Sirs:
A 54-year-old female patient was referred to our hospital for lead extraction of a cardiac device-related infective endocarditis (CDRIE). The patient had diabetes, heart failure with reduced ejection fraction, morbid obesity (BMI 51 kg/m2), coronary artery disease and persistent atrial fibrillation. An originally implanted implantable cardioverter defibrillator (ICD) for primary prevention was upgraded to a cardiac resynchronization system (CRT-D) 2 years ago due to AV-node-ablation for therapy refractory atrial fibrillation. Three years ago, a left atrial appendage occluder (LAAO, Amulet, Abbott, St. Paul, MN) was implanted due to recurrent gynecological bleedings under oral anticoagulation.
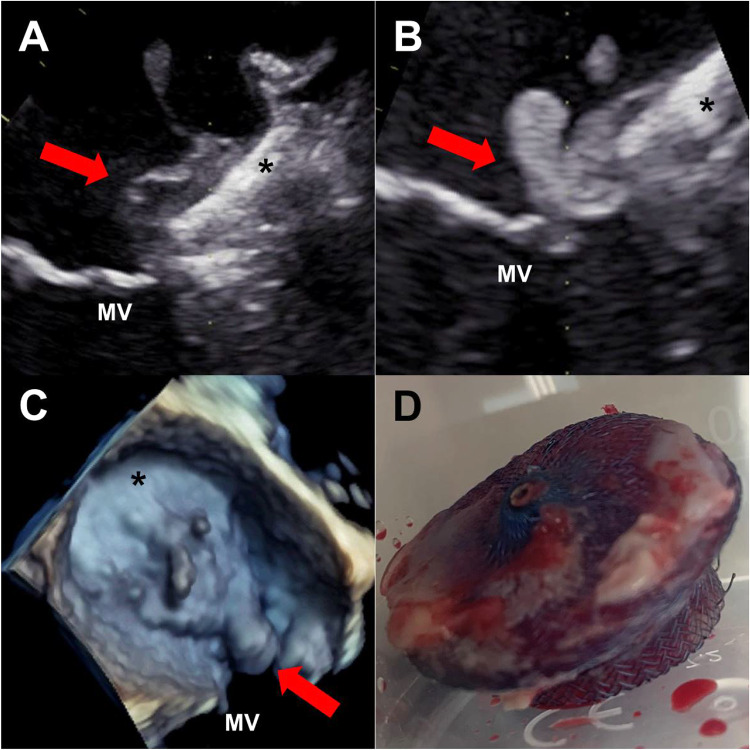
She was admitted to the referring hospital with fever and chills. Blood cultures showed persistent bacteremia with methicillin-resistant Staphylococcus aureus. Transthoracic echocardiography revealed a suspicious mass on the implanted CRT lead. On admission we performed a transesophageal echocardiography (TEE) which confirmed a lead vegetation of 13 × 5 mm size but the exam was stopped prematurely due to respiratory insufficiency and given the presumed identification of the causative focus. Treatment with vancomycin was continued and transvenous lead extraction was performed successfully. Cultures of the pacemaker lead also confirmed methicillin-resistant Staphylococcus aureus. A second TEE was performed following complete device extraction and respiratory stabilization: no signs of valvular vegetations could be found but a gelatinous mass with floating parts (Fig. 1, panel a) was present on the LAAO. The patient was started on intravenous heparin and after 7 days TEE showed no reduction of the mass on the LAAO. Instead the vegetation increased towards the mitral valve (Fig. 1, panel b, c). Given the uncontrolled infection the patient was planned for surgery. The infected LAAO (panel d) was retrieved, the mitral valve replaced and an epimyocardial pacemaker lead was implanted on the left ventricle. Histopathology confirmed highly active purulent infection of the LAAO and the surrounding tissue. Postoperatively the patient remained hemodynamically stable and was discharged to a rehabilitation clinic with ongoing antibiotic therapy.
Fig. 1.
a Transesophageal echo (TEE) post transvenous lead extraction revealing a gelatinous mass (red arrow) on the LAAO (asterisk) in close proximity to the mitral valve (MV). b Control TEE showing persistence of the mass with growth (red arrow) towards the mitral valve (MV). c 3-D-image of the LAAO (asterisk) with the growing mass on the device and in the anterolateral commissure (red arrow) of the MV. d Retrieved device showing endothelial growth and purulent infection
With the TEE confirming lead vegetation and persistent bacteremia with endocarditis-typical specimens the diagnosis endocarditis is made according to Duke criteria [1]. Even though the TEE was terminated prematurely it revealed no signs of valvular endocarditis or right sided abscess formation. Accordingly, transvenous lead extraction in addition to antibiotics is the preferred therapy in patients with CDRIE while a surgical approach remains to be considered in cases of large vegetations [2, 3]. The endocarditic involvement of the LAAO at that point of time remains unclear. To the best of our knowledge no case of an infected LAAO has been reported so far and presumed incidence must be very low. Thrombus formation on an LAAO on the other hand occurs in about 3.7% of cases [4] and antithrombotic treatment was started accordingly. However, the mass did not decrease and the patient was planned for surgery given the uncontrolled infection and the progressive involvement of the mitral valve. Whether earlier surgery would have been possible sparing the mitral valve remains open to speculation but this case should help to create awareness of this rare situation. Especially in the case of an aggressive specimen such as Staphylococcus aureus and given the proximity of the LAA to the mitral valve, a thorough echocardiographic exam should be mandatory.
Acknowledgements
Open Access funding provided by Projekt DEAL.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
References
- 1.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O’Gara P, Taubert KA, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. Circulation. 2015;132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 2.Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, Masoudi FA, Okum EJ, Wilson WR, Beerman LB, Bolger AF, Estes NAM, Gewitz M, Newburger JW, Schron EB, Taubert KA, et al. Update on cardiovascular implantable electronic device infections and their management. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 3.Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, Poole J, Boriani G, Costa R, Deharo J-C, Epstein LM, Saghy L, Snygg-Martin U, Starck C, Tascini C, Strathmore N, Kalarus Z, Boveda S, Dagres N, Rinaldi CA, Biffi M, Gellér L, Sokal A, Birgersdotter-Green U, Lever N, Tajstra M, Kutarski A, Rodríguez DA, Hasse B, Zinkernagel A, Mangoni E. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections—endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2020;57:e1–e31. doi: 10.1093/ejcts/ezz296. [DOI] [PubMed] [Google Scholar]
- 4.Dukkipati SR, Kar S, Holmes DR, Doshi SK, Swarup V, Gibson DN, Maini B, Gordon NT, Main ML, Reddy VY. Device-related thrombus after left atrial appendage closure. Circulation. 2018;138:874–885. doi: 10.1161/CIRCULATIONAHA.118.035090. [DOI] [PubMed] [Google Scholar]