulated immune responses. Blunt arrows indicate the potential targets of the listed drugs. See text for further details.
Table 2.
Drugs used to counteract the acute respiratory distress, cytokine storm and cardiovascular complications.
| Drugs | Approved use | Trial code (NCT)a and/or reference with clinical study results |
|---|---|---|
| Anti-cytokines | ||
| Anti-IL6 receptor | ||
| Tocilizumab | Rheumatoid Arthritis |
[158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168] NCT04335071 NCT04317092 NCT04377659 NCT04306705 NCT04403685 NCT04339712 NCT04361032 NCT04333914 NCT04345445 NCT04310228 NCT04335305 NCT04330638 |
| Sarilumab | Rheumatoid Arthritis |
NCT04324073 NCT04327388 NCT04315298 NCT04357860 NCT04357808 NCT04359901 |
| Anti-IL6 | ||
| Siltuximab | Multicentric Castleman’s disease | NCT04329650 |
| Olokizumab | Chronic idiopathic arthritis | NCT04380519 |
| JAK inhibitors | ||
| Ruxolitinib | Myelofibrosis, Polycythaemia vera, graft-versus-host disease |
NCT04362137 NCT04334044 NCT04366232 NCT04348695 NCT04374149 |
| Baricitinib | Rheumatoid arthritis |
NCT04340232 NCT04421027 NCT04393051 NCT04362943 NCT04399798 NCT04321993 NCT04390464 |
| Recombinant IL1 receptor antagonist | ||
| Anakinra | Rheumatoid arthritis and cryopyrin-associated periodic syndrome (FDA and EMA) Familial Mediterranean fever and Still’s disease (EMA) |
[180], [181], [182], [183], [184] NCT04339712 NCT04330638 NCT04366232 NCT04324021 |
| Anti-Interferon-γ | ||
| Emapalumab | Orphan Drug for haemophagocytic lymphohistiocytosis | NCT04324021 |
| Anti-GM-CSF | ||
| Mavrilimumab | – |
[187] NCT04399980 NCT04397497 |
| Immunomodulating agents | ||
| Interferons | ||
| Interferon-β and α | Multiple Sclerosis, viral hepatitis and cancer |
[133], [199] NCT04350671 NCT04343768 NCT04350684 NCT04276688 NCT04350281 NCT04324463 NCT04385095 NCT04254874 NCT04320238 |
| Interferon-λ | – |
NCT04343976 NCT04388709 NCT04354259 NCT04344600 |
| S1P signaling modulators | ||
| Fingolimod | Multiple Sclerosis |
[205], [209], [210] NCT04280588 |
| Ozanimod | Multiple Sclerosis | NCT04405102 |
| Opaganib | – |
NCT04435106 NCT04414618 |
| CD24Fc | – | NCT04317040 |
| Mesenchymal stem cells | Orphan drug designations: Duchenne muscular dystrophy, amyotrophic lateral sclerosis, anal fistula, epidermolysis bullosa, graft-versus-host disease. |
[223], [224] NCT04366271 NCT04429763 NCT04315987 NCT04366323 NCT04336254 NCT04346368 NCT04382547 |
| Corticosteroids | ||
| Dexamethasone Methylprednisolone Budenosonide Ciclesonide |
Arthritis, asthma, irritable bowel disease/Crohn disease, emesis, multiple sclerosis and various autoimmune diseases |
[225] NCT04381936 NCT04395105 NCT04445506 NCT04360876 NCT04327401 NCT04344730 NCT04325061 NCT04374071 NCT04355247 NCT04273321 NCT04343729 NCT04416399 NCT04355637 NCT04193878 NCT04361474 NCT04374474 |
| Drugs acting at cardiovascular level | ||
| Anti-C5 complement mAbs | ||
| Eculizumab | Paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, generalized myasthenia gravis and neuromyelitis optica spectrum disorder |
NCT04355494 NCT04288713 NCT04346797 |
| Ravulizumab | Paroxysmal nocturnal hemoglobinuria |
NCT04369469 NCT04390464 |
| Antithrombotic and fibrinolytic agents | ||
| Low molecular weight heparin; unfractionated heparin | Prophylaxis and treatment of venous thrombosis and thromboembolism | [236], [237], [238], [239], [240], [244] |
| Tissue-type plasminogen activator | Thrombolytic treatment in acute myocardial infarction; pulmonary embolism; acute ischemic stroke; central venous catheter occlusion | [247], [248] |
| Phosphodiesterase type 5 inhibitor | ||
| Sildenafil | Pulmonary hypertension; erectile dysfunction | NCT04304313 |
| Vasoactive Intestinal Polypeptide analog | ||
| Aviptadil | Orphan drug for the treatment of ARDS, ALI and sarcoidosis |
NCT04311697 NCT04360096 |
| Anti-VEGF-A | ||
| Bevacizumab | Cancer treatment; age-related macular degeneration (off-label) |
NCT04305106 NCT04344782 NCT04275414 |
ARDS: acute respiratory distress syndrome; ALI: acute lung injury.
NCT: ClinicalTrials.gov identifier; data from ClinicalTrials.gov accessed on June 2020. Due to the rapidly evolving situation and the increasing number of clinical trials, the reported list of clinical trials does not mean to be exhaustive.
2. Anti-SARS-CoV-2 drugs: mechanisms of action, approved indications, biological rationale for their use as anti-viral agents
2.1. Drugs that interfere with SARS-CoV-2 entry
The first step in any viral infection entails binding of the virus to a host cell through its target receptor. Both SARS-CoV and SARS-CoV-2 entry into cells requires the interaction of the viral spike (S) glycoprotein (the envelope-associated protein conferring coronaviruses the characteristic crown-like morphology) with the angiotensin-converting enzyme 2 (ACE2) [14], [15], [16]. ACE2 is a dimeric ectoenzyme with dipeptidyl carboxypeptidase activity. Although the ACE2 mRNA has been detected in a variety of tissues [17], the protein has not always been analyzed or detected. The ACE2 protein is expressed at high levels on the surface of the lung alveolar epithelial cells and enterocytes of the small intestine providing an easily accessible route for SARS-CoV-2 infection [18]. The ACE2 protein is also present in smooth muscle, pericytes and endothelial cells of the vasculature, heart, kidney and this might account for the multi-organ dysfunction observed in severe COVID-19 patients [18], [19], [20], [21], [22], [23]. Other tissue sites where the ACE2 protein was detected include, among others, the basal epithelium of the nasal, nasopharynx oral mucosa, the basal cell layer of epidermis, and testis [18], [24]. The viral S glycoprotein is a trimer and each monomer contains two subunits, S1 and S2, of which S1 is responsible for the virus attachment to the host cell surface though the receptor-binding domain (RBD), whereas S2 is required for the fusion of the viral and cellular membranes. After the attachment step, the entry process requires the S protein priming by cellular proteases, consisting in the proteolytic cleavage at the S1-S2 boundary and at a downstream position in S2; this process leads to the exposure of a peptide that is involved in membrane fusion [25], [26]. The S proteins of Coronaviruses can be cleaved by various cellular proteases; in the case of SARS-CoV-2, the transmembrane protease serine 2 protease (TMPRSS2) plays a critical role in S protein priming, whilst the endosomal cysteine protease cathepsin L may replace TMPRSS2 in this function in cells other than those of the lung [27], [28]. Moreover, it has been recently demonstrated that the host cell protease furin can cleave the SARS-CoV-2 S protein at the S1/S2 site cleavage, an essential step for viral entry into lung cells [29]. Since ACE2 is located within lipid rafts, cell infection by SARS-CoV-2 also requires interaction of the viral S protein with different raft components, including sialic-acid-containing gangliosides; this interaction also facilitates the contact of the S protein with the ACE2 receptor [30]. After cleavage of the S protein, SARS-CoV-2 can be induced to fuse at both the plasma membrane and the endosomal membrane [27], [31]. Various endocytic pathways have been described as being used for cell infection by different Coronaviruses, including clathrin-coated vesicles, caveolae as well as clathrin- and caveolae-independent mechanisms [32], [33].
Different antiviral agents or other drugs used for indications unrelated to virus infections have been used to block SARS-CoV-2 entry into the host cells either by i) inhibiting virus attachment and proteolytic cleavage of the S protein, ii) targeting key cellular enzymatic activities or proteins involved in the endocytic processes or iii) using a combination of both mechanisms (Fig. 1).
2.1.1. Umifenovir
Umifenovir (Arbidol) is a small indole-derivative molecule with a broad spectrum of activity against DNA/RNA and enveloped/non-enveloped viruses that prevents viral entry into the host cell (attachment and internalization), behaving as host-targeting and direct-acting antiviral agent [34], [35]. In particular, due to its hydrophobicity, umifenovir displays high affinity for the lipids of the host cell membranes altering their fluidity and rendering them less prone to fusion with the virus. This agent is also able to interact with aromatic residues of the viral glycoproteins involved in the attachment and in the membrane destabilization necessary for the fusion process. Furthermore, umifenovir markedly affects clathrin-mediated endocytosis by hampering the release of clathrin-coated pits from the plasma membrane with consequent slowing of vesicle intracellular trafficking and accumulation of clathrin-coated structures where the viral particles remain trapped [35]. Finally, based on structural similarities between the umifenovir binding sites in the hemagglutinin of the H3N2 influenza virus and the S glycoprotein of SARS-CoV-2, it has been suggested that this drug might block the trimerization of the S glycoprotein, which is essential for the virus cell adherence and entry [36]. Umifenovir is licensed (only in Russia and China) for the prophylaxis and treatment of influenza A and B infections but it has shown in vitro activity against infections by hepatitis C and B viruses (HCV and HBV), Ebola and other viruses [37]. In a clinical pilot trial conducted in sixty-nine COVID-19 patients, oral treatment with umifenovir (n = 36) showed a tendency to reduce viral load and mortality rate as compared to the control group receiving interferon or other non-specified antiviral agents (0% vs 16%) [38]. The results of a retrospective cohort study in patients with COVID-19, without invasive ventilation, who received umifenovir plus lopinavir/ritonavir (n = 16) or lopinavir/ritonavir only (n = 17), showed a potential benefit of the triple combination therapy to reduce viral load and delay disease progression. In fact, after 14 days of treatment, in the umifenovir-treated group nasopharyngeal specimens were negative for SARS-CoV-2 in 94% of patients (vs 53% of the control group) and the chest computed tomography (CT) scans were improved in 69% of cases (vs 29% of the control group) [39]. Subsequently, umifenovir was tested as monotherapy (n = 34) and its activity compared to that of lopinavir/ritonavir (n = 16). On day 14 after treatment, no viral load was detected in the umifenovir group, whereas the virus was still found in 44.1% of patients treated with lopinavir/ritonavir [40]. Conversely, in another study with non-ICU patients (n = 45) umifenovir failed to improve the prognosis and virus clearance compared to the control group receiving symptomatic treatment, including the most appropriate supportive care (n = 36) [41]. A similar conclusion was drawn by an observational cohort study on the real-world efficacy and safety of umifenovir used as single agent or in combination with lopinavir/ritonavir. There was no evidence that adding umifenovir to lopinavir/ritonavir could shorten the time to negative conversion of SARS-CoV-2 nucleic acid in pharyngeal swabs or improve the symptoms [42]. However, a retrospective analysis of adverse drug reactions in 217 Chinese patients with COVID‐19, by a hospital pharmacovigilance system, reported a lower incidence of adverse effects (that were mostly at the gastrointestinal and hepatic level) for umifenovir compared to lopinavir/ritonavir (18.1% vs 63.8%) [43]. A small retrospective cohort study has recently suggested the use of umifenovir for post-exposure prophylaxis, based on the significant reduction of infection risk observed in family members (n = 66 in 27 families) and health care workers (n = 124) who were exposed to patients with confirmed SARS-CoV-2 infection [44]. Further clinical studies are ongoing to evaluate the role of umifenovir in COVID-19 management, used as monotherapy [NCT04260594] or in combination with other antiviral agents [NCT04350684, NCT04273763]. In the randomized, double-blind, placebo-controlled clinical NCT04350684 trial, umifenovir is added to a therapeutic regimen including interferon-β1a, lopinavir/ritonavir and a single dose of hydroxychloroquine plus standard of care.
2.1.2. Baricitinib
Baricitinib is a potent and selective inhibitor of the Janus kinases 1/2 (JAK1/JAK2), currently used in the therapy of rheumatoid arthritis. Based on the results of a BenevolentAI’s knowledge graph, the small-molecule kinase inhibitor baricitinib was predicted to alter virus entry by inhibiting AP2-associated kinase 1 (AAK1) and cyclin G–associated kinase (GAK), which are likely involved in SARS-CoV-2 endocytosis [45]. The BenevolentAI’s knowledge graphical method uses machine learning to integrate the scientific information on the biological processes involved in viral infection with that on the mechanisms of action of available drugs in order to identify potential new pharmacological targets and therapeutic indications. Besides exerting potential direct antiviral effects, baricitinib might prevent the dysregulated production of pro-inflammatory cytokines typically observed in COVID-19 patients via the inactivation of interleukin-6 (IL6)-JAK-signal transducer and activator of transcription (STAT) pathway (this activity will be more deeply discussed in section 3, especially regarding the JAK inhibitor ruxolitinib). Some clinical trials, also including placebo-controlled studies, are evaluating the safety and efficacy of baricitinb, mostly as 2-week add-on therapy in patients with mild to moderate COVID-19. Results from a small study in 12 patients with moderate COVID-19 pneumonia, treated with baricitinib in combination with lopinavir/ritonavir [NCT04358614], indicated that a 2-week oral treatment with the JAK1/JAK2 inhibitor was well tolerated. Moreover, although proper control groups were missing, the authors reported improved clinical and laboratory parameters [46]. A favorable clinical course was also reported in an 87‐year‐old woman, with mild‐to‐moderate COVID‐19, chronically treated with baricitinib for rheumatoid arthritis, who also received other pharmacological treatments to control viral infection (i.e., lopinavir/ritonavir, hydroxychloroquine) [47]. This patient was part of a family cluster of COVID-19, and the three other family members (husband, son and daughter) received the same antiviral therapy with the exception of baricitinib. Interestingly, the patient’s husband (90‐year‐old) and son (59‐year‐old) showed a rapid disease progression and died of respiratory failure. Baricitinib is currently investigated in clinical trials as single agent [NCT04340232, NCT04421027, NCT04393051, NCT04362943, NCT04399798, NCT04321993, NCT04390464] or in combination with hydroxychloroquine, lopinavir/ritonavir, remdesivir [NCT04373044, NCT04346147, NCT04320277, NCT04401579] for moderate to severe COVID-19 pneumonia.
2.1.3. Chloroquine and hydroxychloroquine with or without azithromycin
Chloroquine and hydroxychloroquine are among the most frequently used drugs for the treatment of COVID-19 patients in view of their potential inhibitory activity on virus entry. These quinolines are approved for the prevention and treatment of uncomplicated malaria and for the treatment of chronic inflammatory diseases such as systemic lupus erythematosus and rheumatoid arthritis. Both agents are generally regarded as inhibitors of the endocytic pathways through elevation of the endosomal pH which results in inactivation of lysosomal proteases, thus interfering with the fusion of virus and host cell membranes (reviewed in [48]). However, other mechanisms appear to contribute to their antiviral activity, including impaired receptor recognition by coronaviruses due to altered terminal glycosylation of ACE2 [49] and inhibition of viral attachment to the lipid raft as a consequence of a reduced interaction of the SARS-CoV-2 S protein N-terminal domain with membrane gangliosides [30]. Indeed, in vitro studies have demonstrated that chloroquine is able to block SARS-CoV-2 infection at low-micromolar concentrations [50], [51], [52]. Furthermore, chloroquine and hydroxychloroquine exhibit immunomodulatory activity since they reduce the Toll-like receptor (TLR) signaling (that plays a crucial role in the innate immune system) and production of inflammatory cytokines, as well as the expression of co-stimulatory molecules in T cells (for a comprehensive review see [53]). So far, however, there is not conclusive or robust clinical evidence on the usefulness of quinolines in COVID-19. Starting from mid-February 2020, chloroquine was included in the sixth version of the COVID-19 treatment guidelines by the National Health Commission of the People’s Republic of China. According to these guidelines, the initial recommended chloroquine dose was 500 mg twice daily for no more than 10 days; however, due to safety concerns the maximum therapy course was reduced to 7 days and a lower dose was recommended for patients weighing less than 50 kg. Based on clinical trials conducted in China in more than 10 hospitals, treatment of >100 patients with chloroquine is superior to control treatment in preventing pneumonia exacerbation, improving lung imaging results, accelerating virus-negative conversion, and shortening the disease course [54]. However, detailed information on the study design, patient characteristics or control treatment were not provided. The results of a blinded, randomized, controlled Chinese trial for COVID-19 pneumonia, reported a significant improvement in terms of symptoms and CT findings in patients treated with hydroxychloroquine (n = 31; 400 mg/day for 5 days) compared to the control group (n = 31) [55]. Conversely, in a previous pilot study in 30 treatment-naïve patients with confirmed COVID-19, hydroxychloroquine did not show any clinical benefit [56]. A French study on a cohort of 80 patients with severe COVID-19 treated with hydroxychloroquine (600 mg/day for 10 days plus the macrolide antibiotic azithromycin for 5 days) did not reveal antiviral activity or clinical benefit [57]. A published interim analysis of a double-blind, randomized, phase 2b clinical trial [NCT04323527] performed in Brazil, after enrollment of the first 81 patients with severe ARDS treated with high and low chloroquine doses (i.e., 600 mg/twice/day for 10 days, n = 41; 450 mg twice daily on day 1 and once daily for 4 days, n = 40) indicated that the high-dosage group showed a higher incidence of cardiotoxic effects (QTc interval prolongation) and a higher mortality rate compared to the low-dosage group (39% vs 13%) [58]. All these patients also received the macrolide antibiotic azithromycin that may induce cardiotoxic effects. These preliminary data indicate that high chloroquine dosage should not be recommended for treating critically ill COVID-19 patients. Although hydroxychloroquine is better tolerated than chloroquine, both agents may cause in the long-term life-threatening arrhythmias (an effect increased by the concomitant use of azithromycin), leucopenia, neuropsychiatric effects and retinopathy. In addition, quinoline overdose can lead to cardiovascular collapse, seizures and coma [59]. Therefore, the use of chloroquine/hydroxychloroquine for COVID-19 management requires a careful patient selection and monitoring.
Based on the initial publication in The Lancet of the results of a multinational registry analysis conducted by Surgisphere Corporation, showing that treatment with hydroxychloroquine/chloroquine (with or without a macrolide) in hospitalized COVID-19 patients (n = 14,888) failed to induce clinical benefit and was associated with higher risk of death and cardiovascular complications compared to control treatment (n = 81,144) [60], on May 23, 2020, the WHO temporarily halted the Solidarity Trial arm with chloroquine/hydroxychloroquine. Thereafter, the article was retracted by three of the four co-authors of the original article since Surgisphere (owned by one of the authors) did not make available to a third-party audit the complete dataset used for the study [61]. Thus, on June 3, 2020, the WHO announced that there was no reason to modify the Solidarity trial protocol and the arm with quinolines was resumed. Nevertheless, on the basis of a low benefit/risk ratio, the FDA retracted the Emergency Use Authorization (EUA) previously issued to hydroxychloroquine for use in COVID-19 hospitalized patients outside of clinical trials. To have a clear view on the overall risk–benefit ratio of using chloroquine/hydroxychloroquine especially in severely ill COVID-19 patients we will have to wait for the conclusion of well-designed, multi-center, randomized, controlled studies. Actually, ClinicalTrials.gov lists a number of phase 3 studies testing chloroquine and more frequently hydroxychloroquine, alone [e.g., NCT04364815; NCT04340544; NCT04334382; NCT04342221; NCT04315896; NCT04261517; NCT04420247; NCT04325893] or in association with other therapies (e.g., azithromycin, camostat, lopinavir/ritonavir, favipiravir, convalescent plasma) for mild to moderate or severe COVID-19 [e.g., NCT04328272; NCT04405921; NCT04358081; NCT04339816; NCT04321278; NCT04355052; NCT04353271; NCT04403100; NCT04411433; NCT04332835]. Some of these studies are specifically designed for evaluating the efficacy of hydroxychloroquine treatment in pregnant women who are at high-risk of severe complications and mortality from COVID-19 [NCT04365231; NCT04410562]. A variety of clinical trials are instead evaluating hydroxychloroquine for pre- and post-exposure prophylaxis [NCT04346329; NCT04331834; NCT04328467; NCT04372017; NCT04397328; NCT04330144; NCT04408456; NCT04318015; NCT04349228; NCT04328285: NCT04352933; NCT04341441; NCT04363450; NCT04403100]. Most of these studies are randomized trials vs placebo or vs azithromycin, lopinavir/ritonavir, or standard supportive care.
The first published demonstration of a possible additional benefit deriving from the association of azithromycin with hydroxychloroquine was provided by the results of a French open-label single-arm study with 26 cases treated daily with 600 mg of hydroxychloroquine; six of them also received azithromycin (500 mg on the first day followed by 250 mg daily) to prevent bacterial infection. All patients treated with both drugs showed negative nasopharyngeal SARS-CoV-2 PCR conversion compared to 57.1% of those treated with hydroxychloroquine as single agent and 12.5% of the untreated ones [62]. The results of a French retrospective non-randomized study in a total of 1061 patients treated for at least 3 days with hydroxychloroquine plus azithromycin showed that early treatment with this drug combination was well-tolerated and associated with a very low fatality rate (0.9%) [63]. The mechanism underlying the potential azithromycin activity against SARS-CoV-2 still needs to be clarified; recently, it has been hypothesized that this antibiotic might inhibit CD147, a glycosylated transmembrane protein that would serve as additional receptor for SARS-CoV-2 cell invasion [64]. Furthermore, azithromycin might stimulate immune responses against the virus by inducing the synthesis of type I and III interferons, as demonstrated in epithelial cells collected from patients with chronic obstructive pulmonary disease [65]. As mentioned above, a number of clinical trials are currently evaluating azithromycin mostly in combination with hydroxychloroquine for the treatment of COVID-19 or as prophylaxis.
2.1.4. Nafamostat, camostat, bromhexin and gabexate mesylate as TMPRSS2 inhibitors
Another approach to inhibit SARS-CoV-2 infection consists in inhibiting the protease that cleaves the S protein, thus facilitating viral entry and activation. TMPRSS2 is an androgen-dependent enzyme over-expressed in the prostate cancer tissues and involved in modulating organ inflammation as in the case of pancreatitis. This enzyme is also present on airway epithelial cells, cardiac endothelium, microvascular endothelial cells, kidney, and digestive tract, all possible targets of SARS-CoV-2 infection. Since SARS-CoV-2 may cause endothelial dysfunction (as discussed in Section 3), which can lead to systemic thrombosis and associated complications, targeting of TMPRSS2 might represent a suitable strategy not only to prevent viral infection but also for the treatment of severely ill patients [66]. The non-selective TMPRSS2 inhibitors camostat mesylate and nafamostat mesylate were previously found to hamper cell infection by SARS-CoV infection in preclinical models [67], [68], [69] and both agents also inhibit SARS-CoV-2 infection, with nafamostat showing higher activity [27], [70], [71]. Furthermore, nafamostat and camostat possess anti-inflammatory activity in the airways by reducing inflammatory cytokine production as shown in a model of chronic allergen-induced asthma or of influenza virus infected tracheal epithelial cells [72], [73]. In addition, nafamostat is able to inhibit the coagulation and fibrinolytic systems, the kallikrein-kinin system, the complement cascade, and activation of protease-activated receptors [74]. Therefore, their anti-inflammatory, anti-coagulant and fibrinolytic properties might contribute to attenuate the symptoms and complications occurring in COVID-19 patients. Both agents are approved in Japan for the treatment of pancreatitis, and nafamostat is also used for disseminated intravascular coagulation and as anticoagulant in extracorporeal circulation. Three case reports of elderly COVID-19 patients with pneumonia, all taking antivirals like lopinavir/ritonavir and hydroxychloroquine, showed that the introduction of nafamostat induced clinical and radiological improvement without significant adverse effects [75]. A randomized, double-blind trial as well as another clinical trial testing nafamostat versus placebo or conventional therapy [RACONA study, NCT04352400; NCT04418128] are presently listed in ClinicalTrials.gov. Four clinical trials are evaluating camostat in COVID-19 hospitalized patients and/or outpatients as single agent versus placebo [NCT04321096; NCT04353284] and in combination with hydroxychloroquine versus azithromycin plus hydroxychloroquine [NCT04355052] or versus placebo [NCT04338906].
A mechanism similar to nafamostat and camostat (i.e., non-selective inhibition of TMPRSS2) has been hypothesized for gabexate mesylate which is a serine protease inhibitor marketed in Italy and Japan for the treatment of pancreatitis. Furthermore, bromhexin, an over-the counter mucolytic cough suppressant, has been proposed as selective TMPRSS2 inhibitor for the treatment and prevention of COVID-19 [76]. Based on the results of a molecules’ docking study, bromhexin might also interact with the main protease 3CLpro/Mpro thus potentially altering intracellular steps of SARS-CoV-2 replication [77]. In clinical trials, bromhexin is tested for the prevention of SARS-CoV-2 infection in health care personnel assisting infected patients as singe agent [NCT04405999] or in combination with low-dose hydroxychloroquine [NCT04340349], and for the treatment of COVID-19 pneumonia as add-on therapy with hydroxychloroquine [NCT04355026], interferon-α2b and umifenovir [NCT04273763] or the potassium-sparing diuretic spironolactone [NCT04424134]. The rationale for using spironolactone relies on its ability to increase the plasma levels of a soluble ACE2 form that might sequester SARS-CoV-2, thereby preventing its interaction with membrane-associated ACE2 and virus cell entry [78]. A similar therapeutic strategy for blocking virus entry into the pulmonary cells is to deliver high concentrations of a soluble form of ACE2 [79]. This strategy is tested in a placebo-controlled phase 2 clinical trial [NCT04335136] where APN01, a recombinant human ACE2, is administered to COVID-19 patients.
2.1.5. Teicoplanin
Inhibition of the viral spike protein cleavage by cathepsin L in the late endosome might also result in decreased SARS-CoV-2 entry into the cells [80]. Once SARS-CoV-2 reaches the endosomes, the cysteinyl proteinase cathepsin L is the main protease that cleaves the S1 subunit in the acidic endosome compartment releasing it from the S2 subunit. This process leads to the insertion of a fusion peptide into the endosome membrane, after which the viral and endosome membranes merge allowing the release of the viral RNA into the cytoplasm of the host cells. Thus, inhibition of cathepsin L might block both virus entry and the release of the viral RNA at the intracellular level. Among already available drugs capable of inhibiting cathepsin L, the glycopeptide teicoplanin, an antibiotic used for the treatment of Gram-positive resistant bacteria [e.g., methicillin-resistant Staphylococcus Aureus infections (MRSA)], has shown activity against numerous viruses, including SARS-CoV, MERS-CoV and SARS-CoV-2, in cellular in vitro models [81], [82], [83]. A preliminary observation on the feasibility and tolerability of a teicoplanin-based complementary therapy added to hydroxychloroquine/tocilizumab was reported in a cohort study with 21 patients affected by severe COVID-19 and in ICUs [84]. However, to our knowledge, no clinical trials are currently ongoing with teicoplanin for COVID-19 treatment.
2.2. Inhibitors of translation, processing and replication of SARS-CoV-2
Once inside the cell, SARS-CoV-2, like other coronaviruses, uses two third of its positive-sense single-stranded RNA genome as template to directly translate two open reading frames (ORF1a and ORF1ab), connected by a ribosomal frameshift site, into the two overlapping polyproteins, pp1a and pp1ab, which are afterward cleaved by viral proteases into 16 nonstructural proteins (nsps) [85]. Some nsps (including RNA-dependent RNA polymerase, helicase and other enzymatic activities required for mRNA capping and proofreading) eventually contribute to form the replication-transcription complex, which is anchored to double-membrane vesicles integrated into a reticulovesicular network of modified endoplasmic reticulum membranes, also including convoluted membranes [86], [87]. The nsps include the main protease (Mpro) (nsp5) –a chymotrypsin‐like protease (3CLpro)– and a papain‐like protease (PLpro) (nsp3) that are responsible for the polyprotein cleavage. The remaining viral RNA genome, through the action of the RNA-dependent RNA polymerase, generates a set of subgenomic mRNAs which are translated into accessory proteins and structural proteins which will form the viral particle: spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins. The S, E and M structural proteins are produced in the rough endoplasmic reticulum from where they move along the secretory pathway to the endoplasmic reticulum-Golgi apparatus intermediate compartment (ERGIC) for viral particle assembly. The viral genomic RNA is encapsulated by the nucleocapsid N protein that thereafter buds into the ERGIC and acquires a membrane containing the S, E and M structural proteins. Finally, the virus is released by exocytosis (Fig. 1).
2.2.1. Protease inhibitors
The 3CLpro/Mpro is highly conserved among various coronaviruses, and mutations in 3CLpro/Mpro are often lethal to the virus [88], [89]. Therefore, 3CLpro/Mpro is indispensable for viral replication and thus represents an attractive therapeutic target for inhibiting the coronavirus infection process [89]. This enzyme is a homodimeric cysteine protease whose recognition sequence at most sites of viral polyproteins is Leu-Gln↓(Ser,Ala,Gly). Several previous reports have indicated that the HIV aspartate protease inhibitors lopinavir and ritonavir have the potential to act also as SARS-CoV protease inhibitors through their binding to 3CLpro/Mpro [90], [91], [92], [93]. For HIV treatment, the two drugs are used in combination, but ritonavir is administered at a dose that does not affect HIV protease activity but rather inhibits the cytochrome P450 3A4-mediated metabolism of lopinavir, thus increasing its plasma levels. Both drugs bind to amino acid residues present at the active site of SARS-CoV-2 3CLpro/Mpro, with ritonavir showing higher number of atomic contacts, binding efficiency, and number of key binding residues compared to lopinavir [94]. However, the results of a randomized, controlled, open-label study performed in China (LOTUS trial) with 199 severe COVID-19 hospitalized patients indicated that lopinavir/ritonavir treatment was not more effective than standard of care in terms of time to clinical improvement, reduced mortality, or diminished viral RNA detection [95]. Moreover, lopinavir/ritonavir-based therapy was associated with increased gastrointestinal adverse effects. Interestingly, a post hoc subgroup analysis revealed that the difference in the 28-day mortality rate between lopinavir/ritonavir and standard of care groups was higher when patients treated within 12 days after symptom onset were compared to those treated later (rate differences: −12 vs −5.2) [95]. These findings suggested that the timing of administration after symptom onset is crucial for maximizing lopinavir/ritonavir efficacy [95], [96]. Indeed, in a study with 120 patients, early treatment with lopinavir/ritonavir (within 10 days from symptom onset) significantly reduced viral shedding duration compared to patient who were not treated with the protease inhibitors (median 19 days vs 28 days) [97]. Thus, this drug combination has been proposed as early treatment of mild COVID-19 cases to decrease viral load and to prevent disease worsening [98]. Numerous randomized controlled phase 2/3 trials are testing lopinavir/ritonavir doublet to define its therapeutic role in COVID-19 management, as single agents or in combination for mild, moderate to severe COVID-19 or as prophylaxis. For mild disease, lopinavir/ritonavir is used as single agent compared to hydroxychloroquine [NCT04307693], whereas for moderate to severe cases is tested in association with hydroxychloroquine and interferon-β1b with or without ribavirin [NCT04343768; NCT04276688] (for the combination of lopinavir/ritonavir with interferon see also Section 3). Lopinavir/ritonavir monotherapy is also evaluated as: i) early outpatient treatment for patients with risk factors of poor outcome (i.e., at least one comorbidity among hypertension, diabetes, obesity, cancer, chronic renal disease, immunodeficiency or age >70 years age; NCT04365582) or in adult patients with symptoms of acute respiratory infection for ≤ 6 days [NCT04372628]; ii) as prophylaxis in exposed healthcare workers [NCT04328285] or in adult patients undergoing elective or emergency surgery in a COVID-19 exposed environment to reduce COVID-19 related pulmonary complications [NCT04386070]; and iii) as post-exposure prophylaxis in individuals who have experienced high-risk close contact with a confirmed COVID-19 case during his/her symptomatic period [NCT04321174]. Some of these clinical trials are completed but results have not yet been published or posted in the ClinicalTrials.gov website.
Based on an integrated computational analysis, other HIV protease inhibitors (darunavir and saquinavir) were identified as potential 3CLpro/Mpro inhibitors of SARS-CoV-2 [99] and two clinical trials are testing darunavir in combination with the pharmacoenhancer cobicistat for the treatment of pneumonia caused by SARS-CoV-2 versus conventional treatment [NCT04252274] or versus lopinavir/ritonavir [NCT04425382]. However, a very recent in vitro study has shown that darunavir lacks antiviral activity against SARS-CoV-2 [100].
In the Chemical Abstracts Service (CAS) Registry of Chemical Substances for 3CLpro/Mpro a total number of 2178 potential drug candidates have been listed (https://www.cas.org/) [89]. Furthermore, based on a virtual docking prediction study, some HCV NS3/4A protease inhibitors (e.g., simeprevir, paritaprevir, grazoprevir, boceprevir, telaprevir) might also inhibit the 3CLpro/Mpro [101], [102]. However, none of these agents is currently clinically evaluated for COVID-19 treatment.
Concerning the other coronavirus protease PLpro, although considered another potential therapeutic target since it is crucial for viral replication, the development of SARS-CoV-2 PLpro inhibitors is still at an early stage [103], despite several investigational compounds have been found to efficiently inhibit the corresponding SARS-CoV and MERS-CoV enzyme [104].
2.2.2. RNA-dependent RNA polymerase inhibitors
Another therapeutic strategy for COVID-19 relies on the targeting of the viral RNA-dependent RNA polymerase (nsp 12) that catalyzes the synthesis of all viral RNA molecules, thus playing a central role in SARS-CoV-2 replication and transcription. In particular, the viral polymerase synthesizes a full-length complementary negative-strand RNA as template to produce positive-strand genome for the virus progeny and subgenomic mRNAs deriving from negative-sense RNA intermediates. Since the structure of SARS-CoV-2 polymerase is similar to that of other positive sense RNA viruses and some catalytic amino acid residues in the active site are conserved in most viral polymerases [105], several nucleoside/nucleotide analogues used for the treatment of other viral infections have been repurposed for COVID-19.
2.2.2.1. Remdesivir
The adenosine analogue remdesivir is one the most frequently tested anti-SARS-CoV-2 agents and has been firstly approved in Japan for severe COVID-19. Remdesivir was originally developed for RNA virus infections and tested for Ebola during the 2018 outbreak in Democratic Republic of the Congo but failed to show clinical benefit. It has a broad-spectrum antiviral activity, including MERS-CoV, SARS-CoV and SARS-CoV-2, both in vitro and in vivo in animal models [52], [106], [107], [108], [109]. Remdesivir is a prodrug that, after diffusion into the cells, is metabolized to the alanine metabolite GS-704277 and further converted into a nucleoside monophosphate, which is highly polar and remains trapped within the cell [106]. Host cell kinases eventually convert the monophosphate derivative into a triphosphate nucleotide that is misincorporated into the nascent RNA chain by the RNA dependent RNA polymerase with consequent inhibition of the RNA synthesis [110]. Remdesivir has been found to interact with the SARS-CoV-2 polymerase, competing with the physiological ATP nucleotide, and to behave as delayed-chain-terminator, since RNA synthesis is terminated after the addition of three nucleotides [105], [111], [112]. It should be noted that the efficacy of remdesivir or of other nucleoside/nucleotide-based agents, whose activity relies on their misincorporation into the viral genome, might be counteracted by a coronavirus proofreading exoribonuclease (nsp14) that would enable the virus to evade the pharmacological inhibition [113].
Intravenous remdesivir was used to treat the first COVID-19 patient diagnosed in the US with rapid improvement of the clinical conditions [114] and is regarded as one of the most promising agents for SARS-CoV-2. Presently, the drug is included in the National Institutes of Health (NIH) guidelines for the medical management of severe COVID-19 cases (https://www.covid19treatmentguidelines.nih.gov/whats-new/, accessed June 14, 2020). Interim analysis of a randomized, double-blinded, placebo-controlled trial [Adaptive COVID-19 Treatment Trial, ACTT-1, NCT04280705], conducted by the National Institute of Allergy and Infectious Diseases of NIH and involving 1062 hospitalized patients, showed remdesivir superiority over placebo. In particular, patients treated with remdesivir (n = 538; 200 mg at day 1, followed by 100 mg daily for additional 9 days) had a significant shorter time to recovery (median 11 vs 15 days; p < 0.001) and a lower mortality rate (7.1% vs 11.9) compared to those who received placebo (n = 521) [115]. Following these results, the FDA has issued an emergency use authorization for remdesivir. Thereafter, on June 25, 2020, EMA’s human medicines committee (CHMP) has recommended granting a conditional marketing authorization to remdesivir for treating adults and adolescents (>12 years of age) with pneumonia who require supplemental oxygen. An open-label, phase 3 study on 397 patients with severe COVID-19 did not find differences between 5-day and 10-day courses of remdesivir administered as add-on therapy to standard of care [NCT04292899] [116]. A recent exploratory analysis, performed by the remdesvir’s sponsor and presented at the Virtual COVID-19 Conference (in the context of the 23rd International AIDS Conference), revealed a 62% reduction in the risk of mortality when 312 patients enrolled in the NCT04292899 trial were compared to a retrospective cohort of 818 patients receiving standard of care treatment in the same time period. However, to know the real impact of remdesivir on mortality risk of COVID-19 patients we will need to wait for the results of prospective, placebo-controlled phase 3 studies.
Conversely, in a randomized clinical trial [NCT04257656] conducted in China, the intravenous administration of remdesivir (at the same dose used in the ACTT trial) to adult patients with severe COVID-19 (n = 158) was not associated with significant clinical benefits compared to placebo (n = 79) [117]. In an uncontrolled study where 61 patients (64% receiving mechanical ventilation) were treated with remdesivir on a compassionate-use basis, clinical improvement at 28 days was observed in 68% of patients [118]. However, this trial raised several criticisms on the study design and result interpretation, due to lack of control, small sample size, inappropriate data censoring, high variability of disease severity [119], [120], [121], [122]. In another study, remdesivir was administered as compassionate treatment to 32 hospitalized patients (18 of whom in ICU) and beneficial effects were observed on SARS-CoV-2 pneumonia, mainly in non-critically ill patients [123].
Remdesvir is usually well-tolerated for short courses. However, concerns were raised about its potential toxicity in patients with kidney dysfunction, related not only to the drug-mediated injury of renal tubular epithelial cells, but also to the nephrotoxicity associated with the drug vehicle (i.e., sulfobutylether-β-cyclodextrin) required for the intravenous formulation [124]. Several ongoing randomized phase 3 clinical trials are evaluating remdesivir in patients with severe COVID-19, as single agent versus a) placebo [the above mentioned ACTT trial], b) standard of care [NCT04292899, testing different drug schedules in not mechanically ventilated and mechanically ventilated patients], c) lopinavir/ritonavir, lopinavir/ritonavir plus interferon-β1a, hydroxyquinoline, and standard of care [NCT04315948], or in patients with moderate COVID-19 versus standard of care [NCT04292730; testing two different drug schedules]. In other randomized phase 3 studies, the safety and efficacy of remdesivir is evaluated in combination with different anti-COVID-19 agents: hydroxychloroquine [NCT04321616], tocilizumab [NCT04409262], baricitinib [NCT04401579]. Moreover, one of the arms of the ongoing WHO-promoted Solidarity trial includes intravenous remdesivir.
2.2.2.2. Favipiravir
Another nucleoside analogue used for COVID-19 is favipiravir, which acts as a competitive inhibitor of the RNA-dependent RNA polymerase. This agent was previously approved in Japan for the treatment of influenza A and B and, in particular, for novel or re-emerging influenza viruses [125]. Presently, the drug has been approved in Russia for COVID-19 and is investigated worldwide. Favipiravir, after undergoing intracellular tri-phosphorylation, exerts antiviral effects as a guanosine analogue, through several mechanisms including chain termination, slowed RNA synthesis and lethal mutagenesis (due to C-to-U and G-to-A transitions favored by the low cytosine content of SARS-CoV-2 genome) [126]. The results of an open-label non-randomized clinical study performed in China indicated that orally administered favipiravir (day 1: 1600 mg/twice daily; days 2–14: 600 mg/twice daily) plus aerosol inhaled interferon-α (n = 35) induced faster viral clearance, higher improvement rate in chest imaging and fewer adverse effects compared to lopinavir/ritonavir (n = 45) [127]. However, it should be noted that COVID-19 patients with severe clinical conditions or with chronic liver and kidney diseases at an advanced stage were excluded from this study [127]. Randomized phase 2/3 clinical trials are evaluating the actual therapeutic role of favipiravir in mild/moderate COVID-19 as single agent, versus standard of care and/or placebo [NCT04346628; NCT04336904; NCT04425460; NCT04358549; NCT04349241; NCT04402203; NCT04387760] or versus hydroxychloroquine with or without azithromycin [NCT04373733; NCT04359615], and in combination with hydroxychloroquine or azithromycin [NCT04411433; NCT04392973], or tocilizumab [NCT04310228].
2.2.2.3. Other nucleoside/nucleotide analogues
Based on a molecular docking study, other nucleoside/nucleotide analogues, either approved for different viral infections (i.e., ribavirin, tenofovir, sofosbuvir, telbivudine) or under clinical investigation (i.e., galidesivir, EIDD-2801) were found to bind and potentially inhibit the SARS-CoV-2 RNA dependent RNA polymerase [128], [129]. Ribavirin, is a guanosine analog with a wide antiviral spectrum against RNA and DNA viruses that, besides inhibiting the viral polymerase catalytic activity, interferes with RNA capping and blocks the synthesis of guanosine by inhibiting the inosine monophosphate dehydrogenase, a crucial enzyme involved in the de novo synthesis of purines [130]. The drug is mainly used for the treatment of infections caused by respiratory syncytial virus, HCV, and viral hemorrhagic fever. Ribavirin was also tested in previous SARS-CoV and MERS-CoV outbreaks with conflicting results [131]. Regarding SARS-CoV-2, ribavirin was found to inhibit virus replication at micromolar concentrations [132] and in a completed randomized, phase 2 trial [NCT04276688] recruiting a total of 127 patients with mild to moderate COVID-19 the addition of ribavirin together with interferon-β to lopinavir/ritonavir was more effective than lopinavir/ritonavir [133]; see also Section 3. A still not recruiting clinical trial for COVID-19 patients without comorbidities is planning to evaluate ribavirin in combination with nitazoxanide and ivermectin [NCT04392427], which are both anti-parasitic agents shown to potentiate interferon-α and -β production and consequently immune responses [134]. However, the above-mentioned clinical trials do not allow to clarify the actual contribution of ribavirin on disease course. On the other hand, a phase 1 study is evaluating a ribavirin inhaled formulation as single agent for hospitalized adult patients with respiratory distress due to COVID-19 [NCT04356677].
Tenofovir, an adenosine nucleotide analog approved for the treatment of HIV or HBV chronic infection, was found to be efficiently incorporated by SARS-CoV-2 RNA dependent RNA polymerase terminating the polymerase reaction [135]. Co-formulated with emtricitabine, tenofovir is regarded as a highly effective component of the antiretroviral therapy of HIV infected patients and a first-line option for HIV pre- and post-exposure prophylaxis. In line with the latter therapeutic approach, two phase 3 randomized clinical trials have been planned to assess tenofovir/emtricitabine for pre-exposure prophylaxis of COVID-19 in healthcare workers who are at risk of SARS-CoV-2 infection [NCT04405271; NCT04334928].
Among drugs not yet approved for specific indications that have been considered for SARS-CoV-2, galidesivir is an adenosine analog tested in a phase 1 trial aimed at analyzing its pharmacokinetics, safety and antiviral effects in patients with yellow fever or with moderate-severe (but not critically ill) COVID-19 [NCT03891420]. Another investigational agent for COVID-19, is represented by EIDD-2801, an orally bioavailable prodrug of the ribonucleoside analog EIDD-1931 with broad-spectrum antiviral activity against various unrelated RNA viruses. This compound inhibits the infection by SARS-CoV-2, MERS-CoV and SARS-CoV in primary cultures of human airway epithelial cells [136]. Moreover, administration of EIDD-2801 to mice infected with SARS-CoV or MERS-CoV significantly improved the pulmonary function and reduced the viral load [131]. The antiviral effects exhibited by EIDD-1931 have been attributed to its ability to introduce multiple lethal transition mutations across the viral RNA, but not in the host RNA, that would allow predicting a high barrier to the development of drug resistance [137]. Two randomized phase 2 clinical trials are testing EIDD-2801 safety, tolerability and efficacy on virus clearance in newly hospitalized [NCT04405739] or symptomatic adult outpatients with COVID-19 [NCT04405570].
3. Drugs used to treat the acute respiratory distress, cytokine release syndromes and cardiovascular complications
Especially in elderly patients or in the presence of comorbidities, COVID-19-associated mortality is frequently due to the inability to resolve the viral infection and the overwhelming inflammatory response. Severe lung and systemic inflammation in COVID-19 patients has been attributed to a dysregulated response of the innate immune system to the viral infection with an excessive release of a number of cytokines (“cytokine storm”) [138]. The uncontrolled inflammatory response induces life-threatening damage in the lung tissue and/or dysfunction in both micro and macro-circulation, which may lead to cardiac arrest or myocardial infarction. The cytokine storm is also associated with lymphocytopenia as a result of an inefficient adaptive immune response [139], [140], [141]. Moreover in patients who died from COVID-19, histological examination of the lungs showed bilateral diffuse alveolar damage with proteinaceous edema, reactive type II pneumocyte hyperplasia with multinucleated cells, and mononuclear cell infiltration [142], [143]. The resulting increase of the alveolar exudate contributes to impede alveolar gas exchange worsening hypoxia [144].
Once inside the cell, the viral RNAs are detected by the pattern recognition receptors [PRRs, mostly by TLRs), expressed in the cells of the innate immune system. PRRs/TLRs trigger a downstream cascade of proteins which activate the transcription factor nuclear factor-kB (NF-kB) and interferon regulatory factor 3 (IRF3), leading to the production of type I interferons (α and β) and a variety of pro-inflammatory cytokines [145], [146]. Indeed, patients with severe COVID-19 have increased plasma concentrations of tumor necrosis factor α (TNFα), IL2, IL7, and IL10, granulocyte-colony stimulating factor (G-CSF), monocyte chemoattractant protein 1 (MCP1), MCP3, macrophage inflammatory protein 1 α (MIP1α), and interferon-γ-inducible protein 10 (IP-10), which indicate the occurrence of a cytokine storm [147], [148], [149]. High levels of IL6 and IL8 also contribute to hypercoagulation due to activation of the complement and coagulation cascades, causing disseminated intravascular coagulation [150], [151].
Waiting for an effective antiviral therapy or vaccine against the virus, any treatment that can decrease the severe symptoms of COVID-19 may help to attenuate the mortality rates and to improve the quality of life of severely ill patients. In this regard, several pharmacological therapies, with different mechanisms of action, have been used in order to improve the symptoms related to COVID-19. In the next section, we will consider the agents directed against the: a) cytokine storm and b) cardiovascular damage. The first category includes: i) anti-cytokine drugs, such as tocilizumab, sarilumab, siltuximab, olokizumab, ruxolitinib, baricitinib, anakinra, emapalumab, mavrilimumab; and ii) immunomodulating agents, such as interferon-β, interferon-α or interferon-λ, fingolimod, ozanimod, opaganib, CD24Fc, allogenic mesenchymal stem cells and the lately reappraised dexamethasone. The second group comprises: the anti-C5 complement monoclonal antibodies (mAbs) eculizumab and ravulizumab; anti-thrombotic and fibrinolytic agents; the phosphodiesterase type 5 inhibitor sildenafil; the vasoactive intestinal polypeptide analog aviptadil; and the anti VEGF-A mAb bevacizumab (Fig. 2). However, the effects of the two drug sets are closely interconnected, as many drugs that have an impact on the circulatory system may also reduce circulating inflammatory cytokines.
3.1. Drugs used to counteract the cytokine storm
3.1.1. Anti-cytokine drugs
3.1.1.1. Inhibitors of the IL6 pathway
The first therapeutic agent used to counteract the inflammatory reaction in patients with COVID-19 pneumonia was tocilizumab, a humanized IgG1κ mAb that interacts with membrane (IL6R) or soluble IL6 (sIL6R) receptors preventing the binding of IL6 and the downstream activation of JAK/STAT and Ras/mitogen-activated protein (MAP) kinase pathways. Both IL6 receptors form complexes with the gp130 protein, and, by doing so, IL6R activates the classical (anti-inflammatory) signaling that is restricted to a limited number of cell types (hepatocytes and leukocyte subtypes). Conversely, sIL6R triggers the trans-signaling (pro-inflammatory) pathway in all somatic cells, even in the absence of IL6R, since gp130 is ubiquitously expressed (reviewed in [152]). During inflammation, the sIL6R increases allowing IL6 to stimulate cells, like endothelial and smooth muscle cells, that normally are not activated by the cytokine [153]. Thus, blockage of IL6 binding to its receptors by tocilizumab would reduce both classical and trans-signaling pathways, although the anti-inflammatory effect is expected to derive mostly from inhibition of the trans-signaling pathways [154]. Tocilizumab is currently FDA- and EMA-approved for rheumatoid arthritis, systemic juvenile idiopathic arthritis, polyarticular juvenile idiopathic arthritis, idiopathic multicentric Castleman’s disease, and cytokine release syndrome associated with chimeric antigen receptors (CAR) T-cell therapy. The rationale for using tocilizumab in COVID-19 patients relies on IL6 role in the cytokine storm symptoms and on the finding that high baseline circulating IL6 is associated with COVID-19 severity and further increased cytokine levels correlate with disease exacerbation [155], [156], [157]. Furthermore, since IL6 may favor coagulation activation (see above) blockade of this cytokine might have a favorable impact on severely ill COVID-19 patients with associated disseminated intravascular coagulation or thrombotic microangiopathy.
In ClinicalTrials.gov ~30 phase 2/3 studies are specifically testing tocilizumab in COVID-19 patients with pneumonia or with cytokine release syndrome. In most studies tocilizumab is used as single agent compared [e.g., NCT04335071] or not [e.g., NCT04317092, NCT04377659] to placebo, to standard of care or best supportive care [e.g., NCT04306705; NCT04403685] or to other agents such as the IL1β receptor antagonist anakinra [e.g., NCT04339712], the iron chelator deferoxamine [NCT04361032], the anti-PD1 mAb nivolumab or a chloroquine analog [NCT04333914], and intravenous methylprednisolone [NCT04345445]. In additional studies, the anti-IL6 receptor mAb is tested in combination with other drugs: faviparavir [NCT04310228], the anti-PD-1 mAb pembrolizumab [NCT04335305], anakinra or the anti-IL6 mAb siltuximab (as single agents or in combination) [NCT04330638].
Several studies, mostly Italian and often single-center, have recently reported the results of the clinical experience accumulated so far with the off-label use of tocilizumab for COVID-19 patients, with some contrasting results. Among them, an open-label prospective study in 51 patients with severe COVID-19 pneumonia and high IL6 plasma levels showed that the anti-IL6 receptor mAb rapidly reduced fever and inflammatory markers and restored lymphocytopenia, although failing to affect the clinical outcome [158]. According to a retrospective observational study, higher survival rates (92% vs 42.1%) were observed in patients treated with tocilizumab (n = 62) compared to those who did not have access to this therapy (n = 23; historic control) [159]. A non-significant lower mortality rate (15% vs 33%; p = 0.15) was reported by another Italian retrospective study after 28-day follow-up of tocilizumab-treated patients (n = 32) compared to patients receiving standard of care (n = 33) [160]. Likewise, a pilot prospective open-label, single-arm multicenter study testing tocilizumab administration to 63 patients within 6 days from hospital admission reported an improvement of respiratory function and laboratory parameters and increased likelihood of survival (HR 2.2 95% CI 1.3–6.7, p < 0.05) [161]. A small French prospective study reported a significant decrease in the number of ICU admissions and/or mortality in tocilizumab treated patients (25% vs 72%, P = 0.002) [162]. Furthermore, a larger study on 100 patients with COVID-19 pneumonia accompanied by hyperinflammatory syndrome and acute respiratory failure described improvement or stabilization of the respiratory conditions in 77% of patients [163]. Case reports also supported the potential benefit from tocilizumab treatment [164]. A small Chinese retrospective study in 21 patients showed that tocilizumab is associated with rapid improvement of the clinical symptoms and hypoxemia, and prevention of clinical worsening in severe COVID-19 patients, without serious adverse events [165]. However, tocilizumab did not reduce ICU admission and mortality rates in 21 critically ill patients with severe COVID-19 pneumonia [166]. Tocilizumab treatment was found to be associated with an initial rise in IL6 levels, as an expected consequence of the mAb-mediated inhibition of IL6 interaction with its receptors, followed by a significant decrease of the C-reactive protein inflammatory marker [167]. However, the D-dimer, measured as an indicator of intravascular fibrin formation, remained unaffected suggesting that tocilizumab might have limited effect on the activation of the coagulation cascade [163]. Furthermore, the clinical response to tocilizumab seems to be negatively affected by hyperglycemia, shown to be associated with increased IL6 levels [168]. In regard to tocilizumab safety, concerns have been raised about the risk of candidemia, septic shock and possible occurrence of intestinal perforation (an adverse effects reported in rheumatoid arthritis patients), which may be favored by the altered hemodynamics observed in critically ill COVID-19 patients [163], [169], [170], [171]. Based on these initial data, the efficacy and safety of tocilizumab in severe COVID-19 need to be corroborated by the results of the ongoing randomized controlled clinical trials.
Clinical studies are also testing the other anti-IL6 receptor mAb sarilumab (FDA- and EMA-approved for rheumatoid arthritis) and anti-IL6 mAbs such as siltuximab (FDA- and EMA-approved for multicentric Castleman’s disease) and the investigational mAb olokizumab. Like tocilizumab, sarilumab is able to bind both IL6R and sIL6R, but being a fully human mAb, has a lower risk of inducing neutralizing antibodies and allergic reactions compared to chimeric/humanized mAbs [172]. Phase 2 and 3 clinical trials have been designed in order to evaluate intravenous [NCT04324073, NCT04327388, NCT04315298] or subcutaneous [NCT04357860, NCT04357808, NCT04359901] administration of sarilumab in hospitalized patients with moderate-severe COVID-19. The anti-IL6 mAb siltuximab is evaluated as single agent for COVID19 pneumonia versus intravenous infusion of methylprednisolone [NCT04329650]. In regard to olokizumab, a phase 2/3 study is evaluating the efficacy and safety of this mAb administered subcutaneously versus placebo or RPH-104, a macromolecule capable of sequestering IL1β, in severe COVID-19 patients [NCT04380519].
Since in SARS-CoV-2 infected cells and in immune cells the production and activity of IL6 is strictly dependent on the JAK/STAT pathway [173], [174], JAK inhibitors have also been used in COVID-19 patients with the aim of reducing the excessive inflammatory reaction. Among these, the orally administered ruxolitinib is a JAK1/JAK2 small-molecule inhibitor approved for the treatment of myelofibrosis, polycythemia vera, and graft-versus-host disease [175]. Consistently with its mechanism of action, in patients with myelofibrosis, ruxolitinib was able to reduce IL6 and TNFα levels and was well-tolerated [176]. In ClinicalTrials.gov several studies are recruiting patients with COVID-19 pneumonia or cytokine storm to test ruxolitinib as single agent [e.g., NCT04362137, NCT04334044] or in combination with anakinra [NCT04366232], simvastatin [NCT04348695], or therapeutic plasma exchange [NCT04374149]. Although regarded as a well-tolerated agent, ruxolitinib may increase the risk of opportunistic infections and, in the context of an inflammatory state, JAK inhibition may exacerbate thrombocytopenia and anemia [177]. Furthermore, in COVID-19 patients two case reports of diffuse skin reactions with purpuras and a rapid decrease of hematocrit values were described [178]. Baricitinib is another JAK inhibitor that besides interrupting the JAK1/JAK2-dependent signaling involved in cytokine-mediated inflammatory response to the SARS-Cov-2 infection, might also exert direct antiviral effects by blocking virus entry (see Section 2).
3.1.1.2. IL1 inhibitor
Anakinra is a recombinant, non-glycosylated form of the natural occurring human interleukin-1 receptor antagonist (IL-1Ra) that neutralizes the biological activity of IL1α and IL1β by competitively inhibiting their binding to IL1 type I receptor (IL1RI). IL1β is a cytokine that contributes to the host defenses against infections by activating phagocytes as well as lymphocyte Th1- and Th17-mediated adaptive immune responses. The latter are involved in cell-mediated immunity against intracellular pathogens, including viruses, and in the pathogenesis of inflammatory disorders, respectively [179]. Anakinra is approved by FDA and EMA for rheumatoid arthritis and cryopyrin-associated periodic syndrome, and, by EMA only, for familial Mediterranean fever and Still’s disease, to reduce inflammation. The rationale for the use of anakinra in the treatment of SARS-CoV-2 infection relies on its ability to interfere with the macrophage activation syndrome, i.e. secondary hemophagocytic lymphohistiocytosis (sHLH), which leads to a massive production of IL1β. This syndrome is characterized by pancytopenia, hyper-coagulation, acute kidney injury, and hepatobiliary dysfunction, and is associated with a high mortality rate. The risk of developing this syndrome can be measured by evaluating the hemophagocytosis score (HScore). An European study has shown that intravenous administration of anakinra, followed by subcutaneous injection, to eight COVID-19 patients affected by sHLH improved the respiratory function and reduced the mortality rate compared to historical values reported for patients with sHLH during sepsis (43% vs 67%) [180]. Positive outcomes were described in other studies or case reports of patients with severe/moderate COVID-19 ARDS after intravenous treatment with high doses of anakinra as single agent [181], [182], [183] or in combination with remdesivir [184]. In particular, Cavalli et al. reported a 10% mortality rate (3 out 29 patients) that was significantly lower than that observed in patients receiving standard-of-care treatment (44%; 7 out of 16 patients) [182]. In a proof-of-concept study, subcutaneous injection of anakinra in nine patients with moderate-severe COVID-19 pneumonia produced a favorable outcome in the majority of patients with only one of them requiring immediate treatment discontinuation after the first dose due to respiratory failure [185]. Different clinical trials are recruiting COVID-19 hospitalized patients to test the safety/efficacy of anakinra, usually in comparison with other drugs used off-label or placebo, as single agent or in combination with ruxolitinib [NCT04366232], siltuximab or tocilizumab [NCT04330638, NCT04339712], and emapalumab [NCT04324021]. The latter is a recombinant, human IgG1 mAb directed against interferon-γ, which inhibits its binding to cell surface receptors and the subsequent activation of intracellular pro-inflammatory signaling pathways. Emapalumab is FDA-approved to treat the severe inflammatory condition of primary HLH in which serum interferon-γ levels are elevated [186].
3.1.1.3. Granulocyte-macrophage colony-stimulating factor (GM-CSF) inhibitor
Blockade of GM-CSF, a cytokine capable of stimulating the secretion of a number of pro-inflammatory mediators (e.g., IL1. IL6, TNFα) by the effectors cells of the innate immune system has also been explored for the management of COVID-19 patients with pneumonia and hyper-inflammation using the anti-GM-CSF mAb mavrilimumab [NCT04399980; NCT04397497]. In an Italian single-center study, treatment with mavrilimumab of 13 non-mechanically ventilated patients with severe COVID-19 pneumonia and systemic hyperinflammation was well tolerated and associated with some clinical benefit [187]. In fact, during a 28-day follow-up only 8% of mavrilimumab-treated patients progressed to mechanical ventilation compared with 35% of patients in the control group (9 out 26 patients).
3.1.2. Immunomodulating agents
3.1.2.1. Interferons
During a viral infection, type I and III interferons are generally produced within minutes to hours post-infection. Both interferon types possess antiviral, antiproliferative, and immunomodulatory activities through activation of similar signaling pathways and transcriptional responses. Type I interferons (α, β, ε, κ, ω in humans) exert biological effects after binding to a ubiquitously expressed type I membrane receptor (IFNAR complex containing the IFNAR1/IFNAR2 subunits), whereas type III interferons (intereferon-λ) interact with type III interferon receptor (IFNLR1 that heterodimerizes with IL10Rβ), preferentially expressed on epithelial cells and neutrophils [188]. After ligand binding, both receptors activate the JAK/STAT signaling pathways through recruitment of TYK2 and JAK1 that phosphorylate STAT1 and STAT2. Phosphorylated STAT1/STAT2 forms a complex with IRF9 generating the transcription factor ISGF3, which eventually promotes the expression of interferon‐stimulated genes in infected and neighboring cells activating an intracellular program that limits virus spreading [189]. Among the different genes stimulated by interferons, two of them play a crucial role in their antiviral activity: 2′,5′-oligoadenylate (2-5A) synthase and the protein kinase R (or RNA-activated protein kinase). The 2-5A synthase generates 2′,5′-linked oligoadenylates that activate RNase L, which in turn cleaves both cellular and viral single-stranded RNAs, whereas the protein kinase R inactivates the eukaryotic initiation factor eIF2α, a protein involved in protein synthesis. Thus, the major interferon antiviral effect relies on inhibition of RNA translation. Type I interferons modulate also immune responses against viruses, through enhancing antigen presentation, co-stimulation, and cytokine production by the effector cells of innate immune system, leading to enhanced adaptive immune responses [190]. The response mediated by type I interferons is more potent, rapid, transient, diffuse and inflammatory, whereas the type III interferon response is less potent, slower, sustained, anatomically restricted and less inflammatory [188].
Interestingly, the cytokine storm associated with COVID-19 is due to an uncontrolled response of the immune system to SARS-CoV2 viral infection that leads not to only to an excessive production of cytokines but also a diminished/delayed interferon response [191], [192], [193]. Based on preclinical studies and observations in SARS-CoV or MERS-CoV infected patients, the outcome of the interferon-mediated response to the viral infection seems to depend on the viral load and integrity of the host immune system. In particular, if the initial viral burden is low, type I interferons are promptly released and efficiently clear the infection; conversely, if the viral load is high or in elderly patients the early interferon production is hampered and a delayed interferon-mediated response may not only fail to control the infection but also result in inflammation and lung damage [194]. Thus, exogenously administered type I interferons would have protective effects as prophylaxis or in the early stage of SARS-CoV-2 infection, whereas they may deteriorate tissue injury and pneumonia when administration is delayed. Concerning the potential therapeutic role of interferon-λ, the lack of pro-inflammatory systemic effects would allow its safe administration also in an advanced phase of the infection [194], [195], [196]. Although better tolerated than type I interferons that may cause severe systemic side effects due to the ubiquitous expression of IFNAR, a possible disadvantage of interferon-λ is its lack of antiviral effects on infected alveolar macrophages or endothelial cells that do not express IFNLR1 but may serve as virus reservoir [197].
Presently, only type I interferons are clinically approved (for multiple sclerosis, viral hepatitis and cancer), whereas interferon-λ is investigated for hepatitis D virus infection. For COVID-19 hospitalized patients, several clinical trials are testing type I interferons; in particular: i) parenteral interferon-β1a or interferon-β1b in combination with lopinavir/ritonavir, lopinavir/ritonavir plus hydroxychloroquine and/or umifenovir or plus ribavirin [NCT04350671, NCT04343768, NCT04350684, NCT04276688] or with hydroxychloroquine/chloroquine [NCT04350281], or hydroxychloroquine and azithromycin [NCT04324463]; ii) inhaled pegylated interferon-α2b as single agent versus placebo [NCT04385095] or with umifenovir [NCT04254874]. In an additional trial, interferon-α1b as nasal drops is assessed for low- or high-risk medical staff exposed to SARS-CoV2 infected patients, as single agent or combined with thymosin α1, respectively [NCT04320238]. In regard to interferon-λ, the safety and efficacy of subcutaneous pegylated interferon-λ is tested as immediate/early therapy in non-critically ill hospitalized or ambulatory patients [NCT04343976, NCT04388709, NCT04354259] or as prophylaxis in non-hospitalized individuals exposed to patients with COVID-19 [NCT04344600]. Subcutaneous interferon-β1b plus lopinavir/ritonavir represents one arm in the ongoing WHO-promoted Solidarity trial. Furthermore, inhaled interferon-α in combination with ribavirin is included in the China’s National Health Commission guidelines for COVID-19 treatment. The recently published results of a multicenter, prospective, open-label, randomized, phase 2 trial performed in China, testing early treatment with lopinavir/ritonavir, ribavirin plus interferon-β1b versus lopinavir/ritonavir in patients with mild to moderate COVID-19, showed that the triple combination is safe and more effective in shortening the duration of virus shedding and hospital stay [133]. However, interferon-including therapies pose some concerns based on the recently reported findings showing that ACE2 is an interferon-responsive gene and that in human nasal epithelia and lung tissue this cytokine may up-regulate ACE2 expression potentially favoring SARS-CoV2 infection [198].
3.1.2.2. Sphingosine 1-phosphate (S1P) signaling modulators
Another immunomodulating agent, fingolimod (FTY720), an orally administered drug approved for relapsing-remitting multiple sclerosis, was evaluated in COVID-19 patients [199]. Once absorbed, fingolimod undergoes phosphorylation to form an analog of the naturally occurring S1P, a lipid signaling molecule whose activity is mediated by the interaction with four subtypes of G protein-coupled receptors (S1P1 and S1P3–5) [200], [201]. After binding to S1P1, fingolimod initially activates the receptor and thereafter down-regulates its expression, causing retention of naïve T cells and central memory T cells in the lymph nodes and induction of lymphocytopenia [202]. Nevertheless, fingolimod does not substantially affect memory effector T cells, which play an important role in the defense against infectious agents [203]. Moreover, it does not affect humoral immune responses and does not prevent the generation of virus-specific cytotoxic T cells in the lymph nodes [203]. Thus, it has been hypothesized that patients on S1P modulators might have a reduced risk of complications from SARS-CoV-2 infection. Furthermore, due to the S1P role in lung endothelial cell integrity [204], in COVID-19 patients, fingolimod might reduce vascular permeability and consequent lung injury [205]. In clinical trials with fingolimod for multiple sclerosis, conflicting results were reported showing either no change or increased risk of viral infections (especially herpes virus) compared to controls [206], [207], [208]. Severe COVID-19 cases have been reported in patients with multiple sclerosis on treatment with fingolimod that was stopped upon SARS-CoV-2 diagnosis; in all these cases patients fully recovered from infection [205], [209], [210]. Presently, one single arm non-randomized clinical trial is recruiting 30 COVID-19 patients with severe pneumonia to determine the efficacy of fingolimod administered for three consecutive days [NCT04280588]. The safety and efficacy of another S1P analog, ozanimod, recently approved by FDA and EMA for relapsing-remitting multiple sclerosis, is evaluated in COVID-19 patients requiring oxygen support versus standard of care [NCT04405102]. Modulation of the S1P signaling can also be obtained by using opaganib, a selective inhibitor of the sphingosine kinase isoenzyme SphK2 that is involved in the endogenous biosynthesis of S1P. Opaganib is an orally administered agent endowed with anti-cancer and anti-inflammatory properties. It has been tested in Israel for COVID-19 and showed some benefit in few hospitalized patients who had access to the drug via a compassionate use program [NCT04435106]. Recently, the FDA has approved the application for a phase 2, randomized, double-blind and placebo-controlled study to evaluate opaganib in patients with moderate-to-severe SARS-CoV-2 pneumonia [NCT04414618].
3.1.2.3. CD24Fc
Another molecule that has raised some interest to control the inflammatory response associated with SARS-CoV-2 infection is CD24Fc, a recombinant fusion protein that comprises CD24 attached to the Fc region of human IgG1. CD24 is a glycosylated membrane protein expressed in hematopoietic cells (including immature B and T cells, granulocytes, macrophages and some epithelial cells) that plays a regulatory role on B and T cell homeostasis [211]. In humans, CD24 is able to suppress inflammation upon interaction with the PRR Siglec10 and several danger-associated molecular patterns (DAMPs), helping to reduce the host immune response against proteins released by damaged cells. Preclinical studies demonstrated that the chimeric molecule CD24Fc mitigates the graft-versus-host disease, by decreasing the overall inflammatory response, and, in particular, the release of IL1β, IL6 and TNFα release [212]. These data provided the biological rationale for the clinical testing of CD24Fc also for COVID-19, and a randomized, double-blind, placebo-controlled, phase 3 study is currently recruiting severely ill infected patients [NCT04317040].
3.1.2.4. Mesenchymal stem cells
A cell-based approach to modulate the damage deriving from inflammation and altered activation of the immune system in COVID-19 is represented by allogeneic mesenchymal stem cells (MSCs) which are multipotent cells with reparative and immunomodulatory properties isolated from different tissue types (e.g., adipose tissue, bone marrow, umbilical cord) [213], [214], [215]. The protective effects exerted by MSCs are mediated by their direct regenerative ability and secretion of multiple paracrine factors including anti-inflammatory cytokines such as IL10 [216], [217]. MSCs have been shown to expand the proportion of regulatory T cells and to decrease IL6 and TNFα [218], [219]. In preclinical in vivo models of acute lung injury, MSCs reduced the pulmonary edema, concentrations of pro-inflammatory cytokines in the lung, and mortality rate [220], [221]. Moreover, a recent review of the published results on MSC-based therapies for patients with ARDS (n = 117) has indicated lack of serious adverse events and a favorable trends in terms of improvement of pulmonary function, inflammatory parameters and mortality rate [222]. A Chinese pilot clinical trial indicated that transplantation of allogenic MSCs in 7 patients with COVID-19 pneumonia was safe and significantly improved the functional outcomes, causing a decrease of TNFα and an increase of IL10 serum levels [223]. Furthermore, a prospective nonrandomized open-label cohort study has assessed the safety and efficacy of exosomes derived from allogeneic bone marrow MSCs in 27 patients with moderate-to-severe acute COVID19 [224]. Exosomes from bone marrow MSCs contain a variety of cytokines, growth factors, mRNAs and microRNAs that mediate MSCs anti-inflammatory, regenerative, and immunomodulatory properties. This study reported encouraging results with a survival rate of 83%, significant increase in oxygenation and changes in leukocyte count, inflammatory and coagulation parameters, suggesting a positive impact on the SARS-CoV-2-triggered cytokine storm [224]. More than thirty clinical trials are ongoing in severe COVID-19 patients, to evaluate the therapeutic potential of intravenous allogeneic MSCs, isolated from different tissues (e.g., adipose tissue, umbilical cord, bone marrow, dental pulp, or olfactory mucosa) versus placebo or standard of care [e.g., NCT04366271, NCT04429763 NCT04315987, NCT04366323, NCT04336254, NCT04346368, NCT04382547].
3.1.2.5. Corticosteroids
Dexamethasone is an old corticosteroid, i.e., a drug with broad anti-inflammatory and immunosuppressant activity that reduces cell-mediated immunity and various cytokine production. Its clinical indications span from pain in the joints to asthma, irritable bowel disease/Crohn disease, emesis, multiple sclerosis and various autoimmune diseases, as well as different types of cancer, to name just the most prevalent. It has also been used in the previous SARS-CoV outbreak, with conflicting results. Dexamethasone, like other corticosteroids, has been and is used to relieve the wheezing associated with COVID-19 in many hospital setting and was included in one arm of the Randomized Evaluation of COVid-19 thERapY (RECOVERY) trial [NCT04381936], an adaptive, multiple arm, randomized, controlled trial that in several months has enrolled more than 11,500 patients from over 175 National Health Service hospitals in UK. The treatment arms featured in the trial are: lopinavir/ritonavir; low dose dexamethasone; hydroxychloroquine [now discontinued because of lack of efficacy]; azithromycin; tocilizumab; convalescent plasma; standard of care. Recently, great interest was stirred after the disclosure of the results showing that dexamethasone [6 mg/day for 10 days] significantly reduced 28-day mortality in critically ill COVID-19 patients on mechanical ventilation (29.3% vs 41.4%) and in severe COVID-19 patients receiving oxygen (23.3% vs 26.2%), when compared to standard of care treatment [225]. The analysis was performed on 2104 dexamethasone-treated patients and 4321 patients that had received standard of care. Interestingly, the less severe patients, i.e. not requiring respiratory support, would not gain any benefit by dexamethasone administration, suggesting that choosing the right timing in relation to the hyper-inflammatory status of the patient is of paramount relevance in order to achieve optimal results. However, the relationship between the biological effects of corticosteroids and COVID-19 may not be as straightforward as it seems. A recent study shows an inverse relationship between prognosis and basal endogenous levels of cortisol (the natural corticosteroid produced by the adrenals), measured within 48 hrs since hospital admission in COVID-19 patients [226]. Other studies are evaluating intravenous dexamethasone [NCT04395105; NCT04445506; NCT04360876; NCT04327401; NCT04344730; NCT04325061] or methylprednisolone [NCT04374071; NCT04355247; NCT04273321; NCT04343729] for moderate-severe COVID-19 patients. Moreover, inhaled corticosteroids (i.e., budesonide, ciclesonide) are tested in patients with pneumonia [NCT04416399; NCT04355637; NCT04193878 with formoterol] or with hypo/anosmia [NCT04361474; NCT04374474].
3.2. Drugs used to counteract the cardiovascular complications
Although the clinical manifestations of COVID-19 are dominated by respiratory symptoms, the disease prognosis is largely influenced by the involvement of various organs, including the heart. Cardiovascular complications (i.e., myocardial infarction, acute heart failure and cardiomyopathy, shock and cardiac arrest, dysrhythmias, venous thromboembolic events, acute myocarditis) are associated with a high mortality rate and occur in about 10% of hospitalized patients [227]. Furthermore, patients with pre-existing cardiovascular diseases are predisposed to SARS-CoV-2-induced myocardial injury and infection is associated with a high mortality rate; in these patients, the risk of heart failure and myocardial damage increases to ≥ 35% [228], [229], [230]. The mechanisms involved in the cardiovascular injury of COVID-19 include: i) direct damage upon virus entry through ACE2 present in coronary endothelial cells, cardiomyocytes and cardiac fibroblasts; ii) increased oxygen consumption deriving from fever, enhanced adrenergic tone and tachycardia; iii) increased oxidative stress as a result of ROS production; iv) massive cytokine release and a state of hyperinflammation that contribute to pneumonia/ARDS with consequent acute heart failure, as well as endotheliitis leading to disseminated intravascular coagulation, thrombosis and infarction; v) SARS-CoV-2-induced ACE2 downregulation due to receptor shedding or internalization with consequent increase of angiotensin II [228], [231]. In fact, normally, ACE2 degrades angiotensin II to produce angiotensin 1–7 that is endowed with vasodilating and anti-inflammatory effects. Therefore, a reduction in ACE2 function after viral infection may result in a dysfunctional renin-angiotensin system, associated with an increase of angiotensin II, which would lead to vasoconstriction and inflammation.
3.2.1. Anti-C5 complement mAbs
Dysregulated immunothrombosis (i.e., clot formation triggered by the interaction of innate immune system components, like macrophages, neutrophils and the complement system, with platelets and coagulation factors, that provides a first line defense against infectious agents) with diffuse microvascular thrombi formation has been also described in COVID-19 and involved in multi-organ damage [232], [233]. A proposed therapeutic approach for thrombotic microangiopathy occurring in severe COVID-19 relies on the use of eculizumab, a recombinant humanized IgG2/4k mAb that specifically binds to the C5 protein, inhibiting the terminal pathway in the complement cascade [234], [235]. In fact, eculizumab prevents C5 cleavage with the consequent production of C5a, a potent pro-inflammatory peptide, and C5b that coordinates the formation of the membrane attack complex. Eculizumab is FDA- and EMA- approved for the treatment of paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, generalized myasthenia gravis and neuromyelitis optica spectrum disorder, all diseases associated with a complement-dependent damage. Recent reports of eculizumab administration together with anticoagulant therapy, lopinavir/ritonavir and hydroxychloroquine, ceftriaxone and vitamin C to four COVID-19 patients with severe pneumonia or ARDS resulted in full recovery and drop in inflammatory markers [235]. Three trials are listed in ClinicalTrials.gov where eculizumab is tested in patients with moderate to severe COVID-19 [NCT04355494, NCT04288713 or SOLID-C19 study and NCT04346797]. Another mAb against C5, ravulizumab, approved for paroxysmal nocturnal hemoglobinuria, is evaluated for COVID-19 severe pneumonia, ALI, or ARDS [NCT04369469, NCT04390464].
3.2.2. Antithrombotic and fibrinolytic agents
In patients with severe COVID-19, high rates of venous thromboembolism and disseminated intravascular coagulation, due to dysregulation of the coagulation and fibrinolytic systems are reported. Moreover, increased levels of the pro-coagulant D-dimer marker are associated with poor prognosis. These findings led to the prophylactic use of anti-thrombotic agents, such as low molecular weight heparin (LMWH) or unfractionated heparin (UFH), sometimes used at doses higher (i.e., intermediate-dose or full/therapeutic dose) than those recommended for thromboprophylaxis. However, there is not a general agreement on whether empiric escalation of anticoagulant doses is preferable to standard prophylactic doses [236], [237], [238], [239], [240]. Furthermore, heparin has anti-inflammatory properties by inhibiting IL6, IL8, TNFα release, C-reactive protein and adhesion of neutrophils to endothelial cells [241], [242], [243]. In a retrospective cohort study, the administration of LMWH to COVID-19 patients besides producing anticoagulant effects also reduced IL6 levels and increased lymphocyte counts suggesting a beneficial effect towards controlling the cytokine storm [244]. In addition, UFH and LMWH have been shown to inhibit the binding of the SARS-CoV-2 S protein to its cellular receptor, ACE2, in an in vitro cell system expressing ACE2 and TMPRSS2. These results suggest another mechanism through which heparin may slow down or prevent disease progression in the early phases of COVID-19. [245], [246].
Fibrinolytic drugs, namely tissue-type plasminogen activator (tPA) as systemic intravenous treatment or as lung-targeted nebulizer form, have been proposed for COVID-19 patients [247], [248]. Several clinical trials are currently assessing different heparin regimens, other anticoagulants, systemic and local fibrinolytic approaches, and antiaggregants (e.g., rivaroxaban; defibrotide; clopidogrel, aspirin) (ClinicalTrials.gov).
3.2.3. Phosphodiesterase-5 (PDE-5) inhibitor
The PDE-5 inhibitor sildenafil, a vasodilator that is approved for treating erectile dysfunction and pulmonary arterial hypertension [249], is also evaluated in a phase 3 trial for patients with mild to severe COVID-19 [NCT04304313]. In fact, sildenafil has a wide range of anti-inflammatory, antioxidant, and vasodilatory actions resulting in cardioprotective effects and improved pulmonary circulation [250], [251], [252].
3.2.4. Vasoactive intestinal polypeptide (VIP) analog
Aviptadil is a synthetic form of VIP that increases adenosine cyclase activity with consequent smooth muscle relaxation, approved in combination with phentolamine only in certain countries, for the treatment of erectile dysfunction. Previous studies have demonstrated that VIP induces relaxation and inhibits the proliferation of pulmonary vascular smooth muscle cells derived from patients with pulmonary arterial hypertension [253], [254]. Aviptadil was designated by FDA and EMA as orphan drug for the treatment of ARDS and on this basis it has been suggested it might induce a potential clinical benefit in COVID-19. Two clinical trials are testing aviptadil intravenously together with standard of care for the treatment of hospitalized COVID-19 patients with respiratory failure [NCT04311697], or inhaled in patients with non-acute lung injury without evidence of ARDS in order to evaluate whether the drug might prevent disease progression [NCT04360096].
3.2.5. Anti-vascular endothelial growth factor-A (VEGF-A) mAb
The VEGF-A is considered the most potent inducer of vascular permeability. The potential involvement of VEGF-A in COVID-19 has been related to the excessive production of angiotensin II, consequent to the SARS-CoV-2-mediated down-regulation of ACE2. In fact, angiotensin II is able to increase VEGF-A expression which in turn may exacerbate inflammation stimulating the recruitment of inflammatory cells and the release of proinflammatory cytokines [255], [256], [257]. Numerous studies have confirmed a key role of VEGF-A as potential therapeutic target in ALI and ARDS due do the increased vascular permeability and pulmonary edema [258]. Furthermore, VEGF-A has been involved in disruption of the blood–brain barrier and may contribute to brain inflammation in the course of SARS-CoV-2 infection [259]. Thus, blockage of VEGF-A might have a role in COVID-19 management. Indeed, bevacizumab, a humanized anti-VEGF-A mAb, clinically used for the treatment of a variety of solid tumors, is investigated in patients with severe or critically severe COVID-19 [NCT04305106, NCT04344782, NCT04275414]. However, the therapeutic benefits of bevacizumab may be hampered by its various adverse effects related to the inhibition of the VEGF-A physiological role on regulation of vascular homeostasis (e.g, hypertension, bleeding, delayed wound healing, thromboembolic events).
4. Conclusions
All over the world, the scientific community is racing to evaluate a huge number of drugs or associations of drugs for COVID-19 treatment or prophylaxis, in order to block or at least slow down the current SARS-CoV-2 pandemic. Except for remdesivir and the data on dexamethasone previously discussed, the few concluded clinical trials have not yet identified pharmacological treatments that might be safe and efficacious, particularly in severely ill patients. Recently, passive immunization with plasma collected from convalescent individuals has gained much attention as a promising therapeutic approach on the basis of the marked and rapid clinical improvement reported in a small number of patients [260], [261]. Neutralizing antibodies would prevent viral entry by inhibiting the interaction of the viral S protein with ACE2 or the conformational changes of the S protein required for the viral and host membrane fusion [262]. However, in a recently concluded randomized study in 103 patients with severe life-threatening COVID-19, convalescent plasma failed to induce a statistically significant reduction in time to clinical improvement within 28 days compared to standard treatment [263]. These controversial results point towards some crucial aspect that need to be addressed in the currently ongoing clinical trials, such as: i) the optimal time to collect the convalescent plasma, ii) the total antibody dose, iii) the optimum titer of anti-SARS-CoV-2 neutralizing antibodies, iv) the need of plasma pre-treatment to inactivate the virus as well as other pathogens; v) the role of antibody-dependent enhancement (ADE) in exacerbating clinical symptoms [264]. ADE is a mechanism by which the antigen–antibody complex does not lead to the clearance of the virus but rather provides another route for infection of host cells through the Fc receptor present in monocytes/macrophages. Passive immunization against SARS-CoV-2 might be also obtained with the administration of mAbs directed against specific SARS-CoV-2 epitopes and a human neutralizing mAb that recognizes a conserved epitope of the receptor-binding domain in the S protein has been recently characterized [265].
Despite the enormous efforts put on the task of finding a (better) cure for COVID-19 patients by researchers and clinicians, it is clear that the proliferation of small trials, that we have witnessed thus far, is hardly going to answer the fundamental question: would a drug/combination of drugs work and how much better than standard of care would be? Such questions can be answered only by organizing well-designed, randomized, controlled and, hopefully, multicenter clinical trials that would enroll an adequate number of patients in order to get clear-cut responses. These are not trivial aspects to solve, from both a scientific as well as administrative/legal/ethical perspective. The much welcomed, albeit slow, decrease of the number of COVID-19 cases in countries where the infection initially started and the progressive shift of the pandemic’s epicenter in other countries/continents highlight the importance of data sharing and international collaboration. However, such meritorious efforts should not distract the medical community and the health system organizations as a whole from other issues that deserve proper attention. Indeed, the battle against SARS-CoV-2 has so much stressed the healthcare systems in most countries, such that too many people with debilitating/severe/chronic diseases not only are at higher risk of getting sick but also have been left to themselves for too long.
CRediT authorship contribution statement
Lucia Lisi: Conceptualization, Writing - original draft. Pedro Miguel Lacal: Visualization. Maria Luisa Barbaccia: Conceptualization, Writing - review & editing. Grazia Graziani: Conceptualization, Writing - original draft, Writing - review & editing.
Acknowledgments
We would like to acknowledge the support of the “Fondazione AIRC” to the National Civil Protection to help tackle the COVID-19 emergency in Italy and its commitment to continue supporting cancer research during the challenging times of SARS-CoV-2 pandemic. G. Graziani is Principal Investigator (PI) of the AIRC grant IG 2017 - ID. 20353 project. P.M. Lacal is recipient of a RC18 grant from the Italian Ministry of Health. Due to the large number of recently published studies on COVID-19, we apologize to those authors whose work was not cited in this review.
References
- 1.Bahadur S., Long W., Shuaib M. Human coronaviruses with emphasis on the COVID-19 outbreak. Virusdisease. 2020:1–5. doi: 10.1007/s13337-020-00594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X., Wang X., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heller L., Mota C.R., Greco D.B. COVID-19 faecal-oral transmission: are we asking the right questions? Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172:577–582. doi: 10.7326/m20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain V., Yuan J.M. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int. J. Public Health. 2020:1–14. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D. Rafiq, A. Batool, M.A. Bazaz, Three months of COVID-19: A systematic review and meta-analysis, Rev Med Virol. (2020) e2113, doi: 10.1002/rmv.2113. [DOI] [PMC free article] [PubMed]
- 10.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang B., Zhou X., Qiu Y., Feng F., Feng J., Jia Y., et al. Clinical characteristics of 82 death cases with COVID-19. Lancet Infect. Dis. 2020;20:e102–e107. doi: 10.1101/2020.02.26.20028191. [DOI] [Google Scholar]
- 12.Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K., et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. (Wien) 2020;162:1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent J.L., Taccone F.S. Understanding pathways to death in patients with COVID-19. Lancet Respir Med. 2020;8:430–432. doi: 10.1016/S2213-2600(20)30165-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin M., Sorour M.K., Kasry A. Comparing the binding interactions in the receptor binding domains of SARS-CoV-2 and SARS-CoV. J. Phys. Chem. Lett. 2020;11:4897–4900. doi: 10.1021/acs.jpclett.0c01064. [DOI] [PubMed] [Google Scholar]
- 15.Mahmoud I.S., Jarrar Y.B., Alshaer W., Ismail S. SARS-CoV-2 entry in host cells-multiple targets for treatment and prevention. Biochimie. 2020;175:93–98. doi: 10.1016/j.biochi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik Y.A. Properties of coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020;42:3–11. [PubMed] [Google Scholar]
- 17.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 18.Hamming W., Timens M.L., Bulthuis A.T., Lely G., van Navis H. Goor, tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 20.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 21.Tikellis C., Johnston C.I., Forbes J.M., Burns W.C., Burrell L.M., Risvanis J., et al. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41:392–397. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- 22.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noureddine F.Y., Altara R., Fan F., Yabluchanskiy A., Booz G.W., Zouein F.A. Impact of the renin-angiotensin system on the endothelium in vascular dementia: unresolved issues and future perspectives. Int. J. Mol. Sci. 2020;21:E4268. doi: 10.3390/ijms21124268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan C., Li K., Ding Y., Lu W.L., Wang J. ACE2 expression in kidney and testis may cause kidney and testis damage after 2019-nCoV infection. medRxiv. 2020 doi: 10.1101/2020.02.12.20022418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madu I.G., Roth S.L., Belouzard S., Whittaker G.R. Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide. J. Virol. 2009;83:7411–7421. doi: 10.1128/JVI.00079-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Version 2. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann M., Kleine-Weber H., Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 2020;78:779–784.e5. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fantini J., Di Scala C., Chahinian H., Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkard C., Bloyet L.M., Wicht O., van Kuppeveld F.J., Rottier P.J., de Haan C.A., et al. Dissecting virus entry: replication-independent analysis of virus binding, internalization, and penetration using minimal complementation of β-galactosidase. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glebov O.O. Understanding SARS-CoV-2 endocytosis for COVID-19 drug repurposing. FEBS J. 2020 doi: 10.1111/febs.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaising J., Lévy P.L., Polyak S.J., Stanifer M., Boulant S., Pécheur E.I. Arbidol inhibits viral entry by interfering with clathrin-dependent trafficking. Antiviral Res. 2013;100:215–219. doi: 10.1016/j.antiviral.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Blaising J., Polyak S.J., Pécheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vankadari N. Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int. J. Antimicrob. Agents. 2020;28 doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pécheur E.I., Borisevich V., Halfmann P., Morrey J.D., Smee D.F., Prichard M., et al. The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. J. Virol. 2016;90:3086–3092. doi: 10.1128/JVI.02077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Z. Wang, B. Yang, Q. Li, L. Wen, R. Zhang, Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China, Clin Infect Dis. (2020) ciaa272, doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed]
- 39.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J. Infect. 2020;81:e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T., et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020;81:e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lian N., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin. Microbiol. Infect. 2020;26:917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen C.Y., Xie Z.W., Li Y.P., Deng X.L., Chen X.T., Cao Y., et al. Real-world efficacy and safety of lopinavir/ritonavir and arbidol in treating with COVID-19: an observational cohort study. Zhonghua Nei Ke Za Zhi. 2020;59:E012. doi: 10.3760/cma.j.cn112138-20200227-00147. [DOI] [PubMed] [Google Scholar]
- 43.Sun J., Deng X., Chen X., Huang J., Huang S., Li Y., et al. Incidence of Adverse drug reactions in COVID-19 patients in china: an active monitoring study by hospital pharmacovigilance system. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J.N., Wang W.J., Peng B., Peng W., Zhang Y.S., Wang Y.L., et al. Potential of arbidol for post-exposure prophylaxis of COVID-19 transmission-a preliminary report of a retrospective cohort study. Curr. Med. Sci. 2020:1–6. doi: 10.1007/s11596-020-2203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J. Infect. 2020;S0163–4453(20):30228. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo Caputo S., Corso G., Clerici M., Santantonio T.A. Baricitinib: a chance to treat COVID-19? J. Med. Virol. 2020 doi: 10.1002/jmv.26033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J., Chen Y., Fan X., Wang X., Han Q., Liu Z. Advances in the use of chloroquine and hydroxychloroquine for the treatment of COVID-19. Postgrad. Med. 2020;1–10 doi: 10.1080/00325481.2020.1778982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.X. Yao, F. Ye, M. Zhang, C. Cui, B. Huang, P. Niu, et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin Infect Dis. (2020) ciaa237, doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed]
- 52.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 54.Gao J., Tian Z., Yang X.u. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BST. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.03.22.20040758. [DOI] [Google Scholar]
- 56.Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L., et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., et al. CloroCovid-19 Team. Effect of High vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3(e208857) doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Juurlink D.N. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020;192:E450–E453. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Mehra M.R., Ruschitzka F., Patel A.N. Retraction-Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;395:1820. doi: 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Million M., Lagier J.C., Gautret P., Colson P., Fournier P.E., Amrane S., et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020;101738 doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ulrich H., Pillat M.M. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem. Cell Rev. Rep. 2020;16:434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menzel M., Akbarshahi H., Bjermer L., Uller L. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci. Rep. 2016;6:28698. doi: 10.1038/srep28698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strope J.D., PharmD C.H.C., Figg W.D. TMPRSS2: potential biomarker for COVID-19 outcomes. J. Clin. Pharmacol. 2020;60:801–807. doi: 10.1002/jcph.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J. Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Y., Vedantham P., Lu K., Agudelo J., Jr Carrion R., Nunneley J.W., et al. Protease inhibitors targeting coronavirus and filovirus entry. Version 2. Antiviral Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamamoto M., Matsuyama S., Li X., Takeda M., Kawaguchi Y., Inoue J.I., et al. Identification of nafamostat as a potent inhibitor of middle east respiratory syndrome coronavirus s protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob. Agents Chemother. 2016;60:6532–6539. doi: 10.1128/AAC.01043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob. Agents Chemother. 2020;64:e00754–e820. doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamamoto M., Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M., et al. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12:E629. doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin C.C., Lin L.J., Wang S.D., Chiang C.J., Chao Y.P., Lin J., et al. The effect of serine protease inhibitors on airway inflammation in a chronic allergen-induced asthma mouse model. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/879326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamaya M., Shimotai Y., Hatachi Y., Lusamba Kalonji N., Tando Y., Kitajima Y., et al. The serine protease inhibitor camostat inhibits influenza virus replication and cytokine production in primary cultures of human tracheal epithelial cells. Pulm. Pharmacol. Ther. 2015;33:66–74. doi: 10.1016/j.pupt.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim H.S., Lee K.E., Oh J.H., Jung C.S., Choi D., Kim Y., et al. Cardiac arrest caused by nafamostat mesilate. Kid. Res. Clin. Pract. 2016;35:187–189. doi: 10.1016/j.krcp.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jang S., Rhee J.Y. Three cases of treatment with Nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy. Int. J. Infect. Dis. 2020;96:500–502. doi: 10.1016/j.ijid.2020.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Depfenhart M., de Villiers D., Lemperle G., Meyer M., Di Somma S. Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy? Intern. Emerg. Med. 2020:1–12. doi: 10.1007/s11739-020-02383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huynh T., Wang H., Luan B. In silico exploration of the molecular mechanism of clinically oriented drugs for possibly inhibiting SARS-CoV-2's main protease. J. Phys. Chem. Lett. 2020;11:4413–4420. doi: 10.1021/acs.jpclett.0c00994. [DOI] [PubMed] [Google Scholar]
- 78.Cadegiani F.A. Can spironolactone be used to prevent COVID-19-induced acute respiratory distress syndrome in patients with hypertension? Am. J. Physiol. Endocrinol. Metab. 2020;318:E587–E588. doi: 10.1152/ajpendo.00136.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. (Lond). 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 80.Liu T., Luo S., Libby P., Shi Cathepsin G.P. L-selective inhibitors: a potentially promising treatment for COVID-19 patients. Pharmacol. Ther. 2020;213:107587. doi: 10.1016/j.pharmthera.2020.107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C., et al. Glycopeptide antibiotics potently inhibit cathepsin l in the late endosome/lysosome and block the entry of ebola virus, middle east respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J. Biol. Chem. 2016;291:9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baron S.A., Devaux C., Colson P., Raoult D., Rolain J.M. Teicoplanin: an alternative drug for the treatment of COVID-19? Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J., Ma X., Yu F., Liu J., Zou F., Pan T., et al. Teicoplanin potently blocks the cell entry of 2019-nCoV. BioRxiv. 2020 doi: 10.1101/2020.02.05.935387. [DOI] [Google Scholar]
- 84.Ceccarelli G., Alessandri F., d’Ettorre G., Borrazzo C., Spagnolello O., Oliva A., et al. Intensive care COVID-19 study group of sapienza university. Is teicoplanin a complementary treatment option for COVID-19? The question remains. Int. J. Antimicrob. Agents. 2020;106029 doi: 10.1016/j.ijantimicag.2020.106029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Romano M., Ruggiero A., Squeglia F., Maga G., Berisio R. A structural view of SARS-CoV-2 RNA replication machinery: RNA Synthesis Proofreading and Final Capping. Cells. 2020;9:E1267. doi: 10.3390/cells9051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knoops K., Kikkert M., Worm S.H., Zevenhoven-Dobbe J.C., van der Meer Y., Koster A.J., et al. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6 doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hagemeijer M.C., Verheije M.H., Ulasli M., Shaltiël I.A., de Vries L.A., Reggiori F., et al. Dynamics of coronavirus replication-transcription complexes. J. Virol. 2010;84:2134–2149. doi: 10.1128/JVI.01716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pillaiyar T., Manickam M., Namasivayam V., Hayashi Y., Jung S.H. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J. Med. Chem. 2016;59:6595–6628. doi: 10.1021/acs.jmedchem.5b01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goyal B., Goyal D. Targeting the dimerization of the main protease of coronaviruses: a potential broad-spectrum therapeutic strategy. ACS Comb. Sci. 2020;22:297–305. doi: 10.1021/acscombsci.0c00058. [DOI] [PubMed] [Google Scholar]
- 90.Jenwitheesuk E., Samudrala R. Identifying inhibitors of the SARS coronavirus proteinase. Bioorg. Med. Chem. Lett. 2003;13:3989–3992. doi: 10.1016/j.bmcl.2003.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamamoto N., Yang R., Yoshinaka Y., Amari S., Nakano T., Cinatl J., et al. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 2004;318:719–725. doi: 10.1016/j.bbrc.2004.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X.W., Yap Y.L. Old drugs as lead compounds for a new disease? Binding analysis of SARS coronavirus main proteinase with HIV, psychotic and parasite drugs. Bioorg. Med. Chem. 2004;12:2517–2521. doi: 10.1016/j.bmc.2004.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nukoolkarn V., Lee V.S., Malaisree M., Aruksakulwong O., Hannongbua S. Molecular dynamic simulations analysis of ritonavir and lopinavir as SARS-CoV 3CL(pro) inhibitors. J. Theor. Biol. 2008;254:861–867. doi: 10.1016/j.jtbi.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nutho B., Mahalapbutr P., Hengphasatporn K., Pattaranggoon N.C., Simanon N., Shigeta Y., et al. Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? Atomistic Insights into the Inhibitory Mechanisms. Biochemistry. 2020;59:1769–1779. doi: 10.1021/acs.biochem.0c00160. [DOI] [PubMed] [Google Scholar]
- 95.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Owa A.B., Owa O.T. Lopinavir/ritonavir use in Covid-19 infection: is it completely non-beneficial? J. Microbiol. Immunol. Infect. 2020;S1684–1182(20):30128–30136. doi: 10.1016/j.jmii.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan D., Liu X.Y., Zhu Y.N., Huang L., Dan B.T., Zhang G.J., et al. Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection. Eur. Respir. J. 2020;56:2000799. doi: 10.1183/13993003.00799-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klement-Frutos E., Burrel S., Peytavin G., Marot S., Lê M.P., Godefroy N., et al. Early administration of ritonavir-boosted lopinavir could prevent severe COVID-19. J. Infect. 2020;S0163–4453(20):30318–30322. doi: 10.1016/j.jinf.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan S.A., Zia K., Ashraf S., Uddin R., Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- 100.De Meyer S., Bojkova D., Cinatl J., Van Damme E., Meng C.B., Van Loock M., et al. Lack of antiviral activity of darunavir against SARS-CoV-2. Int. J. Infect. Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bafna K., Krug R.M., Montelione G.T. Structural similarity of SARS-CoV2 Mpro and HCV NS3/4A proteases suggests new approaches for identifying existing drugs useful as COVID-19 therapeutics. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12153615. [DOI] [Google Scholar]
- 102.Eleftheriou P., Amanatidou D., Petrou A., Geronikaki A. In silico evaluation of the effectivity of approved protease inhibitors against the main protease of the novel SARS-CoV-2 virus. Molecules. 2020;25:E2529. doi: 10.3390/molecules25112529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ghosh A.K., Brindisi M., Shahabi D., Chapman M.E., Mesecar A.D. Drug development and medicinal chemistry efforts toward SARS-coronavirus and covid-19 therapeutics. ChemMedChem. 2020;15:907–932. doi: 10.1002/cmdc.202000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pillaiyar T., Meenakshisundaram S., Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov. Today. 2020;25:668–688. doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. Mechanism of inhibition of ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11:326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.A.J. Pruijssers, A.S. George, A. Schäfer, N.M.A. Okba, R. van Haperen, A.D.M.E. Osterhaus, et al. Remdesivir potently inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice, bioRxiv. (2020), doi: https://doi.org/10.1101/2020.04.27.064279. [DOI] [PMC free article] [PubMed]
- 108.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U.S.A. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J., et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S., et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci. 2020;6:672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang L., Zhou R. Structural basis of potential binding mechanism of remdesivir to SARS-CoV-2 RNA dependent RNA polymerase. J. Phys. Chem. B. 2020 doi: 10.1021/acs.jpcb.0c04198. [DOI] [PubMed] [Google Scholar]
- 113.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.00221-18. e00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.J.H. Beigel, K.M. Tomashek, L.E. Dodd, A.K. Mehta, B.S. Zingman, A.C. Kalil, et al., Remdesivir for the Treatment of Covid-19 - Preliminary Report. N Engl J Med. (2020) NEJMoa2007764, doi: 10.1056/NEJMoa2007764. [DOI] [PubMed]
- 116.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R., et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bonovas S., Piovani D. Compassionate use of remdesivir in Covid-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2015312. [DOI] [PubMed] [Google Scholar]
- 120.Fätkenheuer G., Lundgren J. Compassionate use of remdesivir in Covid-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2015312. [DOI] [PubMed] [Google Scholar]
- 121.Hoffmann C. Compassionate use of remdesivir in Covid-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2015312. [DOI] [PubMed] [Google Scholar]
- 122.Wu J., Wu B., Lai T. Compassionate use of remdesivir in Covid-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2015312. [DOI] [PubMed] [Google Scholar]
- 123.Antinori S., Cossu M.V., Ridolfo A.L., Rech R., Bonazzetti C., Pagani G., et al. Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: Clinical outcome and differences in post-treatment hospitalisation status. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Adamsick M.L., Gandhi R.G., Bidell M.R., Elshaboury R.H., Bhattacharyya R.P., Kim A.Y., et al. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J. Am. Soc. Nephrol. 2020;31:1384–1386. doi: 10.1681/ASN.2020050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shiraki K., Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020;209 doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shannon, B. Selisko, N. Le, J. Huchting, F. Touret, G. Piorkowski, et al., Favipiravir strikes the SARS-CoV-2 at its Achilles heel, the RNA polymerase, bioRxiv. (2020) doi: 10.1101/2020.05.15.098731. [DOI] [PMC free article] [PubMed]
- 127.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Elfiky A.A., Ribavirin Remdesivir, SofosbuvirGalidesivir and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kandeel M., Al-Nazawi M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020;251 doi: 10.1016/j.lfs.2020.117627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khalili J.S., Zhu H., Mak N.S.A., Yan Y., Zhu Y. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID-19. J. Med. Virol. 2020;92:740–746. doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kelleni M.T. Nitazoxanide/azithromycin combination for COVID-19: a suggested new protocol for early management. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chien M., Anderson T.K., Jockusch S., Tao C., Kumar S., Li X., et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase. bioRxiv. 2020 doi: 10.1101/2020.03.18.997585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., Leist S.R., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020;125883 doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Agostini M.L., Pruijssers A.J., Chappell J.D., Gribble J., Lu X., Andres E.L., et al. Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J. Virol. 2019;93:e01348–e1419. doi: 10.1128/JVI.01348-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun Y., Dong Y., Wang L., Xie H., Li B., Chang C., et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: The Beijing experience. J. Autoimmun. 2020;112:102473. doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vardhana S.A., Wolchok J.D. The many faces of the anti-COVID immune response. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Manjili R.H., Zarei M., Habibi M., Manjili M.H. COVID-19 as an acute inflammatory disease. J. Immunol. 2020;205:12–19. doi: 10.4049/jimmunol.2000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lacy J.M., Brooks E.G., Akers J., Armstrong D., Decker L., Gonzalez A., et al. COVID-19: postmortem diagnostic and biosafety considerations. Am. J. Forensic Med. Pathol. 2020 doi: 10.1097/PAF.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tian S., Xiao S.Y. Pathology of 2019 novel coronavirus pneumonia: a dynamic disease process. J. Thorac. Oncol. 2020;15:e67–e68. doi: 10.1016/j.jtho.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? Version 2. J. Transl. Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Honda K., Takaoka A., Taniguchi T. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 146.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.England J.T., Abdulla A., Biggs C.M., Lee A.Y.Y., Hay K.A., Hoiland R.L., et al. Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes. Blood. Rev. 2020;15 doi: 10.1016/j.blre.2020.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med. Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Y. Yang, C. Shen, J. Li, J. Yuan, M. Yang, F. Wang, et al., Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome, medRxiv. (2020), doi: https://doi.org/10.1101/2020.03.02.20029975.
- 150.Levi M., van der Poll T., Buller H.R. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9. [DOI] [PubMed] [Google Scholar]
- 151.Bester J., Matshailwe C., Pretorius E. Simultaneous presence of hypercoagulation and increased clot lysis time due to IL-1β, IL-6 and IL-8. Cytokine. 2018;110:237–242. doi: 10.1016/j.cyto.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 152.Garbers C., Heink S., Korn T., Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17:395–412. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 153.Calabrese L.H., Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat. Rev. Rheumatol. 2014;10:720–727. doi: 10.1038/nrrheum.2014.127. [DOI] [PubMed] [Google Scholar]
- 154.Nishimoto N., Kishimoto T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb. Exp. Pharmacol. 2008:151–160. doi: 10.1007/978-3-540-73259-4_7. [DOI] [PubMed] [Google Scholar]
- 155.T. Liu, J. Zhang, Y. Yang, H. Ma, Z. Li, J. Zhang, et al., The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. (2020) e12421, doi: 10.15252/emmm.202012421. [DOI] [PMC free article] [PubMed]
- 156.Zhang C., Wu Z., Li J., Zhao H., Wang G. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang S., Li L., Shen A., Chen Y., Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig. 2020;40:511–518. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Morena V., Milazzo L., Oreni L., Bestetti G., Fossali T., Bassoli C., et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur. J. Int. Med. 2020;76:36–42. doi: 10.1016/j.ejim.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Capra R., De Rossi N., Mattioli F., Romanelli G., Scarpazza C., Sormani M.P., et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Int. Med. 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N., et al. TOCI-RAF Study Group. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Int. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sciascia S., Aprà F., Baffa A., Baldovino S., Boaro D., Boero R., et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin. Exp. Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- 162.Klopfenstein T., Zayet S., Lohse A., Balblanc J.C., Badie J., Royer P.Y., et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020;S0399–077X(20):30129–30133. doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020;19:102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Di Giambenedetto S., Ciccullo A., Borghetti A., Gambassi G., Landi F., Visconti E., et al. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U.S.A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Colaneri M., Bogliolo L., Valsecchi P., Sacchi P., Zuccaro V., Brandolino F., et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020;8:E695. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Antwi-Amoabeng D., Kanji Z., Ford B., Beutler B.D., Riddle M.S., Siddiqui F. Clinical outcomes in COVID-19 patients treated with tocilizumab: an individual patient data systematic review. J. Med. Virol. 2020 doi: 10.1002/jmv.26038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marfella R., Paolisso P., Sardu C., Bergamaschi L., D'Angelo E.C., Barbieri M., et al. Negative impact of hyperglycaemia on tocilizumab therapy in Covid-19 patients. Diabetes Metab. 2020;S1262–3636(20):30082–30083. doi: 10.1016/j.diabet.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Antinori S., Bonazzetti C., Gubertini G., Capetti A., Pagani C., Morena V., et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.P. G. Guardiola, J.Á. Díez Ares, N. Peris Tomás, J.C. Sebastián Tomás, S. Navarro Martínez, Intestinal perforation in patient with COVID-19 infection treated with tocilizumab and corticosteroids. Report of a clinical case, Cir Esp. (2020) S0009-739X(20)30167-6, doi:10.1016/j.ciresp.2020.04.030. [DOI] [PMC free article] [PubMed]
- 171.Vikse J., Henry B.M. Tocilizumab in COVID-19: Beware the risk of intestinal perforation. Int. J. Antimicrob. Agents. 2020;106009 doi: 10.1016/j.ijantimicag.2020.106009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Scott L.J. Sarilumab: first global approval. Drugs. 2017;77:705–712. doi: 10.1007/s40265-017-0724-2. [DOI] [PubMed] [Google Scholar]
- 173.Yeung Y.T., Aziz F., Guerrero-Castilla A., Arguelles S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des. 2018;24:1449–1484. doi: 10.2174/1381612824666180327165604. [DOI] [PubMed] [Google Scholar]
- 174.Seif F., Aazami H., Khoshmirsafa M., Kamali M., Mohsenzadegan M., Pornour M., et al. JAK inhibition as a new treatment strategy for patients with COVID-19. Int. Arch. Allergy Immunol. 2020:1–9. doi: 10.1159/000508247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Roskoski R., Jr Properties of FDA-approved small molecule protein kinase inhibitors: a 2020 update. Pharmacol. Res. 2020;152 doi: 10.1016/j.phrs.2019.104609. [DOI] [PubMed] [Google Scholar]
- 176.Arana Yi C., Tam C.S., Verstovsek S. Efficacy and safety of ruxolitinib in the treatment of patients with myelofibrosis. Future Oncol. 2015;11:719–733. doi: 10.2217/fon.14.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Saeed, McLornan D., Harrison C.N. Managing side effects of JAK inhibitors for myelofibrosis in clinical practice. Expert Rev. Hematol. 2017;10:617–625. doi: 10.1080/17474086.2017.1337507. [DOI] [PubMed] [Google Scholar]
- 178.V. Gaspari, C. Zengarini, S. Greco, V. Vangeli, A. Mastroianni, Side effects of ruxolitinib in patients with SARS-CoV-2 infection: two case reports, Int J Antimicrob Agents. (2020) 106023, doi:10.1016/j.ijantimicag.2020.106023. [DOI] [PMC free article] [PubMed]
- 179.Deng J., Yu X.Q., Wang P.H. Inflammasome activation and Th17 responses. Mol. Immunol. 2019;107:142–164. doi: 10.1016/j.molimm.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 180.Dimopoulos G., de Mast Q., Markou N., Theodorakopoulou M., Komnos A., Mouktaroudi M., et al. Favorable anakinra responses in severe covid-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28:117–123.e1. doi: 10.1016/j.chom.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pontali E., Volpi S., Antonucci G., Castellaneta M., Buzzi D., Tricerri F., et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J. Allergy Clin. Immunol. 2020;146:213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyper-inflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Filocamo G., Mangioni D., Tagliabue P., Aliberti S., Costantino G., Minoia F., et al. Use of anakinra in severe COVID-19: a case report. Int. J. Infect. Dis. 2020;96:607–609. doi: 10.1016/j.ijid.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Franzetti M., Pozzetti U., Carugati M., Pandolfo A., Molteni C., Faccioli P., et al. Interleukin-1 receptor antagonist anakinra in association with remdesivir in severe Coronavirus disease 2019: a case report. Int. J. Infect. Dis. 2020;97:215–218. doi: 10.1016/j.ijid.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Aouba A., Baldolli L., Geffray R., Verdon E., Martin-Silva Bergot N., et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 186.Al-Salama Z.T. Emapalumab: first global approval. Drugs. 2019;79:99–103. doi: 10.1007/s40265-018-1046-8. [DOI] [PubMed] [Google Scholar]
- 187.De Luca G., Cavalli G., Campochiaro C., Della-Torre E., Angelillo M.P., Tomelleri A., et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lazear H.M., Schoggins J.W., Diamond M.S. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50:907–923. doi: 10.1016/j.immuni.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., et al. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.González-Navajas J.M., Lee J., David M., Raz E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Acharya D., Liu G., Gack M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020:1–2. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Pere H., et al. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. Science. 2020 doi: 10.1101/2020.04.19.20068015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.ParkIwasaki A. Type I and Type III Interferons - Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe. 2020;27:870–887. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Andreakos E., Tsiodras S. COVID-19: lambda interferon against viral load and hyperinflammation. EMBO Mol. Med. 2020;12 doi: 10.15252/emmm.202012465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.T.R. O'Brien, D.L. Thomas, S.S. Jackson, L. Prokunina-Olsson, R.P. Donnelly, R. Hartmann, Weak Induction of Interferon Expression by SARS-CoV-2 Supports Clinical Trials of Interferon Lambda to Treat Early COVID-19, Clin Infect Dis. (2020) ciaa453, doi: 10.1093/cid/ciaa453. [DOI] [PMC free article] [PubMed]
- 197.Prokunina-Olsson L., Alphonse N., Dickenson R.E., Durbin J.E., Glenn J.S., Hartmann R., et al. COVID-19 and emerging viral infections: The case for interferon lambda. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang T., Tian X., Chen C.Y., Ma L.Y., Zhou S., Li M., et al. The efficacy and safety of fingolimod in patients with relapsing multiple sclerosis: a meta-analysis. Br. J. Clin. Pharmacol. 2020;86:637–645. doi: 10.1111/bcp.14198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chun J., Hartung H.P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 2010;33:91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Derfuss T., Mehling M., Papadopoulou A., Bar-Or A., Cohen J.A., Kappos L. Advances in oral immunomodulating therapies in relapsing multiple sclerosis. Lancet Neurol. 2020;19:336–347. doi: 10.1016/S1474-4422(19)30391-6. [DOI] [PubMed] [Google Scholar]
- 202.MarciniakCamp S.M., Garcia J.G.N., Polt R. An update on sphingosine-1-phosphate receptor 1 modulators. Bioorg. Med. Chem. Lett. 2018;28:3585–3591. doi: 10.1016/j.bmcl.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pinschewer D.D., Brinkmann V., Merkler D. Impact of sphingosine 1-phosphate modulation on immune outcomes. Neurology. 2011;76:S15–S19. doi: 10.1212/WNL.0b013e31820d9596. [DOI] [PubMed] [Google Scholar]
- 204.Natarajan V., Dudek S.M., Jacobson J.R., Moreno-Vinasco L., Huang L.S., Abassi T., et al. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2013;49:6–17. doi: 10.1165/rcmb.2012-0411TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Foerch C., Friedauer L., Bauer B., Wolf T., Adam E.H. Severe COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cohen J.A., Barkhof F., Comi G., Hartung H.P., Khatri B.O., Montalban X., et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 207.Kappos L., Radue E.W., O'Connor P., Polman C., Hohlfeld R., Calabresi P., et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 208.Juif P.E., Kraehenbuehl S., Dingemanse J. Clinical pharmacology, efficacy, and safety aspects of sphingosine-1-phosphate receptor modulators. Expert Opin. Drug Metab. Toxicol. 2016;12:879–895. doi: 10.1080/17425255.2016.1196188. [DOI] [PubMed] [Google Scholar]
- 209.Barzegar M., Mirmosayyeb O., Nehzat N., Sarrafi R., Khorvash F., Maghzi A.H., et al. COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol. Neuroimmunol. Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Valencia-Sanchez C., Wingerchuk D.M. A fine balance: Immunosuppression and immunotherapy in a patient with multiple sclerosis and COVID-19. Mult. Scler Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen G.Y., Tang J., Zheng P., Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Toubai T., Rossi C., Oravecz-Wilson K., Zajac C., Liu C., Braun T., et al. Siglec-G represses DAMP-mediated effects on T cells. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mei S.H., Haitsma J.J., Dos Santos C.C., Deng Y., Lai P.F., Slutsky A.S., et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 214.Lv F.J., Tuan R.S., Cheung K.M., Leung V.Y. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 215.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gupta N., Su X., Popov B., Lee J.W., Serikov V., Matthay M.A. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J. Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 217.Gupta N., Krasnodembskaya A., Kapetanaki M., Mouded M., Tan X., Serikov V., et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Prevosto C., Zancolli M., Canevali P., Zocchi M.R., Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 219.Li J., Li D., Liu X., Tang S., Wei F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J. Inflamm. (Lond.) 2012;9:33. doi: 10.1186/1476-9255-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chimenti L., Luque T., Bonsignore M.R., Ramírez J., Navajas D., Farré R. Pre-treatment with mesenchymal stem cells reduces ventilator-induced lung injury. Eur. Respir. J. 2012;40:939–948. doi: 10.1183/09031936.00153211. [DOI] [PubMed] [Google Scholar]
- 221.McIntyre L.A., Moher D., Fergusson D.A., Sullivan K.J., Mei S.H., Lalu M., et al. Canadian critical care translational biology group efficacy of mesenchymal stromal cell therapy for acute lung injury in preclinical animal models: a systematic review. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Qu W., Wang Z., Hare J.M., Bu G., Mallea J.M., Pascual J.M., et al. Cell-based therapy to reduce mortality from COVID-19: Systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem Cells Transl. Med. 2020 doi: 10.1002/sctm.20-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.The RECOVERY Collaborative Group, Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report, N Engl J Med. (2020) July 17, doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed]
- 226.Tan T., Khoo B., Mills E.G., Phylactou M., Patel B., Eng P.C., et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabet. Endocrinol. 2020;8:659–660. doi: 10.1016/S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tomasoni D., Italia L., Adamo M., Inciardi R.M., Lombardi C.M., Solomon S.D., et al. COVID 19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur. J. Heart Fail. 2020 doi: 10.1002/ejhf.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 2020;45:100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhu H., Rhee J.W., Cheng P., Waliany S., Chang A., Witteles R.M., et al. Cardiovascular complications in patients with COVID-19: consequences of viral toxicities and host immune response. Curr. Cardiol. Rep. 2020;22:32. doi: 10.1007/s11886-020-01292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Henry B.M., Vikse J., Benoit S., Favaloro E.J., Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin. Chim. Acta. 2020;507:167–173. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.McGonagle D., Plein S., O'Donnell J.S., Sharif K., Bridgewood C. Increased cardiovascular mortality in African Americans with COVID-19. Lancet Respir Med. 2020;8:649–651. doi: 10.1016/S2213-2600(20)30244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Campbell C.M., Kahwash R. Will complement inhibition be the new target in treating COVID-19-related systemic thrombosis? Circulation. 2020;141:1739–1741. doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- 235.Diurno F., Numis F.G., Porta G., Cirillo F., Maddaluno S., Ragozzino A., et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 236.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C., Fogerty A.E., Waheed A., et al. COVID and coagulation: bleeding and thrombotic manifestations of SARS-CoV2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention antithrombotic therapy, and follow-up. J. Am. Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cattaneo M., Bertinato E.M., Birocchi S., Brizio C., Malavolta D., Manzoni M., et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb. Haemost. 2020 doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mousavi S., Moradi M., Khorshidahmad T., Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015 doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Qian Y., Xie H., Tian R., Yu K., Wang R. Efficacy of low molecular weight heparin in patients with acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. COPD. 2014;11:171–176. doi: 10.3109/15412555.2013.831062. [DOI] [PubMed] [Google Scholar]
- 243.Liu Y., Mu S., Li X., Liang Y., Wang L., Ma X. Unfractionated heparin alleviates sepsis-induced acute lung injury by protecting tight junctions. J. Surg. Res. 2019;6:175–185. doi: 10.1016/j.jss.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 244.Shi C., Wang C., Wang H., Yang C., Cai F., Zeng F., et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: a retrospective clinical study. medRxiv. 2020 doi: 10.1101/2020.03.28.20046144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.L.J. Partridge, L.R. Green, P.N. Monk. Unfractionated heparin potently inhibits the binding of SARS-CoV-2 spike protein to a human cell line BioRxiv. (2020), doi: 10.1101/2020.05.21.107870.
- 246.Mycroft-West C.J., Su D., Pagani I., Rudd T.R., Elli S., Guimond S.E., et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the surface protein (spike) S1 receptor binding domain with heparin. BioRxiv. 2020 doi: 10.1101/2020.04.28.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang J., Hajizadeh N., Moore E.E., McIntyre R.C., Moore P.K., Veress L.A., et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J. Thromb. Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Whyte C.S., Morrow G.B., Mitchell J.L., Chowdary P., Mutch N.J. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J. Thromb. Haemost. 2020;18:1548–1555. doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Parikh V., Bhardwaj A., Nair A. Pharmacotherapy for pulmonary arterial hypertension. J. Thorac Dis. 2019;11:S1767–S1781. doi: 10.21037/jtd.2019.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kukreja R.C. Sildenafil and cardioprotection. Curr. Pharm. Des. 2013;19:6842–6847. doi: 10.2174/138161281939131127110156. [DOI] [PubMed] [Google Scholar]
- 251.Rogosnitzky M., Berkowitz E., Jadad A.R. Delivering benefits at speed through real-world repurposing of off-patent drugs: the COVID-19 pandemic as a case in point. JMIR Public Health Surveill. 2020;6 doi: 10.2196/19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Solaimanzadeh Acetazolamide. Nifedipine and phosphodiesterase inhibitors: rationale for their utilization as adjunctive countermeasures in the treatment of coronavirus disease 2019 (COVID-19) Cureus. 2020;12 doi: 10.7759/cureus.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mucke H.A. Pulmonary arterial hypertension: on the way to a manageable disease. Curr. Opin. Invest. Drugs. 2008;9:957–962. [PubMed] [Google Scholar]
- 254.Lythgoe M.P., Rhodes C.J., Ghataorhe P., Attard M., Wharton J., Wilkins M.R. Why drugs fail in clinical trials in pulmonary arterial hypertension, and strategies to succeed in the future. Pharmacol. Ther. 2016;164:195–203. doi: 10.1016/j.pharmthera.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 255.Rizkalla B., Forbes J.M., Cooper M.E., Cao Z. Increased renal vascular endothelial growth factor and angiopoietins by angiotensin II infusion is mediated by both AT1 and AT2 receptors. J. Am. Soc. Nephrol. 2003;14:3061–3071. doi: 10.1097/01.asn.0000099374.58607.c9. [DOI] [PubMed] [Google Scholar]
- 256.Yoshiji H., Kuriyama S., Noguchi R., Fukui H. Angiotensin-I converting enzyme inhibitors as potential anti-angiogenic agents for cancer therapy. Curr. Cancer Drug Targets. 2004;4:555–567. doi: 10.2174/1568009043332790. [DOI] [PubMed] [Google Scholar]
- 257.Radin D.P., Krebs A., Maqsudlu A., Patel P. Our ACE in the HOLE: justifying the use of angiotensin-converting enzyme inhibitors as adjuvants to standard chemotherapy. Anticancer Res. 2018;38:45–49. doi: 10.21873/anticanres.12190. [DOI] [PubMed] [Google Scholar]
- 258.Medford A.R., Millar A.B. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax. 2006;61:621–626. doi: 10.1136/thx.2005.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yin X.X., Zheng X.R., Peng W., Wu M.L., Mao X.Y. Vascular endothelial growth factor (VEGF) as a vital target for brain inflammation during the COVID-19 outbreak. ACS Chem. Neurosci. 2020;11:1704–1705. doi: 10.1021/acschemneuro.0c00294. [DOI] [PubMed] [Google Scholar]
- 260.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U.S.A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically Ill patients with COVID-19 With convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Abraham J. Passive antibody therapy in COVID-19. Nat. Rev. Immunol. 2020;12:1–3. doi: 10.1038/s41577-020-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.L. Li, W. Zhang, Y. Hu, X. Tong, S. Zheng, J. Yang, et al., Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial, JAMA. (2020) e2010044, doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed]
- 264.Franchini M., Del Fante C., Klersy C., Glingani C., Percivalle E., Baldanti F., et al. Challenges in the production of convalescent hyperimmune plasma in the age of COVID-19. Semin. Thromb. Hemost. 2020 doi: 10.1055/s-0040-1713433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang C., Li W., Drabek D., Okba N.M.A., van Haperen R., Osterhaus A.D.M.E., et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11:2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]