Abstract
American bolloworm, Helicoverpa armigera Hubner (Noctuidae: Lepidoptera) is considered as a major pest of various crops all over the world. It is mainly controlled by indiscriminate use of synthetic insecticides in the world due to which this pest developed resistance to most of the available insecticides. Therefore, in the current study, the efficacy of virulent strain of HaNPV (0.5 × 109 PIB/ml) alone and in combination with recommended doses of spintoram (20 g/100 L of water) and emamectin benzoate (200 ml/100 L of water) was tested in field. The combination of HaNPV with spintoram and emamectin benzoate 100% reduced the larval population as compared to emamectin benzoate and HaNPV alone. This suggested that the combination of spintoram and emamectin benzoate with HaNPV could be used in field to manage the infestation of H. armigera.
Keywords: NPV, Cotton, Entomopathogenic virus, Resistance
1. Introduction
American Bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) is a major pest of several field crops and vegetables causing losses of several billions dollars annually. It has wide hosts range including approximately all the commercially grown plant families. This could be one of the reason of evolution of resistance in this pest against different insecticides (Wang and Qin, 2007). The other reasons for quick resistance development in H. armigera could be its short life cycle, high fecundity and favorable environmental conditions prevailing in the area (Denholm et al. 1998).
In some countries, the problem of insecticide resistance in H. armigera has worsened causing 20–30% crop loss (Bhargava et al., 2008). The indiscriminate use of chemical insecticides to control. this pest led to development of resistance .to several insecticides groups including pyrethroids (>800 folds), organophosphates (>700 folds), carbamates (>300 folds) and a low level of resistance (8 folds) (Ahmad et al., 1995, Ahmad et al., 2002, Faheem et al., 2013, Yang et al., 2013) and serious harmful effects on the environment (Sabir et al. 2011). Thus usage of synthetic insecticide should be minimized and replaced with environmentally benign methods to reduce insecticide resistance and delay development of resistance in insect pests (Bielza 2008).
Therefore, there is a dire need to find some alternative methods and compounds for the sustainable management of H. armigera without damaging the non-target organisms and the environment. The use of entomopathogenic micro-organisms could be an effective option for H. armigera but a few products are available in the market for its management (Qayyum et al. 2020). Although, Bacillus thuringiensis (Bt) crops significantly reduced the problem of cotton bollworms but some pests like H. armigera have developed resistance to these Bt crops (Tabashnik et al. 2008, 2009; Alvi et al., 2012). At the same time, heavy usage of non-selective chemical insecticides for sucking pests has led to decline in population of biological control agents, allowing H. armigera to resurge.
Another option for the management of H. armigera is the use of nuclear polyhedrosis virus (NPV) which species-specific and have almost no harm to the beneficial natural enemies of pests and the environment (Caballero et al., 1992, Cory and Hails, 1997). These NPV have been successfully used against some lepidopterous pest species (Liu et al. 2006; Inceoglu et al. 2006; Kumar et al. 2011; Ahmad et al. 2018; Ayyub et al. 2019), but there are few studies in which authors have evaluated field efficacy NPV against H. armigera. Some authors have reported increased in the efficacy of NPV when they are mixed with sub-lethal doses of synthetic insecticides both in laboratory and field conditions (Charati et al. 1999; Bret et al. 1997; Ayyub et al. 2019). To the best of our knowledge, none of any studies reported the impact of NPV along with insecticides against H. armigera. In the current study, efficacy of HaNPV along with two insecticides (spintoram and emamectin benzoate) was assessed against H. armigera under field conditions. We hypothesized that the combination of NPV with insecticide will be more effective as compared to sole insecticide or NPV against H. armigera.
2. Materials and methods
2.1. Study site and layout of experiment
The cotton field comprised of 594 × 480 sq.ft (6 Acres) was selected and divided into four similar blocks each of 594 × 110 sq.ft (1.5 acres). There were six treatments: Spintoram, Emamectin benzoate; lab selected HaNPV; Spintoram + HaNPV; Emamectin benzoate + HaNPV and Control and each treatment was replicated four times. The plot size per treatment was 90 × 100 sq.ft and plot to plot distance was 9 feet whereas block to block distance was 10 feet. However, on boundaries of North south 4.5ft was untreated area and from east west boundaries 5 ft were untreated area. The experiment was laid out in randomized complete block design.
2.2. Cultivation of cotton
The field was lazer leveled before seed bed preparation. The seed bed preparation was made by two ploughings followed by one deep ploughing, 1 rotavater and again two ploughings to make the soil good for cotton seed germination. The sowing was made on April 29, 2014 by planting 8 kg seed/Acre of MNH-886 by planter with row to row distance 2.5feet and plant to plant distance 0.75ft. Soon after sowing, irrigation was made and till germination irrigation was made after every 3 days and after that at every 7 days interval. However, the irrigation was skipped if rainfall occurred at the time of irrigation. The gap flailing was made after 3rd irrigation. The thinning was done at 6 leaf stage. 1 bag of DAP and 1 bag of CAN per acre was added at the time of seed bed preparation and then half bag of urea at every alternate irrigation through fertigation was used till 10th of October 2014. Pest scouting was started on 30th may 2014 and continued till October 31st, 2014 after 7 days interval. Sucking pests were controlled by applying relevant insecticides.
2.3. Field spraying and data recording
Field doses of each treatment, HaNPV @ 0.5 × 109 PIB/ml alone and in combination with recommended doses of spintoram (20 g/100 L of water) and emamectin benzoate (200 ml/100 L of water) were prepared and sprayed in cotton field at dusk so that larvae have enough time to pick up NPV from foliage. The most virulent strain of HaNPV was used in field experiments (Abid et al. 2020). The data of H. armigera population was recorded 24 h before spraying each field and then at 24 h interval for a total of eight days. During data recording, plants were carefully observed for dead larvae that were collected and brought to laboratory for confirmation of the NPV infection.
2.4. Data analysis
The data of reduction (%) of H. armigera population in different treatments was subjected to randomized complete block design (RCBD) analysis of variance (ANOVA) and means were separated by Tukey’s HSD test. All tests were performed using Statistix 8.1v (Analytical software, 2005).
3. Results
The number of larvae observed per 25 plants at several intervals after application are presented in Table 1. There was significant.difference among the treatments after 1 day exposure (F = 2.85, DF = 5, P = 0.05). After 2 days post application, there were no significant differences in the treatments compared with the control (F = 1.59, df = 5, P = 0.22). After 3 days post application, the number of larvae varied from 1.75 to 2.25 in spintoram, emamectin benzoate, NPV and their mixture treatments which were significantly less than the control treatment where 5.00 larvae/25 plants were observed (F = 4.89, df = 5, P = 0.01).
Table 1.
Average number of Helicoverpa armigera larvae after application of spintoram, emamectin benzoate, NPV and their mixtures.
| Treatment | Number of larvae / 25 plants |
|||||||
|---|---|---|---|---|---|---|---|---|
| After 1 day exposure | After 2 day exposure | After 3 day exposure | After 4 day exposure | After 5 day exposure | After 6 day exposure | After 7 day exposure | After 8 day exposure | |
| Control | 2.50 ± 1.04b | 2.75 ± 0.48ab | 5.00 ± 0.91a | 6.50 ± 0.64a | 8.50 ± 1.04a | 9.75 ± 0.48a | 9.75 ± 0.85a | 12.25 ± 0.48a |
| Spintoram | 3.25 ± 0.48b | 2.75 ± 0.25ab | 2.25 ± 0.25b | 1.00 ± 0.41bc | 0.50 ± 0.29bc | 0.50 ± 0.29b | 0.00 ± 0.00b | 0.00 ± 0.00c |
| Emamectin benzoate | 2.25 ± 0.25b | 3.00 ± 0.41a | 2.00 ± 0.41b | 2.00 ± 0.41b | 1.50 ± 0.29b | 0.75 ± 0.48b | 0.50 ± 0.29b | 1.50 ± 0.29b |
| NPV | 3.00 ± 0.41b | 1.75 ± 0.25b | 1.75 ± 0.63b | 1.25 ± 0.48bc | 0.75 ± 0.48bc | 0.50 ± 0.29b | 0.25 ± 0.25b | 0.50 ± 0.29c |
| Spintoram + NPV | 2.75 ± 0.25b | 2.00 ± 0.71ab | 1.75 ± 0.25b | 0.25 ± 0.25c | 0.00 ± 0.00c | 0.00 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00c |
| Emamectin benzoate + NPV | 5.50 ± 1.04a | 2.00 ± 0.41ab | 2.00 ± 0.41b | 1.00 ± 0.41bc | 0.50 ± 0.29bc | 0.50 ± 0.29b | 0.50 ± 0.29b | 0.00 ± 0.00c |
Values within columns followed by the same letter do not differ significantly at the P > 0.05.
Similarly, the number of larvae in all the treatments (except control) were significantly lower viz. 0.25–2.00 after 4 days exposure which were significantly higher than the control containing 6.50 larvae (F = 25.52, df = 5, P < 0.01). After 5 days of exposure, no population was observed in Spintoram + NPV, while higher population was observed in control viz. 8.50 larvae/25 plants (F = 51.31, df = 5, P < 0.01). After 6 days of treatment, larval population in control increased to 9.75 larvae/25 plants while larval population was significantly lower in all other treatments varying from 0.00 to 0.75 larvae/25 plants (F = 133.62, df = 5, P < 0.01). After 7 days post-treatment, larval population in control increased to 9.75 larvae/25 plants while larval population was significantly lower in all other treatments varying from 0.00 to 0.50 larvae/25 plants (F = 92.08, df = 5, P < 0.01). Finally, larval population reduced to 0.00 in spintoram, spintoram + NPV and emamectin benzoate + NPV while in control population increased to 12.25 larvae.25 plants after 8 days exposure (F = 359.91, df = 5, P < 0.01).
3.1. Number of larvae in control treatment
The live number of larvae in the control treatment were significantly increased with the passage of time (F = 19.33, df = 7, P < 0.001, Fig. 1).
Fig. 1.

Number of larvae recorded in the control treatment at different intervals. Bars with different letters show significant differences at p < 0.05.
3.2. Number of larvae in spintoram treatment
The live number of larvae of H. armigera in the spintoram treatment were significantly reduced after eight days exposure to spintoram (F = 25.65, df = 7, P < 0.001). There were no larvae found after 7 and 8 days exposure to spintoram (Fig. 2).
Fig. 2.

Number of larvae recorded in the spintoram treatment at different intervals. Bars with different letters show significant differences at p < 0.05.
3.3. Number of larvae in emamectin benzoate treatment
The live number of larvae of H. armigera after 6 and 7 days exposure to emamectin benzoate were significantly lower compared with the exposure to emamectin benzoate after 1st, 2nd, 3rd, 4th, 5th and 8th days (F = 4.96, df = 7, P = 0.002). However, there were no significant differences in larvae after first 5th and 8th day exposure to emamectin benzoate (Fig. 3).
Fig. 3.

Number of larvae recorded in the emamectin benzoate treatment at different intervals. Bars with different letters show significant differences at p < 0.05.
3.4. Number of larvae in NPV treatment
The live number of larvae of H. armigera in the NPV treatment were significantly decreased (F = 4.62, df = 7, P = 0.003) with the passage of time to exposure (Fig. 4).
Fig. 4.

Number of larvae recorded in the NPV treatment at different intervals. Bars with different letters show significant differences at p < 0.05.
3.5. Number of larvae in spintoram + NPV treatment
live number of larvae of H. armigera in the spintoram + NPV treatment were highly significantly decreased (F = 14.09, df = 7, P < 0.001) with the passage of time to exposure (Fig. 5). There were no larvae found 4 days after exposure to spintoram + NPV mixture.
Fig. 5.
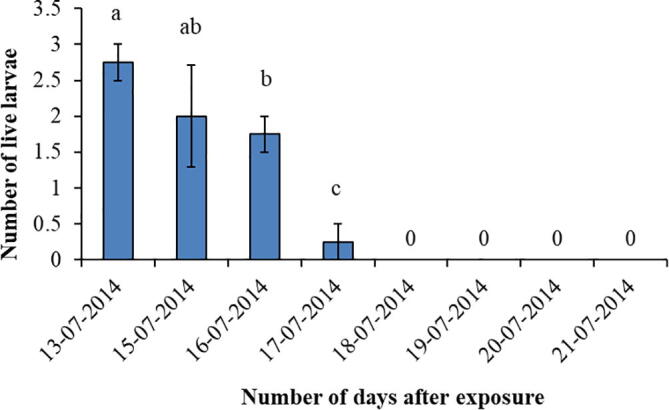
Number of larvae recorded in the spintoram + NPV treatment at different intervals. Bars with different letters show significant differences at p < 0.05.
3.6. Number of larvae in emamectin benzoate + NPV treatment
The live number of larvae of H. armigera in the emamectin benzoate + NPV treatment were highly significantly decreased (F = 13.89, df = 7, P < 0.001) with the passage of time to exposure (Fig. 6). There were no larvae found 8 days after exposure to emamectin benzoate + NPV mixture.
Fig. 6.
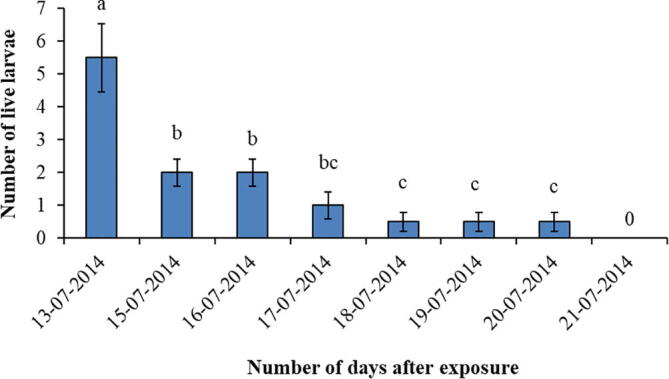
Number of larvae recorded in the emamectin benzoate + NPV treatment at different intervals. Bars with different letters show significant differences at p < 0.05.
4. Discussion
In Pakistan, farmers use different insecticides as a major tool for the management of various lepidopteran pests. However, extensive use of insecticides has created the resistance in lepidopterous insect pests (Kaur and Dilawari, 2011, Shad et al., 2012, Faheem et al., 2013, Abbas et al., 2014, Jan et al., 2015, Qayyum et al., 2015 Feb). Authors have reported resistance in H. armigera to a wide range of insecticides and might be the main reason for sporadic out breaks of this pest (Alvi et al., 2012, Faheem et al., 2013, Qayyum et al., 2015 Feb). In the current study, field efficacy of NPV alone and its combination with two new chemistry insecticides (spintoram and emamectin benzoate) was evaluated. The results indicated that the combination of NPV + spintoram caused 100% reduction of H. armigera from cotton field followed by the combination of NPV + emamectin benzoate and NPV alone. However, larval population was increased in control plots.
The higher reduction of larval population in plots where NPV + spintoram was sprayed due to additive effect of spintoram when mixed with NPV. Spintoram insecticide is extracted from Saccharopolyspora spinosa, a soil bacterium is an effective bio-pesticide with very low mammalian and beneficial insect toxicity. The spintoram has a dual mode. of action, the nicotinic acetylcholine receptor, and GABA receptor allosteric modulators. Spintoram has been used effectively for the .management of different insect pests especially lepidopterans (Abbas et al., 2015, Jan et al., 2015; Ullah et al. 2016). However, emamectin benzoate has been derived from Streptomyces avermitilis and is a glutamate-gated. chloride .channel allosteric modulator (Sparks et al., 2012, Sparks and Nauen, 2015). In the current study, spintoram, as well as emamectin benzoate significantly lower the number of ABW larvae under field trials to 100% after one week of application.
In the current study, NPV significantly reduced the number of H. armigera larvae under field applications, when applied alone. Previously, NPV as an effective microbial insecticide has been reported in H. armigera (Gupta et al., 2007, Marzban et al., 2009, Pugalenthi et al., 2013), Spodoptera littoralis (Sutanto et al. 2014) and Spodoptera litura (Maqsood et al. 2017). Several hurdles have been faced due to low persistent, slow action and needed repeated applications when the microbial agents are used individually in integrated pest management. Combined application of microbes with bio-pesticides may help to control H. armigera, when these mixtures may enhance their virulence than expected in a single application. In the present study, the mixtures of NPV and spintoram/emamectin benzoate significantly reduced the number of H. armigera larvae under field conditions. These results are in agreement with the results of previous studies where a combination of NPV and flubendiamide insecticide significantly reduced the lepidopteran larvae (Shaurub et al., 2014, Maqsood et al., 2017). Similar results were obtained by Nawaz et al. (2019) who reported NPV as effective tool for the management of H. armigera under laboratory conditions.
5. Conclusion
In conclusion, NPV, spintoram and emamectin benzoate are bio-rational insecticides and have the potential to be effectively used in integrated pest management strategies as a key component to minimize the insecticides resistance developed in this pest. The present results suggest that NPV with a combination of insecticides should be used for suppression of the ABW larvae under field conditions.
Declaration of Competing Interest
The authors declare no potential conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas N., Ijaz M., Shad S.A., Khan H. Stability of field-selected resistance to conventional and newer chemistry insecticides in the house fly, Musca domestica L. (Diptera: Muscidae) Neotropical Entomol. 2015;44:402–409. doi: 10.1007/s13744-015-0290-9. [DOI] [PubMed] [Google Scholar]
- Abbas N., Khan H.A.A., Shad S.A. Cross-resistance, genetics, and realized heritability of resistance to fipronil in the house fly, Musca domestica (Diptera: Muscidae): a potential vector for disease transmission. Parasitol. Res. 2014;113:1343–1352. doi: 10.1007/s00436-014-3773-4. [DOI] [PubMed] [Google Scholar]
- Abid A.D., Saeed S., Zaka S.M., Ali M., Shahzad M.S., Khan K.A., Iqbal N. Manifold passages in an assorted infection in a host could improve virulence of Helicoverpa armigera Nucleopolyhedrovirus (HaNPV) Saudi J. Biol. Sci. 2020;27(6):1419–1422. doi: 10.1016/j.sjbs.2020.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M., Arif I.M., Ahmad Z. Monitoring insecticide resistance of Helicoverpa armigera (Lepidoptera: Noctuidae) in Pakistan. J. Econ. Entomol. 1995;88(4):771–776. [Google Scholar]
- Ahmad M., Arif M.I., Ahmad Z., Denholm I. Cotton Whitefly (Bemisia tabaci) resistance to organophosphate and pyrethroid insecticides in Pakistan. Pest Manag. Sci. 2002;58:203–208. doi: 10.1002/ps.440. [DOI] [PubMed] [Google Scholar]
- Alvi A.K.H., Sayyed H.A., Naeem M., Ali M. Field evolved resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) to Bacillus thuringiensis toxin Cry1Ac in Pakistan. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0047309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Analytical software, 2005. Statistix 8.1 for Windows. Analytical Software, Tallahassee,
- Bhargava, M. C., Choudhary, R. K., Jain, P. C., 2008. Genetic Engineering of plants for insect resistance. In: Entomology: Novel Approaches (Jain, P.C. and Bhargava, M. C. eds.). New India Publishing, New Delhi, India. 133-144 pp.
- Bielza P. Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis. Pest Manag. Sci. 2008;64:1131–1138. doi: 10.1002/ps.1620. [DOI] [PubMed] [Google Scholar]
- Caballero P., Aldebis H.K., Varga Osuna E., SantiagoAlvarez C. Epizootics caused by a nuclear polyhedrosis virus in populations of Spodoptera litura in southern Spain. Biol. Sci. Technol. 1992;2:35–38. [Google Scholar]
- Charati S.N., Pawar V.M., Sakhare M.V. Potentiation of Helicoverpa armigera nucleopoly hedrosis virus with different insecticides. J. Maharashtra Agric. Univ. 1999;24(1):119–120. [Google Scholar]
- Cory, J.S., Hails, R., 1997. The ecology and biosafety of baculoviruses. Curr. Opinion Biotechnol. 8, 323–327. [DOI] [PubMed]
- Denholm I., Cahill M., Dennehy T.J., Horowitz A.R. Challenges with managing insecticide resistance in agricultural pests, exemplisfied by the whitefly Bemisia tabaci. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998;353(1376):1757–1767. [Google Scholar]
- Faheem U., Nazir T., Saleem M., Yasin M., Bakhsh M. Status of insecticide resistance in Helicoverpa armigera (Hübner) in Southern Punjab, Pakistan. Sarhad J. Agric. 2013;29:563–572. [Google Scholar]
- Gupta R.K., Raina J.C., Monobrullah M.D. Optimization of in vivo production of nucleopolyhedro virus in homologous host larvae of Helicoverpa armigera. J. Entomol. 2007;4:279–288. [Google Scholar]
- Jan M.T., Abbas N., Shad S.A., Rafiq M., Saleem M.A. Baseline susceptibility and resistance stability of Earias vittella Fabricius (Lepidoptera: Noctuidae) to cypermethrin, deltamethrin and spinosad. Phytoparasitica. 2015;43:577–582. [Google Scholar]
- Kaur P., Dilawari V. Inheritance of resistance to Bacillus thuringiensisCry1Ac toxin in Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) from India. Pest Manag. Sci. 2011;67:1294–1302. doi: 10.1002/ps.218. [DOI] [PubMed] [Google Scholar]
- Maqsood S., Afzal M., Aqueel M.A., Raza A.B.M., Wakil W., Babar M.H. Efficacy of nuclear polyhedrosis virus and flubendiamide alone and in combination against Spodoptera litura F. Pakistan. J. Zool. 2017;49:1783–1788. [Google Scholar]
- Marzban R., He Q., Liu X.X., Zhang Q.W. Effects of Bacillus thuringiensis toxin Cry1Ac and Cytoplasmic polyhedrosis virus of Helicoverpa armigera (Hübner) (HaCPV) on Cotton bollworm (Lepidoptera: Noctuidae) J. Invertebr. Pathol. 2009;101:71–76. doi: 10.1016/j.jip.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Nawaz, A., Ali, H., Sufyana, M., Gogi, M.D., Arif, M.J. Ranjha, M.H., Arshida, M., Waseema, M., Mustafa, T., Qasim, M., Rizwan M., Zaynab, M., Khan, K.A. Ghramh, H.A., 2019. Comparative bio-efficacy of nuclear polyhedrosis virus (NPV) and Spinosad against American bollwormm, Helicoverpa armigera (Hubner). Revista Brasileira de Entomologia 63, 277–282.
- Pugalenthi P., Dhanasekaran S., Elumali K., Krishnappa K. Bio-efficacy of NPV tested against American bollworm, Helicoverpa armigera (Hub.) (Lepidoptera: Noctuidae) and protection of cotton boll damage. Int. J. Renew. Environ. Sci. 2013;1(3):22–26. [Google Scholar]
- Qayyum M.A., Wakil W., Arif M.J., Sahi S.T., Saeed N.A., Russell D.A. Multiple resistances against formulated organophosphates, pyrethroids, and newer-chemistry insecticides in populations of Helicoverpa armigera (Lepidoptera: Noctuidae) from Pakistan. J. Econ. Entomol. 2015 Feb;108(1):286–293. doi: 10.1093/jee/tou037. [DOI] [PubMed] [Google Scholar]
- Qayyum M.A., Saleem M.A., Saeed S., Wakeel W. Integration of entomopathogenic fungi and eco-friendly insecticides for management of red palm weevil, Rhynchophorus ferrugineus (Olivier) Saudi J. Biol. Sci. 2020 doi: 10.1016/j.sjbs.2019.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shad S.A., Sayyed A.H., Fazal S., Saleem M.A., Zaka S.M., Ali M. Field evolved resistance to carbamates, organophosphates, pyrethroids, and new chemistry insecticides in Spodoptera litura Fab. (Lepidoptera: Noctuidae) J. Pest. Sci. 2012;85:153–162. doi: 10.1007/s10340-011-0404-z. [DOI] [Google Scholar]
- Shaurub E.H., Meguid A.A., Aziz N.M.A. Effect of individual and combined treatment with Azadirachtin and Spodoptera littoralis multicapsid nucleopolyhedro virus (SpliMNPV, Baculoviridae) on the Egyptian cotton leafworm Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) Ecol. Balkanica. 2014;6:93–100. [Google Scholar]
- Sparks T.C., Dripps J.E., Watson G.B., Paroonagian D. Resistance and cross-resistance to the spinosyns: a review and analysis. Pestic. Biochem. Physiol. 2012;102:1–10. [Google Scholar]
- Sparks T.C., Nauen R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015;121:122–128. doi: 10.1016/j.pestbp.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Sutanto K.D., Salamouny S.E., Dawood A.S. Affectivity of Spodoptera littoralis nucleopolyhedro virus (SpliNPV) against first and second instar larvae of the cotton leafworm, Spodoptera littoralis (Boisd.) Afr. J. Microbiol. Res. 2014;8:337–340. doi: 10.5897/AJMR2013.5352. [DOI] [Google Scholar]
- Tabashnik B.E., Gassmann A.J., Crowder D.W., Carrière Y. Insect resistance to Bt crops: evidence versus theory. Nat. Biotechnol. 2008;26(2):199. doi: 10.1038/nbt1382. [DOI] [PubMed] [Google Scholar]
- Wang C.Z., Qin J.D. Insect-plant co-evolution: multitrophic interactions concerning Helicoverpa species. Chinese Bull. Entomol. 2007;44(3):311–319. [Google Scholar]
- Yang Y., Li Y., Wu Y. Current status of insecticide resistance in Helicoverpa armigera after 15 years of Bt cotton planting in China. J. Econ. Entomol. 2013;106:375–381. doi: 10.1603/ec12286. [DOI] [PubMed] [Google Scholar]


