Abstract
Background
Prior authorization of prescription medications is a policy tool that can potentially impact care quality and patient safety.
Objective
To examine the effectiveness of a mandatory peer‐review program in reducing antipsychotic prescriptions among Medicaid‐insured children, accounting for secular trends that affected antipsychotic prescribing nationally.
Data Source
Medicaid Analytical eXtracts (MAX) with administrative claims for health services provided between January 2006 and December 2011.
Study Design
This retrospective, observational study examined prescription claims records from Washington State (Washington) and compared them to a synthetic control drawing from 20 potential donor states that had not implemented any antipsychotic prior authorization program or mandatory peer review for Medicaid‐insured children during the study period. This method provided a means to control for secular trends by simulating the antipsychotic use trajectory that the program state would have been expected to experience in the absence of the policy implementation.
Principal Findings
Before the policy implementation, antipsychotic use prevalence closely tracked those of the synthetic control (6.17 per 1000 in Washington vs. 6.21 in the synthetic control group). Within two years after the policy was implemented, prevalence decreased to 4.04 in Washington and remained stable in the synthetic control group (6.47), corresponding to an approximately 38% decline.
Conclusion
Prior authorization program designs and implementations vary widely. This mandatory peer‐review program, with an authorization window and two‐stage rollout, was effective in moving population level statistics toward safe and judicious use of antipsychotic medications in children.
Keywords: antipsychotic medications, children and adolescents, mandatory peer review, Medicaid, prior authorization, synthetic control method
What This Study Adds.
This study contributes to growing evidence that mandatory peer review of antipsychotic medications is effective in reducing antipsychotic medication utilization among Medicaid‐insure children.
This study adjusts for national secular trends in recent stabilization of antipsychotic utilization by using data from other state systems to create a synthetic control that simulates the anticipated trajectory of antipsychotic use had the mandatory peer review not been implemented in this state.
1. INTRODUCTION
Safe and effective use of antipsychotic medications for children and adolescents [hereafter, children] in the United States remains a pressing treatment issue in behavioral health care. Antipsychotic medication use is substantially higher for children in the United States than in most other developed countries. 1 , 2 , 3 At the turn of the twenty‐first century, rapid growth in pediatric antipsychotic medication treatment occurred in the United States. 4 , 5 Recent evidence suggests a national trend toward stabilization in antipsychotic prescribing among Medicaid‐insured children. 6 However, concerns persist about antipsychotic medication prescribing rates among Medicaid‐insured children, whose prescription rates for antipsychotics are more than twofold those of privately insured children. 6
In response to these concerns, 31 state Medicaid programs employed authorization programs specific to antipsychotic medication treatment among all or a subset of children 18 years of age and younger by 2012. 7 , 8 , 9 Fifteen of the 31 states implemented authorization with a mandatory peer review by physicians, most frequently child and adolescent psychiatrists. 7 All but one of these 31 authorization programs were implemented since 2008. 1 , 7 Rapid growth of these authorization programs nationally likely reflects a concerted effort of the Centers for Medicare and Medicaid Services to bring attention to the safe and judicious use of antipsychotic medications among pediatric populations 10 and calls for increased monitoring efforts from state Medicaid programs. 11
Prior authorization programs have various design features or parameters such as varying age of the recipient, number of drugs concurrently used, or frequency of reviews. Another variation across these programs involves the variation in strategies employed to avoid restricting access when the medication is clinically necessary. Programs range from providing the “hard stop” at time of dispensing, to establishing an authorization window that allows medications to be dispensed for a time‐delineated period while awaiting adjudication. In January 2009, Washington State (Washington) implemented a mandatory collegial review that still allows for a 60‐day authorization window it labels as a “child in crisis” fill for dispensing the medication. To draw a distinction, we therefore refer to the program in Washington as a “mandatory peer review” because the 60‐day window means that authorization is not actually required prior to dispensing (ie, prior authorization). While authorization windows may address access barriers incurred during a hard edit, these windows may also allow for the initiation of high start doses (which may entail risk of a dystonic reaction or marked sedation) or undetected drug interaction concerns, and may be less effective than prior authorization in promoting safe and judicious antipsychotic use.
Given the widespread implementation of authorization programs—including mandatory peer reviews—since 2008, these programs may, in part, explain stabilization or declines in antipsychotic medication treatment among Medicaid‐insured children. 6 A recent systematic review, which identified studies evaluating 10 Medicaid prior authorization programs, found that all but one of these prior authorization programs were associated with reductions in antipsychotic prescribing among the targeted age cohorts. 12 However, designs of prior research studies limit our ability to attribute reductions in antipsychotic prescribing to the prior authorization programs themselves. Of the 10 authorization programs previously evaluated, only one of these programs was evaluated using a comparison population. 12 Conducted by Stein et al, this study found a null effect of the prior authorization program on antipsychotic prescribing among young children (0 to 5). 13 Lack of control groups limits our ability to discern whether reported reductions in antipsychotic prescribing post‐implementation are attributable to the authorization programs or to national efforts encouraging safe and judicious antipsychotic use.
The present study seeks to contribute to this evidence base by evaluating the mandatory collegial review in Washington, controlling for secular trends in a select set of comparison states. We employ the synthetic control method, 14 which uses a data‐driven and objective procedure for selecting the comparison group. Our application of a synthetic control generates a hypothetical counterfactual region—the comparison region—that uses the Washington data prior to implementation of the mandatory program to draw a weighted average from the states without monitoring programs during the study period. Data from comparison states were then used to simulate the prevalence of an antipsychotic use trajectory that Washington would have witnessed in the absence of the policy and to measure the policy's impact on antipsychotic prescribing rates. While prior evaluations of the mandatory peer review find reductions in antipsychotic prescribing post‐implementation of the mandatory peer review, 15 this paper investigates whether the early evidence of effectiveness persists when controlling for secular trends in antipsychotic prescribing among children in the United States.
2. METHODS
In April 2008, the Washington State Health Care Authority (HCA), University of Washington faculty, and Seattle Children's Hospital collaborated to establish the Partnership Access Line (or “PAL”), an elective consultation service seeking to improve access to child and adolescent psychiatric expertise for primary care settings. 16 The PAL provided elective consultations for all primary care providers on diagnostic clarification, medication adjustment, and treatment planning, including consultation on antipsychotic initiation and monitoring. 17 In January 2009, the HCA subsequently initiated a mandatory peer‐review program for Medicaid‐insured children (17 years of age and younger) for the dispensing of antipsychotic medications that exceed the maximum dose for the enrollees’ age group, as set forth by the HCA. Then, in July 2012 HCA implemented additional mandatory review triggers in an effort to reduce poly‐pharmacy. The program endorsed a sixty‐day authorization window during which time the medication would be temporarily dispensed awaiting the outcome of the mandatory peer review. 12 The mandatory secondary collegial review program relied upon the same child psychiatric consulting team as in the voluntary PAL consult program for more easily scheduled telephone discussions and greater consistency of best practice educational messaging. The Washington approach therefore initially provided opportunities for elective psychiatric consultation prior to implementing a mandatory peer‐review process. Guidance available to state systems references the incremental approach of providing educational opportunities prior to mandatory peer review as a promising practice. 12
2.1. Data sources and inclusion criteria
This study used state level estimates of monthly point prevalence of antipsychotic prescribing in 21 states including Washington as well as 20 states that did not implement any antipsychotic mandatory peer review or prior authorization program for Medicaid‐insured children during the study period. The outcome variable, monthly point prevalence of antipsychotic use, represents the number of children (aged 1 to 17) with an active prescription for at least one antipsychotic medication on the 15th of each month (index date), per 1000 children. The prescription was considered active as of the index date if that date was included within the days‐supply of a prior prescription, and the enrollee was Medicaid‐eligible on that day. Data were generated from eligibility and utilization data available in Medicaid Analytical eXtracts (MAX) from the years 2006 to 2011.
Potential donor states for the synthetic control group were limited to states with no indication of an authorization program during the study period (2006‐2011). Identification of states without authorization programs drew from three prior studies examining the “state of the states” in pediatric antipsychotic monitoring programs, including prior authorization. 8 , 9 , 18 Twenty‐four states had no indication of authorization in the three independently fielded state surveys. Of these 24 states, four states (Connecticut, Hawaii, Kansas, and Massachusetts) were excluded due to missing data or other data anomalies documented within the Medicaid Analytic eXtracts. 19 , 20 This resulted in a balanced panel of 21 states, including Washington, with 72 monthly observations for each state. The Institutional Review Board at [institution withheld to preserve anonymity of the authors] reviewed and approved the study protocol.
Table 1 summarizes the potential predictors of antipsychotic use included in the synthetic control model. We included two variables that are indicative of children's ability to access qualified mental health professionals who can deliver evidence‐based psychosocial interventions to substitute for antipsychotic medications. Third, prescriber feedback has been shown to reduce antipsychotic medication use in some settings, particularly for regimes with limited empirical support. 21 Fourth, we included the percentage of population living in urban areas for each state, as accessibility, availability, and acceptability of mental health care varies significantly across rural and urban areas. 22 Temporal shocks in the number of children served (due to, eg, changes in eligibility criteria and documentation requirements) impact per capita system resources; thus, we included the number of Medicaid‐eligible children on the 15th of each month, aged 1 to 17 (in logarithmic scale). Furthermore, we added the ratio of females to the population served and the ratio of the youngest children (age 1‐5) as time‐varying covariates.
TABLE 1.
Predictors included in the synthetic control construction
| Variable | Source | Average | Standard deviation |
|---|---|---|---|
| (1) Receipt of mental health services among children (percentage). |
Denominator: Children aged 2‐17 years who had an ongoing emotional, developmental, or behavioral problem that, in the view of their parents, required treatment or counseling. Numerator: Children that received mental health services in the past year. Based on nationally representative survey of parents, conducted by Maternal and Child Health Bureau, Health Resources and Services Administration (HRSA). 27 |
62.1 | 8.2 |
| (2) Mental health professional shortage (percentage). |
Denominator: Minimum number of providers necessary in the state to meet the mental health needs, determined by HRSA. Numerator: Gap between minimum necessary and actual number of providers (lower percentages reflected less shortage). 28 |
34.4 | 14.9 |
| (3) Clinically informed prescriber feedback system |
Availability of a prescriber feedback systems such as prescription reviews, prescribing performance letters, based National Alliance on Mental Illness survey of mental health authorities. 29 Five‐point Likert scale (0‐4), smaller numbers representing limited efforts (eg, limited programs and feedback that is not prescriber specific). |
3 | 1.4 |
| (4) Percent living in urban areas | US 2010 Census 30 | 74.1 | 14.9 |
Average and standard deviations were presented for all 50 states and District of Columbia.
The pre‐intervention period was defined as January 2006 to March 2008, the period in which Washington was not exposed to any intervention that targets antipsychotic prescriptions (ie, prior to implementation). The post‐intervention period was defined as February 2009 (first month after the implementation of mandatory reviews) to December 2011 (many of the donor states implemented a prior authorization program in 2012).
2.2. Analyses
Our approach was to compare the antipsychotic use prevalence trajectories from Washington to the trajectories of a synthetic control. The synthetic control was composed of a weighted average of the 20 states. The procedure assigned weights to these 20 states that were non‐negative, ranging from 0 to 1, and summed to 1 across the 20 comparison states. The algorithm then iterated through many combinations of weights until it found a single set of weights for the donor units that produced maximum resemblance between the synthetic control and Washington prior to implementation. To operationalize maximum resemblance, the weights that define the synthetic control cohort were chosen from all possible weights that minimize the synthetic model's mean squared prediction error in the pre‐intervention period. Weights were chosen so that the hypothetical counterfactual region best reproduces Washington's outcome path between January 2006 and March 2008 and its relation to the predictor variables.
As a specification test for the robustness of the model, we conducted placebo tests (also known as falsification tests), proposed by Abadie, Diamond, and Hainmueller. 14 This allowed us to assess whether the effect estimated by the synthetic control was large relative to the effect estimated for a state chosen at random. The tests are akin to the classic framework for permutation inference, where the synthetic control method was applied to every potential control state in the sample. The models and tests were implemented using the synth package written and updated for Stata 15.1. 23
3. RESULTS
The synthetic control algorithm determined that a weighted average of four states generated the hypothetical counterfactual region with maximum resemblance to Washington's outcome path between January 2006 and March 2008 and its relation to the predictor variables: California (40.4%), Louisiana (27.5%), New Mexico (30.7%), and South Dakota (1.4%). All other states were assigned zero weight by the estimator. The synthetic control's point prevalence for antipsychotics resembled the Washington data—reflecting the balance between Washington and the synthetic control (Table 2). For example, in January 2006, the monthly point prevalence of antipsychotic use in Washington was 5.2 (per 1000), 5.3 in the synthetic control. Similar differences were observed throughout the pre‐period. At the outset of the year when the authorization program was initially implemented in Washington (January 2008), the monthly point prevalence of antipsychotic use was 6.2 in Washington and the synthetic control.
TABLE 2.
Predictor variables and monthly point prevalence of antipsychotic use
| WA | Synthetic control | |
|---|---|---|
| Receipt of mental health services among children | 53.9 | 54.8 |
| Mental health professional shortage | 21.1 | 25.1 |
| Clinically informed prescriber feedback system | 1.0 | 3.1 |
| Proportion living in urban areas (%) | 84.1 | 84.0 |
| Log (population served) | 13.2 | 13.7 |
| % Female | 49.4 | 49.5 |
| % Aged 1‐5 | 39.8 | 40.0 |
| Monthly point prevalence of antipsychotic use | ||
| Jan‐06 | 5.19 | 5.32 |
| Jan‐07 | 5.65 | 5.76 |
| Jan‐08 | 6.17 | 6.21 |
| Jan‐09 | 5.43 | 6.31 |
| Jan‐10 | 4.28 | 6.18 |
| Jan‐11 | 4.18 | 6.14 |
| Dec‐11 | 4.04 | 6.47 |
The synthetic control's predictor variables also resembled the Washington data. For example, receipt of mental health services varied less than one percentage point (53.9 vs. 54.8). Standard deviation for this variable was 8.2 percentage points. Mental health provider shortages were less severe in Washington (21.1), four percentage points larger for synthetic Washington (25.1). Standard deviation for this variable was 14.9 percentage points. Balance was not achieved for clinically informed prescriber feedback system score. This may also be because of the lack of variation in the score (14 out of 20 states scored four points). It is also likely that the prescriber feedback, with its limited variation in this subset of states, was not a strong predictor of monthly prevalence.
The actual rates in Washington declined post‐implementation, diverging from the relatively stable rates of the synthetic control (Figure 1). Within two years after the authorization program was fully implemented (December 2011), the prevalence in Washington decreased from 6.17 per 1000 to 4.04 per 1000. The prevalence rate, however, remained flat for the synthetic control, as shown in Figure 1 and Table 1. Figure 2 presents the gap in monthly point prevalence between Washington and the synthetic control, demonstrating the divergence in monthly antipsychotic point prevalence between the two units, a difference of 2.43 per 1000 children between the two samples at the end of the observation period (2011).
FIGURE 1.
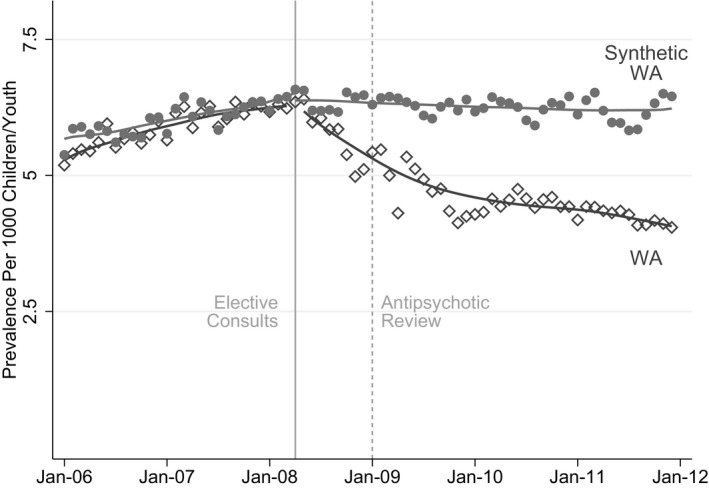
Monthly Rates of Antipsychotic Prescription Fills
FIGURE 2.
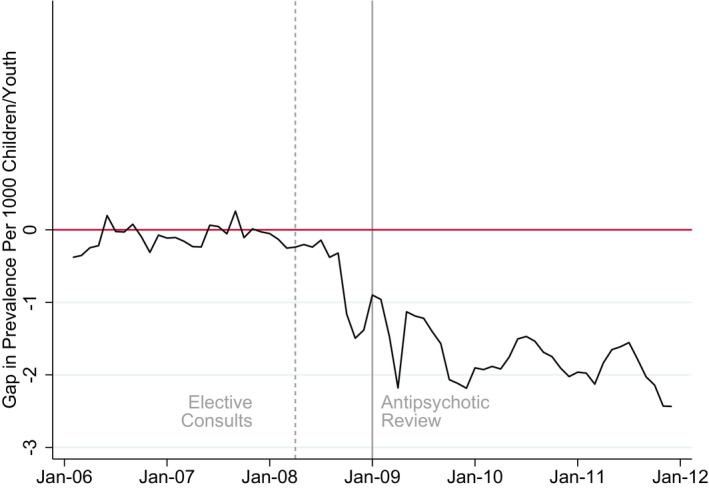
Difference between WA and Synthetic WA in Monthly Rates of Antipsychotic Prescription Fills [Color figure can be viewed at wileyonlinelibrary.com]
Notes: The plot represent the monthly gap between prevalence rates for Washington State and its synthetic control.
To evaluate the significance of this difference, we posed the question of whether this result can be entirely driven by chance. To answer this question, we ran falsification test (placebo studies) by applying the synthetic control method to each of the 20 other states, shifting Washington to the donor pool, and plotting the gap between prevalence data for each state and its synthetic control. This iterative procedure is akin to the classic framework for permutation inference and provides us with a distribution of estimated gaps for the states where no intervention took place. 14 Figure 3 displays the results for the placebo test. The gray lines represent the gap associated with each of the 20 states. The black line represents the gap for Washington and synthetic Washington. As the figure illustrates, the estimated gap for Washington during the post‐implementation period was unusually large relative to the distribution of the gaps for each of the 20 states in the donor pool. In other words, if one was to relabel the implementation state in the dataset at random, the probability of obtaining results of the magnitude of those obtained for Washington would be small, <0.05 (1 out of 21).
FIGURE 3.
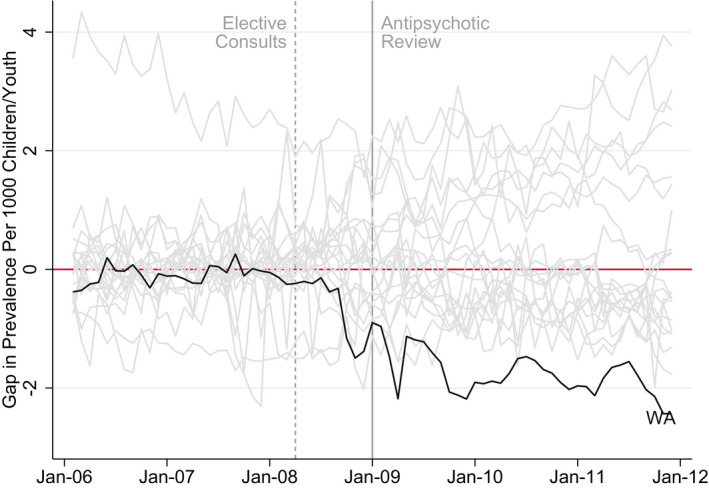
Falsification Tests [Color figure can be viewed at wileyonlinelibrary.com]
Notes: Falsification test procedure applies synthetic control method to each of the 20 states, shifting Washington to the donor pool. Each gray plot represents the gap between prevalence rates for a state and its synthetic control. The combination of these gray plots provides us a distribution of estimated gaps for the states where no intervention took place. The black plot represents the gap between prevalence rates for Washington State and WA’s synthetic control
4. DISCUSSION
The well‐documented and rapid expansion of Medicaid prior authorization programs for pediatric antipsychotic use since 2009 warrants rigorous study. 7 , 8 , 9 While previous studies find that recent stabilization may be associated with widespread implementation of prior authorization programs, the ability to draw causal inference is limited because the majority of evaluations lack a comparison group to control for secular trends (eg, the release of antipsychotic practice parameters by professional organizations and federal efforts to impact antipsychotic use). 12 Our state level analyses employ a synthetic control to address these limitations. In comparative case studies, there is some degree of ambiguity about how comparison units are chosen. Researchers investigating state policies often select comparison states based on proximity in geography, demographics, or other state level parameters. The method we utilize employs a data‐driven procedure rather than a subjective methods to construct comparison group and reduces discretion in the choice of the comparison units. 14 In addition to transparency in choosing comparisons, a second advantage of the synthetic control method relative to traditional regression methods (eg, difference‐in‐differences) is the safeguard against extrapolation. Because the weights are restricted to be positive and sum to one, the synthetic control method provides a safeguard against extrapolation. 14
Public policymakers and private insurers use prior authorization programs to improve patient safety, care quality, and to reduce low‐value care. The Washington Medicaid mandatory peer‐review program had two design features worth comment. First, the program employed a 60‐day authorization window in which the child could be dispensed the antipsychotic medication while the order was under adjudication. This design feature is under‐studied in the extant literature. Prior studies suggest that prescribing clinicians, caregivers, and families express concern that a hard stop may result in the inability to dispense the medications when needed and potentially increase the risk of psychiatric decompensation or present safety concerns (eg, aggression). 12 , 24 Therefore, the authorization window in Washington is responsive to the concerns of some stakeholders. By providing a window within which the authorization process may occur, the program intends to mitigate the potential unintended consequence of limiting access to clinically indicated and appropriate antipsychotic medications.
On the other hand, a 60‐day authorization window could potentially compromise the effectiveness of the intervention. Our study finds that, even with the authorization window, reduction in antipsychotic medications occurred after implementation of the Washington mandatory peer‐review program. However, our study is unable to answer whether medications dispensed before secondary peer review (during the 60s) hold potential for adverse effects, such as a dystopic reaction, marked sedation, or potential drug interactions. Additional investigation is warranted on the extent of prescribing that occurs during the authorization window and implications for child well‐being.
The second feature was the incremental rollout of collegial secondary review: In the first 12 months, prescribing physicians had access to child psychiatrist consultations (Partnership Access Line), and the consultations were elective. The mandatory collegial secondary review in the adjudication process was implemented only after the first 12 months. Our results show that the decline rate in the elective period was substantially similar to the decline rate in the first 12 months of mandatory review.
Prior authorization policies are often highly contested. According to a physician survey conducted by the American Medical Association (AMA), prior authorization procedures are burdensome and costly for health care providers. 25 , 30 These concerns have generated national advocacy efforts from professional organizations to reform prior authorization program desings. 26 Washington's program design has potential to relax some aspects of the administrative burden. Incremental rollout is responsive to recommendations from expert consensus groups that prior authorizations be implemented in collaboration with other clinical supports (eg, consultative access lines and investment in psychosocial therapeutic services) to facilitate evidence‐based prescribing. 12
Our study has several limitations. While the donor states did not implement any authorization program, there is the potential for other state level policies to have been implemented during the study period. However, other mechanisms are not likely to be as impactful on antipsychotic prescribing as a hard edit implemented through a prior authorization program. While our dataset represents the population of Medicaid‐insured children, some aberrations may exist in managed care encounter data within Medicaid programs. We leverage the strength of Medicaid claims‐based data to capture prescription drug utilization, which represent medications dispensed at point‐of‐sale. As with other studies using claims data, these measures reflect filled prescriptions rather than actual ingestion of medications; however, since the focus of the study is on prescribing patterns rather than the clinical effects of the medications, filled prescriptions represent an appropriate measure of the practices targeted by the intervention.
Prior authorization programs are valuable policy tools for public and private insurers, for improving patient safety and care quality. We provided evidence on a prior authorization program with design features, including a 60‐day authorization window, secondary “peer” review via an access line, and incremental rollout. The program was effective in moving population level statistics toward safe and judicious use of antipsychotic medications in children.
Supporting information
Supplementary Material
Table S1
ACKNOWLEDGMENT
Joint Acknowledgment/Disclosure Statement: Supported by PCORI grant IHS‐1409‐23194 and AHRQ grant R01‐HS026001; Dr Crystal's effort is additionally supported by NCATS award UL1TR003017.
Akincigil A, Mackie TI, Cook S, Hilt RJ, Crystal S. Effectiveness of mandatory peer review to reduce antipsychotic prescriptions for Medicaid‐insured children. Health Serv Res. 2020;55:596–603. 10.1111/1475-6773.13297
REFERENCES
- 1. Zito JM, Safer DJ, Berg L, et al. A three‐country comparison of psychotropic medication prevalence in youth. Child Adolesc Psychiatry Ment Health. 2008;2(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zoëga H, Baldursson G, Hrafnkelsson B, Almarsdóttir AB, Valdimarsdóttir U, Halldórsson M. Psychotropic drug use among Icelandic children: A nationwide population‐based study. J Child Adolesc Psychopharmacol. 2009;19(6):757‐764. 10.1089/cap.2009.0003 [DOI] [PubMed] [Google Scholar]
- 3. Rani F, Murray ML, Byrne PJ, Wong ICK. Epidemiologic features of antipsychotic prescribing to children and adolescents in primary care in the United Kingdom. Pediatrics. 2008;121(5):1002‐1009. [DOI] [PubMed] [Google Scholar]
- 4. Olfson M, Blanco C, Liu S‐M, Wang S, Correll CU. National trends in the office‐based treatment of children, adolescents, and adults with antipsychotics. Arch Gen Psychiatry. 2012;69(12):1247‐1256. [DOI] [PubMed] [Google Scholar]
- 5. Matone M, Localio R, Huang Y‐S, dos Reis S, Feudtner C, Rubin D. The relationship between mental health diagnosis and treatment with second‐generation antipsychotics over time: a National Study of U.S. Medicaid‐Enrolled Children. Health Serv Res. 2012;47(5):1836‐1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crystal S, Mackie T, Fenton MC, et al. Rapid growth of antipsychotic prescriptions for children who are publicly insured has ceased. But Concerns Remain. Health Affairs. 2016;35(6):974‐982. [DOI] [PubMed] [Google Scholar]
- 7. Schmid I, Burcu M, Zito JM. Medicaid prior authorization policies for pediatric use of antipsychotic medications. JAMA. 2015;313(9):966‐968. [DOI] [PubMed] [Google Scholar]
- 8. dos Reis S, Tai M‐H, Camelo WC, Reeves G. A national survey of state Medicaid psychotropic‐monitoring programs targeting youths. Psychiatr Serv. 2016;67(10):1146‐1148. [DOI] [PubMed] [Google Scholar]
- 9. Mackie TI, Hyde J, Palinkas LA, Niemi E, Leslie LK. Fostering psychotropic medication oversight for children in foster care: a national examination of states’ monitoring mechanisms. Adm Policy Ment Health. 2017;44(2):243‐257. [DOI] [PubMed] [Google Scholar]
- 10. CMS Medicaid Integrity Program . Atypical Antipsychotic Medications: Use in Pediatric Patients. 2013. https://www.cms.gov/medicare‐medicaid‐coordination/fraud‐prevention/medicaid‐integrity‐education/pharmacy‐education‐materials/downloads/atyp‐antipsych‐pediatric‐factsheet.pdf. Accessed February 22, 2019.
- 11. CMS . A Review of State Medicaid Approaches on Child AntipsychoticMonitoring Programs. https://www.medicaid.gov/medicaid‐chip‐program‐information/by‐topics/prescription‐drugs/downloads/state‐medicaid‐dur‐summaries.pdf. Accessed February 22, 2019.
- 12. Mackie TI, Larson J, Lee S, Huang L. Steering Committee Members. Best Practices in Promoting Safe and Judicious Antipsychotic Prescribing for Children and Adolescents. Rockville, MD: Substance Abuse and Mental Health Services Administration; https://store.samhsa.gov/sites/default/files/d7/priv/pep19‐antipsychotic‐bp_508.pdf [Google Scholar]
- 13. Stein BD, Leckman‐Westin E, Okeke E, et al. The effects of prior authorization policies on Medicaid‐enrolled children’s use of antipsychotic medications: Evidence from two mid‐Atlantic States. J Child Adolesc Psychopharmacol. 2014;24(7):374‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abadie A, Diamond A, Hainmueller J. Synthetic control methods for comparative case studies: Estimating the effect of California’s tobacco control program. J Am Stat Assoc. 2010;105(490):493‐505. [Google Scholar]
- 15. Barclay RP, Penfold RB, Sullivan D, Boydston L, Wignall J, Hilt RJ. Decrease in statewide antipsychotic prescribing after implementation of child and adolescent psychiatry consultation services. Health Serv Res. 2017;52(2):561‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hilt RJ. Telemedicine for child collaborative or integrated care. Child Adolesc Psychiatr Clin N Am. 2017;26(4):637‐645. [DOI] [PubMed] [Google Scholar]
- 17. Seattle Children’s Hospital . Partnership Access Line. https://www.seattlechildrens.org/healthcare‐professionals/access‐services/partnership‐access‐line/. Accessed February 22, 2019.
- 18. Zito JM, Burcu M, McKean S, Warnock R, Kelman J. Pediatric use of antipsychotic medications before and after Medicaid peer review implementation. JAMA Psychiatry. 2018;75(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Zhu Y, Chen C, et al. Internal validation of Medicaid Analytic eXtract (MAX) data capture for comprehensive managed care plan enrollees from 2007 to 2010. Pharmacoepidemiol Drug Saf. 2018;27:1067‐1076. [DOI] [PubMed] [Google Scholar]
- 20. Byrd VLH, Dodd AH. Assessing the usability of encounter data for enrollees in comprehensive managed care across MAX 2007–2009. Mathematica Policy Res. 2012. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3983721/ [Google Scholar]
- 21. Hazra M, Uchida H, Sproule B, Remington G, Suzuki T, Mamo DC. Impact of feedback from pharmacists in reducing antipsychotic polypharmacy in schizophrenia: reducing antipsychotic polypharmacy. Psychiatry Clin Neurosci. 2011;65(7):676‐678. [DOI] [PubMed] [Google Scholar]
- 22. Popat S. The 2014 Update of the Rural‐Urban Chartbook. 2014:153 https://ruralhealth.und.edu/projects/health‐reform‐policy‐research‐center/pdf/2014‐rural‐urban‐chartbook‐update.pdf
- 23. Hainmueller J. Synth Package. https://web.stanford.edu/~jhain/synthpage.html. Accessed August 27, 2018.
- 24. Hawke JM, Ratliff S, Walker J.A Report on Family Experiences with the Use and Monitoring of Antipsychotic Medications for Their Children. Family Run Executive Director Leadership Association; 2018. https://www.fredla.org/wp‐content/uploads/2018/10/FREDLA‐FOCUS‐GROUP‐ON‐ANTIPSYCHOTIC‐MEDICATIONS‐REPORT‐_Final‐5‐8‐18.pdf. Accessed April 28, 2019.
- 25. American Medical Association. 2017 AMA Prior Authorization Physician Survey. Published 2018. Accessed November 1, 2019. https://www.ama‐assn.org/sites/ama‐assn.org/files/corp/media‐browser/public/arc/prior‐auth‐2017.pdf
- 26. American Medical Association. Consensus Statement on Improving the Prior Authorization Process. Accessed November 1, 2019. https://www.ama‐assn.org/sites/ama‐assn.org/files/corp/media‐browser/public/arc‐public/prior‐authorization‐consensus‐statement.pdf
- 27. U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau . The Health and Well‐Being of Children: A Portrait of States and the Nation, 2011–2012. Rockville, Maryland: Department of Health and Human Services; 2014. [Google Scholar]
- 28. Bureau of Health Workforce . Health Resources & Service Administration Shortage Designation. https://bhw.hrsa.gov/shortage‐designation. Accessed February 22, 2019.
- 29. Aron L, Honberg R, Duckworth K. Grading the States: A Report on America’s Health Care System for Serious Mental Illness. Arlington, VA: National Alliance on Mental Illness; 2009. https://www.nami.org/getattachment/About‐NAMI/Publications/Reports/NAMI_GTS2009_FullReport.pdf. Accessed August 20, 2018. [Google Scholar]
- 30. US Census Bureau . American Fact Finder. https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed August 18, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Table S1


