Abstract
Salinity intrusion is one of the biggest problems in the context of sustainable agricultural practices. The major concern and challenge in developing salt-resistance in cultivated crops is the genetic complexity of the trait and lack of natural variability for stress-responsive traits. In this context, tomato wild relatives are important and have provided novel alleles for breeding abiotic stress tolerance including salt tolerance. We provide here a case study, involving tomato wild relative Solanum chilense and cultivated variety Solanum lycopersicum, carried out under high salt stress to investigate comparative transcriptional regulation mediating ROS homeostasis and other physiological attributes. Salt dependent oxidative stress in S. lycopersicum was characterized by a relatively higher H2O2 content, generation of O2•−, electrolytic leakage and lipid peroxidation whereas reduced content of both ascorbate and glutathione. On the contrary, the robust anti-oxidative system in the S. chilense particularly counteracted the salt-induced oxidative damages by a higher fold change in expression profile of defense-related salt-responsive genes along with the increased activities of anti-oxidative enzymes. We conclude that S. chilense harbours novel genes or alleles for salt stress-related traits that could be identified, characterized, and mapped for its possible introgression into cultivated tomato lines.
Keywords: Salt stress, Antioxidants, Tomato, Wild halophytes, Solanum chilense
1. Introduction
Crop productivity is prominently affected by various abiotic and biotic stresses. Among the abiotic stresses, soil salinity is one of the greatest challenges for food production (Albaladejo et al., 2017). Approximately 12 billion USD are lost worldwide every year due to the salt stress (Zahedi et al., 2019). About 10% of the total land area (950 Mha) and 50% of the total irrigated area (230 Mha) of the world is salt-affected, and this threat will increase frequently in upcoming years (Abiala et al., 2018). Despite several studies and bulk of literature available on the salinity tolerance mechanism, there is a lack of clear-cut understanding of salt-stress response mechanisms and key genes controlling salt tolerance in certain agriculturally important crops (Mishra and Tanna, 2017). Tomato (Solanum lycopersicum L.; family Solanaceae) is the second most agro-economically important vegetable crop after potato (Solanum tuberosum L.) (Ronga et al., 2017). Tomato is extremely sensitive to salinity, and severe loss of overall yield and economic productivity has been recorded due to salt stress (Albaladejo et al., 2017). At molecular and physiological level, salt stress imposes reactive oxygen species (ROS) induced oxidative stress that leads to ionic and nutritional imbalances, and osmotic changes in plants (Ashraf et al., 2018). These changes may lead to oxidative damages, perturbation in metabolic pathways and important physiological processes in plants. These changes include reduced photosynthesis (destruction of photosynthetic apparatus), impairment of electron transport, stomatal closure, ion toxicity and decreased cellular growth (Assaha et al., 2017, Rahman et al., 2019). Further, prolonged exposure to saline conditions might result in hyper ionic and/or hyperosmotic stress, in addition to dehydration response (Fricke, 2019). Overall, these changes result in reduced crop productivity. However, plants have mechanisms to counteract salt stress. The major counteracting mechanisms include changes occurring at morphological, biochemical, physiological and developmental level (Acosta-Motos et al., 2015, Hossain and Dietz, 2016). This comprises of ROS homeostasis, increased antioxidant defense mechanism as well as activation of ROS scavenging pathways, compartmentation of toxic ions, osmolyte biosynthesis, ion homeostasis, change in photosynthetic parameters, and hormonal changes (Flowers and Colmer, 2015). Further, ROS scavenging system consists of several non-antioxidant molecules as well as anti-oxidative defense enzymes and/or their isoforms located in different cellular compartments, particularly, isoforms that exist in mitochondria, chloroplast, and peroxisomes (Assaha et al., 2017, Rahman et al., 2019). Apart from antioxidative defense mechanism, salt stress avoidance is also achieved through accumulation of osmolytes and other non-enzymatic antioxidants that facilitate ROS scavenging and absorption of water, and decrease the osmotic potential of cytoplasm (Latef and Chaoxing, 2014, Puniran-Hartley et al., 2014, Rakhmankulova et al., 2015).
Solanum chilense, wild relative of tomato is native to most salt affected arid area of the Atacama Desert and is naturally present in salt-affected regions of Northern Chile (Chetelat et al., 2009). It has extreme potential to grow well in diverse and fluctuating environments. The tolerance of this wild species to such harsh environment is possibly due to presence of essential genes that govern key tolerance traits against various abiotic and biotic stresses (Martínez et al., 2014, Thapa et al., 2015). Interestingly, biochemical and physiological basis of salt resistance in S. chilense has been less explored when compared to other wild relatives such as Solanum cheesmanii, Solanum pennellii, and Solanum pimpinellifolium (Almeida et al., 2014). Therefore, a comparative study on the response of cultivated tomato S. lycopersicum and its wild-relative S. chilense would be useful in identification and characterization of mechanisms, essential genes, proteins, gene regulatory pathways, and novel signaling mechanism involved in salt tolerance in S. chilense. In the background of this, the present study was carried out to examine the qRT–PCR-based relative quantification of differentially expressed salt stress responsive genes in S. lycopersicum and S. chilense at vegetative stage or pre-reproductive phase. Further, we measured the biochemical response of different antioxidant enzymes and evaluated the physiological response to salinity to assess and characterise salt tolerance in S. chilense. We believe that the information generated from this study would be useful to improve the salinity tolerance in cultivated tomatoes through crop breeding programs.
2. Materials and methods
2.1. Plant materials, growth conditions and salinity imposition
The experiments were conducted in the greenhouse facility available at the ICAR-Indian Institute of Vegetable Research, Varanasi, India (25.3521°N, 82.9502°E) during spring-summer period. Tomato species, viz., S. chilense (accession LA1972) and S. lycopersicum (cv. Kashi Amrit) were used for the study. Seeds were sown and seedlings were raised in nursery trays. Twenty days old seedlings were transplanted into plastic pots (one plant/pot) containing mixture of soil vermiculite-perlite and farmyard manure. Plants were grown under 25 °C/15 °C day/night temperature, 50–65% relative humidity, and optimal photoperiod of 16 h/8h (day/night). The salt stress was imposed twenty days after transplanting through addition of NaCl for 7, 14 and 21 days. A non-stress or control treatment (0 days) was carried out without NaCl (EC of 3.8 dSm−1) whereas, saline treatments received 500 mmol of NaCl for 7, 14, and 21 days (EC of 26.8 dSm−1). The fully-open upper third leaves were collected at different days of salt exposure. The collected leaf samples were instantly frozen in liquid nitrogen and preserved in −80 °C.
2.2. Determination of electrolyte leakage (EL) and relative water content (RWC)
At 0, 7, 14 and 21 days of salt stress, EL and RWC of plant leaf tissues were estimated by the method of Khare et al. (2010). To determine the RWC, ten leaf discs (fully expanded fresh leaf) were weighed to note down the fresh mass (FM). The leaf discs were rehydrated in water (50 ml) in a Petri dish for 16 h (at 25 °C) until the leaf became fully turgid. The leaf discs were reweighed to obtain their turgid mass (TM) after the end of imbibition time. The leaf discs were then fully dried in an oven and reweighed to obtain the dry mass (DM). The RWC was calculated following the equation: RWC (%) = [(FM − DM)/(TM − DM)] × 100. EL was estimated with the help of a portable conductivity meter (CM-180, Elico, India). The EL was calculated by the equation: EL (%) = x/y × (1 0 0).
2.3. Measurement of chlorophyll content index (CCI), chlorophyll fluorescence, chlorophyll and carotenoid content
Chlorophyll content meter (CCM-200, Apogee Instruments, Inc. USA) was used for measurement of CCI. Photosynthetic efficiency was determined by using three fully expanded upper leaves with the help of a portable plant efficiency analyzer (Hansatech Instruments Ltd, UK). PSII (quantum efficiency) was calculated according to Maxwell and Johnson (2000). Carotenoid, Chl a and Chl b content were estimated by extracting the fresh leaf sample (300 mg) in 80% v/v acetone and after centrifugation at 8000 rpm for 20 min, absorbance of supernatant was recorded at 480, 510, 645, and 663 nm (Lichtenthaler and Buschmann, 2001) with a UV–visible spectrophotometer (Bio-Rad Smart Spec™ Plus, USA). Both chlorophyll and carotenoid content were measured as defined by Arnon (1949) and the outcomes were expressed as mg g−1 FW.
2.4. Measurement of hydrogen peroxide (H2O2) and superoxide anion (O2•−)
Measurement of H2O2 content in leaf sample was done as per the method described by Jana and Choudhuri (1981). 200 mg of fresh leaf sample was homogenized in 50 mM phosphate buffer (pH 6.5) to extract H2O2. To 3 ml of the supernatant, 1 ml of 0.1% titanium sulphate was added and centrifuged at 8000g for 15 min. The measurement of intensity of the yellow color mixture was done at 410 nm. Using the extinction coefficient of 0.28 mmol−1 cm−1, the H2O2 concentration was measured and expressed as μmol g−1 FW. Estimation of superoxide (O2•−) radicals were executed as per the procedure described by Misra and Fridovich (1972). 200 mg fresh leaf sample was homogenized in 50 mM potassium phosphate buffer (pH 7.5) premixed with 250 mM sucrose, 10 mM MgCl2, and 1 mM diethyldithiocarbamate. In a final volume, the assay mixture contained the supernatant along with 3 mM epinephrine in phosphate buffer (pH 7.5) and 0.3 mM NADH. The end product’s optical density was determined at 540 nm and the superoxide radical’s formation was expressed as ΔA540 min−1 mg−1 protein.
2.5. Estimation of proline (Pro) and malondialdehyde (MDA) content
The proline level was assessed as per the technique described by Bates et al. (1973). For this, 200 mg of fresh leaf sample was extracted in 5 ml of sulfosalicylic acid (3%). 2 ml of supernatant was mixed to 2 ml of acid ninhydrin. 2 ml of glacial acetic acid was then blended with the mixture followed by boiling at 100 °C for 1 h in a water bath. Further, 4 ml of toluene was added in the mixture in a test tube by thorough mixing and chromophore absorbance at 520 nm was noted down and expressed as μg g−1 FW. The procedure given by Heath and Packer (1968) was followed for the assessment of malondialdehyde (MDA). 200 mg of leaves were homogenized in 5 ml of 10% trichloroacetic acid (TCA) having 0.25% of 2-thiobarbituric acid (TBA). The mixture was promptly cooled down after heating at 90 °C for 30 min followed by centrifugation at 8000g for 15 min. The specific absorbance of the supernatant was measured at 532 nm and 600 nm. The concentration of MDA was assessed and expressed as μmol g−1 FW using an extinction coefficient of 155 mM−1 cm−1.
2.6. Assay of antioxidant enzymes activities
The method of McKersie et al. (1990) was followed for assessment of catalase (CAT, EC 1.11.1.6) activity. 200 mg of fresh leaf tissue was homogenized in 50 mM Tris-NaOH buffer (pH 8.0) containing 0.5 mM EDTA, 0.5% (v/v) Triton X-100, and 2% (w/v) polyvinyl pyrrolidone (PVP). Using an extinction coefficient of 0.036 mM−1 cm−1 and by a progressive decrease in absorbance for 5 min, the decomposition of H2O2 level was noted down at 240 nm. In order to express the enzyme-specific activity, mmol of H2O2 oxidized mg−1 (protein) min−1 was used. The ascorbate peroxidase (APX; EC: 1.11.1.11) activity was calculated as suggested by Nakano and Asada (1981). 5 ml of 50 mM potassium phosphate buffer of pH 7.8 that contained 1 mM EDTA, 1 mM ascorbic acid, 1% PVP; and 1 mM phenylmethylsulphonyl fluoride, 200 mg of fresh leaf sample was blended at 4 °C. By the reduction in absorbance at 290 nm (extinction coefficient of 2.8 mM−1 cm−1) up to 5 min, the rate of ascorbate oxidation was documented. The enzyme specific activity of ascorbate peroxidase was estimated as mmol ascorbate oxidized mg−1 (protein) min−1. The activity of glutathione reductase (GR; EC 1.6.4.2) was estimated following Sánchez-Rodríguez et al. (2010). 200 mg of fresh leaf sample was homogenized with 5 ml of 100 mM Tris-HCl buffer (pH 7.8). In a final volume of 2 ml, the reaction mixture comprised of Tris-HCl buffer (100 mM, pH 7.8), oxidized glutathione (0.5 mM, GSSG), MgCl2 (3 mM), NADPH (0.2 mM), and 200 µl of enzyme extract at 25 °C. With the addition of NADPH at ambient temperature, the reaction was commenced; and for the next 5 min, the reduction in the absorbance of NADPH at 340 nm (extinction coefficient 6.2 mM−1 cm−1) was noted down, in the absence of GSSG, amelioration was made for NADPH oxidation. The enzyme specific activity was expressed as mmol NADPH oxidized mg−1 (protein) min−1. The assay of superoxide dismutase (SOD, EC 1.15.1.1) activity was done following the procedure of Nahakpam and Shah (2011). 200 mg fresh leaf samples were mixed with 100 mM potassium phosphate buffer (5 ml, pH 7.8), including 0.1 mM EDTA, 0.1% (v/v) Triton X-100, and 2% (w/v) of soluble polyvinyl pyrrolidone (PVP). During the following 5 min, the formation of adrenochrome was recorded at 470 nm. Under experimental conditions, the magnitude of enzyme needed to stimulate 50 per cent of epinephrine oxidation is described as one unit of SOD activity.
200 mg fresh leaf sample was ground with liquid nitrogen followed by homogenization in 5 ml of 5% (w/v) meta-phosphoric acid at 4 °C. Following the procedure of Owens and Belcher (1965), both total glutathione and reduced glutathione (GSH) were assayed. The reduced glutathione is then oxidized by 5,5-dithiobisnitrobenzoic acid (DTNB), and by the action of GR and NADPH, there is reduction of the oxidized glutathione (GSSG) into GSH. The absorbance of the resultant reaction mixture was recorded at 412 nm following the incubation for 4 min at room temperature. In the same resultant mixture, total glutathione was assayed by adding 0.25 mM NADPH and 0.5 units of glutathione reductase. With reference to a standard curve of 1–5 µg, the concentration of GSH and total glutathione were measured. As per the method of Law et al. (1983), DHA, total AsA, and reduced AsA were assayed. Dithiothreitol (DTT) was used for estimation of total AsA through a reduction of DHA to AsA. The reading was then recorded at 525 nm and the total AsA content was measured using the reference of a plot of standard AsA. With substitution of 100 µl of DTT with 100 µl of distilled water, the reduced AsA was enumerated in a similar method as that of the earlier procedure. The analysis of DHA was undertaken from the difference between the reduced AsA and total AsA and it was expressed as mg g−1 FW. Guaiacol peroxidase (EC 1.11.1.7) activity was measured according to the procedure of Shah et al. (2001). 200 mg of fresh leaf sample was homogenized in 5 ml of 60 mM sodium phosphate buffer (pH 7.0). At 470 nm (extinction coefficient of 26.6 mM−1 cm−1) up to 5 min, the increase in absorbance was recorded and the enzyme-specific activity is expressed as mmol H2O2 reduced mg−1 (protein) min−1. The activity of dehydroascorbate reductase (EC 1.8.5.1) and monodehydroascorbate reductase (MDHAR; EC 1.6.5.4) activity were measured following the procedure provided by Sánchez-Rodríguez et al. (2010). 200 mg of fresh leaf sample was mixed in 5 ml of 25 mM sodium phosphate buffer (pH 7.0). Monitoring the increase in absorbance at 265 nm for 3 min (extinction coefficient of 14 mM−1 cm−1) caused due to the formation of ascorbate in reaction mixture, the DHAR activity was assayed. The specific activity of enzyme was expressed as mmol NADPH oxidized mg−1 (protein) min−1. 200 mg of fresh leaf sample was homogenized in 5 ml of 100 mM HEPES-HCl buffer (pH 7.6). To detect the oxidation rate of NADPH, the change in absorbance at 340 nm (extinction coefficient of 6.2 mM−1 cm−1) up to 3 min was recorded. The enzyme specific activity was expressed as mmol ascorbate oxidized mg−1 (protein) min−1.
2.7. RNA extraction, cDNA synthesis, and real-time quantitative PCR detection
Total RNA was extracted from 200 mg of fresh leaves of tomato plants using TRI Reagent (Invitrogen-Ambion, USA) following the manufacturer's recommendation. First-strand of cDNA was synthesized from 1 μg of total RNA using the iScript™ cDNA synthesis kit (Bio-Rad Laboratories, USA) following the manufacturer's recommendations. qRT-PCR amplification reactions of expression analysis were performed using SsoFast™ EvaGreen® Supermix (BioRad Laboratories, USA) according to the manufacturer's instructions with an iQ5 thermal cycler (BioRad Laboratories, USA) using iQ5 software. Primers were designed by Primer 3 software (Supplementary Table 1) (version 0.4.0, http://www.bioinfo.ut.ee). Heat-maps were produced using data obtained from qRT-PCR with the help of Bioconductor R software (http://www.bioconductor.org). The relative level of gene expression quantification was detected by using the 2−ΔΔCT method (Livak and Schmittgen, 2001), and then Ct data value were normalized to the Actin gene transcript level which was used as an internal control.
2.8. Statistical analysis
The experiment was carried out with 3 replications. The analysis of variance (ANOVA) and Duncan's multiple range test (DMRT) was performed to compare the mean values of treatments (P ≤ 0.05 considered as a significant difference). The principal component analysis (PCA) of multivariate analysis was also performed. All these statistical analysis were performed using SPSS software (SPSS Inc., Version 21.0).
3. Results
Wilting symptoms in the S. chilense plants were delayed; the plants remained green and continued to grow after 14 days of salt-stress. The yellowing of leaves was recorded in the S. lycopersicum plants. Even after 21 d of salt-stress, the upper 4 to 5 leaves of the S. chilense plants remained green, while all the leaves, except the upper two leaves of S. lycopersicum plants became yellow.
3.1. Electrolytic leakage, relative water content, chlorophyll content index and chlorophyll fluorescence
A significantly lesser extent of EL was found in S. chilense than S. lycopersicum under all the three salt treatments. On exposure to the salt treatments, S. lycopersicum showed a large increase in EL (Table 1), as indicated by higher relative conductivity (84–248%), whereas S. chilense noted lower increase in EL (34–131%) after exposure for 7 to 21 days of salt stress. The S. chilense had 39, 34 and 44% lower EL than S. lycopersicum after 7, 14, and 21 days of salt treatment, respectively. S. chilense always exhibited a higher potential of holding tissue water than S. lycopersicum (Table 1). As compared to the non-stress S. chilense, , the reduction in RWC for S. chilense was 11, 24 and 30% respectively, while for S. lycopersicum it was 24, 39 and 55%, respectively at 7, 14 and 21 days of salinity treatment. The S. chilense were capable of holding more water content (28, 38 and 71%) than S. lycopersicum after 7, 14 and 21 days salt treatment, respectively. Significantly higher Fv/Fm was reported in S. chilense than S. lycopersicum which were up to 55–73% higher after 14 and 21 days of salt stress (Table 1). The reduction in Fv/Fm for S. chilense was 12, 22 and 22%, respectively, and for S. lycopersicum, it was 18, 29 and 36% at 7, 14 and 21 days of salt stress, respectively. S. chilense exhibited significantly higher value of CCI (51% at 21 days of stress) compared to S. lycopersicum. After 21 days of salt stress treatment, S. lycopersicum exhibited almost 56% loss of CCI, whereas S. chilense experienced up to 38% loss in CCI (Table 1).
Table 1.
Effects of 0, 7, 14, and 21 d of salt stress conditions on Fv/Fm, relative water content (RWC), electrolyte leakage (EL), hydrogen peroxide (H2O2) and superoxide anion (O2•−) in Solanum lycopersicum and Solanum chilense.
| Treatments | Physiological parameter and free radical generation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fv/Fm |
RWC |
EL |
H2O2 |
O2•− |
||||||
| DVRT-1 | Chilense | DVRT-1 | Chilense | DVRT-1 | Chilense | DVRT-1 | Chilense | DVRT-1 | Chilense | |
| 0 d | 0.65 ± 0.03bcd | 0.93 ± 0.02a | 81.3 ± 3.3ab | 89.1 ± 5.6a | 21.4 ± 1.5e | 17.8 ± 1.7e | 27.1 ± 4.8bc | 25.5 ± 2.00c | 0.48 ± 0.14cd | 0.25 ± 0.01d |
| 7 d | 0.53 ± 0.03cde | 0.81 ± 0.02ab | 61.5 ± 3.8cd | 78.9 ± 1.6ab | 39.6 ± 3.8c | 24.0 ± 3.1de | 34.8 ± 1.6bc | 28.5 ± 1.2bc | 0.82 ± 0.08c | 0.27 ± 0.01d |
| 14 d | 0.46 ± 0.07de | 0.72 ± 0.03bc | 48.8 ± 3.7de | 67.6 ± 3.6bc | 51.2 ± 2.0b | 33.8 ± 3.9cd | 53.7 ± 3.9a | 32.4 ± 2.5bc | 1.43 ± 0.24b | 0.35 ± 0.02cd |
| 21 d | 0.41 ± 0.10e | 0.72 ± 0.01bc | 36.0 ± 3.9e | 61.9 ± 5.0cd | 74.8 ± 3.1a | 41.4 ± 1.8bc | 64.1 ± 1.7a | 38.7 ± 4.3b | 2.28 ± 0.18a | 0.76 ± 0.13cd |
Mean (+SE) was calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying the DMRT test.
3.2. H2O2 content and O2•− generation rate
The formation rate of H2O2 content augmented progressively with increasing days of exposure of salt stress in both the S. chilense and S. lycopersicum. Despite this, H2O2 level (1.9–2.35 times) was significantly higher during 14 days as well as 21 days of salt stress in S. lycopersicum, compared to S. chilense, where increase of H2O2 level (1.2–1.5 times) was noted (Table 1). A higher degree of superoxide anion formation rate was recorded in salt exposed leaves of S. chilense and S. lycopersicum under both salt stress as well as non-stress conditions. Superoxide anion (O2•−) formation rate gradually increased over the salt stress conditions by 1.0 times (7 days), 1.3 times (14 days) to 2.9 times (21 days) for S. chilense (Table 1). Contrary to this, in S. lycopersicum, a significantly increased superoxide anion (O2•−) formation rate of 1.6 times (7 days), 2.9 times (14 days) to 4.6 times (21 days) were registered (Table 1).
3.3. Chlorophyll, carotenoids, lipid peroxidation, and proline content
S. chilense maintained 60, 89, and 188% higher chlorophyll compared to S. lycopersicum after exposure to salt stress time period that is 7, 14, and 21 days, respectively (Table 2). S. lycopersicum exhibited almost 73% loss of chlorophyll after 21 days of salt stress, whereas S. chilense experienced up to 37% loss in chlorophyll. The S. chilense maintained significantly higher carotenoids content in all the salt treatments as well as non-salt treatment plants after 21 days of salt stress (Table 2). The carotenoids content of S. lycopersicum was reduced to 55% after 21 days of salt stress while the S. chilense experienced 16% carotenoid reduction. In S. chilense and S. lycopersicum, MDA concentration significantly differed prior to the salt treatments. The S. chilense produced 39, 47, and 61% lower MDA than S. lycopersicum after 7, 14 and 21 days of salt treatment, respectively (Table 2). S. chilense exposed from 7 to 21 days of salt stress showed about 32 to 136% increase in the MDA level with respect to the non-stress treatment, while this elevation of MDA level in S. lycopersicum was 85–422% indicating lower lipid peroxidation in S. chilense (Table 2). The content of proline increased at all stages of salt stress except 21 days of stress in both S. chilense as well as S. lycopersicum with the increased duration of salt stress. However, at 7, 14 and 21 days of salt stress, higher level of proline was noted for the S. chilense (1.8–2.1 folds) compared to the S. lycopersicum (Table 2).
Table 2.
Effects of 0, 7, 14, and 21 d of salt stress conditions on proline, lipid peroxidation (LPO), chlorophyll colour index (CCI), total chlorophyll (Total Chl) and carotenoids in Solanum lycopersicum and Solanum chilense.
| Treatments | Metabolites and pigment content |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Proline (µ g−1 FW) |
LPO (mm g−1 FW) |
CCI |
Total Chl (mg g-1FW) |
Carotenoids (mg g−1 FW) |
||||||
| DVRT-1 | Chilense | DVRT-1 | Chilense | DVRT-1 | Chilense | DVRT-1 | Chilense | DVRT-1 | Chilense | |
| 0 d | 11.5 ± 1.28 d | 13.8 ± 1.6d | 1.28 ± 0.03e | 1.09 ± 0.19e | 47.6 ± 2.4ab | 50.6 ± 1.0a | 3.1 ± 0.15b | 3.8 ± 0.31a | 0.55 ± 0.04a | 0.59 ± 0.02a |
| 7 d | 56.8 ± 1.45c | 104.1 ± 2.0b | 2.39 ± 0.25cd | 1.44 ± 0.23de | 42.0 ± 2.4bc | 46.6 ± 1.5abc | 2.0 ± 0.07cd | 3.3 ± 0.01ab | 0.40 ± 0.01c | 0.55 ± 0.03a |
| 14 d | 112.2 ± 1.50b | 215.9 ± 5.9a | 3.91 ± 0.43b | 2.06 ± 0.21cde | 32.6 ± 1.9d | 39.0 ± 2.9cd | 1.7 ± 0.05d | 3.2 ± 0.15ab | 0.42 ± 0.02bc | 0.53 ± 0.02ab |
| 21 d | 98.8 ± 2.26b | 208.2 ± 6.8a | 6.72 ± 0.36a | 2.58 ± 0.13c | 20.6 ± 1.7e | 31.3 ± 1.6d | 0.82 ± 0.08e | 2.3 ± 0.11c | 0.24 ± 0.008d | 0.49 ± 0.04abc |
Mean (+SE) was calculated from three replicates for each treatment. Values with different letters are significantly different at P ≤ 0.05 applying the DMRT test.
3.4. Assessment of antioxidant enzymes activities
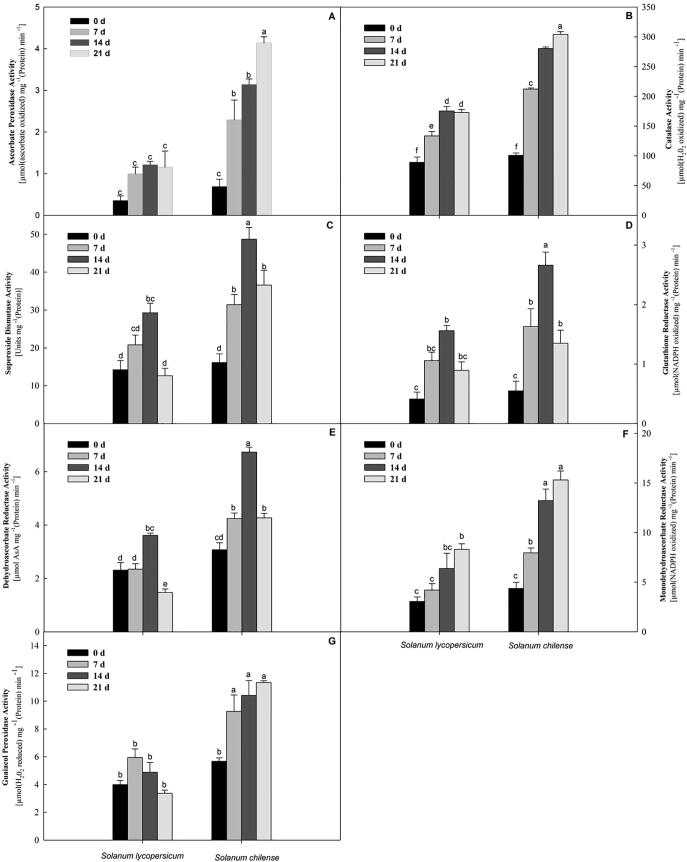
Ascorbate peroxidase enzymatic activity in S. chilense augmented up to 21 days of salt stress condition, and APX activity in S. lycopersicum augmented considerably up to 14 days of stress. Afterwards, a sharp drop was recorded (Fig. 1A). S. chilense and S. lycopersicum differ in specific activity of APX under non-stress circumstances. At 7 and 14 days of salt stress, an elevated activity of APX was noted in the S. chilense (∼130% and ∼158%) compared to S. lycopersicum which increased to ∼258% after 21 days of salt exposure. The enzymatic activity of catalase was augmented up to 14 days of salt stress condition in S. chilense and S. lycopersicum and afterwards, a steady reduction was recorded in S. lycopersicum but activity of CAT enzyme increased constantly up to 21 days of salt stress in S. chilense (Fig. 1B). The scale of this intensification became gradually much higher with the duration of salt stress (1.5-fold at 14 days and 1.7-fold at 21 days in S. chilense) compared to S. lycopersicum. SOD enzymatic activities in S. chilense and S. lycopersicum were comparable but, S. chilense displayed higher activities of SOD than the S. lycopersicum under salt stress (Fig. 1C); the degree of this intensification became gradually greater with the duration of salinity (51% at 7 days, 66% at 14 days, and 189% at 21 days in S. chilense). The activity of superoxide dismutase was the maximum in 14 days of salinity in both S. chilense as well as S. lycopersicum (Fig. 1C). GSSG (oxidized glutathione) was converted into GSH (reduced glutathione) by NADPH-dependent process with the help of glutathione reductase (GR). The GR activity in S. chilense was higher significantly compared to S. lycopersicum under control treatments. However, activity of GR in S. chilense and S. lycopersicum increased up to 14 days of salinity and declined afterwards (Fig. 1D). After 7 days of salinity, the activity of GR was noted to be ∼54% higher compared to S. lycopersicum, further increased to ∼70% at 14 days. At 21 days of salt stress, the S. chilense exhibited 51% higher GR activity than S. lycopersicum counterparts.
Fig. 1.
Activities of key antioxidant enzymes in Solanum lycopersicum and Solanum chilense at 0, 7, 14, and 21 d of salt stress conditions. (A) ascorbate peroxidase activity, (B) catalase activity, (C) superoxide dismutase activity, (D) glutathione reductase activity, (E) dehydroascorbate reductase activity, (F) monodehydroascorbate reductase activity, and (G) guaiacol peroxidase activity. The data are mean of three replicates ± SE. * Bars with distinct letters are significantly different at P ≤ 0.05 applying the DMRT test.
Under control (non-stress), the activity of DHAR differed considerably between S. chilense and S. lycopersicum (Fig. 1E). The DHAR activity increased in both S. chilense and S. lycopersicum up to 14 days of salt stress, but we observed a sharp decline at 21 days of salinity (Fig. 1E). The S. chilense showed significantly higher enzyme activity of DHAR than S. lycopersicum, and this was augmented with the salinity duration (80% at 7 days, 86% at 14 days, and 190% at 21 days in S. chilense). The specific MDHAR enzymatic activity gradually augmented with the periods of salt stress condition in S. chilense and also S. lycopersicum (Fig. 1F). At 7 days of salt stress, S. chilense recorded 89% higher activity of MDHAR as compared to S. lycopersicum; this consistently increased to 107% at 14 days, but dropped abruptly to 84% at 21 days. The S. chilense showed 42% higher enzymatic activity of GPX compared to S. lycopersicum under non-stress circumstances. The increasing days of salt stress affected a significant decline in the activity of GPX in S. lycopersicum after 7 days of stress but the activity of GPX enzyme in S. chilense was constantly increased up to 21 days of salinity (Fig. 1G). At 7 days of salt stress condition, S. chilense recorded 55% greater activity compared to S. lycopersicum; this alteration increased to 113% at 14 days, and 237% at 21 days (Fig. 1G).
3.5. Ascorbate (AsA) and glutathione (GSH) levels
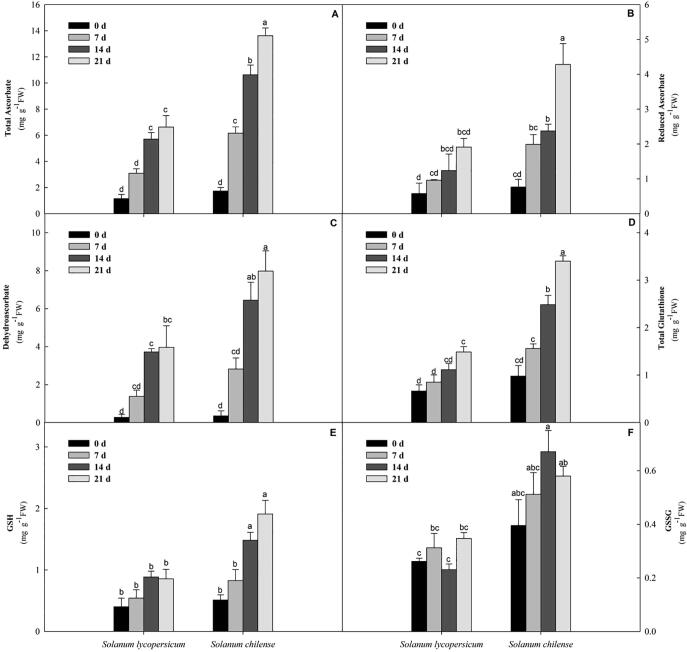
Significantly higher total AsA level (99–105%) was noted in S. chilense compared to S. lycopersicum (Fig. 2A). The reduced ascorbate, recorded an increase of 107 to123% at all stages of salt stress in the S. chilense compare to S. lycopersicum (Fig. 2B). The dehydroascorbate (DHA) level was comparable among the S. chilense and S. lycopersicum at 7, 14, and 21 days of salt stress condition and level of DHA was significantly higher (101–103%) in S. chilense compared to S. lycopersicum (Fig. 2C). The glutathione accumulation was detected to be considerably greater in the S. chilense as compared to the S. lycopersicum during the salt stress time, and it augmented progressively with increasing time duration of salt stress condition (82% greater at 7 days, and 122–128% higher at 14 and 21 days; Fig. 2D). The GSH accumulation level in S. chilense followed a parallel pattern and significantly augmented at 21 days (123%) from slight increase of 52% at 7 days and 67% at 14 days of salt stress (Fig. 2E). On the other hand, the GSSG concentration scale in the S. chilense followed a diverse trend and after that it was reduced with the salt stress severity. The values of GSSG were 64% (7 days) and 192% (14 days) higher compared to S. lycopersicum, and subsequently it displayed a decline (67% at 21 days) (Fig. 2F).
Fig. 2.
Estimation of (A) total ascorbate (AsA), (B) reduced ascorbate (RAsA), (C) dehydroascorbate (DHA), (D) total glutathione (Glut), (E) reduced glutathione (GSH), and (F) oxidized glutathione (GSSG) in Solanum lycopersicum and Solanum chilense at 0, 7, 14, and 21 d of salt stress conditions. The data are mean of three replicates ± SE. * Bars with distinct letters are significantly different at P ≤ 0.05 applying the DMRT test.
3.6. Principal component analysis (PCA)
The analyzed data produced three different PCs that yielded about 95.1% of total variance. PCA executed on physiological factors resulted in 95% variance. PC1 (84.3%) was mainly determined by RWC, carotenoid, total chlorophyll, Fv/Fm, and chlorophyll color index (CCI) with significantly positive value whereas EL, MDA, H2O2, and superoxide ion showed strong negative values (Fig. S1a), while PC2 (11.4%) presented strong positive value with proline. For physio-chemical properties, the PC1 and PC2 showed an eigenvalue of 8.4 and 1.1, respectively (Fig. S1b). PCA for the different enzymatic activities provided total variance of 94.2%, where PC1 (84.2%) was mainly governed by CAT, APX, MDHAR, GSH, GSSG, GPX, total ascorbate, total glutathione and dehydroascorbate with strong positive value. PC2 (10%) had a strong positive value for GR, DHAR, and SOD despite reduced ascorbate (Fig. S1C). For enzymatic activities, the PC1 and PC2 displayed eigenvalue of 10.9 and 1.3 which governed 84.2% and 10.2% of the total variability of data sets correspondingly (Fig. S1D). PCA analysis for the salt stress period gave total 96.3% variance where PC1 (78.2%) was largely determined by 14 days and 21 days of salt stress period of S. chilense; conversely PC2 (18.0%) was related with 0 days (non-stress) of S. lycopersicum and S. chilense (Fig. S1E).
3.7. Study of antioxidant genes at transcript levels
The consequence of salt stress on the gene transcriptional level of different detoxification-related genes has been presented in Fig. S2. Transcript gene expression pattern of different SOD isoforms such as SOD, CuZn-SOD, and Fe-SOD increased significantly in all the three-stages of salt treatment. Compared to S. lycopersicum, the expression level of CuZn-SOD and Fe-SOD isoforms increased at 7, 14, and 21 days (2.4, 3.1, and 4.7-fold; and 1.7, 2.0, and 2.4-fold, respectively) in the S. chilense. The expression level of SOD isoform in S. chilense also displayed a differential response and augmented up to three fold at 21 days of salt stress than S. lycopersicum (Fig. S2). The transcript level of APX and its isoforms such as chloroplast stromal ascorbate peroxidase and cytosolic ascorbate peroxidase 1 increased stably in S. chilense compared to its S. lycopersicum at 14 and 21 days of salt treatment (3.1 fold, 3.7 fold in APX; 1.4 fold, 2.9 fold in APXchl; and 2.3 fold, 4.0 fold in APXcyto, respectively). Expression level of GR, DHAR 1, DHAR 2, and MDHAR transcripts was also prominently increased up to 14 days of salt stress period in S. chilense (4.0, 3.1 fold, 2.6, and 2.2 fold, respectively) compared to its S. lycopersicum counterparts. There after expression level of these genes were down-regulated after 21 days of salinity in both the lines (Fig. S2). The expression level of CAT1 and CAT 2 transcript displayed a significantly higher level in S. chilense compared to the S. lycopersicum at all three-stages of saline treatment (3.69 and 4.56-fold at 21 days, respectively).
4. Discussion
Soil salinity is one of the leading abiotic factors responsible for delimiting the economic productivity. It has been suggested that the reduction in plant growth, biomass, yield and hence the productivity under saline conditions could be directly impacted with the inhibition of cell elongation and cell division, ROS generation, decreased enzymic activities, hormonal imbalances, reduced mineral uptake (Ahmad et al., 2012, Yousuf et al., 2016). Due to increased salinity, accumulation of excessive Na+ and Cl- ions near the roots leads into generation of ionic as well as osmotic stress, activating signals that inhibits cell division and plant growth and developmental signaling (Deinlein et al., 2014). Enhanced production of ROS under salinity response have detrimental effects on cellular metabolism and physiological aspects including DNA damage, lipid peroxidation of cell membranes, protein denaturation, oxidation of carbohydrates, breakdown of pigments, and inhibition and/or impairment in enzymic activities (Jajic et al., 2015, Mittler, 2017). ROS molecules including hydroxyl radical (OH•−), O2•−, and H2O2 produced during high salt stress have been reported as product of impaired physiological processes like impaired electron transport progressions in mitochondria and chloroplast as well as altered photo-respiratory pathways (García-Caparrós et al., 2019). In the results of our study, we report increased ROS accumulation and H2O2 production in S. lycopersicum than S. chilense, reflecting strong antioxidative defense mechanism involved in S. chilense both at biochemical and molecular level.
Cellular water deficit is a major physiological consequence of salt stress which reduces the accessibility of water for dynamic cellular functions necessary for plant survival and growth (Rahman et al., 2019). During prolonged salt stress hyperosmotic and hyper-ionic conditions generate physiological water deficit and accumulation of Na+ and Cl- and, increasingly, displace other mineral nutrients including K+, Ca+2, NO3− (Campestre et al., 2016). Under the saline environment plants having greater water holding capacity will have higher relative water content (RWC) and show better tolerance in stressed condition. RWC could be used for evaluating the overall metabolic changes, and estimates the plant water status in terms of cellular hydration and is highly dependent on both leaf water potential and osmotic adjustment (Meena and Kaur, 2019). In fact, the reduction in RWC under salt stress could be attributed to the decreased water and mineral uptake (Ahmad et al., 2016). We found better RWC in S. chilense than cultivated S. lycopersicum variety. Osmotic adjustment during salt stress in S. lycopersicum might result in the turgor loss point occupying at lower leaf water potential (Suárez, 2011). Martínez et al. (2014) reported that higher water-retaining ability on leaf dry weight basis during salt stress condition is an important tolerance strategy and could be correlated with higher stress tolerance in S. chilense than S. lycopersicum.
Electrolyte leakage (EL) is an assurance of stress response in whole plant cells and could be measured almost immediately after the imposition of a stress. It has been reported that ROS generated during oxidative stress reacts with polyunsaturated fatty acids (PUFA) to form lipid hydroperoxides, and thereby, decreasing permeability of cell membrane that ultimately results into in increased electrolyte leakage (Alzahrani et al., 2019). The most common reason attributed for EL is the efflux of K+ and so-called counterions (Cl–, NO3– HPO42–, malate2–, citrate3–) that transfer to balance the efflux of potassium ions (Alzahrani et al., 2019). In our results, we found higher EL in S. lycopersicum compared to S. chilense. One of the important reasons for this trend is that halophytic species accrue greater concentration of Na+ in its aerial parts than S. lycopersicum without compromising K+ homeostasis (Gharbi et al., 2017a). It has been found that S. chilense displayed lesser osmotic potential than S. lycopersicum. Under high salinity S. chilense behaved as includer species accommodating higher Na+ in shoots than S. lycopersicum which showed excluder behavior towards salt stress. Generally, salt stress response in plants is governed by high retention of K+ ions and exclusion of Na+ from shoots (Shabala, 2017). Similarly, positive correlation between salinity tolerance and K+ retention have been evidenced in many salt sensitive plants like alfalfa (Guo et al., 2016), mustard (Chakraborty et al., 2016), wheat (Wu et al., 2015), cucumber (Redwan et al., 2016) and cotton (Wang et al., 2017). It was suggested that Na+ absorption pathway is highly dependent on K+ channels and K+ homeostasis under high NaCl concentration in wild species could be attributed due to tolerate high Na+ endogenous concentration (Gharbi et al., 2018). In a recent study, demonstrated the osmotic adjustment response of S. lycopersicum and S. chilense under 125 mM salt stress for 7 days, and reported contrasting behavior in the two with respect to modalities of osmotic adjustment and phyto-hormonal profiling. ROS induced membrane lipid degradation and accumulation of MDA is a potential biomarker to assess salt stress in plants (Meena and Kaur, 2019). The lower lipid peroxidation in S. chilense uncovered the role of more efficient anti-oxidative defense system which is expressed, at least in part, by the significantly highly inherited and salt induced activities of SOD and APX and highly induced activities of CAT and MDHAR. We found higher MDA in S. lycopersicum than S. chilense indicating a strong anti-oxidative defense mechanism and resistance against salinity in S. chilense. Similarly, Martínez et al. (2014) recorded the better potential of S. chilense with significantly lower MDA content than S. lycopersicum under salt stress. ROS induced cellular damages also lead to destruction of photosynthetic machinery including chlorophyll and carotenoid content. The effect of salinity on photosynthetic activity and destruction of chlorophyll pigments is highly dependent on concentration, plant genotype and duration of the salt stressed environment (Alzahrani et al., 2019). However, S. chilense showed lesser destruction profile comparatived to cultivated species, particularly, at 14 and 21 days of treatment. Our finding are in line with Kordrostami et al. (2017) who also noted a decrease in the total content of Chl and carotenoids under stressed condition.
It has been reported that under salt stressed environment decreased pigment content could be associated with distortion in chlorophyll ultrastructure, inhibition of Rubisco, pigment protein complex abnormalities, activation of chlorophyllase enzyme (involved in chlorophyll degradation), stomata closure leading to decreased leaf intercellular CO2 pressure and restricted uptake of minerals such as Mg2+ (Ahmad et al., 2018). The actual mechanism of photosynthetic reduction in cultivated tomato compared to wild species is not clear. However, due to lesser destruction profile of photosynthetic pigments in S. chilense, photosynthetic activity is not/or less disturbed under salt stress environment. But higher destruction of photosynthetic pigments in S. lycopersicum lead into photosynthetic inhibition. In our result, the decreased Fv/Fm values for S. lycopersicum indicated a decline in the rate of capture and conversion in excitation energy by PSII reaction centre, and so, reduced PSII photochemical efficiency through perturbed organization of PS II reaction centres under salt stress. The reduced PSII performance might be associated with dissipation of excessive harvested energy as consumed by Calvin cycle to counteract the ROS induced oxidative stresses in cellular system. Non photo chemical quenching (NPQ) was always found to be higher in S. lycopersicum than S. chilense as down-regulation of photosynthesis in S. lycopersicum causes an imbalance of energy in the reaction centre of PSII, and therefore, preventing photo-inhibition (Petridis et al., 2012). In this way, S. lycopersicum rapidly disperse much of an energy in order to preserve an adequate balance between and carbon metabolism and photosynthetic electron transport (Gharbi et al., 2017a). Conversely, S. chilense maintains excess energy distribution reflected in the form of extremely high irradiance that actually exist in natural habitat, and therefore, maintain high photochemical quenching (qP) values. Apart from this, NaCl induced photosynthetic maintenance could also be linked, at least partly, to salinity induced increase in stomatal conductance, and therefore, higher gas exchange (Gharbi et al., 2017b). For the period of salinity accumulation of osmolytes, particularly, proline has been positively correlated with abiotic tolerance in many plant species (Escalante-Magaña et al., 2019). Proline can also have several other functions under stress environments, such as an eliminator of harmful free radicals, ROS scavenger, and a suitable buffer to maintain cellular redox potential. Higher accumulation of proline under salt stress is accompanied by higher enzymic activities of CAT, SOD and APX which suggest the activation of defense-related anti-oxidative system by increased production of proline (de Freitas et al., 2018). S. chilense plants kept accumulating the proline with the increased severity of salt stress and presented two fold higher values of proline comparatived to S. lycopersicum. The elevated proline level in S. chilense contributed to cellular osmotic balance and enhanced salt tolerance. Higher accumulation of proline under salt stress has been well demonstrated in tomato (Gharsallah et al., 2016) and bean seedlings (Farhangi-Abriz and Torabian, 2017). As an exposer of salinity, ROS formation is enhanced in S. chilense as well as in S. lycopersicum but S. chilense recorded lower H2O2 concentration and O2•− formation than S. lycopersicum counterparts, representing a well-adjusted ROS formation in the S. chilense under salt stress condition. This reduction in H2O2 level and O2•− formation rate is indicative of enhanced ROS cellular homeostasis level in S. chilense.
Antioxidant enzymes are the key elements essentially required for defense mechanism through neutralization and amendment of ROS causing oxidative stress. In our results, we found the differential expression of anti-oxidative defense related transcripts with up-regulation of superoxide dismutases (FeSOD and Cu/ZnSOD), catalase (Catalase 1and 2), ascorbate peroxidase (APXchl and APXcyto), dehydroascorbate reductase 1and 2 (DHAR 1and 2), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), and glutathione peroxidase (GPX) transcripts. It is well known that salinity induces changes at the level of transcripts encoding for antioxidant enzymes (Gupta et al., 2019). Recently, Csiszár et al. (2014) noted an up-regulation of the transcript level expression pattern of defense-related enzymes in S. lycopersicum against the salt stress injury. Superoxide ion (O2•−) is catalyzed and removed by metalloenzyme SOD in cells, where oxidation of one O2•− forms O2 and another one is reduced to H2O2 (Amjad et al., 2019). In the present study, it was found that significantly higher SOD activity in salt stress exposed S. chilense compared to their S. lycopersicum counterparts in all three-stages of salt stress. Furthermore, we have evaluated the expression level of two different isoforms of SOD in both plants expression level of Fe-SOD and Cu/Zn-SOD increased significantly for 2.48 and 4.47 times in salt-treated, respectively. In our results, during the increased NaCl stress the activities of SOD increased in both plants. Further, increased H2O2 content was accompanied by decreased activity of SOD (H2O2 producer). In contrast, the activities of H2O2 detoxifying enzymes were reported to be constant or even decreased in S. chilense. Since salt stress affected the concentration of AsA and GSH, increased H2O2 content can be attributed to non‐enzymatic reduction of superoxide by AsA and GSH (Mittova et al., 2015). Our result were consistent with the Frary et al. (2010) who reported that salt tolerant wild genotypes of S. pennellii exhibited significantly higher SOD activity than the cultivated tomato under salt stress. In this study, highest CAT enzyme activity was noted in S. chilense plants than S. lycopersicum indicating a better scavenging ability of H2O2 by the tolerant genotypes. Increased CAT activity also has been found in wild tomato genotype S. pennellii after exposure to salinity condition (Mittova et al., 2002, Mittova et al., 2003). In our study, S. chilense recorded significantly higher activity of APX compared to S. lycopersicum during 14 as well as 21 days of salt stress. Under Ash-GSH and water-water cycles, APX is well-known to be convoluted H2O2 scavenger and consumes AsA to efficient electron donor. Increase in APX activities under salt stress has been evidenced in several studies and literatures which is line with the our result (Gharsallah et al., 2016). The observed alleviation of oxidative stress in S. chilense could be attributed to salt‐induced higher activities of the mitochondrial SOD and APX. However, NaCl dependent increase in APX activity (both matrix and membrane bound isoenzymes) was found to be higher than SOD which indicated that the production of H2O2 is higher than H2O2 detoxification in S. chilense. In this way, an increase in APX activity detoxify the generated H2O2 at the cost of its production and therefore, regulates the lipid peroxidation, and lower the H2O2 induced oxidative stress in S. chilense. Apart from SOD, CAT, and APX the other versatile anti-oxidant enzyme in cellular defense against salt stresses is Glutathione reductase (GR) catalyzing the oxidative form of glutathione GSSG to reduced form GSH (Bartoli et al., 2017). APX is the key antioxidant enzyme in plants whilst GR play an indispensable role in in maintaining the reduced glutathione pool during stress. GR actually, increases the ratio of NADP+ /NADPH, thereby, diverting the photosynthetic electron flow to NADP+ rather than O2 and O2•− to generate oxygenic free radicals. In previous studies it has been reported that during NaCl stress the salt-tolerant genotypes exhibit higher antioxidative enzymic activities compared to salt sensitive genotypes (Noreen et al., 2017, Zafar et al., 2017). The results recorded in this study revealed that after imposition of salt for 7 and 14 days, the S. chilense plants displayed a greater level of activity of GR compared to the S. lycopersicum and suggested that the S. chilense are more salt tolerant than S. lycopersicum. Regulation of ascorbate redox state DHAR plays a key part as it participates with GSH produced by AsA for reduction of DHA (Foyer and Noctor, 2003), which is precarious for oxidative stress. In the current study, enhanced levels of DHAR activities were noted in the S. chilense during the salt-exposed period. DHAR expression was observed to be highest after 14 days of salt stress. Several studies reported an increase in DHAR activity level against salinity and other abiotic stresses which was further supported of our result (Mittler, 2002, Ushimaru et al., 2006). In contrast, MDHAR is responsible for regeneration of AsA and this process is totally NAD(P)H-dependent (Shalata et al., 2001), which is further consumed as a source of detoxifying H2O2 with the help of APX. Outcomes of this study proved that the MDHAR activity was increased in S. chilense than the S. lycopersicum.
Physiological and biochemical characteristic in S. chilense and S. lycopersicum, together with control conditions as well as salt stress conditions displayed a significant correlation. CCI, total Chl, and carotenoid were correlated positively with RWC and negatively with LPO, EL, H2O2, and O2•− during both control and stress conditions (Supplementary Table 2). LPO showed a positive and significant correlation with EL, H2O2, and O2•− and a negative correlation with total Chl, CCI, RWC and carotenoid (Supplementary Table 2). There was a strong positive significant relationship among the mean values of constituents of the enzymatic antioxidant structure such as SOD, CAT, APX, GR, DHAR, MDHAR, GPX, Glut, GSSG, AsA, DHA, RAsA, and GSH at all three-stages of saline conditions (Supplementary Table 3). Similarly, other findings were supportive to our results which stated that the greater antioxidant enzyme activities contributed to mitigate the action of ROS and led to an improved osmotic modification to salt stress (Bhatnagar-Mathur et al., 2009). Principal component analysis provides an important tool for identifying and characterizing the important traits and crucial components involved in salt tolerance among the genotypes and under different conditions (Negrão et al., 2017). This multidimensional preference analysis allows the identification of parameters that are best characterized based on tolerance capabilities under the effect of response variables and identifies the principle variables which explain the pattern of correlations within the measured salt stress component traits (Jan et al., 2017). PCA performed on physiological and ROS attributes displayed four separate clusters represented as first group (RWC and CCI), second group (Fv/Fm, total Chl and Car), third group (EL, MDA, H2O2, and O2•−) and the fourth group (Pro), which clearly demonstrated various responses of different attributes related to salt stress conditions and indicated that these attributes are controlled in a synchronous way (Fig. S1).
5. Conclusions
It was found that salt stress decreases the growth, biomass yield, pigment content and RWC, but increases H2O2, MDA content, EL, and anti-oxidant enzymes such as SOD, CAT, and GR in S. lycopersicum. In contrast, S. chilense showed a lower impact in growth and biomass yield along with other physiological and biochemical attributes compared to S. lycopersicum. The salt-tolerant genotypes showed higher CAT, SOD, GR and proline content in S. chilense than S. lycopersicum reflecting better anti-oxidative system in S. chilense than S. lycopersicum. In S. chilense, the capacity to amend the Ascorbate-Glutathione cycle to survive the salt stress environment is better than the S. lycopersicum, and comparably higher accumulation of ROS detoxifying antioxidant enzymes was observed to keep the cellular apparatus in normalized conditions under the salt stress. We believe that the study would provide a basis for selection of more wild tomato genotypes for salinity tolerance.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2020.06.032.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abiala M.A., Abdelrahman M., Burritt D.J., Tran L.S.P. Salt stress tolerance mechanisms and potential applications of legumes for sustainable reclamation of salt-degraded soils. Land Degrad. Dev. 2018;29:3812–3822. doi: 10.1002/ldr.3095. [DOI] [Google Scholar]
- Acosta-Motos J.R., Diaz-Vivancos P., Álvarez S., Fernández-García N., Sánchez-Blanco M.J., Hernández J.A. NaCl-induced physiological and biochemical adaptative mechanisms in the ornamental Myrtus communis L. plants. J. Plant Physiol. 2015;183:41–51. doi: 10.1016/j.jplph.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Ahmad P., Abdel Latef A.A., Hashem A., Abd Allah E.F., Gucel S., Tran L.-S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016;7:347. doi: 10.3389/fpls.2016.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad P., Alyemeni M., Ahanger M., Egamberdieva D., Wijaya L., Alam P. Salicylic acid (SA) induced alterations in growth, biochemical attributes and antioxidant enzyme activity in faba bean (Vicia faba L.) seedlings under NaCl toxicity. Russ. J. Plant Physiol. 2018;65:104–114. doi: 10.1134/S1021443718010132. [DOI] [Google Scholar]
- Ahmad P., Kumar A., Ashraf M., Akram N.A. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.) Afr. J. Biotechnol. 2012;11:2694–2703. doi: 10.5897/AJB11.3203. [DOI] [Google Scholar]
- Albaladejo I., Meco V., Plasencia F., Flores F.B., Bolarin M.C., Egea I. Unravelling the strategies used by the wild tomato species Solanum pennellii to confront salt stress: from leaf anatomical adaptations to molecular responses. Environ. Exp. Bot. 2017;135:1–12. doi: 10.1016/j.envexpbot.2016.12.003. [DOI] [Google Scholar]
- Almeida P., de Boer G.-J., de Boer A.H. Differences in shoot Na+ accumulation between two tomato species are due to differences in ion affinity of HKT1; 2. J. Plant Physiol. 2014;171:438–447. doi: 10.1016/j.jplph.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Alzahrani S.M., Alaraidh I.A., Migdadi H., Alghamdi S., Khan M.A., Ahmad P. Physiological, biochemical, and antioxidant properties of two genotypes of Vicia faba grown under salinity stress. Pak. J. Bot. 2019;51:786–798. [Google Scholar]
- Amjad M., Ameen N., Murtaza B., Imran M., Shahid M., Abbas G., Naeem M.A., Jacobsen S.E. Comparative physiological and biochemical evaluation of salt and nickel tolerance mechanisms in two contrasting tomato genotypes. Physiol. Plant. 2019 doi: 10.1111/ppl.12930. [DOI] [PubMed] [Google Scholar]
- Arnon D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M.A., Akbar A., Parveen A., Rasheed R., Hussain I., Iqbal M. Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol. Biochem. 2018;123:268–280. doi: 10.1016/j.plaphy.2017.12.023. [DOI] [PubMed] [Google Scholar]
- Assaha D.V., Ueda A., Saneoka H., Al-Yahyai R., Yaish M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017;8:509. doi: 10.3389/fphys.2017.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli C.G., Buet A., Grozeff G.G., Galatro A., Simontacchi M. Ascorbic Acid in Plant Growth, Development and Stress Tolerance. Springer; 2017. Ascorbate-glutathione cycle and abiotic stress tolerance in plants; pp. 177–200. [DOI] [Google Scholar]
- Bates L., Waldren R., Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bhatnagar-Mathur P., Devi M.J., Vadez V., Sharma K.K. Differential antioxidative responses in transgenic peanut bear no relationship to their superior transpiration efficiency under drought stress. J. Plant Physiol. 2009;166:1207–1217. doi: 10.1016/j.jplph.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Campestre M.P., Antonelli C., Calzadilla P.I., Maiale S.J., Rodríguez A.A., Ruiz O.A. The alkaline tolerance in Lotus japonicus is associated with mechanisms of iron acquisition and modification of the architectural pattern of the root. J. Plant Physiol. 2016;206:40–48. doi: 10.1016/j.jplph.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Chakraborty K., Sairam R., Bhaduri D. Effects of different levels of soil salinity on yield attributes, accumulation of nitrogen, and micronutrients in Brassica spp. J. Plant Nutr. 2016;39:1026–1037. doi: 10.1080/01904167.2015.1109105. [DOI] [Google Scholar]
- Chetelat R.T., Pertuzé R.A., Faúndez L., Graham E.B., Jones C.M. Distribution, ecology and reproductive biology of wild tomatoes and related nightshades from the Atacama Desert region of northern Chile. Euphytica. 2009;167:77–93. doi: 10.1007/s10681-008-9863-6. [DOI] [Google Scholar]
- Csiszár J., Horváth E., Váry Z., Gallé Á., Bela K., Brunner S., Tari I. Glutathione transferase supergene family in tomato: salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol. Biochem. 2014;78:15–26. doi: 10.1016/j.plaphy.2014.02.010. [DOI] [PubMed] [Google Scholar]
- de Freitas P.A.F., de Souza Miranda R., Marques E.C., Prisco J.T., Gomes-Filho E. Salt tolerance induced by exogenous proline in maize is related to low oxidative damage and favorable ionic homeostasis. J. Plant Growth Regul. 2018;37:911–924. doi: 10.1007/s00344-018-9787-x. [DOI] [Google Scholar]
- Deinlein U., Stephan A.B., Horie T., Luo W., Xu G., Schroeder J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014;19:371–379. doi: 10.1016/j.tplants.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Magaña C., Aguilar-Caamal L.F., Echevarría-Machado I., Medina-Lara F., Cach L.S., Martínez-Estévez M. Contribution of glycine betaine and proline to water deficit tolerance in pepper plants. HortScience. 2019;54:1044–1054. doi: 10.21273/HORTSCI13955-19. [DOI] [Google Scholar]
- Farhangi-Abriz S., Torabian S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017;137:64–70. doi: 10.1016/j.ecoenv.2016.11.029. [DOI] [PubMed] [Google Scholar]
- Flowers T.J., Colmer T.D. Plant salt tolerance: adaptations in halophytes. Ann. Bot. 2015;115:327–331. doi: 10.1093/aob/mcu267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C.H., Noctor G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 2003;119:355–364. doi: 10.1034/j.1399-3054.2003.00223.x. [DOI] [Google Scholar]
- Frary A., Göl D., Keleş D., Ökmen B., Pınar H., Şığva H.Ö., Yemenicioğlu A., Doğanlar S. Salt tolerance in Solanum pennellii: antioxidant response and related QTL. BMC Plant Biol. 2010;10:58. doi: 10.1186/1471-2229-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke W. Energy costs of salinity tolerance in crop plants: night-time transpiration and growth. New Phytol. 2019 doi: 10.1111/nph.15555. [DOI] [PubMed] [Google Scholar]
- García-Caparrós P., Quiróz A.L., Teresa Lao M. Water and nutrient uptakes efficiencies in rosemary plants under different fertigation treatments. J. Plant Nutr. 2019:1–8. doi: 10.1080/01904167.2019.1628978. [DOI] [Google Scholar]
- Gharbi E., Lutts S., Dailly H., Quinet M. Comparison between the impacts of two different modes of salicylic acid application on tomato (Solanum lycopersicum) responses to salinity. Plant Signal. Behav. 2018;13:e1469361. doi: 10.1080/15592324.2018.1469361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbi E., Martínez J.-P., Benahmed H., Hichri I., Dobrev P.I., Motyka V., Quinet M., Lutts S. Phytohormone profiling in relation to osmotic adjustment in NaCl-treated plants of the halophyte tomato wild relative species Solanum chilense comparatively to the cultivated glycophyte Solanum lycopersicum. Plant Sci. 2017;258:77–89. doi: 10.1016/j.plantsci.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Gharbi E., Martínez J.-P., Benahmed H., Lepoint G., Vanpee B., Quinet M., Lutts S. Inhibition of ethylene synthesis reduces salt-tolerance in tomato wild relative species Solanum chilense. J. Plant Physiol. 2017;210:24–37. doi: 10.1016/j.jplph.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Gharsallah C., Fakhfakh H., Grubb D., Gorsane F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants. 2016;8 doi: 10.1093/aobpla/plw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P., Wei H., Zhang W., Bao Y. Physiological responses of alfalfa to high-level salt stress: root ion flux and stomatal characteristics. Int. J. Agric. Biol. 2016;18 doi: 10.17957/IJAB/15.0073. [DOI] [Google Scholar]
- Gupta S., Dong Y., Dijkwel P.P., Mueller-Roeber B., Gechev T.S. Genome-wide analysis of ROS antioxidant genes in resurrection species suggest an involvement of distinct ROS detoxification systems during desiccation. Int. J. Mol. Sci. 2019;20:3101. doi: 10.3390/ijms20123101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hossain M.S., Dietz K.-J. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front. Plant Sci. 2016;7:548. doi: 10.3389/fpls.2016.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajic I., Sarna T., Strzalka K. Senescence, stress, and reactive oxygen species. Plants. 2015;4:393–411. doi: 10.3390/plants4030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan S.A., Bibi N., Shinwari Z.K., Rabbani M.A., Ullah S., Qadir A., Khan N. Impact of salt, drought, heat and frost stresses on morpho-biochemical and physiological properties of Brassica species: An updated review. J. Rural Dev. Agric. 2017;2:1–10. [Google Scholar]
- Jana S., Choudhuri M.A. Glycolate metabolism of three submersed aquatic angiosperms: effect of heavy metals. Aquat. Bot. 1981;11:67–77. doi: 10.1016/0304-3770(81)90047-4. [DOI] [Google Scholar]
- Khare N., Goyary D., Singh N.K., Shah P., Rathore M., Anandhan S., Sharma D., Arif M., Ahmed Z. Transgenic tomato cv. Pusa Uphar expressing a bacterial mannitol-1-phosphate dehydrogenase gene confers abiotic stress tolerance. Plant Cell Tissue Org. Cult. (PCTOC) 2010;103:267–277. doi: 10.1007/s11240-010-9776-7. [DOI] [Google Scholar]
- Kordrostami M., Rabiei B., Kumleh H.H. Biochemical, physiological and molecular evaluation of rice cultivars differing in salt tolerance at the seedling stage. Physiol. Mol. Biol. Plants. 2017;23:529–544. doi: 10.1007/s12298-017-0440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latef A.A.H.A., Chaoxing H. Does inoculation with Glomus mosseae improve salt tolerance in pepper plants? J. Plant Growth Regul. 2014;33:644–653. doi: 10.1007/s00344-014-9414-4. [DOI] [Google Scholar]
- Law M., Charles S.A., Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem. J. 1983;210:899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler H.K., Buschmann C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protocols Food Anal. Chem. 2001 doi: 10.1002/0471142913.faf0403s01. [DOI] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Martínez J.P., Antúnez A., Araya H., Pertuzé R., Fuentes L., Lizana X.C., Lutts S. Salt stress differently affects growth, water status and antioxidant enzyme activities in Solanum lycopersicum and its wild relative Solanum chilense. Aust. J. Bot. 2014;62:359–368. doi: 10.1071/BT14102. [DOI] [Google Scholar]
- Maxwell K., Johnson G.N. Chlorophyll fluorescence a practical guide. J. Exp. Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- McKersie B.D., Hoekstra F.A., Krieg L.C. Differences in the susceptibility of plant membrane lipids to peroxidation. Biochimica et Biophysica Acta (BBA)-Biomembr. 1990;1030:119–126. doi: 10.1016/0005-2736(90)90246-K. [DOI] [PubMed] [Google Scholar]
- Meena Y.K., Kaur N. Towards an understanding of physiological and biochemical mechanisms of drought tolerance in plant. Annu. Res. Rev. Biol. 2019:1–13. doi: 10.9734/arrb/2019/v31i230042. [DOI] [Google Scholar]
- Mishra A., Tanna B. Halophytes: potential resources for salt stress tolerance genes and promoters. Front. Plant Sci. 2017;8:829. doi: 10.3389/fpls.2017.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R. ROS are good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittova V., Tal M., Volokita M., Guy M. Salt stress induces up-regulation of an efficient chloroplast antioxidant system in the salt-tolerant wild tomato species Lycopersicon pennellii but not in the cultivated species. Physiol. Plant. 2002;115:393–400. doi: 10.1034/j.1399-3054.2002.1150309.x. [DOI] [PubMed] [Google Scholar]
- Mittova V., Theodoulou F.L., Kiddle G., Gómez L., Volokita M., Tal M., Foyer C.H., Guy M. Coordinate induction of glutathione biosynthesis and glutathione-metabolizing enzymes is correlated with salt tolerance in tomato. FEBS Lett. 2003;554:417–421. doi: 10.1016/S0014-5793(03)01214-6. [DOI] [PubMed] [Google Scholar]
- Mittova V., Volokita M., Guy M. Springer; 2015. Antioxidative Systems and Stress Tolerance: Insight from Wild and Cultivated Tomato Species, Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants; pp. 89–131. [DOI] [Google Scholar]
- Nahakpam S., Shah K. Expression of key antioxidant enzymes under combined effect of heat and cadmium toxicity in growing rice seedlings. Plant Growth Regul. 2011;63:23–35. doi: 10.1007/s10725-010-9508-3. [DOI] [Google Scholar]
- Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- Negrão S., Schmöckel S., Tester M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017;119:1–11. doi: 10.1093/aob/mcw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreen S., Siddiq A., Hussain K., Ahmad S., Hasanuzzaman M. Foliar application of salicylic acid with salinity stress on physiological and biochemical attributes of sunflower (Helianthus annuus L.) crop. Acta Scientiarum Polonorum-Hortorum Cultus. 2017;16:57–74. [Google Scholar]
- Owens C., Belcher R. A colorimetric micro-method for the determination of glutathione. Biochem. J. 1965;94:705. doi: 10.1042/bj0940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridis A., Therios I., Samouris G., Koundouras S., Giannakoula A. Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage and antioxidant activity of four Greek olive (Olea europaea L.) cultivars. Plant Physiol. Biochem. 2012;60:1–11. doi: 10.1016/j.plaphy.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Puniran-Hartley N., Hartley J., Shabala L., Shabala S. Salinity-induced accumulation of organic osmolytes in barley and wheat leaves correlates with increased oxidative stress tolerance: in planta evidence for cross-tolerance. Plant Physiol. Biochem. 2014;83:32–39. doi: 10.1016/j.plaphy.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Rahman M.M., Mostofa M.G., Rahman M.A., Miah M.G., Saha S.R., Karim M.A., Keya S.S., Akter M., Islam M., Tran L.-S.P. Insight into salt tolerance mechanisms of the halophyte Achras sapota: an important fruit tree for agriculture in coastal areas. Protoplasma. 2019;256:181–191. doi: 10.1007/s00709-018-1289-y. [DOI] [PubMed] [Google Scholar]
- Rakhmankulova Z., Shuyskaya E., Shcherbakov A., Fedyaev V., Biktimerova G.Y., Khafisova R., Usmanov I.Y. Content of proline and flavonoids in the shoots of halophytes inhabiting the South Urals. Russ. J. Plant Physiol. 2015;62:71–79. doi: 10.1134/S1021443715010112. [DOI] [Google Scholar]
- Redwan M., Spinelli F., Marti L., Weiland M., Palm E., Azzarello E., Mancuso S. Potassium fluxes and reactive oxygen species production as potential indicators of salt tolerance in Cucumis sativus. Funct. Plant Biol. 2016;43:1016–1027. doi: 10.1071/FP16120. [DOI] [PubMed] [Google Scholar]
- Ronga D., Zaccardelli M., Lovelli S., Perrone D., Francia E., Milc J., Ulrici A., Pecchioni N. Biomass production and dry matter partitioning of processing tomato under organic vs conventional cropping systems in a Mediterranean environment. Sci. Hortic. 2017;224:163–170. doi: 10.1016/j.scienta.2017.05.037. [DOI] [Google Scholar]
- Sánchez-Rodríguez E., Rubio-Wilhelmi M.M., Cervilla L.M., Blasco B., Rios J.J., Rosales M.A., Romero L., Ruiz J.M. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. 2010;178:30–40. doi: 10.1016/j.plantsci.2009.10.001. [DOI] [Google Scholar]
- Shabala S. Signalling by potassium: another second messenger to add to the list? J. Exp. Bot. 2017;68:4003–4007. doi: 10.1093/jxb/erx238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K., Kumar R.G., Verma S., Dubey R. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 2001;161:1135–1144. doi: 10.1016/S0168-9452(01)00517-9. [DOI] [Google Scholar]
- Shalata A., Mittova V., Volokita M., Guy M., Tal M. Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: The root antioxidative system. Physiol. Plant. 2001;112:487–494. doi: 10.1034/j.1399-3054.2001.1120405.x. [DOI] [PubMed] [Google Scholar]
- Suárez N. Effects of short-and long-term salinity on leaf water relations, gas exchange, and growth in Ipomoea pes-caprae. Flora-Morphol. Distrib. Funct. Ecol. Plants. 2011;206:267–275. doi: 10.1016/j.flora.2010.05.006. [DOI] [Google Scholar]
- Thapa S.P., Miyao E.M., Davis R.M., Coaker G. Identification of QTLs controlling resistance to Pseudomonas syringae pv. tomato race 1 strains from the wild tomato, Solanum habrochaites LA1777. Theor. Appl. Genet. 2015;128:681–692. doi: 10.1007/s00122-015-2463-7. [DOI] [PubMed] [Google Scholar]
- Ushimaru T., Nakagawa T., Fujioka Y., Daicho K., Naito M., Yamauchi Y., Nonaka H., Amako K., Yamawaki K., Murata N. Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J. Plant Physiol. 2006;163:1179–1184. doi: 10.1016/j.jplph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Wang N., Qi H., Qiao W., Shi J., Xu Q., Zhou H., Yan G., Huang Q. Cotton (Gossypium hirsutum L.) genotypes with contrasting K+/Na+ ion homeostasis: implications for salinity tolerance. Acta Physiol. Plant. 2017;39:77. doi: 10.1007/s11738-017-2381-1. [DOI] [Google Scholar]
- Wu H., Shabala L., Liu X., Azzarello E., Zhou M., Pandolfi C., Chen Z.-H., Bose J., Mancuso S., Shabala S. Linking salinity stress tolerance with tissue-specific Na+ sequestration in wheat roots. Front. Plant Sci. 2015;6:71. doi: 10.3389/fpls.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf P.Y., Ahmad A., Ganie A.H., Iqbal M. Salt stress-induced modulations in the shoot proteome of Brassica juncea genotypes. Environ. Sci. Pollut. Res. 2016;23:2391–2401. doi: 10.1007/s11356-015-5441-3. [DOI] [PubMed] [Google Scholar]
- Zafar Z.U., Manzoor H., Rasul S., Noreen S., Ali Q., Iqbal M., Javed M., Gul H.S., Ahmad Z., Shahzad F. Strategies to improve crop salt and drought tolerance: Success and limitations. Agrobios (India) 2017;11:265–298. [Google Scholar]
- Zahedi S.M., Abdelrahman M., Hosseini M.S., Hoveizeh N.F., Tran L.-S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019;253:246–258. doi: 10.1016/j.envpol.2019.04.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.