Abstract
Testicular torsion and detorsion (TTD) is a serious urological condition affecting young males that is underlined by an ischemia reperfusion injury (tIRI) to the testis as the pathophysiological mechanism. During tIRI, uncontrolled production of oxygen reactive species (ROS) causes DNA damage leading to germ cell apoptosis (GCA). The aim of the study is to explore whether inhibition of NADPH oxidase (NOX), a major source of intracellular ROS, will prevent tIRI-induced GCA and its association with endoplasmic reticulum (ER) stress. Sprague-Dawley rats (n = 36) were divided into three groups: sham, tIRI only and tIRI treated with apocynin (a NOX inhibitor). Rats undergoing tIRI endured an ischemic injury for 1 h followed by 4 h of reperfusion. Spermatogenic damage was evaluated histologically, while cellular damages were assessed using real time PCR, immunofluorescence staining, Western blot and biochemical assays. Disrupted spermatogenesis was associated with increased lipid and protein peroxidation and decreased antioxidant activity of the enzyme superoxide dismutase (SOD) as a result of tIRI. In addition, increased DNA double strand breaks and formation of 8-OHdG adducts associated with increased phosphorylation of the DNA damage response (DDR) protein H2AX. The ASK1/JNK apoptosis signaling pathway was also activated in response to tIRI. Finally, increased immuno-expression of the unfolded protein response (UPR) downstream targets: GRP78, eIF2-α1, CHOP and caspase 12 supported the presence of ER stress. Inhibition of NOX by apocynin protected against tIRI-induced GCA and ER stress. In conclusion, NOX inhibition minimized tIRI-induced intracellular oxidative damages leading to GCA and ER stress.
Keywords: Testicular ischemia Reperfusion Injury, NADPH oxidase, Oxidative stress, Germ cell apoptosis, ER stress
Abbreviations: ANOVA, analysis of variance; ASK1, apoptosis signaling kinase 1; ATF, activating transcription factor; ATM, ataxia telangiectasia mutated; BSA, bovine serum albumin; BTB, blood-testis barrier; cDNA, complementary DNA; Chk, checkpoint kinase; CHOP, CCAAT-enhancer-binding protein homologous protein; DAPI, diamidino phenylindole; DDR, DNA damage response; DMSO, dimethyl sulfoxide; DNA, deoxyribonucleic acid; ECL, electrochemiluminescence; eIF2α1, eukaryotic initiation factor 2α1; ELISA, enzyme-linked immunosorbent assay; ER, endoplasmic reticulum; GCA, germ cell apoptosis; gDNA, genomic DNA; GRP78, glucose-related protein 78; H&E, hematoxylin and eosin; H2AX, histone variant; H2O2, hydrogen peroxide; i.p., intraperitoneal; IAP, inhibitors of apoptosis; IF, immunofluorescence; IRE1, inositol requiring kinase 1; JNK, c-Jun N-terminal Kinase; kDa, kilodalton; MDA, malondialdehyde; mRNA, messenger ribonucleic acid; NADP, nicotinamide adenine dinucleotide phosphate; NOX, NADPH oxidase; O2, molecular oxygen; O2−, superoxide anion; OS, oxidative stress; p-, phosphorylated; PARP, poly ADP-ribose polymerase; PCC, protein carbonyl content; PCR, polymerase chain reaction; PERK, pancreatic ER kinase; phox, phagocyte oxidase; PVDF, polyvinylidene difluoride; RIPA, radioimmunoprecipitation assay; RNA, ribonucleic acid; ROS, reactive oxygen species; RT, reverse transcription; SD, standard deviation; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; SOD, superoxide dismutase; ST, seminiferous tubule; TOS, testicular oxidative stress; TRAF-2, tumor-necrosis-factor receptor-associated factor 2; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; UPR, unfolded protein response; γ-H2AX, 139 serine-phosphorylated histone variant; 8-OHdG, 8-hydroxy-2′-deoxyguanosine
1. Introduction
Testicular ischemia reperfusion injury (tIRI) is the suggested underlying mechanism for testicular torsion and detorsion (TTD), which is a urological emergency that commonly affects young males with a possibility of future infertility issues (Kessler and Bauml, 2009). It is well documented that TTD/tIRI results in oxidative DNA damage, seminiferous tubule (ST) atrophy and germ cell apoptosis (GCA) (Asadi et al., 2017). Deciphering the cellular pathways following TTD/tIRI is critical in understanding male infertility issues since the abnormal spermatozoa of infertile men are commonly linked to oxidative stress (Baker and Aitken, 2005). If the intracellular antioxidant system is impaired and/or the production of reactive species (ROS) exceeds the cellular defense capacity, this will create oxidative stress (OS) (Masella et al., 2005).
NADPH oxidase (NOX) is one of the main endogenous sources of ROS. It is composed of two membrane-bound subunits (gp91phox and p22phox), three cytosolic subunits (p67phox, p47phox and p40phox) and a guanine nucleotide-binding (G) protein (Babior, 2004). Subunit assembly of the enzyme is required to catalyze the electron transfer from NADPH to oxygen and releasing the superoxide anion according to the following reaction: NADPH + 2O2 → NADP+ + H+ + 2O2−. The physiological importance of NOX was emphasized by its association with chronic diseases or diseases associated with excessive NOX-generated ROS (Lambeth, 2007). In the testes, NOX5 has been identified in spermatocytes, which play an essential role in germ cell proliferation and fertilization (Banfi et al., 2001). There is also growing evidence showing enhanced ROS generation by NOX during endoplasmic reticulum (ER) stress (Haynes et al., 2004). Physiologically, the ER produces ROS during cell metabolism, however, it increases during ER stress due to elevation in improper protein folding (Santos et al., 2009, Li et al., 2010). It was found that some ER chaperones have functional interactions with NOX in distinct cell types (Laurindo et al., 2012). Furthermore, post-translational modifications of some NOX subunits are carried out by glucose-incorporating enzymes within the ER membrane (DeLeo et al., 2000). Patients born with a congenital deficiency of such enzymes suffer from neutrophil dysfunction in association with compromised NOX activity (Hayee et al., 2011). It has also been speculated that although some short and immature NOX isoforms maintain their ability to produce ROS, however, they lack the domains responsible for traffic and membrane anchoring, which render them retained in the ER (Laurindo et al., 2014). These reports are indicative of the possible role of ER in cellular redox physiology and pathophysiology.
Excessive accumulation of unfolded or misfolded proteins in the ER disturbs protein homeostasis generating ER stress, which provokes its quality control system namely the unfolded protein response (UPR) pathway (Guzel et al., 2017). In normal conditions, three ER transmembrane transducers are found bound to the chaperone glucose-regulated protein 78 (GRP78), a chaperone protein that regulates the folding and trafficking of proteins and is upregulated during ER stress. The three transducers are inositol requiring kinase 1 (IRE1), pancreatic ER kinase (PERK) and activating transcription factor 6 (ATF6) that are maintained inactive by their attachment to GRP78. Under ER stress, GRP78 overexpression prompts the release of these transducers resulting in their activation and initiating the UPR pathway (Amodio et al., 2018).
ER stress has also been associated with triggering the apoptosis cascades. ER-mediated apoptosis is mediated by the activation of: (1) PERK/ATF6-induced expression of the transcription factor C/EBP homologous protein (CHOP), (2) CHOP-activated ER-associated caspase 12, and (3) the IRE1-induced ASK1/JNK apoptosis pathway (Garcia de la Cadena and Massieu, 2016, Choy et al., 2018). During ER stress, procaspase 12 becomes activated by homodimerization and elicits a cascade of reactions leading to the activation of caspases 9 and 3 to eventually achieve a cytochrome c-independent apoptosis (Morishima et al., 2002, Morishima et al., 2002, Garcia de la Cadena and Massieu, 2016).
Thus, we hypothesize that NOX-derived ROS plays a role in tIRI-induced oxidative DNA damage and GCA in association with activation of the UPR pathway as a result of ER stress. The objective of this study is to examine the role of NOX in ROS generation during tIRI using its selective inhibitor, apopcynin, and prior to reperfusion of the ischemic testis and establish a direct link between ER stress and NOX activity.
2. Materials & methods
2.1. Animals and ethics
Eight weeks old male Sprague Dawley (SD) rats (300–380 g) (Charles River, Waltham, MA, USA) were equally divided into three groups: sham (n = 12), unilateral tIRI (n = 12), and tIRI-treated with apocynin (n = 12). Rats were kept at a 12 h’ light and dark cycle with food and water provided at all times. The animals were housed and cared for by the animal house at the Health Science Center, Kuwait University. The surgical protocol was approved by the ethics committee on animal research at Kuwait University.
2.2. Surgery
Prior to surgery, the anesthetic was prepared by mixing ketamine (50 mg/kg; Tekam, Hikma Pharmaceuticals, Jordan) and xylazine (2 mg/kg; Rompun, Bayer GMP, Germany), which was administered intraperitoneally (i.p.). When sedated, the inguinal region was shaved and sterilized with Betadine and 70% ethanol. The incision was made at the left ipsilateral side while the right contralateral testis side remained intact serving as a positive internal control (Al-Maghrebi & Renno, 2016). The first group underwent a sham operation whereby the left testis was exposed for 1 h before placing it back into the scrotal sac, the incision was closed with surgical clips and rats were allowed to recover for 4 h. The other two groups endured an ischemic injury by clamping the testicular artery using a straight bulldog clamp (700 g pressure; Roboz Surgical Instrument Co., Gaithersburg, MD, USA) to block blood flow to the left ipsilateral testis for 1 h. After 30 min from inducing ischemia, the tIRI only-subjected rats were i.p. injected with 250 µl of the vehicle dimethyl sulfoxide (DMSO). The third group was i.p. injected with apocynin (50 mg/kg dissolved in DMSO; Selleckchem, Houston, TX, USA). The clamp was removed to allow for blood re-flow. The reperfusion period lasted for 4 h after which all animals were sacrificed by decapitation. Both testes were harvested from each rat, one half from each testis was stored at −80 °C for molecular and biochemical assays, while the other half was fixed in Bouin’s solution (50% saturated picric acid, 35% distilled water, 10% formalin and 5% acetic acid) for histological analysis.
The dosage and i.p. administration of apocynin (50 mg/kg) were selected based on earlier studies using the same drug to investigate cerebral IRI (Zhang et al 2015), striatum neurotoxicity (Dang et al 2016) and spinal cord injury (Sun et al 2017). The timing of apocycin injection during tIRI was decided based on the acute nature of the injury and its relation to the clinical settings of TTD.
2.3. Histological analysis
Fixed testes were processed prior to embedding in paraffin blocks. Tissue sections (4 µm) were stained with hematoxylin and eosin (H&E) and were examined using the Zeiss LSM 700 light microscope (Carl Zeiss Microscopy Ltd. Jena, Germany) at different magnifications (10×, 20× and 40×). For spermatogenesis evaluation, images captured at 40× magnification were analyzed by the Johnsen scoring system (Johnsen, 1970). Thirty STs per testis per group were evaluated in a blinded manner.
Immunofluorescence (IF) staining of tissue sections (4 µm) was carried out as previously described (Al-Kandari et al, 2019) using the following primary antibodies: survivin and p-JNK (Santa Cruz Biotechnology, Dallas, TX, USA), p-ASK1 (Bioss Antibodies, Woburn, MA, USA), eIF2α1 (phospho S51), CHOP, GRP78 and caspase 12 (Abcam, Cambridge, UK) and γ-H2AX (Cell Signaling, Danvers, MA, USA). Slides were visualized with the Zeiss LSM 700 confocal microscope (Carl Zeiss Microscopy Ltd. Jena, Germany) and the fluorescence intensity of 30 STs/testis/group was measured using the ZEN black and ZEN blue software. A double sequential IF staining was performed for the phosphorylated forms of ASK1 and JNK using their respective antibodies and the above-mentioned dilutions.
The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed using the in situ cell death detection kit (Roche, Basel, Switzerland) following the manufacturer’s protocol. Stained slides were examined under the Zeiss LSM 700 confocal microscope (Carl Zeiss Microscopy Ltd. Jena, Germany) and images were processed with the ZEN Black and ZEN Blue software (Carl Zeiss Microscopy Ltd. Jena, Germany). The fluorescent nuclei from 30 STs per testis per group were counted.
2.4. Biochemical assays
Total protein extracts were prepared from frozen testes using the radio-immunoprecipitation assay (RIPA) lysis buffer (Santa Cruz, Dallas, TX, USA) in the presence of protease inhibitors.
All assays were performed following the manufacturers’ protocols. A standardized protein concentration was individually determined for use in each of the following biochemical assays.
The activity of the enzyme superoxide dismutase (SOD) was determined indirectly using the SOD Determination Kit (Sigma-Aldrich, St. Louis, MO, USA). Malondialdehyde (MDA) was assayed using the BIOXYTECH® LPO-586™ kit (Oxis Research, Portland, OR, USA). The level of cellular protein carbonylation was measured using the protein carbonyl content (PCC) Assay kit (BioVision, Milpitas, CA, USA). The NADP/NADPH Quantification Kit (Sigma-Aldrich, St. Louis, MO, USA) was used as an indirect measure for NOX activity. The enzyme activity of poly ADP-ribose polymerase (PARP) was determined using the PARP universal colorimetric assay kit (R&D Systems, Minneapolis, MN, USA). The caspase-9 colorimetric assay kit (BioVision, Milpitas, CA, USA) and the caspase 3 assay kit (Sigma-Aldrich, St. Louis, MO, USA) were used to measure the enzyme activity of caspases 9 and 3, respectively. Survivin protein concentration in testicular tissue was quantified using the competitive rat survivin enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource, San Diego, CA, USA).
2.5. Molecular assays
Genomic DNA (gDNA) was extracted from frozen testicular tissue by using the DNeasy blood and tissue kit (Qiagen, Venlo, Netherlands). The concentration of 8-hydroxy-2′-deoxyguanosineis (8-OHdG) was quantified using the EpiQuik™ 8-OHdG DNA damage quantification direct kit (Epigentek, Farmingdale, NY, USA). The absolute concentration of 8-OHdG was calculated using a standard curve made of purified 8-OHdG samples provided in the kit.
RNA isolation, reverse transcription and realtime PCR was performed as previously described (Al-Maghrebi & Renno, 2016). Standardized gene-specific Taqman assays for Birc5 (survivin; Rn00574012_m1), and β-actin (Rn00667869_m1) were purchased (Applied Biosystems, Foster City, California, USA). Reactions were carried out using the ABI Prism Sequence Detection System (SDS) 7500 (Applied Biosystems, Foster City, CA, USA) using the following real-time PCR conditions: 50 °C for 2 min, 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Relative mRNA expression was quantified using the 2−ΔΔCt method (Livak and Schmittgen, 2001) by setting the gene expression in sham samples to 1.00 (calibrator) and calculating the fold change in expression in tIRI and apocynin-treated groups.
2.6. Western blot
The primary antibodies for GRP78, eIF2α1 (phospho S51), DDIT3 (CHOP) and caspase-12 were purchased from Abcam, Cambridge, UK. In brief, 50 μg from each protein sample were resolved by a 10% SDS-PAGE, transferred to a PVDF membrane and incubated overnight with the above antibodies individually at 4 °C. Secondary antibodies and an ECL kit (Amersham, GE Healthcare, Chicago, IL, USA) were used to visualize the specified proteins. Band intensities were measured with the ChemiDoc™ imaging system, which were quantitated by the image lab software (Bio-Rad Laboratories, Hercules, CA, USA).
2.7. Statistical analysis
Results were statistically analyzed using the GraphPad Prism software (GraphPad Software, San Diego, CA, USA). One-way analysis of variance (ANOVA) followed by the Holm-Sidak multiple comparisons test were used for the analysis of raw data and for in between group comparisons. Data are presented as the mean ± standard deviation (SD) and were considered significant if p was <0.05.
3. Results
3.1. Effect of apocynin on spermatogenesis
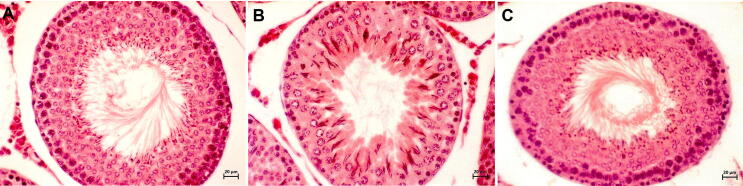
The tIRI-subjected testes had STs with disrupted morphology, few number of spermatids with a low Johnsen score of 5.67 ± 0.72 as compared to sham (9.93 ± 0.25 *p < 0.0001) and apocynin-treated rats (9.67 ± 0.48 #p < 0.0001) indicating an arrest of spermatogenesis (Fig. 1). Normal histological features and spermatogenesis was observed for the STs of ipsilateral testes of sham and apocynin-treated rats as well as contralateral testes from all experimental groups (p > 0.05).
Fig. 1.
Effect of apocynin on spermatogenesis. Rat ipsilateral testicular tissue sections stained with H&E demonstrating normal histological features of the STs and normal spermatogenesis in both sham (A) and apocynin-treated (C). The sections from the tIRI group (B) displayed disrupted germ layers indicating damage to spermatogenesis. Testicular sections were photographed at 10× (A, C and E) and 40× (B, D and F) magnifications.
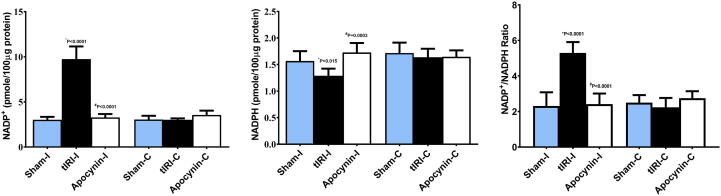
3.2. Apocynin inhibits NOX activity
The activity of NOX was determined indirectly by measuring the levels of NADP, NADPH and their ratio. A significant elevation in NADP levels was measured (pmole/100 μg protein) in ipsilateral tIRI (9.7 ± 1.4) compared to sham (3.0 ± 0.32, *p < 0.0001) and apocynin-treated (3.3 ± 0.37, #p < 0.0001) (Fig. 2). Inversely, NADPH concentration (pmole/100 μg protein) was significantly decreased in tIRI-subjected ipsilateral testes (1.3 ± 0.14) in contrast to sham (1.6 ± 0.19, *p = 0.015) and apocynin-treated groups (1.7 ± 0.2, #p = 0.0003). The calculated NADP/NADPH ratio showed a significant increase in ipsilateral tIRI-subjected testes (5.3 ± 0.61) compared to sham (2.3 ± 0.7, *p < 0.0001) and apocynin-treated rats (2.4 ± 0.6, #p < 0.0001) (Fig. 2).
Fig. 2.
Apocynin activates NOX during tIRI. Bar Graphs representing the concentrations of (A) NADP+, (B) NADPH and (C) their ratio. While NADP displayed a significant increase in NADP+ concentration during tIRI, NADPH levels were reduced as compared to sham and apocynin-treated groups. This resulted in a significant increase in the NADP+/NADPH ratio during tIRI indirectly suggesting NOX activation during tIRI. No significant difference was obtained between contralateral testes (P < 0.05). Data are presented as mean ± SD (n = 6/group). *tIRI compared to sham and #apocynin compared to tIRI. I = Ipsilateral and C = Contralateral.
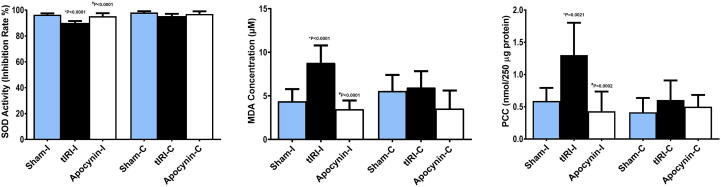
3.3. Apocynin prevents testicular oxidative stress
The accumulation of ROS because of tIRI had its prominent effect on significantly decreasing SOD activity (90.1 ± 1.45) in contrast to sham (96.4 ± 1.07, *p < 0.0001) and apocynin-treated rats (95.2 ± 2.38, #p < 0.0001) (Fig. 3). Moreover, a significant increase in MDA concentration was measured in ipsilateral tIRI-subjected testes (8.8 ± 2.0) in contrast to sham (4.4 ± 1.4, *p < 0.0001) and apocynin-treated (3.5 ± 1.0, #p < 0.0001) (Fig. 3). Similarly, PCC was remarkably high in the tIRI ipsilateral testes (1.3 ± 0.5) in comparison to sham (0.59 ± 0.20, *p = 0.0021) and apocynin-treated groups (0.43 ± 0.31, #p = 0.0002) (Fig. 3). There was no significant difference in the levels of SOD, MDA and PCC in contralateral testes (p > 0.05).
Fig. 3.
Apocynin prevents testicular oxidative stress. Ipsilateral tIRI-subjected rats had significantly (A) low SOD activity (%), (B) increased MDA concentration (μM) and (C) heightened PCC levels (nmole) as compared to sham and apocynin-treated groups, which showed baseline levels of SOD, MDA and PCC. No significant difference was measured among contralateral testes (p > 0.05). Data are presented as mean ± SD (n = 6/group). *tIRI compared to sham and #apocynin-treated compared to tIRI. I = Ipsilateral and C = Contralateral.
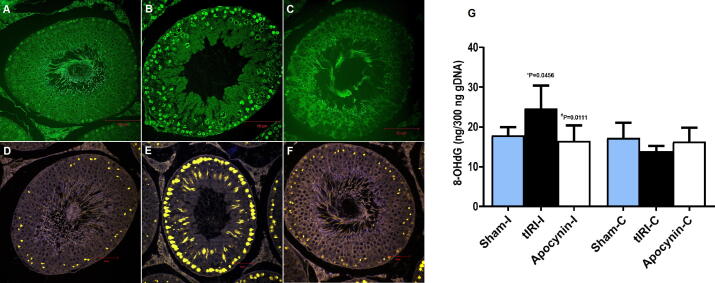
3.4. DNA damage and repair is diminished by apocynin
Single and double DNA strand breaks were detected by the TUNEL assay (Fig. 4). The fluorescently labeled free 3′ DNA ends were visualized using confocal microscopy. The ipsilateral testes of tIRI rats displayed significant DNA strand breaks (51 ± 10) in comparison to sham (0.0 ± 0.0, *p < 0.0001) and apocynin-treated rats (0.9 ± 1.3, #p < 0.0001). Accumulation of 8-OHdG, a DNA adduct, was found to be higher in the ipsilateral testes of tIRI (25 ± 5.7) compared to sham (18 ± 2.1 *p = 0.0456) and apocynin-treated rats (17 ± 3.8, #p = 0.0111) (Fig. 4). No significant difference was observed between contralateral testes for DNA strand breaks (p > 0.05). PARP activity, a sensor of DNA damage and repair, was increased in tIRI-subjected testes (0.13 ± 0.02) in contrast to sham and apocynin-treated (0.08 ± 0.02 *p = 0.0003, 0.09 ± 0.01 #p = 0.0019, respectively) (Fig. 4). Expression of the DNA damage response protein, γ-H2AX, was detected by IF staining (Fig. 4). A significant increase in γ-H2AX immuno-expression was mainly detected in the spermatogonia of tIRI ipsilateral testes with an IF intensity of 1216 ± 112 compared to sham (728 ± 56, *p < 0.0001) and apocynin-treated rats (793 ± 67, #p < 0.0001). No significant difference was observed among contralateral testes for both PARP activity and γ-H2AX (p > 0.05).
Fig. 4.
DNA damage and repair is attenuated by apocynin. (A-C) Rat ipsilateral testicular tissue sections labeled by TUNEL showing increased DNA damage in tIRI testes in comparison to sham and apocynin-treated groups at 40x magnifications. (D-F) Ipsilateral testicular tissue sections subjected to IF staining for γ-H2AX (yellow) showing increased immune-expression in the spermatogonia of tIRI compared to sham and apocynin-treated groups at 40x magnification. (G) Bar Graph representing 8-OHdG concentration (ng) showing that ipsilateral testes of tIRI had significantly higher concentration of 8-OHdG in comparison to sham and apocynin-treated groups. No significant difference was obtained among contralateral testes (p-value > 0.05). Data are presented as mean ± SD (n = 6/group). *tIRI compared to sham and #apocynin-treated compared to tIRI. I = Ipsilateral and C = Contralateral.
3.5. Apocynin attenuates germ cell apoptosis
The activities of caspases 9 and 3 were increased by 66% and 53% in ipsilateral tIRI rats, respectively as compared to sham (p < 0.0001) and apocynin-treated (p < 0.0001) (Table 1). There was no notable activation of caspases 9 and 3 in contralateral testes of the three experimental groups (p > 0.05).
Table 1.
Expression of apoptosis and DNA damage markers.
| Sham - I | tIRI - I | tIRI + Apocynin - I1 | Sham - C | tIRI - C | tIRI + Apocynin - C1 | |
|---|---|---|---|---|---|---|
| Caspase 9 (A405) | 0.03 ± 0.00 | 0.05 ± 0.01* | 0.03 ± 0.006# | 0.03 ± 0.01 | 0.03 ± 0.004 | 0.03 ± 0.01 |
| Caspase 3 (mmol pNA/min/ml) | 15 ± 4.5 | 33 ± 7.1* | 16 ± 5.4# | 16 ± 6.4 | 16 ± 3.8 | 13 ± 2.4 |
| Survivin (pg/ml) | 968 ± 31 | 591 ± 91* | 1079 ± 133# | 898 ± 116 | 1128 ± 121 | 1035 ± 249 |
| Birc5 (Normalized fold expression) | 1.0 ± 0.0 | 0.39 ± 0.07* | 0.90 ± 0.17# | 1.0 ± 0.0 | 1.1 ± 0.09 | 0.90 ± 0.17 |
| p-ASK1 (IF intensity) | 225 ± 15 | 1,028 ± 123 | 319 ± 62 | 357 ± 39 | 393 ± 49 | 392 ± 44 |
| p-JNK (IF intensity) | 280 ± 29 | 861 ± 125 | 198 ± 42 | 350 ± 26 | 301 ± 52 | 236 ± 24 |
| γ-H2AX (IF intensity) | 728 ± 56 | 1216 ± 112 | 793 ± 67 | 745 ± 85 | 784 ± 120 | 814 ± 97 |
Data are presented as mean ± SD (n = 6).
Data analysis was determined by the one-way analysis of variance (ANOVA) accompanied by the Holms-Sidak multiple comparisons test.
Rats received an i.p. injection of apocynin (50 mg/kg) 30 min prior to reperfusion.
tIRI compared to sham.
tIRI + Apocynin compared to tIRI.
The protein expression for survivin, an inhibitor of apoptosis, was visualized and quantified by real-time PCR and ELISA (pg/ml) (Table 1). Survivin protein levels were notably higher by 64% and 82% in ipsilateral sham and apocynin-treated groups, respectively, in contrast to tIRI (*p = 0.0038 and #p = 0.0002, respectively). The tIRI-suppressed survivin protein expression was due to a transcriptional down regulation of its encoding gene Birc5 (Table 1). The relative expression of Birc5 mRNA was down-regulated by a fold change of 1.5 and 1.3 in comparison to sham and apocynin-treated groups (p < 0.0001). No significant difference was obtained for the survivin mRNA and protein expression in the contralateral testes from the three experimental groups (p > 0.05).
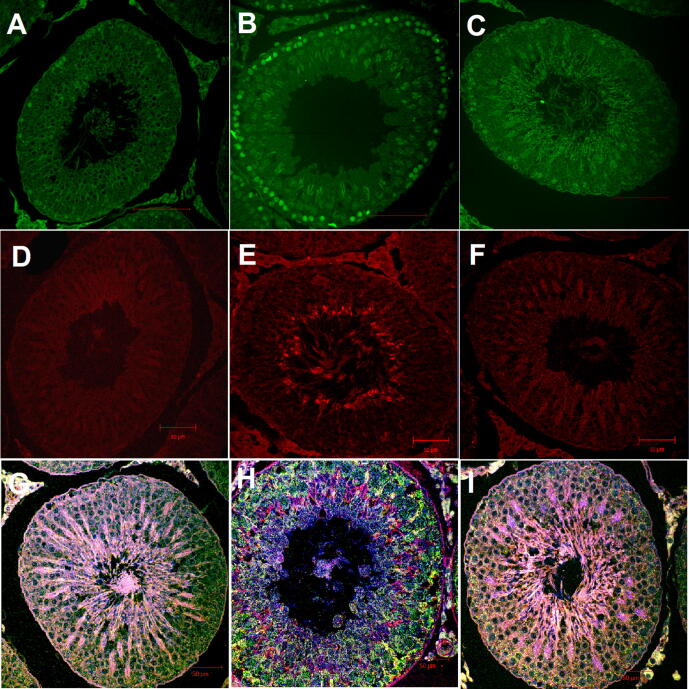
The activation of ASK1 and JNK was confirmed by an increase in the phosphorylation of ASK1 within spermatogonia and spermatocytes, while JNK phosphorylation was mostly localized to spermatocytes only (Fig. 5 and Table 1). Ipsilateral tIRI-subjected testes were found to have significantly higher p-ASK1 IF intensity when compared to sham and apocynin-treated groups (81%, *p < 0.0001 and 73%, #p < 0.0001, respectively). Similarly, p-JNK displayed high fluorescence intensity among ipsilateral tIRI-induced testes in contrast to sham (67%, *p < 0.0001) and apocynin-treated (77%, #p < 0.0001). A double immunofluorescence staining for simultaneous detection of ASK1 and p-JNK was performed displaying an overlapping expression (yellow) in the ipsilateral tIRI testicular sections (Fig. 5). In contralateral testes, phosphorylation of both kinases was similar to sham levels with no significant difference (p-value > 0.05).
Fig. 5.
Apocynin inhibits activation of the ASK1/JNK signaling pathway. Ipsilateral testicular tissue sections subjected to IF staining against the phosphorylated forms of (A-C) ASK1 (green) and (D-F) JNK (red) showing an increased immune-expression in germ cells and spermatocytes of tIRI, respectively, compared to sham and apocynin-treated groups. (G-I) Sequential double IF staining of ipsilateral testis combining p-ASK1, p-JNK and DAPI where the overlap is displayed as yellow color. Images were captured at 40x magnification.
3.6. Apocynin modulates the expression of ER stress markers
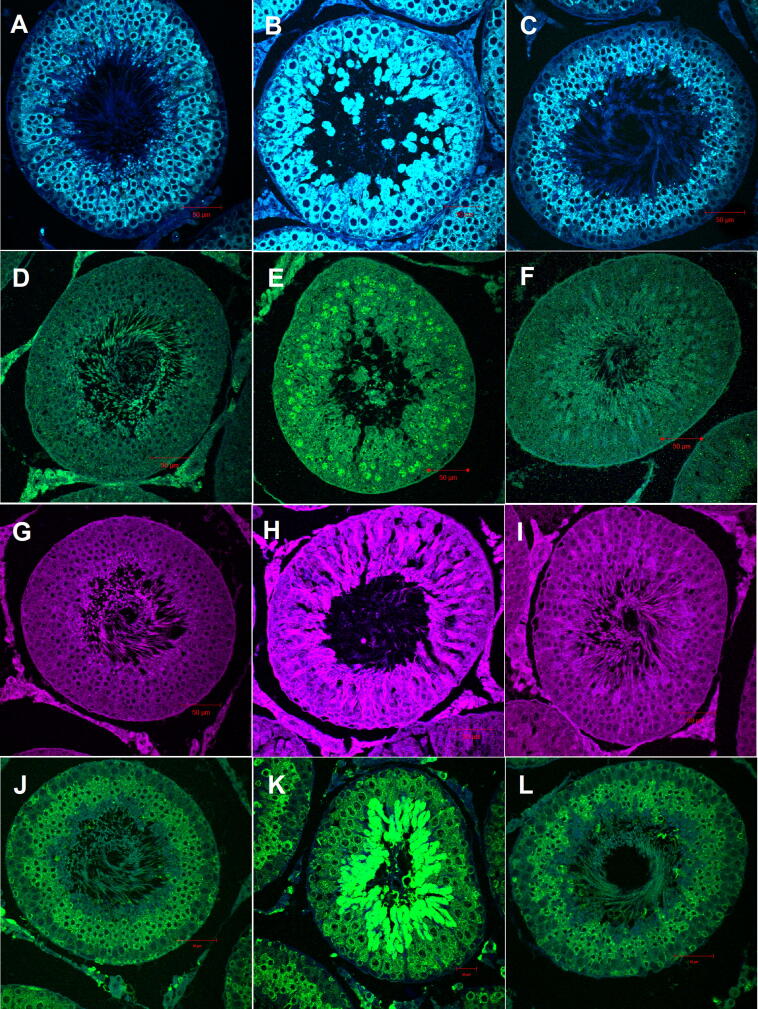
The protein expression of the key proteins within the PERK mediator of UPR pathway: GRP78, eIF2α1 (phospho S51), caspase 12 and CHOP, was determined by IF staining and WB analysis (Fig. 6 and Table 1). All four proteins were significantly expressed during tIRI in comparison to sham and apocynin-treated groups (p < 0.0001) with different cellular localization within the STs. The GRP78 protein expression was found to be remarkably increased in spermatocytes and spermatids of tIRI-subjected rats in contrast to sham by 42% and apocynin-treated rats by 36% (*p < 0.0001 and #p < 0.0001, respectively). The phosphorylated eIF2α1 was detected in the spermatocytes of ipsilateral tIRI-subjected testes as it displayed high fluorescence intensity compared to sham and apocynin-treated groups (38%, p < 0.0001). No significant phosphorylation was observed within contralateral testes of the three experimental groups (p > 0.05). Likewise, CHOP IF expression was also elevated in the spermatocytes of ipsilateral testes of tIRI in contrast to sham by 43% and apocynin-treated group by 45% (p < 0.0001). Whereas, contralateral testes showed almost equal CHOP IF expression (p > 0.05). Moreover, caspase 12 was found to be remarkably upregulated in the spermatocytes of ipsilateral tIRI-subjected rats when compared to sham and apocynin-treated groups (46% and 50%, respectively, p < 0.0001). No significant difference was seen in the contralateral testes of sham, tIRI or apocynin-treated groups (p > 0.05).
Fig. 6.
Apocynin modulates the expression of ER stress markers by IF staining. NOX-induced oxidative and ER stresses through activation of the UPR pathway components in tIRI-subjected testes. Significant upregulation of UPR proteins is demonstrated as high fluorescence intensity in tIRI (B, E, H, K) compared to baseline expression in sham (A, D, G, J) and apocynin-treated groups (C, F, I, L). GRP78 (A-C, blue), p-eIF2α (D-F, green), CHOP (G-I, magenta), and caspase 12 (J-L, green). Images were captured at 40× magnification.
Except for CHOP, the immune-expression of the above UPR proteins were detected by WB analysis (Fig. 7 and Table 1). During tIRI, the expression of GRP78, p-eIF2α1 and caspase 12 was augmented by 57%, 52% and 54% in comparison to sham, respectively, and higher by 35%, 21% and 46% in comparison to apocynin-treated rats, respectively. No significant difference was obtained among contralateral testes (p-value > 0.05).
Fig. 7.
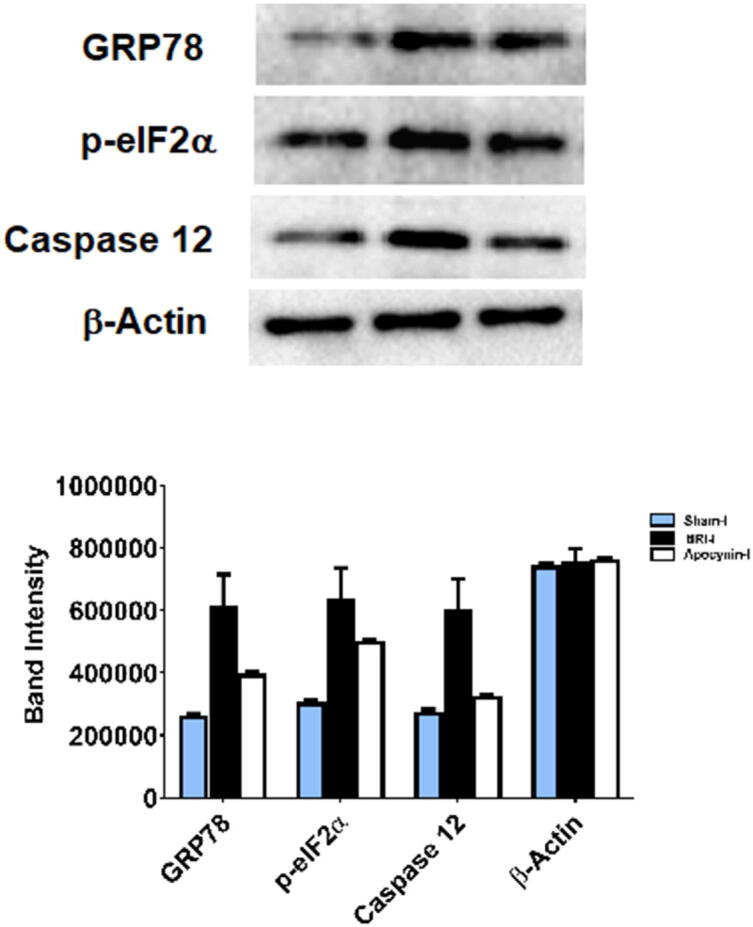
Detection of ER stress markers by Western Blot. During tIRI, the immune-expression of GRP78, p-eIF2α and caspase 12 was increased by 57%, 52% and 54% as compared to sham (p > 0.001) and was higher by 35, 21% and 46% in comparison to apocynin-treated group (p > 0.001). Data are presented as mean ± SD (n = 6/group). *tIRI compared to sham and #apocynin-treated compared to tIRI. I = Ipsilateral.
4. Discussion
The testis is the essential male reproductive organ responsible for spermatogenesis. Thus, any aberrations in sperm production can cause male infertility issues. Oxidative stress is suggested to be the major cause for such spermatogenic abnormalities. Since NOX is one of the major endogenous sources of cellular ROS, its increased activity has been suggested as one mechanism for ROS-mediated death signals in spermatozoa. The current study demonstrates NOX’s regulation of spermatogenesis, TOS, redox status and GCA.
In this study, the tIRI-subjected testes demonstrated clear damage to spermatogenesis reflected by the reduction in germ cell layers and atrophy to seminiferous tubule overall structure, which was associated with increased lipid and protein peroxidation products. These damages were prevented by the use of apocynin, a plant-derived medicinal herb that interferes with the assembly of the functional NOX complex (Touyz, 2008). The inhibitory and therapeutic effect of apocynin was demonstrated in many cells and organs including cancer and has been commonly utilized as a regular NOX inhibitor in research investigations (Suzuki et al., 2013, Qin et al., 2017, Min et al., 2018, Du et al., 2019). In male-factor infertility, oxidative stress is thought to affect the fluidity of the sperm plasma membrane and ROS-induced DNA damage, which may accelerate the process of germ cell apoptosis leading to the decline in sperm counts (Agarwal et al., 2003). Thus, male infertility has been connected to increased ROS production (Aitken and Baker, 2004). NOX has been suggested as a possible candidate for physiological ROS generation in spermatozoa (Baker et al., 2004). NOX-derived ROS was proposed to be vital for spermatocyte maturation and acrosome formation and capacitation through a NOX-dependent apoptosis (Bedard and Krause, 2007). In Nox1- deficient mouse spermatogonial stem cells (SSCs), ROS levels were depleted and SSCs had reduced self-renewal divisions through activation of the p38 MAPK and JNK pathways (Morimoto et al., 2013). Later, the same group identified NOX3 as another ROS contributor to SSCs self-renewal by using short hairpin RNA (Morimoto et al., 2015). ROS generated by NOX4 in male germ cells was also reported to function as a second messenger in signaling pathways and regulating gene expression (Galardo et al., 2014).
Increased oxidative DNA damage was clearly demonstrated in this study by the detection of single and double DNA strand breaks and the formation of 8-OHdG adducts, which were attenuated by NOX inhibition with apocynin. The importance of NOX enzymes’ contribution to oxidative DNA damage is well established by several studies. Using specific NOX siRNA or apocynin hindered the interaction of p47phox and gp91phox subunits, thus considerably reducing DNA damage in esophageal cells (Stefanska and Pawliczak, 2008). The H2O2 generated from NOX isoforms serves as a signaling molecule downstream of the oxidative DNA damage response (DDR) pathways, 8-oxoguanine DNA glycosylase1 (OGG1) and ataxia telangiectasia-mutated (ATM) (Choudhary et al., 2016). Interestingly, it was also recently shown that changes in redox homeostasis could be induced by UVB radiation leading to NOX1 activation, which can prime a transient surge in intracellular ROS that will trigger a series of redox reactions (Raad et al., 2017). Inhibition of UVB-induced NOX activity in both human keratinocytes and in SKH-1 hairless mice led to the quenching of DNA damage and stimulation of the DDR pathway to promote cell survival. Although it is considered as the first line of defense in preserving genomic integrity, The ATM/DDR pathway is also involved in regulating cellular redox status, mitochondrial function and metabolic control. In the presence of DNA double strand breaks, ATM is auto phosphorylated at Ser 1981 to release 2 activated ATM monomers, which will in turn phosphorylate downstream proteins like histone 2A family member X (H2AX), p53, checkpoint kinase 2 homolog (Chk-2) (Bakkenist and Kastan, 2003, Matsuoka et al., 2007). The phosphorylation of ATM at Ser 1981 is induced by TGF-β1 through several redox-sensitive mechanisms using the NOX1/NOX2/NOX4 pathway in rat kidney fibroblasts (Overstreet et al., 2015). In phagocytes, the ATM kinase inactivates NOX2 activity by phosphorylation of Ser 486 in its active site (Beaumel et al., 2017). Under physiological conditions, H2AX activation is thought to be secondary to indirect DNA double strand breaks produced by cellular process such as DNA replication and repair as well as directly due to early oxidative DNA damage (Sedelnikova et al., 2010). A novel ROS signaling pathway was established where the overexpression of γ–H2AX upregulates Nox1-mediated ROS generation after DNA damage (Kang et al., 2012). It was also revealed that ROS generation was increased by both DNA damage and γ–H2AX overexpression, which creates a signaling loop that, will eventually trigger apoptosis. In addition, ROS-mediated H2AX activation was reported to be a consequence of apoptosis induction rather than the cause of caspase activation (Bekeschus et al., 2019). This was confirmed by the abrogation of both H2AX phosphorylation and apoptosis upon inhibition of caspase activity. In conclusion, γ-H2AX is greatly involved in cell death signaling as demonstrated in this study during tIRI-induced apoptosis. In response to γ radiation, PARP deficient cells exhibit increased DNA damage and ATM-kinase activity associated with decreased γ-H2AX formation (Aguilar-Quesada et al., 2007). Activation of PARP was also found to inhibit the phosphorylation of p53, an ATM substrate (Watanabe et al., 2004). This is in agreement with our previous finding (Al-Maghrebi and Renno, 2016) and further supports our current results since NOX inhibition suppresses PARP activity, deters DNA damage and prevents H2AX phosphorylation during tIRI.
The source of ROS-triggering oxidative stress and its signaling pathways during tIRI is not well defined. Recently, ER stress has been associated with various physiological and chronic pathological conditions but with several gaps in other acute conditions such as TTD-induced tIRI (Malhotra and Kaufman, 2007, Ron and Walter, 2007). It was previously proposed that NOX-derived ROS could participate in ER stress (Li et al., 2010, Santos et al., 2014). The ER adapts to oxidative stress by activation of the three UPR pathways: IRE1, PERK and ATF6, which is initiated by upregulation of the GRP78 protein (Guzel et al., 2017). The PERK transducer phosphorylates eIF2α that will eventually lead to upregulation of CHOP transcription (Huang et al., 2018). In the current study, the immuno-expression of GRP78, p-eIF2α and CHOP was significantly increased as a consequence of tIRI. The tIRI-induced UPR activation was repressed by apocynin inhibition of NOX, thus, establishing a direct link between NOX activity and induction of ER stress. Using spontaneously hypertensive rat (SHR) revealed that PERK is Nox1-regulated while IRE1 is Nox4-regulated in vascular smooth muscle cells (VSMC) (Camargo et al., 2018). This differential modulation of ER stress pathways was confirmed to contribute to the irreversible oxidation of the VSMC proteome and vascular dysfunction in hypertension. Cerebral ischemia/reperfusion (I/R) revealed pronounced brain damage, massive neuronal loss and DNA fragmentation in association with induced ER stress reflected by the enhanced expression of GRP78, caspase-12 and CHOP (Nakka et al., 2010). The activation of the UPR pathway was counteracted by the use of a selective inhibitor of eIF2α dephosphorylation that led to reduced brain damage after I/R injury. In Nox2-/- macrophage cells, NOX was found to play a major role in triggering ER stress through the CHOP/Ca+/CaMKII/JNK apoptosis pathway (Li et al., 2010). By using Nox2-/- mice, lack of NOX2 attenuated ER stress–induced renal cell apoptosis, CHOP expression and renal dysfunction (Li et al., 2010). These studies are in agreement with the current findings confirming the direct regulation of ER stress by JNK.
If ER stress persists and the UPR fails to adapt, ER stress-mediated apoptosis will be activated (Amodio et al., 2018). The present study demonstrated the phosphorylation of ASK1/JNK apoptosis pathway and the ER stress related caspase 12 during tIRI while suppressing the expression of the inhibitor of apoptosis (IAP), survivin. These modulations were normalized by apocynin-inhibition of NOX. To our knowledge, this is the first study to prove the activation of ER-stress mediated apoptosis due to NOX-induced OS following tIRI. The involvement of ER stress-induced apoptosis of both male and female reproductive cells and different diseases has been reported and reviewed in several studies (Liu et al., 2016, Karna et al., 2019). It was also reported that cadmium-induced GCA resulted from mitochondrial cytochrome-c release, upregulation of GRP67 and CHOP and phosphorylation of eIF2α and JNK in testes (Ji et al., 2011). Mouse testes subjected to repeated cycles of hypothermia led to the activation of the UPR signaling pathway causing spermatocytes to undergo apoptosis (Kim et al., 2013). Testes from hypoxic rats exhibited enhanced protein expression of p22/p47phox, NOX2 and PERK, which were all normalized by NOX inhibition with apocynin and raisanberine, thus, preventing testicular hypogonadism by ER stress suppression (Zhang et al., 2013). Using a common NOX inhibitor other than apocynin exerted a similar protective effect on attenuating apoptosis (Coyoy et al., 2008, Dong et al., 2017, Kim et al., 2019). This indicates that NOX’s inhibition by itself is necessary to suppress GCA regardless of the type of inhibitor. The ER transmembrane proteins ATF6, IRE1α and PERK are also recognized as mediators of apoptosis (Szegezdi et al 2006). During chronic ER stress, the IRE1α-TRAF2 complex is formed that will activate the ASK1/JNK apoptosis signaling pathway (Urano et al., 2000, Nishitoh et al., 2002). Furthermore, CHOP, JNK and caspase 3 were shown to be activated through the PERK/ATF6, IRE1α/TRAF2/ASK1 and caspase 12, respectively (Lai et al., 2007). Activation of ASK1/JNK signaling pathway have been recently shown to induce GCA via modulation of the thioredoxin system (Al-Kandari et al., 2019). Caspase 12, an ER stress apoptosis mediator, activates caspase 9 that further cleaves caspase 3 to execute apoptosis in a cytochrome-c manner (Morishima et al., 2002, Morishima et al., 2002, Huang et al., 2014, Zhang et al., 2016). Down regulation of survivn, a well-known IAP and a mitotic regulator, was previously associated with the induction of male reproductive diseases including TTD/tIRI (Weikert et al., 2004, Weikert et al., 2005, Al-Maghrebi et al., 2010). Interestingly, survivin expression was also found to be regulated by alterations in the redox status of the cell (Hu et al., 2007). Augmented ROS levels leading to apoptosis were shown to be associated with survivin down regulation in many systems (White-Gilbertson et al., 2009, Ahamed et al., 2011). Nox4-transduced VSMC primary cultures caused the downregulation of survivin protein and mRNA expression (McCrann et al., 2009). In human breast tumors, elevated expression of NOX1/p67/p47 was associated with reduced survivin expression (Pervin et al., 2013). Human colon carcinoma cell line HCT116 transfected with NOX4 siRNA provided evidence that NOX4 is directly involved in the expression of the survivin-associated radio-adaptive responses (Murley et al., 2018). These studies including the current one clearly indicate the critical involvement of NOX-induced ER stress in triggering cell death/survival mechanisms in testicular and non-testicular tissues. Collectively, increased NOX activity during tIRI led to activation of GRP78, which phosphorylated eIF2α1 that through ATF4 up-regulates the gene expression of CHOP. This indicates that the PERK sensor of UPR is activated. While activation of ASK1/JNK pathway is indicative of activation of the IRE1 sensor of UPR. Such activation of UPR is deactivated by NOX inhibition with apocynin treatment during ischemia prior to reperfusion.
5. Conclusion
Our findings demonstrated a novel direct link between the NOX-induced testicular oxidative stress during tIRI and the incidence of ER stress and its involvement in induction of GCA. Future studies are needed to understand the interaction mechanism between the UPR pathway components and triggering GCA cascade. In addition, the efficacy of apocynin as a therapeutic agent was validated in many research papers (Chiang et al., 2011, Uysal et al., 2015, Deng et al., 2016) including the current study, which could also be helpful in reducing the adverse effects of tIRI as a result of TTD.
Funding
This research was funded by Kuwait University OMICSRU/RCF Project grant # SRUL02/13.
CRediT authorship contribution statement
Farah Al-Saleh: Methodology, Writing - review & editing. Farah Khashab: Methodology, Writing - review & editing. Fatemah Fadel: Methodology, Writing - review & editing. Nora Al-Kandari: Methodology, Writing - review & editing. May Al-Maghrebi: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Acknowledgments
The authors would like to acknowledge the excellent technical support of Mrs. Shabeeba Patillath.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agarwal A., Saleh R.A., Bedaiwy M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79(4):829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- Aguilar-Quesada R., Muñoz-Gámez J.A., Martín-Oliva D., Peralta A., Valenzuela M.T., Matínez-Romero R., Quiles-Pérez R., Menissier-de Murcia J., de Murcia G., Ruiz de Almodóvar M., Oliver F.J. Interaction between ATM and PARP-1 in response to DNA damage and sensitization of ATM deficient cells through PARP inhibition. BMC Mol. Biol. 2007;8:29–36. doi: 10.1186/1471-2199-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahamed M., Akhtar M.J., Raja M., Ahmad I., Siddiqui M.K., AlSalhi M.S., Alrokayan S.A. ZnO nanorod-induced apoptosis in human alveolar adenocarcinoma cells via p53, survivin and bax/bcl-2 pathways: role of oxidative stress. Nanomedicine. 2011;7(6):904–913. doi: 10.1016/j.nano.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Aitken R.J., Baker M.A. Oxidative stress and male reproductive biology. Reprod. Fertil. Dev. 2004;16(5):581–588. doi: 10.10371/RD03089. [DOI] [PubMed] [Google Scholar]
- Al-Kandari N., Fadel F., Al-Saleh F., Khashab F., Al-Maghrebi M. The thioredoxin system is regulated by the ASK-1/JNK/p38/survivin pathway during germ cell apoptosis. Molecules. 2019;24(18) doi: 10.3390/molecules24183333. e3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Maghrebi M., Kehinde E.O., Anim J.T. Long term testicular ischemia-reperfusion injury-induced apoptosis: involvement of survivin down-regulation. Biochem. Biophys. Res. Commun. 2010;395(3):342–347. doi: 10.1016/j.bbrc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Al-Maghrebi M., Renno W.M. Altered expression profile of glycolytic enzymes during testicular ischemia reperfusion injury is associated with the p53/TIGAR pathway: effect of fructose 1,6-diphosphate. PeerJ. 2016;4 doi: 10.7717/peerj.2195. e2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio G., Moltedo O., Faraonio R., Remondelli P. Targeting the endoplasmic reticulum unfolded protein response to counteract the oxidative stress-induced endothelial dysfunction. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/4946289. e4946289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi N., Bahmani M., Kheradmand A., Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. J. Clin. Diagn. Res. 2017;11(5):IE01-IE05. doi: 10.7860/JCDR/2017/23927.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B.M. NADPH oxidase. Curr. Opin. Immunol. 2004;16(1):42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Baker M.A., Aitken R.J. Reactive oxygen species in spermatozoa: methods for monitoring and significance for the origins of genetic disease and infertility. Reprod. Biol. Endocrinol. 2005;3:67–75. doi: 10.1186/1477-7827-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M.A., Krutskikh A., Curry B.J., McLaughlin E.A., Aitken R.J. Identification of cytochrome P450-reductase as the enzyme responsible for NADPH-dependent lucigenin and tetrazolium salt reduction in rat epididymal sperm preparations. Biol. Reprod. 2004;71(1):307–318. doi: 10.1095/biolreprod.104.027748. [DOI] [PubMed] [Google Scholar]
- Bakkenist C.J., Kastan M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421(6922):499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Banfi B., Molnar G., Maturana A., Steger K., Hegedus B., Demaurex N., Krause K.H. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001;276(40):37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- Beaumel S., Picciocchi A., Debeurme F., Vives C., Hesse A.M., Ferro M., Grunwald D., Stieglitz H., Thepchatri P., Smith S.M.E., Fieschi F., Stasia M.J. Down-regulation of NOX2 activity in phagocytes mediated by ATM-kinase dependent phosphorylation. Free Radic. Biol. Med. 2017;113:1–15. doi: 10.1016/j.freeradbiomed.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bekeschus S., Schütz C.S., Nießner F., Wende K., Weltmann K.D., Gelbrich N., von Woedtke T., Schmidt A., Stope M.B. Elevated H2AX phosphorylation observed with kINPen plasma treatment is not caused by ROS-mediated DNA damage but is the consequence of apoptosis. Oxid. Med. Cell Longev. 2019;2019 doi: 10.1155/2019/8535163. e8535163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo L.L., Harvey A.P., Rios F.J., Tsiropoulou S., Da Silva R.N.O., Cao Z., Graham D., McMaster C., Burchmore R.J., Hartley R.C., Bulleid N., Montezano A.C., Touyz R.M. Vascular Nox (NADPH Oxidase) compartmentalization, protein hyperoxidation, and endoplasmic reticulum stress response in hypertension. Hypertension. 2018;72(1):235–246. doi: 10.1161/HYPERTENSIONAHA.118.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C.H., Chuang C.H., Liu S.L. Apocynin attenuates ischemia-reperfusion lung injury in an isolated and perfused rat lung model. Transl. Res. 2011;158(1):17–29. doi: 10.1016/j.trsl.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Choudhary S., Boldogh I., Brasier A.R. Inside-out signaling pathways from nuclear reactive oxygen species control pulmonary innate immunity. J. Innate Immun. 2016;8(2):143–155. doi: 10.1159/000442254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K.W., Murugan D., Mustafa M.R. Natural products targeting ER stress pathway for the treatment of cardiovascular diseases. Pharmacol. Res. 2018;132:119–129. doi: 10.1016/j.phrs.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Coyoy A., Valencia A., Guemez-Gamboa A., Moran J. Role of NADPH oxidase in the apoptotic death of cultured cerebellar granule neurons. Free Radic. Biol. Med. 2008;45(8):1056–1064. doi: 10.1016/j.freeradbiomed.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Dang D.K., Shin E.J., Nam Y., Ryoo S., Jeong J.H., Jang C.G., Nabeshima T., Hong J.S., Kim H.C. Apocynin prevents mitochondrial burdens, microglial activation, and pro-apoptosis induced by a toxic dose of methamphetamine in the striatum of mice via inhibition of p47phox activation by ERK. J Neuroinflammation. 2016;18:13–112. doi: 10.1186/s12974-016-0478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo F.R., Burritt J.B., Yu L., Jesaitis A.J., Dinauer M.C., Nauseef W.M. Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J. Biol. Chem. 2000;275(18):13986–13993. doi: 10.1074/jbc.275.18.13986. [DOI] [PubMed] [Google Scholar]
- Deng W., Abliz A., Xu S., Sun R., Guo W., Shi Q., Wang W. Severity of pancreatitis associated intestinal mucosal barrier injury is reduced following treatment with the NADPH oxidase inhibitor apocynin. Mol. Med. Rep. 2016;14(4):3525–3534. doi: 10.3892/mmr.2016.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Li Z., Chen Y., Zhang L., Ye Z., Liang H., Liang X. NADPH oxidase inhibitor, diphenyleneiodonium prevents necroptosis in HK-2 cells. Biomed. Rep. 2017;7(3):226–230. doi: 10.3892/br.2017.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z.D., Yu S., Qi Y., Qu T.F., He L., Wei W., Liu K., Gong S.S. NADPH oxidase inhibitor apocynin decreases mitochondrial dysfunction and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging model in rats. Neurochem. Int. 2019;124:31–40. doi: 10.1016/j.neuint.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Galardo M.N., Regueira M., Riera M.F., Pellizzari E.H., Cigorraga S.B., Meroni S.B. Lactate regulates rat male germ cell function through reactive oxygen species. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0088024. e88024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de la Cadena S., Massieu L. Caspases and their role in inflammation and ischemic neuronal death. Focus on caspase-12. Apoptosis. 2016;21(7):763–777. doi: 10.1007/s10495-016-1247-0. [DOI] [PubMed] [Google Scholar]
- Guzel E., Arlier S., Guzeloglu-Kayisli O., Tabak M.S., Ekiz T., Semerci N., Kayisli U.A. Endoplasmic reticulum stress and homeostasis in reproductive physiology and pathology. Int. J. Mol. Sci. 2017;18(4):792–817. doi: 10.3390/ijms18040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayee B., Antonopoulos A., Murphy E.J., Rahman F.Z., Sewell G., Smith B.N., McCartney S., Furman M., Hall G., Bloom S.L., Haslam S.M., Morris H.R., Boztug K., Klein C., Winchester B., Pick E., Linch D.C., Gale R.E., Smith A.M., Dell A., Segal A.W. G6PC3 mutations are associated with a major defect of glycosylation: a novel mechanism for neutrophil dysfunction. Glycobiology. 2011;21(7):914–924. doi: 10.1093/glycob/cwr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes C.M., Titus E.A., Cooper A.A. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell. 2004;15(5):767–776. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Hu Y., Rahlfs S., Mersch-Sundermann V., Becker K. Resveratrol modulates mRNA transcripts of genes related to redox metabolism and cell proliferation in non-small-cell lung carcinoma cells. Biol. Chem. 2007;388(2):207–219. doi: 10.1515/BC.2007.023. [DOI] [PubMed] [Google Scholar]
- Huang H., An Y., Jiao W., Wang J., Li S., Teng X. CHOP/caspase-3 signal pathway involves in mitigative effect of selenium on lead-induced apoptosis via endoplasmic reticulum pathway in chicken testes. Environ. Sci. Pollut. Res. Int. 2018;25(19):18838–18845. doi: 10.1007/s11356-018-1950-1. [DOI] [PubMed] [Google Scholar]
- Huang Y., Li X., Wang Y., Wang H., Huang C., Li J. Endoplasmic reticulum stress-induced hepatic stellate cell apoptosis through calcium-mediated JNK/P38 MAPK and Calpain/Caspase-12 pathways. Mol. Cell. Biochem. 2014;394(1–2):1–12. doi: 10.1007/s11010-014-2073-8. [DOI] [PubMed] [Google Scholar]
- Ji Y.L., Wang H., Zhao X.F., Wang Q., Zhang C., Zhang Y., Zhao M., Chen Y.H., Meng X.H., Xu D.X. Crosstalk between endoplasmic reticulum stress and mitochondrial pathway mediates cadmium-induced germ cell apoptosis in testes. Toxicol. Sci. 2011;124(2):446–459. doi: 10.1093/toxsci/kfr232. [DOI] [PubMed] [Google Scholar]
- Johnsen S.G. Testicular biopsy score count - a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- Kang M.A., So E.Y., Simons A.L., Spitz D.R., Ouchi T. DNA damage induces reactive oxygen species generation through the H2AX-Nox1/Rac1 pathway. Cell Death Dis. 2012;3(1) doi: 10.1038/cddis.2011.134. e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karna K.K., Shin Y.S., Choi B.R., Kim H.K., Park J.K. The role of endoplasmic reticulum stress response in male reproductive physiology and pathology: a review. World J Mens Health. 2019;37 doi: 10.5534/wjmh.190038. e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler C.S., Bauml J. Non-traumatic urologic emergencies in men: a clinical review. West J. Emerg. Med. 2009;10(4):281–287. [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Park S.J., Kim T.S., Park H.J., Park J., Kim B.K., Kim G.R., Kim J.M., Huang S.M., Chae J.I., Park C.K., Lee D.S. Testicular hyperthermia induces Unfolded Protein Response signaling activation in spermatocyte. Biochem. Biophys. Res. Commun. 2013;434(4):861–866. doi: 10.1016/j.bbrc.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Kim S.K., Rho S.J., Kim S.H., Kim S.Y., Song S.H., Yoo J.Y., Lee S.H. Protective effects of diphenyleneiodonium, an NADPH oxidase inhibitor, on lipopolysaccharide-induced acute lung injury. Clin. Exp. Pharmacol. Physiol. 2019;46(2):153–162. doi: 10.1111/1440-1681.13050. [DOI] [PubMed] [Google Scholar]
- Lai E., Teodoro T., Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology. 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- Lambeth J.D. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007;43(3):332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurindo F.R., Araujo T.L., Abrahao T.B. Nox NADPH oxidases and the endoplasmic reticulum. Antioxid. Redox Signal. 2014;20(17):2755–2775. doi: 10.1089/ars.2013.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurindo F.R., Pescatore L.A., Fernandes Dde C. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic. Biol. Med. 2012;52(9):1954–1969. doi: 10.1016/j.freeradbiomed.2012.02.037. [DOI] [PubMed] [Google Scholar]
- Li G., Scull C., Ozcan L., Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 2010;191(6):1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Peng Z., Cheng W., Dai C., Tong H. Endoplasmic reticulum stress-induced apoptosis in the development of reproduction. J. Reprod. Contracept. 2016;27(1):51–59. doi: 10.7669/j.issn.1001-7844.2016.01.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Malhotra J.D., Kaufman R.J. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007;18(6):716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masella R., Di Benedetto R., Vari R., Filesi C., Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005;16(10):577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Ballif B.A., Smogorzewska A., McDonald E.R., Hurov K.E., Luo J., Bakalarski C.E., Zhao Z., Solimini N., Lerenthal Y., Shiloh Y., Gygi S.P., Elledge S.J. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Sci. 2007;316(5828):1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- McCrann D.J., Yang D., Chen H., Carroll S., Ravid K. Upregulation of Nox4 in the aging vasculature and its association with smooth muscle cell polyploidy. Cell Cycle. 2009;8(6):902–908. doi: 10.4161/cc.8.6.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S., Wu D., Li L., Sun X., Xie W., Li X. Effect of pretreatment with the NADPH oxidase inhibitor apocynin on the therapeutic efficacy of human placenta-derived mesenchymal stem cells in intracerebral hemorrhage. Int. J. Mol. Sci. 2018;19(11):E3679. doi: 10.3390/ijms19113679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto H., Iwata K., Ogonuki N., Inoue K., Atsuo O., Kanatsu-Shinohara M., Morimoto T., Yabe-Nishimura C., Shinohara T. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell. 2013;12(6):774–786. doi: 10.1016/j.stem.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Morimoto H., Kanatsu-Shinohara M., Shinohara T. ROS-Generating Oxidase Nox3 Regulates the Self-Renewal of Mouse Spermatogonial Stem Cells. Biol. Reprod. 2015;92(6):147. doi: 10.1095/biolreprod.114.127647. [DOI] [PubMed] [Google Scholar]
- Morishima N., Nakanishi K., Takenouchi H., Shibata T., Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 2002;277(37):34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- Morishima N., Nakanishi K., Takenouchi H., Shibata T., Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 277. 2002;37:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- Murley J.S., Arbiser J.L., Weichselbaum R.R., Grdina D.J. ROS modifiers and NOX4 affect the expression of the survivin-associated radio-adaptive response. Free Radic. Biol. Med. 2018;123:39–52. doi: 10.1016/j.freeradbiomed.2018.04.547. [DOI] [PubMed] [Google Scholar]
- Nakka V.P., Gusain A., Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox. Res. 2010;17(2):189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., Hori S., Kakizuka A., Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16(11):1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet J.M., Samarakoon R., Cardona-Grau D., Goldschmeding R., Higgins P.J. Tumor suppressor ataxia telangiectasia mutated functions downstream of TGF-β1 in orchestrating profibrotic responses. FASEB J. 2015;29(4):1258–1268. doi: 10.1096/fj.14-262527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervin S., Tran L., Urman R., Braga M., Parveen M., Li S.A., Chaudhuri G., Singh R. Oxidative stress specifically downregulates survivin to promote breast tumour formation. Br. J. Cancer. 2013;108(4):848–858. doi: 10.1038/bjc.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y.Y., Li M., Feng X., Wang J., Cao L., Shen X.K., Chen J., Sun M., Sheng R., Han F., Qin Z.H. Combined NADPH and the NOX inhibitor apocynin provides greater anti-inflammatory and neuroprotective effects in a mouse model of stroke. Free Radic. Biol. Med. 2017;104:333–345. doi: 10.1016/j.freeradbiomed.2017.01.034. [DOI] [PubMed] [Google Scholar]
- Raad H., Serrano-Sanchez M., Harfouche G., Mahfouf W., Bortolotto D., Bergeron V., Kasraian Z., Dousset L., Hosseini M., Taieb A., Rezvani H.R. NADPH oxidase-1 plays a key role in keratinocyte responses to UV radiation and UVB-induced skin carcinogenesis. J. Invest. Dermatol. 2017;137(6):1311–1321. doi: 10.1016/j.jid.2016.12.027. [DOI] [PubMed] [Google Scholar]
- Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Santos C.X., Tanaka L.Y., Wosniak J., Laurindo F.R. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid. Redox. Signal. 2009;11(10):2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- Santos C.X., Nabeebaccus A.A., Shah A.M., Camargo L.L., Filho S.V., Lopes L.R. Endoplasmic reticulum stress and Nox-mediated reactive oxygen species signaling in the peripheral vasculature: potential role in hypertension. Antioxid. Redox Signal. 2014;20(1):121–134. doi: 10.1089/ars.2013.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelnikova O.A., Redon C.E., Dickey J.S., Nakamura A.J., Georgakilas A.G., Bonner W.M. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat. Res. 2010;704(1–3):152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanska J., Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008 doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Gong F., Yin J., Wang X., Wang X., Sun Q., Zhu Z., Su X., Zheng J., Liu L., Li Y., Hu X., Li J. Therapeutic effect of apocynin through antioxidant activity and suppression of apoptosis and inflammation after spinal cord injury. Exp. Ther. Med. 2017;13(3):952–960. doi: 10.3892/etm.2017.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Pitchakarn P., Sato S., Shirai T., Takahashi S. Apocynin, an NADPH oxidase inhibitor, suppresses progression of prostate cancer via Rac1 dephosphorylation. Exp. Toxicol. Pathol. 2013;65(7–8):1035–1041. doi: 10.1016/j.etp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Szegezdi E., Logue S.E., Gorman A.M., Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz R.M. Apocynin, NADPH oxidase, and vascular cells: a complex matter. Hypertension. 2008;51(2):172–174. doi: 10.1161/HYPERTENSIONAHA.107.103200. [DOI] [PubMed] [Google Scholar]
- Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P., Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Uysal A., Sahna E., Ozguler I.M., Burma O., Ilhan N. Effects of apocynin, an NADPH oxidase inhibitor, on levels of ADMA, MPO, iNOS and TLR4 induced by myocardial ischemia reperfusion. Perfusion. 2015;30(6):472–477. doi: 10.1177/0267659114559260. [DOI] [PubMed] [Google Scholar]
- Watanabe F., Fukazawa H., Masutani M., Suzuki H., Teraoka H., Mizutani S., Uehara Y. Poly(ADP-ribose) polymerase-1 inhibits ATM kinase activity in DNA damage response. Biochem. Biophys. Res. Commun. 2004;319(2):596–602. doi: 10.1016/j.bbrc.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Weikert S., Schrader M., Muller M., Krause H., Miller K. Expression of the apoptosis inhibitor survivin in testicular tissue of infertile patients. Int. J. Andro. 2004;27(3):161–165. doi: 10.1111/j.1365-2605.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- Weikert S., Schrader M., Muller M., Schulze W., Krause H., Miller K. Expression levels of the inhibitor of apoptosis survivin in testes of patients with normal spermatogenesis and spermatogenic failure. Fertil. Steril. 2005;83(1):1100–1105. doi: 10.1016/j.fertnstert.2004.12.010. [DOI] [PubMed] [Google Scholar]
- White-Gilbertson S.J., Kasman L., McKillop J., Tirodkar T., Lu P., Voelkel-Johnson C. Oxidative stress sensitizes bladder cancer cells to TRAIL mediated apoptosis by down-regulating anti-apoptotic proteins. J. Urol. 2009;182(3):1178–1185. doi: 10.1016/j.juro.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.L., Dai D.Z., Zhang C., Dai Y. Apocynin and raisanberine alleviate intermittent hypoxia induced abnormal StAR and 3β-HSD and low testosterone by suppressing endoplasmic reticulum stress and activated p66Shc in rat testes. Reprod. Toxicol. 2013;36:60–70. doi: 10.1016/j.reprotox.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Zhang H.F., Li T.B., Liu B., Lou Z., Zhang J.J., Peng J.J., Zhang X.J., Ma Q.L., Peng J., Luo X.J. Inhibition of myosin light chain kinase reduces NADPH oxidase-mediated oxidative injury in rat brain following cerebral ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(9):953–963. doi: 10.1007/s00210-015-1125-2. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Liu J., Chen S., Liu J., Liu L., Liu G., Yuan X. Caspase-12 is involved in stretch-induced apoptosis mediated endoplasmic reticulum stress. Apoptosis. 2016;21(4):432–442. doi: 10.1007/s10495-016-1217-6. [DOI] [PubMed] [Google Scholar]