Abstract
Lipoprotein Lipase (LPL) is known to be a key enzyme for lipid metabolism specifically in an enzymatic glycoprotein which provide tissues without fatty-acids and eliminates triglycerides (TG) by the circulation. Mutations in LPL were proven to cause alteration in fractions within lipoprotein, causing the development of atherosclerosis which predispose to weakening coronary artery disease (CAD) and stroke. We examined the linkage between genetic variant HindIII in LPL on lipoprotein fractions, stroke occurrences and CAD. In this case-control study, we have recruited 315 CAD cases and 205 age-matched controls. A total of 520 genomic DNA was digested with the purified PCR products for restriction fragment length polymorphism with HindIII restriction enzyme. The distribution of genotypes in a decreasing order were TT, 148 (47%), GT 135 (42.9%) and GG 32 (10.2%) in CAD groups of the study while the pattern in controls were GT 91 (44.4%), TT 86 (42%) and GG 28 (13.7%). None of all the allele or genotype frequencies were found to be significant in our study (p greater than 0.05), while the biochemical levels for both TG and LDL-c were shown to be prone in CAD patients when compare with the controls. Furthermore, the occurence of strokes were more in CAD groups vs. controls: 72 (22.9%) vs. 7 (3.4%) [p 0.000]. This could indicate the influence of HindIII variant on plasma lipid levels, and the possibility of considering it a risk factor for atherosclerosis leading to CAD and stroke occurrence.
Keywords: Coronary artery disease, Stroke, Lipoprotein lipase, Rs320 and HindIII
1. Introduction
Mammalian lipase family plays a vital role in sustaining normal plasma levels, accountable for involving numerous enzymes such as hepatic, endothelial, pancreatic, gastric and lipoprotein lipases (LPL) (Nimonkar et al., 2020). The LPL deficiency is infrequently inherited disease characterized by chylomicronemia, severe hypertriglyceridemia, and risk of recurrent pancreatitis (Han et al., 2020). One of the key enzymes in LPL will regulate triglyceride (TG) metabolism along with high density lipoprotein-cholesterol (HDL-C) (Kochetova et al., 2019). LPL promotes modifies the lipids between very low-density lipoprotein to HDLC (Su and Peng, 2020). Previous studies have discovered dyslipidemia as a risk factor in Coronary artery disease (CAD), which have been explored the genetic role of polymorphisms in the LPL gene (Gerodias et al., 2019). LPL plays the role of non-catalytic bridging as one of the ligands in lipoprotein cell surface interaction in uptake of lipoproteins (Laufs et al., 2020). Enzymatic glycoprotein is known to be as LPL, which involves 448 amino-acids with a very well significant role in hydrolyzing triglycerides (TG) (Yamada, 2020). Genetic modification in the LPL gene in which LPL low levels are associated with one of the increased CAD risk factors (Xu et al., 2019). The critical elevation in TG values were associated with molecular variants in the LPL gene which lower the LPL activities and due to this LPL is linked with specific metabolic syndrome as type 2 diabetes mellitus (T2DM), obesity and non-alcoholic fatty liver disease (de Andrade Júnior, 2018, Khan et al., 2015). LPL plays an essential role in lipoprotein metabolism as well as in chylomicrons. As per the results, free fatty acids and monoglycerides are formed for usage in energy production and storage. The LPL gene consists of 10 exons and 11 introns, which spans about 35 kilobases in size; the accurate chromosomal location is at 8p22 and till now, more than 100 mutations have been identified. (Alinaghian et al., 2019).
Cardiac diseases are the combined combination of CAD and hypertension for major causes of death (Kumar et al., 2020). The CAD has been confirmed through the epidemiological studies as multigene cum multifactorial regulated human disease (Fan et al., 2020). The estimated mortality rate will be about 23 million by 2030 (Zhang et al., 2019). Nine million deaths have been recorded in all ages of subjects with CAD and known to be the one of the cause of mortality global-wide (Zheng et al., 2020). The major cause of CAD is atherosclerosis which further precede for coronary heart disease (CHD). The appearance of atherosclerosis will be symptomatic phase and can confirmed as coronary artery calcification in the CAD (Pechlivanis et al., 2020). CAD is one type of cardiovascular disease that is affected due to a low amount of oxygen and the appearance of blood in the heart (Elnaggar et al., 2019). The combination of genetic and environmental factors plays a significant role in the progression and pathogenesis of CAD (Matam et al., 2015). Physical activity, diet and smoking are the major flaws connected with environmental causes (Yuan et al., 2020, Melam et al., 2016). T2DM is one of the risk factor CAD, stroke and CHD events atleast through two-to-three folds and simultaneously, T2DM have the maximum chances of developing CAD which is highly tends to be a complex disease (Khan et al., 2015, Naito and Kasai, 2015). The etiology of CAD is remains to be obscure and heritability rate was documented to be between 40 and 60% as per family and twin studies (Lu et al., 2020).
Genome-wide association studies have identified numerous loci for the CAD and one of the main susceptibility loci is placed at chromosome 15q26.1 which exists the single nucleotide polymorphism in the form of rs17514846 (Yang et al., 2020). Several case-control and meta-analysis studies have shown the relation between LPL gene and CAD (Xu et al., 2019, Javorsky et al., 2007, Daoud et al., 2013, Fischer et al., 2018, Ma et al., 2018, Shahid et al., 2017, Xie, 2017). The accurate genetic and molecular or any other mechanism which affects the LPL and human disease is poorly understood and LDL was interrelated towards certain heterogenous disorders which involve limited cardiovascular disorders (Hartley et al., 2020). The rs320 polymorphism has been studied globally in CAD, stroke, dyslipidemia (Javorsky et al., 2007, Nejati et al., 2018, Sagoo et al., 2008, Samgina et al., 2016, Tetik Vardarli et al., 2017, Velasquez Pereira et al., 2016) and this polymorphism is located between the intron 6–8 and important role of rs320 polymorphism could elevate the atherosclerosis risk through influencing plasma lipid levels (Nagrani et al., 2020). LPL variants, Ser447stop (S447X) are connected to a decline in LPL activity while Asn291Ser (N291S) and Asp9Asn (D9N) with an elevation in LPL activity. Thus, these variants could affect lipid profile levels and lead to CAD eventually (Bhanushali, 2010). A Prior study results from Saudi Arabia have showed that significant linkage occur between HindIII polymorphism (rs320), Ser447Ter Pvull polymorphisms and changes in plasma lipids levels in CAD patients (Daoud et al., 2013). Although the results from previous studies have highlighted the role of polymorphisms at LPL gene in causing CAD, not all of the polymorphisms have showed significant correlations to CAD. Here, we study the effects of the genetic variant, HindIII at the position 481 in intron 8 (T → G), on lipid profile levels and its association to CAD occurrence.
2. Materials and methods
2.1. Study subjects
In this case-control study, we have selected 520 subjects confirmed as 315 cases and 205 controls recruited during the time-phrase of September-2013 to May-2015 from King Abdullah Medical city (KAMC) and Al-Noor specialist hospitals appears at the western region of Saudi Arabia. The inclusion criteria of the CAD cases were based on complete investigation achieved in the hospital premises. The age range of the involved cases were in between 30 and 85 years of age with 50% higher than the stenosis of coronary arteries. T2DM patients were confirmed through American Diabetes Association criteria (Khan et al., 2014), while dyslipidemia cases were confirmed by prior studies (Cleeman and Grundy, 1997). Body Mass Index was calculated as per the described study (Khan et al., 2016) and hypertension was determined as systolic blood pressure as ≥ 140 mmHg and diastolic blood pressure as ≥ 90 mmHg (Alharbi et al., 2015, Poornima et al., 2015). The age and gender controls were opted without any histories of peripheral vascular disease, CAD and stroke. Patients with malignancies and other auto-immune diseases were also excluded from our study. Ethical grant was obtained from Umm Al-Qura University-43430838 in 05/05/1434H and KAMC-13–043. All the involved participants have signed the informed consent form. All chosen methods were executed in accordance with the relevant guidelines and regulations of Umm Al-Qura University and King Abdullah Medical City. Documented and enlightened consent was acquired from every study participant. Data on diverse parameters such as age, sex, smoking, diet, height, and weight were enrolled.
2.2. Biochemical assays
From each patient, 10 mL of blood sample was collected and divided into serum and EDTA vacutainers (Khan et al., 2015). Five mL of the serum sample was used for fasting glucose, lipid profiles and cardiac markers; whereas the remaining 5 mL of the peripheral blood was collected to extract the DNA. Biochemical analysis was carried out with the described studies (Matam et al., 2015, Khan et al., 2014).
2.3. Molecular assays
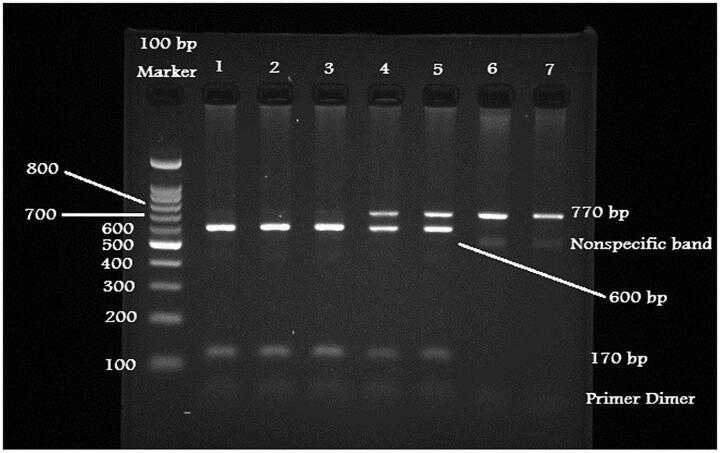
GeneJet-DNA extraction purification kit was used to isolate the genomic DNA through the described protocol and stored in the −20 °C for further usage. NanoDrop spectrophotometer was used to measure the quality and quantity of the genomic DNA (Khan et al., 2015). Genotyping was performed using polymerase chain reaction followed with restriction fragment length polymorphism analysis (Poornima et al., 2019). The primers for intron-8 region of LPL gene was adopted from the prior studies (Javorsky et al., 2007, Ahmadi et al., 2015, Lee et al., 2004). Amplification was performed using GoTaq colorless master mix (Promega Co. Ltd) in a thermal cycler (Khan et al., 2015). Primers yielded 770 bp as the PCR fragment size. Next, undigested PCR products were digested using 10U of HindIII restriction enzyme (New England BioLabs Ltd) was added to make the final volume digested products for 25 µL and incubated at room-temperature for 60 min. PCR products further were digested with 3% ethidium bromide stained in the agarose gel (Khan et al., 2015). GG homozygotes were found to be 770 bp; TT homozygotes were divided into 600/170 bp and heterozygotes were found to be 770/600/170 bp respectively (Fig. 1).
Fig. 1.
Digested PCR products for HindIII polymorphism.
2.4. Data analysis
Statistical data was analysed using SPSS (version 23.0) and Openepi softwares as described (Khan et al., 2019). Clinical data was expressed as mean ± standard deviation. Genotype and allele frequencies were calculated as per the presence of difference alleles observed and complete number of alleles inspected using odds ratio’s (OR’s) and 95% confidence intervals (CI’s). Fischer’s exact t-test were also attained. Non-parametric Krushkal Wallis test was used to compare the mean ranks of skewed quantitative variables in relation to the three products of) LPL gene G > T locus. (TT, GT & GG). Significance was set-up as p < 0.05 (Khan et al., 2015).
3. Results
The study recruited 315 coronary artery diseases patients with more than 50% stenosis confirmed on coronary angiography and 205 aged-matched controls. The characteristics of the participants (Table 1) revealed fairly equaled distribution by age as the patients had mean age and SD of [59.7 (10.87)] versus that of controls of [58.4 (9.28)] with a p value of 0.157. In addition, patients largely demonstrated more characteristics than controls in the following parameters: body mass index 29.26 (4.11) vs. 26.26 (6.49) [p 0.000], smoking status 150 (47.6%) vs. 58 (28.3%) [p 0.000], exercise habits 72 (22.9%) vs. 67 (32.7%) [p 0.009], diabetes mellitus 187 (59.4%) vs. 35 (17.1%) [p 0.000], and blood glucose levels 140.64 mg/dl vs. 83.56 mg/dl [p 0.000].
Table 1.
Clinical characteristics features between CAD cases and controls.
| Variables | CAD cases (n = 315) | Controls (n = 205) | Pvalues |
|---|---|---|---|
| Age (Mean ± SD) | 59.7 (10.87) | 58.4 (9.28) | 0.157 |
| BMI (Mean ± SD) | 29.26 (4.11) | 26.26 (6.49) | 0.000 |
| Gender (Male%) | 206 (65.4%) | 119 (58%) | 0.055 |
| Smoking (%) | 150 (47.6%) | 58 (28.3%) | 0.000 |
| Exercise (%) | 72 (22.9%) | 67 (32.7%) | 0.009 |
| Diabetes (%) | 187 (59.4%) | 35 (17.1%) | 0.000 |
| Fasting glucose (mg/dl) | 140.64 | 83.56 | 0.000 |
| Hypertension (%) | 221 (70.2%) | 41 (20%) | 0.000 |
| SBP (mmHg) | 146.47 | 122.03 | 0.000 |
| DBP (mmHg) | 91.84 | 74.25 | 0.000 |
| Event Stroke (%) | 72 (22.9%) | 7 (3.4%) | 0.000 |
| Total Cholesterol (mg/dl) | 154.17 (23.6) | 140.88 (18.09) | 0.000 |
| HDL-c (mg/dl) | 39.95 (8.05) | 41.14 (8.65) | 0.111 |
| LDL-c (mg/dl) | 115.89 (38.28) | 88.6 (30.18) | 0.000 |
| Triglycerides (mg/dl) | 145.97 (60.27) | 120.12 (22.71) | 0.000 |
The presence of hypertension and events of stroke in patients as compared to controls were statistically: hypertension 221 (70.2%) vs. 41 (20%) [p 0.000], systolic pressure 146.47 vs. 122.03 mmHg (p 0.000), diastolic pressure 91.84 vs. 74.25 mmHg (p 0.000), and events of stroke 72 (22.9%) vs. 7 (3.4%) [p 0.000]. Furthermore, lipid profile revealed significant difference in measured total cholesterol, LDL, triglycerides and curiously the seemingly protective HDL showed no dissimilarity between patients and controls.
The distribution of genotypes (Table 2) were in decreasing order TT, 148 (47%), GT 135 (42.9%) and GG 32 (10.2%) in the cases of the study while the pattern in controls were GT 91 (44.4%), TT 86 (42%) and GG 28 (13.7%). Fig. 1 depicted the position of G and T alleles on intron 8 of the LPL gene. Cases were likely than controls to have all the genotypes in this study. Among the two of the genotypes of HindIII, none were statistically significant as the odd ratios between the cases and controls were as follows: TT [OR = 1.506, 95% CI (0.849–2.670), p 0.182] and GT [OR = 1.298, 95% CI (0.732–2.301), p 0.227]. Alleles distribution among cases and controls were more with T than G, however they were not statistically significant as the OR = 0.826, 95%CI (0.635–1.074), p0.087.
Table 2.
Genotype and allele frequencies between CAD cases and control subjects.
|
Case (3 1 5) |
Control (2 0 5) |
OR | 95% CI | P | ||||
|---|---|---|---|---|---|---|---|---|
| Genotypes | No. | % | No. | % | ||||
| GG | 32 | 10.2 | 28 | 13.7 | 1 | |||
| GT | 135 | 42.9 | 91 | 44.4 | 1.298 | (0.732–2.301) | 0.227 | |
| TT | 148 | 47 | 86 | 42 | 1.506 | (0.849–2.670) | 0.182 | |
| Alleles | ||||||||
| G | 199 | 31.59 | 147 | 35.85 | 0.826 | (0.635–1.074) | 0.087 | |
| T | 431 | 68.41 | 263 | 64.15 | ||||
Comparison of risk parameters across the genotypes (Table 3) demonstrated insignificance except for BMI which showed differential change towards the GG genotype (p = 0.032). Analysis of the effects of HDL-c across all patients (Table 4) revealed statistically significant difference for males (p = 0.000), diabetics (p = 0.000) and triglycerides < 150 mg/dl (p = 0.033). While no differences were noted for obesity and smoking. Regression analysis of associated factors in Table 5 demonstrated significance of G Allele and other parameters except for age (p = 0.513), smoking (p = 0.176) and exercise (p = 0.329).
Table 3.
Risk factors distribution across genotypes.
| No. (%) | TT | GT | GG | P |
|---|---|---|---|---|
| Age [mean(SD)] | 59.42 (11.28) | 59.56 (10.55) | 61.59 (10.40) | 0.581 |
| BMI (Kg/m2) [mean(SD)] | 28.83 (3.9) | 29.33 (4.11) | 30.92 (4.74) | 0.032 |
| Gender (male %) | 105 (33.33%) | 85 (26.98%) | 16 (5.08%) | 0.057 |
| Smoking | 76 (24.13%) | 64 (20.32%) | 10 (3.17%) | 0.118 |
| Exercise (%) | 35 (11.11%) | 30 (9.52%) | 7 (2.22%) | 0.951 |
| Hypertension (%) | 99 (31.43%) | 98 (31.11%) | 24 (7.62%) | 0.474 |
| Systolic BP (mmHg) | 146.47 (12.66) | 147.04 (12.12) | 144.01 (12.07) | 0.462 |
| Diastolic BP (mmHg) | 92.03 (10.17) | 90.58 (9.46) | 92.70 (9.09) | 0.349 |
| Diabetes (%) | 88 (27.94%) | 77 (24.44%) | 22 (6.98%) | 0.479 |
| Blood glucose (mg/dl) | 141.46 (21.36) | 140.32 (20.36) | 138.18 (19.84) | 0.701 |
| Stroke (%) | 41 (13.02%) | 22 (6.98%) | 9 (2.86%) | 0.065 |
| TC (mg/dL) | 155.37 (22.48) | 152.38 (24.79) | 156.17 (23.70) | 0.500 |
| LDLc (mg/dL) | 117.35 (43.35) | 113.80 (24.79) | 117.97 (45.72) | 0.701 |
| HDLc (mg/dL) | 39.78 (8.55) | 39.97 (7.50) | 40.65 (8.13) | 0.859 |
| TG (mg/dL) | 143.31 (59.34) | 146.34 (59.91) | 156.75 (66.50) | 0.519 |
Table 4.
Effects of environmental factors on plasma HDL-c levels.
| Factor | N | HDL-c | Mean difference | -t | P | |
|---|---|---|---|---|---|---|
| Gender | Male | 206 | 37.43 ± 7.2 | − 7.275 | − 8.442 | 0.000 |
| Female | 109 | 44.71 ± 7.4 | ||||
| Obesity | BMI < 30 kg/m2 | 185 | 40.06 ± 8.1 | −0.268 | −0.291 | 0.772 |
| BMI ≥ 30 kg/m2 | 130 | 39.79 ± 8 | ||||
| Smoking | Yes | 150 | 39.51 ± 7.7 | 0.844 | 0.933 | 0.353 |
| No | 165 | 40.35 ± 8.3 | ||||
| Diabetes | Yes | 187 | 37.83 ± 7.6 | −5.23 | −5.968 | 0.000 |
| No | 128 | 43.06 ± 7.7 | ||||
| TG | TG < 150 mg/dl | 161 | 40.89 ± 7.8 | 1.93 | 2.14 | 0.033 |
| TG ≥ 150 mg/dl | 154 | 38.97 ± 8.2 |
Table 5.
Regression analysis with associated risk factors.
| Variables | B | Beta | t | P |
|---|---|---|---|---|
| LPL G Allele | − 0.001 | − 0.008 | − 0.459 | 0.046 |
| Age | 0.001 | 0.012 | 0.654 | 0.513 |
| BMI | 0.004 | 0.049 | 2.674 | 0.008 |
| Smoking | 0.31 | 0.031 | 1.355 | 0.176 |
| Exercise | − 0.019 | − 0.017 | − 0.976 | 0.329 |
| Hypertension | 0.056 | 0.058 | 2.686 | 0.007 |
| Systolic BP | 0.008 | 0.256 | 11.114 | 0.000 |
| Diastolic BP | 0.007 | 0.191 | 8.972 | 0.000 |
| DM | 0.044 | 0.044 | 2.188 | 0.029 |
| Fasting Glucose conc | 0.007 | 0.472 | 18.896 | 0.000 |
| TG | 0.001 | 0.060 | 3.334 | 0.001 |
| TC | 0.001 | 0.060 | 3.341 | 0.001 |
| LDL-c | 0.001 | 0.064 | 3.462 | 0.001 |
| HDL-c | − 0.001 | − 0.012 | − 0.687 | 0.049 |
4. Discussion
The aim of the present study was to investigate the genetic association of rs320 polymorphism in LPL gene in the diagnosed CAD cases in Saudi population. The results of this study confirm the negative association either with allele or genotype frequencies (p > 0.05) between the CAD cases and control subjects.
LPL is a key-enzyme for the lipid metabolism and it can hydrolyze the TG of chylomicrons and VLDL to provide free fatty-acids for oxidation and exploitation in heart and other tissues for storing in adipose tissues. LPL is a pathophysiological procedure for chylomicronemia, diabetes, obesity and atherosclerosis. LPL gene is cDNA translated into 475 amino-acid proteins which involves signal peptide of 27 amino-acids. RFLP in LPL gene has confirmed BamHI, BstNI, HindIII, PvuII and Ser447X sites. HindIII is one of the polymorphisms amongst in which plasma lipids were associated and lowers the plasma LPL activities; further subordinates with high TG and low HDL-c levels which may contribute in the CAD patients. The HindIII variant exists 495 bp from the intron-8 towards the splice site and effect the RNA splicing. The H-allele in this variant may enhance the enzyme activity (Xie, 2017). However, other studies have confirmed various discrepancies regarding the TG level, incidence of myocardial and rate of acute ischemic strokes in different geographical locations and ethnicities.
Our study located in western part of Saudi Arabia revealed elevated total cholesterol, low density cholesterol and triglycerides among patients with coronary artery disease as compared. This is conformity of the results in central province of Saudi Arabia where CAD patients demonstrated drastically greater TG, TC and LDL-c levels than controls in patients with HindIII among other genotypes. Furthermore, similar to our study, the genotype was linked with significantly reduced HDL-c levels. Both the studies have recognized the connection between HindIII variant and high plasma levels in TC, TG and LDL-c with low HDL-c levels (Daoud et al., 2013).
Despite the significant differences in lipid profile between cases and controls, the HindIII polymorphism did not exert an independent vulnerability to the development of CAD. Our study revealed insignificant odd risk ratios among the haplotypes in both cases and controls. The findings were similar to studies done among Saudi population in central province of the country. Furthermore, the frequencies of HindIII in our study (TT, 47.0; GT, 42.9 and GG, 10.2%) were similar in these studies as Daoud et al. (Daoud et al., 2013) recorded (TT, 45.1; TG, 35.8 and GG, 19%) while Abu Amero et al. (Abu-Amero et al., 2003) reported slightly similar (TT, 53.7; TG, 39.2 and GG, 7.1%). These studies proved the fact that LPL-HindIII polymorphism could not be used as an independent genetic risk factor for CAD in Saudi Arab populations. Both the genetic and environmental factors like HTN, diabetes, obesity and smoking are important risk-factors for the development of CAD. In North Africa, the polymorphisms assessed by restriction assay in 100 each of diabetic and non-diabetic Egyptian MI patients and 100 healthy controls demonstrated that individuals with the H2H2 genotype or S2 allele three times more likely to suffer from MI than those carrying the H1H1 or S1S1 (El-Aziz et al., 2011). Despite cultural linkage with the Saudi population, our study and others did not show significant association between myocardial infarction in patients with diabetes across all haplotypes of HindIII polymorphism (Daoud et al., 2013, Abu-Amero et al., 2003).
In the Mediterranean area, a Spanish study which analysed various genetic variants including HindIII in a large, well-characterized population, illustrated their association with TG levels. The results demonstrated a liberated effect of the polymorphisms studied, notably TG-lowering HindIII case (Ariza et al., 2010). The protective LPL variants HindIII were also shown in other populations from southern Europe. In contrast, our study reported raised levels of triglycerides. The differences may well be due to the effects of geographical and ethnic dissimilarities between populations of the studies. In conformity with our finding of dyslipidemia, an Iranian study which evaluated 725 children and adolescents demonstrated the relationship of LPL HindIII [rs320] and abnormal lipid levels (Marateb et al., 2018).
In an appraisal of the associations between seven LPL polymorphisms, lipid fractions and CHD risks in a population-based cohort, case-control, and cross-sectional studies, Sagoo et al (Sagoo et al., 2008) analysed populations comprising 22,734 CHD cases and 50,177 controls. The study reviewed the relationship between various polymorphisms including HindIII to TG, HDL-c, myocardial infarction, or coronary stenosis. It was demonstrated that carriers of the less common allele of HindIII depicted modestly advantageous profiles. Furthermore, subset assessment of 8186 participants revealed that carriers of the less common allele of the HindIII polymorphism higher HDL-c levels and lower TG levels than non-carriers. The findings of our study conformed with higher HDL-c but at variance with lower triglyceride levels as the meta-analysis was composed of large population of participants and varying geographic settings of white European continental ancestry, East Asian, including Mexican-American and Turkish populations.
The event of acute stroke in our study though a small population of participants was 22.9% in cases and 3.4% in controls with a p Value of 0.000. In Colombia, Velasquez et al (Velasquez Pereira et al., 2016), did not record any association between LPL gene polymorphisms including HindIII and acute ischemic stroke in the population studied (Velásquez Pereira and Vargas Castellanos, 2016). In addition, the allele and genotypic frequencies of the studied polymorphisms were similar in cases and controls. However, in a meta-analysis of studies in five Chinese and Japanese population, it was revealed that LPL-HindIII was protective factor against stroke risk. Further stratification showed the protective role played simultaneously for both hemorrhagic and ischemic subtypes. It was postulated that rs320 produces a significant impact on precursor RNA which may be a source of the protective effect of the polymorphism in stroke propensity (Nejati et al., 2018). Lipid profile in Colombian study were not significantly different between cases and controls despite the lack of association between HindIII and the stroke risk. However, the controls had statistically significant high levels of HDL-c than the cases (Velásquez Pereira and Vargas Castellanos, 2016).
The limitations of our study are; (i) we couldn’t provide the validation results (ii) participants were recruited from couple of hospitals placed in the western region and (iii) we miss the plasma levels for LPL through Elisa. A multi-centre study would have in addition, as in case-control studies, selection bias would have been difficult to be avoided. The strength of our study is we have opted good sample size with age and gender matched controls. Our study couldn’t conclude the positive association with HindIII polymorphism in CAD patient and controls. However, other global studies have confirmed as biomarker with rs320 or HindIII polymorphism. At other angle, our study showed HindIII polymorphism in LPL gene was associated with raised TG levels and stroke. However, no associations were recorded with all haplotypes on the risk of myocardial infarction, lipid profile and other established diseases associated with myocardial infarction. Furthermore, the statistical power of the study might be affected as cost was a mitigating factor in ensuring a ratio of 1:3 for cases and controls.
Funding
This project was funded by Umm Al-Qura University, Faculty of Medicine, Umm Al-Qura University to Dr. Neda M Bogari.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We all are deeply appreciated for Dr. Ahmed Fawzy, Dr. Dareen ibrahim Rednah, Dr. Saad Alghamdi, Dr. Khalid Faruqui, Dr. Enas Alharthi, Dr. Elaf Almatrouk, Dr. Noor Balto, Mr. Abdulmonim Gowda, Mr. Hussain Banni and Mr. Ehab Melibary. We are thankful to Faculty of Medicine, UQU, National Science, Technology, and Innovation Plan, (MAARIFAH)- King Abdulaziz City for Science and Technology- the Kingdom of Saudi Arabia for their support. Monzino Heart Center, University of Milan in Italy, King Fahd Armed Forces Hospitals and KAMC, Makkah.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Neda M. Bogari, Email: nmbogari@uqu.edu.sa.
Reem M. Allam, Email: rmallam@medicine.zu.edu.eg.
References
- Nimonkar A.V., Weldon S., Godbout K., Panza D., Hanrahan S., Cubbon R. A lipoprotein lipase–GPI-anchored high-density lipoprotein–binding protein 1 fusion lowers triglycerides in mice: Implications for managing familial chylomicronemia syndrome. 2020;295:2900–2912. doi: 10.1074/jbc.RA119.011079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P., Wei G., Cai K.e., Xiang X.i., Deng W.P., Li Y.B., Kuang S., Dong Z., Zheng T., Luo Y., Liu J., Guan Y., Li C., Dey S.K., Liao Z., Banerjee S. Identification and functional characterization of mutations in LPL gene causing severe hypertriglyceridaemia and acute pancreatitis. J. Cell. Mol. Med. 2020;24(2):1286–1299. doi: 10.1111/jcmm.v24.210.1111/jcmm.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochetova O., Avzaletdinova D., Sharipova L., Korytina G., Akhmadishina L., Morugova T. An analysis of the associations of polymorphic variants of the LEPR (rs1137100), LRP5 (rs3736228), and LPL (rs320) genes with the risk of developing type 2 diabetes. Mellitus. 2019;55:495–503. [Google Scholar]
- Su X, Peng DJCCA. The exchangeable apolipoproteins in lipid metabolism and obesity. 2020. [DOI] [PubMed]
- Gerodias F.R., Posas F.E.B., Baclig M.O., Repotente E.C.T., Pelat J.F.B., Rogelio G.G. Association of lipoprotein lipase gene polymorphisms with coronary artery disease among Filipinos. Int. J. Mol. Epidemiol. Genet. 2019;10:77–84. [PMC free article] [PubMed] [Google Scholar]
- Laufs U., Parhofer K.G., Ginsberg H.N. Hegele RAJEhj. Clin. Rev. Triglycerides. 2020;41:99–109c. doi: 10.1093/eurheartj/ehz785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y. Elsevier; Clinical Molecular Medicine: 2020. Molecular basis of stroke; pp. 189–216. [Google Scholar]
- Xu N., Xu H., Zhao M., Xu Y., Huang L. Associations of systemic, serum lipid and lipoprotein metabolic pathway gene variations with polypoidal choroidal vasculopathy in China. PLoS One. 2019;14 doi: 10.1371/journal.pone.0226763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade Júnior MC. iMedPub Journals. 2018.
- Khan I.A., Vattam K.K., Jahan P., Mukkavali K.K., Hasan Q., Rao P. Correlation between KCNQ1 and KCNJ11 gene polymorphisms and type 2 and post-transplant diabetes mellitus in the Asian Indian population. Genes Dis. 2015;2:276–282. doi: 10.1016/j.gendis.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alinaghian N., Abdollahi E., Torab M., Khodaparast M., Zamani F., Rahimi-Moghaddam P. Gender-related relation between metabolic syndrome and S447X and HindIII polymorphisms of lipoprotein lipase gene in northern Iran. Gene. 2019;706:13–18. doi: 10.1016/j.gene.2019.04.069. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kumar V, Kim JJ. Sarcomeric Gene Variants and Their Role with Left Ventricular Dysfunction in Background of Coronary Artery Disease. Biomolecules. 2020;10. [DOI] [PMC free article] [PubMed]
- Fan Q., Zhu Y., Zhao F. Association of rs2230806 in ABCA1 with coronary artery disease: an updated meta-analysis based on 43 research studies. Medicine (Baltimore). 2020;99 doi: 10.1097/MD.0000000000018662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Lian Z., Zhang W., Cui Y., Wang W., Wu J. Association between interleukin-8 gene -251 A/T polymorphism and the risk of coronary artery disease: a meta-analysis. Medicine (Baltimore). 2019;98 doi: 10.1097/MD.0000000000017866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Ma Y., Chen S., Che Q., Chen D. The integrated landscape of biological candidate causal genes in coronary artery disease. Front. Genet. 2020;11:320. doi: 10.3389/fgene.2020.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechlivanis S., Moebus S., Lehmann N., Erbel R., Mahabadi A.A., Hoffmann P. Genetic risk scores for coronary artery disease and its traditional risk factors: their role in the progression of coronary artery calcification-results of the heinz nixdorf recall study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnaggar I.Z., Hussein S., Amin M.I., Abdelaziz E.A. Association of 584C/T polymorphism in endothelial lipase gene with risk of coronary artery disease. J. Cell. Biochem. 2019;120:14414–14420. doi: 10.1002/jcb.28697. [DOI] [PubMed] [Google Scholar]
- Matam K., Khan I.A., Hasan Q. Rao PJJoKSU-S. Coronary artery disease and the frequencies of MTHFR and PON1 gene polymorphism studies in a varied population of Hyderabad, Telangana region in south. India. 2015;27:143–150. [Google Scholar]
- Yuan S., Lin A., He Q.Q., Burgess S., Larsson S.C. Circulating interleukins in relation to coronary artery disease, atrial fibrillation and ischemic stroke and its subtypes: A two-sample Mendelian randomization study. Int. J. Cardiol. 2020 doi: 10.1016/j.ijcard.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melam G.R., Alhusaini A.A., Buragadda S., Kaur T., Khan I.A. Impact of brisk walking and aerobics in overweight women. J. Phys. Therapy Sci. 2016;28:293–297. doi: 10.1589/jpts.28.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I.A., Poornima S., Jahan P., Rao P., Hasan Q. Type 2 diabetes mellitus and the association of candidate genes in Asian Indian population from Hyderabad, India. J. Clin. Diagnostic Res. : JCDR. 2015;9:Gc01-5. doi: 10.7860/JCDR/2015/14471.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito R, Kasai TJWjoc. Coronary artery disease in type 2 diabetes mellitus: recent treatment strategies and future perspectives. 2015;7:119. [DOI] [PMC free article] [PubMed]
- Lu S., Wang Y., Wang Y., Hu J., Di W., Liu S. The IL-6 rs1800795 and rs1800796 polymorphisms are associated with coronary artery disease risk. J. Cell Mol. Med. 2020 doi: 10.1111/jcmm.15246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yang W., McVey D.G., Zhao G., Hu J., Poston R.N. FURIN expression in vascular endothelial cells is modulated by a coronary artery disease-associated genetic variant and influences monocyte transendothelial migration. J. Am. Heart Association. 2020;9 doi: 10.1161/JAHA.119.014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javorsky M., Gasperikova D., Ukropec J., Sedlakova B., Riecansky I., Krizanova O. Lipoprotein lipase HindIII polymorphism influences HDL-cholesterol levels in statin-treated patients with coronary artery disease. Wiener klinische Wochenschrift. 2007;119:476–482. doi: 10.1007/s00508-007-0824-1. [DOI] [PubMed] [Google Scholar]
- Daoud M.S., Ataya F.S., Fouad D., Alhazzani A., Shehata A.I., Al-Jafari A.A. Associations of three lipoprotein lipase gene polymorphisms, lipid profiles and coronary artery disease. Biomed Rep. 2013;1:573–582. doi: 10.3892/br.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S., Pinto S.P., Lins L., Bianco H.T., Monteiro C.M.C., Pinheiro L.F.M. Association of multiple genetic variants with the extension and severity of coronary artery disease. Arquivos brasileiros de cardiologia. 2018;110:16–23. doi: 10.5935/abc.20170177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W.Q., Wang Y., Han X.Q., Zhu Y., Liu N.F. Associations between LPL gene polymorphisms and coronary artery disease: evidence based on an updated and cumulative meta-analysis. Biosci. Rep. 2018 doi: 10.1042/BSR20171642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid S.U., Shabana Cooper J.A., Beaney K.E., Li K., Rehman A. Genetic risk analysis of coronary artery disease in Pakistani subjects using a genetic risk score of 21 variants. Atherosclerosis. 2017;258:1–7. doi: 10.1016/j.atherosclerosis.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Xie L, Li YM. Lipoprotein Lipase (LPL) Polymorphism and the Risk of Coronary Artery Disease: A Meta-Analysis. International journal of environmental research and public health. 2017;14.
- Hartley Taila, Lemire Gabrielle, Kernohan Kristin D., Howley Heather E., Adams David R., Boycott Kym M. New diagnostic approaches for undiagnosed rare genetic diseases. Annu. Rev. Genom. Hum. Genet. 2020;21(1) doi: 10.1146/annurev-genom-083118-015345. [DOI] [PubMed] [Google Scholar]
- Nejati M., Atlasi M.A., Karimian M., Nikzad H., Azami Tameh A. Lipoprotein lipase gene polymorphisms as risk factors for stroke: a computational and meta-analysis. Iranian J. Basic Medical Sci. 2018;21:701–708. doi: 10.22038/IJBMS.2018.29009.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoo G.S., Tatt I., Salanti G., Butterworth A.S., Sarwar N., van Maarle M. Seven lipoprotein lipase gene polymorphisms, lipid fractions, and coronary disease: a HuGE association review and meta-analysis. Am. J. Epidemiol. 2008;168:1233–1246. doi: 10.1093/aje/kwn235. [DOI] [PubMed] [Google Scholar]
- Samgina T.A., Bushueva O.Y., Nazarenko P.M., Polonikov A.V. Association of the HindIII lipoprotein lipase gene polymorphism with the development of the non-biliary acute pancreatitis: a pilot study. Bull. Exp. Biol. Med. 2016;161:79–82. doi: 10.1007/s10517-016-3350-1. [DOI] [PubMed] [Google Scholar]
- Tetik Vardarli A., Harman E., Bozok Cetintas V., Kayikcioglu M., Vardarli E., Zengi A. Polymorphisms of lipid metabolism enzyme-coding genes in patients with diabetic dyslipidemia. Anatolian J. Cardiology. 2017;17:313–321. doi: 10.14744/AnatolJCardiol.2016.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez Pereira L.C., Vargas Castellanos C.I., Silva Sieger F.A. Polymorphisms of the lipoprotein lipase gene as genetic markers for stroke in colombian population: a case control study. Colombia Medica (Cali, Colombia) 2016;47:189–195. [PMC free article] [PubMed] [Google Scholar]
- Nagrani R., Foraita R., Gianfagna F., Iacoviello L., Marild S., Michels N. Common genetic variation in obesity, lipid transfer genes and risk of metabolic syndrome: results from IDEFICS/I. Family study and meta-analysis. 2020;10:1–14. doi: 10.1038/s41598-020-64031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanushali AA, Das BRJJocg. Genetic variants at the APOE, lipoprotein lipase (LpL), cholesteryl ester transfer protein (CETP), and endothelial nitric oxide (eNOS) genes and coronary artery disease (CAD): CETP Taq1 B2B2 associates with lower risk of CAD in Asian Indians. 2010;1:55-62. [DOI] [PMC free article] [PubMed]
- Daoud M.S., Ataya F.S., Fouad D., Alhazzani A., Shehata A.I. Al-Jafari AAJBr. Associations of three lipoprotein lipase gene polymorphisms, lipid profiles and coronary artery disease. 2013;1:573–582. doi: 10.3892/br.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I.A., Movva S., Shaik N.A., Chava S., Jahan P., Mukkavali K.K. Investigation of Calpain 10 (rs2975760) gene polymorphism in Asian Indians with gestational diabetes mellitus. Meta Gene. 2014;2:299–306. doi: 10.1016/j.mgene.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeman J.I., Grundy S.M.J.C. National Cholesterol Education Program recommendations for cholesterol testing in young adults: a science-based approach. 1997;95:1646–1650. doi: 10.1161/01.cir.95.6.1646. [DOI] [PubMed] [Google Scholar]
- Khan I.A., Vattam K.K., Jahan P., Hasan Q., Rao P. Importance of glucokinase -258G/A polymorphism in Asian Indians with post-transplant and type 2 diabetes mellitus. Intractable Rare Dis Res. 2016;5:25–30. doi: 10.5582/irdr.2015.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi K.K., Kashour T.S., Al-Hussaini W., Nbaheen M.S., Hasanato R.M., Mohamed S. Screening for genetic mutations in LDLR gene with familial hypercholesterolemia patients in the Saudi population. Acta Biochimica Polonica. 2015;62:559–562. doi: 10.18388/abp.2015_1015. [DOI] [PubMed] [Google Scholar]
- Poornima S., Subramanyam K., Khan I.A., Hasan Q. The insertion and deletion (I28005D) polymorphism of the angiotensin I converting enzyme gene is a risk factor for osteoarthritis in an Asian Indian population. J. Renin-angiotensin-aldosterone system : JRAAS. 2015;16:1281–1287. doi: 10.1177/1470320314547403. [DOI] [PubMed] [Google Scholar]
- Khan I.A., Jahan P., Hasan Q. Rao PJJoHS. Relationship between PTEN and gestational diabetes in Asian Indians womens. 2015;3:184. [Google Scholar]
- Khan I.A., Jahan P., Hasan Q., Rao P. Angiotensin-converting enzyme gene insertion/deletion polymorphism studies in Asian Indian pregnant women biochemically identifies gestational diabetes mellitus. J. renin-angiotensin-aldosterone system : JRAAS. 2014;15:566–571. doi: 10.1177/1470320313502106. [DOI] [PubMed] [Google Scholar]
- Khan I.A., Shaik N.A., Pasupuleti N., Chava S., Jahan P., Hasan Q. Screening of mitochondrial mutations and insertion–deletion polymorphism in gestational diabetes mellitus in the. Asian Indian population. 2015;22:243–248. doi: 10.1016/j.sjbs.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poornima S, Subramanyam K, Khan IA, G S, Hasan Q. Role of SREBP2 gene polymorphism on knee osteoarthritis in the South Indian Hyderabad Population: A hospital based study with G595C variant. Journal of orthopaedics. 2019;16:293-7. [DOI] [PMC free article] [PubMed]
- Ahmadi Z., Senemar S., Toosi S., Radmanesh S. The Association of Lipoprotein Lipase Genes, HindIII and S447X Polymorphisms With Coronary Artery Disease in Shiraz City. J. Cardiovascular Thoracic Res. 2015;7:63–67. doi: 10.15171/jcvtr.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Tan C.S., Chia K.S., Tan C.E., Chew S.K., Ordovas J.M. The lipoprotein lipase S447X polymorphism and plasma lipids: interactions with APOE polymorphisms, smoking, and alcohol consumption. J. Lipid Res. 2004;45:1132–1139. doi: 10.1194/jlr.M400016-JLR200. [DOI] [PubMed] [Google Scholar]
- Khan IA, Kamineni V, Poornima S, Jahan P, Hasan Q, Rao PJJorh, et al. Tumor necrosis factor alpha promoter polymorphism studies in pregnant women. 2015;1:18-22.
- Khan I.A., Jahan P., Hasan Q., Rao P. Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diab. Metabolic Syndrome. 2019;13:688–694. doi: 10.1016/j.dsx.2018.11.035. [DOI] [PubMed] [Google Scholar]
- Khan IA, Jahan P, Hasan Q, Rao PJI, research rd. Validation of the association of TCF7L2 and SLC30A8 gene polymorphisms with post-transplant diabetes mellitus in Asian Indian population. 2015;4:87-92. [DOI] [PMC free article] [PubMed]
- Abu-Amero K.K., Wyngaard C.A., Al-Boudari O.M., Kambouris M. Dzimiri NJAop, medicine l. Lack of association of lipoprotein lipase gene polymorphisms with coronary artery disease in the Saudi Arab population. 2003;127:597–600. doi: 10.5858/2003-127-0597-LOAOLL. [DOI] [PubMed] [Google Scholar]
- El-Aziz T.A.A., Mohamed R.H., Hashem R.M.J.M. biochemistry c. Association of lipoprotein lipase and apolipoprotein C-III genes polymorphism with acute myocardial infarction in diabetic patients. 2011;354:141–150. doi: 10.1007/s11010-011-0813-6. [DOI] [PubMed] [Google Scholar]
- Ariza M.-J., Sánchez-Chaparro M.-Á., Barón F.-J., Hornos A.-M., Calvo-Bonacho E., Rioja J. Additive effects of LPL, APOA5 and APOEvariant combinations on triglyceride levels and hypertriglyceridemia: results of the ICARIA genetic sub-study. 2010;11:66. doi: 10.1186/1471-2350-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marateb H.R., Mohebian M.R., Javanmard S.H., Tavallaei A.A., Tajadini M.H., Heidari-Beni M. Prediction of dyslipidemia using gene mutations, family history of diseases and anthropometric indicators in children and adolescents. The CASPIAN-III study. 2018;16:121–130. doi: 10.1016/j.csbj.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoo G.S., Tatt I., Salanti G., Butterworth A.S., Sarwar N., vanMaarle M. Seven lipoprotein lipase gene polymorphisms, lipid fractions, and coronary disease: a HuGE association review and meta-analysis. 2008;168:1233–1246. doi: 10.1093/aje/kwn235. [DOI] [PubMed] [Google Scholar]
- Velásquez Pereira L.C., Vargas Castellanos C.I. Silva Sieger FAJCM. Polymorphisms of the lipoprotein lipase gene as genetic markers for stroke in colombian population: a case control study. 2016;47:189–195. [PMC free article] [PubMed] [Google Scholar]
- Nejati M., Atlasi M.A., Karimian M., Nikzad H. Tameh AAJIjobms. Lipoprotein lipase gene polymorphisms as risk factors for stroke: a computational and meta-analysis. 2018;21:701. doi: 10.22038/IJBMS.2018.29009.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]