Abstract
Objective
The objective of this study is to explore the protective effect of erythropoietin (EPO) on brain injury induced by intrauterine infection in premature infants and its related mechanism, so as to provide reference for clinical medication.
Methods
Intrauterine infection model is established by injecting lipopolysaccharide into pregnant mice, and HE staining of mouse placenta is used to judge whether the model of intrauterine infection is successful or not. Fifteen female rats are successfully pregnant and divided into intrauterine infection group (10 rats) and control group (5 rats). The mice in the intrauterine infection group are intraperitoneally injected with lipopolysaccharide (LPS) at a dose of 0.3 mg/kg. After delivery, 16 newborn mice in the control group are randomly selected as blank control group. 32 newborn mice in the intrauterine infection group are selected as model group, and then divided into infection group and EPO treatment group, 16 mice in each group. After birth, mice in the blank control group are intraperitoneally injected with 0.2 mL saline daily. The infected mice are intraperitoneally injected with 0.2 mL saline daily. The mice in the EPO treatment group are intraperitoneally injected with recombinant human erythropoietin (rhEPO) 5000 IU/kg daily. HE staining results, EPOR protein and NMDAR1 mRNA expression in brain tissue of three groups of neonatal mice were compared.
Results
Firstly, the blood vessels of the mice in the intrauterine infection group are markedly hyperemic and edematous, and the infiltration of neutrophils is increased. The white matter structure of the neonatal mice in the intrauterine infection group is loose and stained lightly. The nerve fibers in the brain are different in thickness and disordered in arrangement. The nucleus is small and dark stained. The number of glial cells in brain tissue increases significantly. Secondly, the EPOR protein expression and physiological level of neonatal mice in intrauterine infection group increase significantly at 3, 7 and 14 days after birth. Compared with the blank control group, the difference is statistically significant (P < 0.05). On the 3rd day after birth, the expression level of EPOR protein in the EPO treated group is significantly higher than that in the intrauterine infection group (P < 0.05). Thirdly, the expression level of NMDA R1mRNA in brain tissue of neonatal mice at birth, on the 3rd and 7th day after EPO treatment is significantly lower than that of intrauterine infection group (P < 0.05).
Conclusion
EPO can promote the proliferation and differentiation of brain endogenous neural stem cells, and has a certain therapeutic effect on brain injury of premature mice caused by intrauterine infection.
Keywords: Erythropoietin, Intrauterine infection, Premature mice, Brain hypoxia, Brain injury
1. Introduction
Perinatal intrauterine infection is an important cause of brain injury in premature infants. White matter lesion (WMI) of premature infants is the most common and most harmful to the health of newborns, which is one of the risk factors leading to neurological sequelae (Ping et al., 2018). There are three ways of intrauterine infection. The first is that maternal blood enters the fetus through the placenta and causes infection. The second is the infection of endometrium or uterine appendages caused by maternal infection, which leads to placental choriontitis and fetal infection. The third is that the pathogen of pregnancy goes up through the vagina and infects the fetus (Yoo et al., 2017). The mechanism of brain injury caused by intrauterine infection is cytokine-mediated immune inflammation, which makes placental blood circulation disorder and seriously damages brain tissue. In addition, when the fetal body temperature is higher than the maternal body temperature caused by inflammation, the increase of brain temperature will aggravate the body's hypoxia symptoms, and the production of peroxides and the release of oxygen free radicals will further aggravate brain damage (Zhang et al., 2017).
Studies have shown that as an excitatory neurotransmitter of the central system, excitatory amino acid (EAA) plays a major toxic role in brain injury caused by intrauterine infection and participates in the occurrence and development of brain tissue injury (Sun et al., 2019). N-methyl-D-aspartic acid (NMDA) receptor is a subtype of ionic glutamate receptor, which not only plays an important physiological role in the development of nervous system, but also participates in the formation of neuronal circuits. After intrauterine infection, extracellular Ca ion influx caused by NMDA receptor destroys the cell structure and induces nerve structural damage, which leads to the release of oxygen free radicals and programmed neuronal death, eventually leading to brain damage (Peng et al., 2019). Therefore, how to start with the mechanism of brain injury and effectively treat the brain injury of premature infants with intrauterine infection by drugs is a hot research topic at present.
Erythropoietin (EPO) is a human endogenous glycoprotein hormone that can stimulate erythropoiesis. It can promote the proliferation and differentiation of erythroid progenitor cells. It is often used to treat anemia with renal insufficiency or as a preventive drug for anemia (Asada et al., 2017). Studies have shown that erythropoietin receptor (EPOR) and EPO are widely expressed in neurons, glial cells, vascular endothelial cells, spinal cord and peripheral nervous system (Bo et al., 2018). When exposed to hypoxia, trauma and other stimuli, the expression of EPO and its receptors are up-regulated, inhibiting inflammation and playing an important neuroprotective role.
At present, there are many studies on the application of EPO in the brain injury of full-term infants caused by intrauterine infection, but few reports on the treatment of premature infants. In this study, preterm mice with intrauterine infection are treated with erythropoietin to explore the protective effect of EPO on brain injury caused by intrauterine infection of preterm infants and its related mechanisms, so as to provide reference for clinical medication.
2. Protective effect of erythropoietin on brain injury induced by intrauterine infection
Premature delivery is one of the common conditions in pregnancy, and its causes are numerous. However, a large number of studies have shown that intrauterine infection is the main cause of early spontaneous premature delivery. Through clinical investigation, it is found that more than 90% of premature births occurring between 24 and 28 weeks are related to infection, and the younger the gestational age, the more affected the premature births are by infection factors (Schwartz, 2017). Premature delivery caused by intrauterine infection is easy to be accompanied by multiple organ damage, which is particularly serious for brain damage. This is mainly related to cytokine-mediated inflammatory response, immune inflammatory response, the role of excitatory amino acid (EAA) and the release of reactive oxygen species (ROS) free radicals. White matter damage (WMD) in premature infants is a unique form of brain damage in premature infants, which is characterized by coagulation necrosis of white matter. If not effectively treated, it will lead to periventricular leukomalacia (PVL) and neurological sequelae in children, which is also a risk factor for cerebral palsy, intellectual impairment and so on. Studies have shown that intrauterine infection causes the increase of white blood cells, mononuclear macrophages, cytokines, and immune inflammation, which leads to extensive damage of white matter in the brain (Mcdougall et al., 2017).
EPO is an important hematopoietic stimulator. It belongs to the superfamily of type I cytokine receptors and is synthesized and secreted by fetal liver and adult kidney (Saidu et al., 2017). EPO regulates the proliferation and differentiation of erythroid progenitor cells by acting on its receptors. It has been found that EPO, besides its important hematopoietic function, can also act as a protective cytokine in the inflammatory response of the central system, and exert neuroprotective effects by affecting the synthesis of brain cytokines. WMD usually occurs in the early stage of myelin formation. Activation of macrophages, oligodendrocytes (OL) and astroglia are involved in white matter injury (Sukenik-Halevy et al., 2017). Among them, oligodendrocyte is an important component of glial cells in the brain, which participates in the formation of white matter myelin sheath, so it plays an important role in the occurrence of periventricular leukomalacia. EPO can promote the regeneration of oligodendrocytes and neurons, thus reducing the damage of white matter.
Existing studies have shown that EPO may act as a protective cytokine in the inflammatory and pathological changes of the central nervous system and have a protective effect on the immune response in the brain. This effect may be achieved by affecting the synthesis of cytokines in the brain and exerting biological effects (Doty et al., 2018). Juul has confirmed through research that the injection of recombinant human erythropoietin (rhEPo) into the peripheral vein or abdominal cavity can directly produce neuroprotection in the brain through the blood-brain barrier (Juul, 2002). In addition, the expression of endogenous EPO in brain tissue increases briefly under the effects of cerebral ischemia, hypoxia or traumatic brain injury. As a multifunctional neuroprotective factor, EPO can protect brain tissue to a certain extent, and it can be used as an immunoregulatory factor to promote the reconstruction of brain tissue structure and function after injury (Perreault and Venters, 2018).
3. Materials and methods
3.1. Establishment of premature mice model of brain injury induced by intrauterine infection
Forty adult mice are selected, 20 males and 20 females. The experimental mice were purchased from the Animal Center of Southwest Medical University, which complies with the national standards for animal experiments. Also, all the procedures of this experiment were carried out in accordance with animal experiment standards. Weight is controlled between 20 and 25 g. All mice are fed in cages at constant temperature and humidity. The ambient temperature is controlled at 22–24 °C and the humidity is controlled at 45–55%. The ratio of light to dark at 12:12 is used to illuminate all mice. After one week of adaptive feeding, mice are mated in cages according to the distribution principle of one male rat and one female rat. The next day, the vaginal smears of the mother mice need to be examined. Under an optical microscope, the field of vision is filled with sperm to indicate pregnancy. After pregnancy, the female mice are kept in isolation.
A total of 15 female rats are successfully conceived. Mice are divided into intrauterine infection group (10 mice) and control group (5 mice) in a ratio of 2:1. After 15 days of gestation, mice in the intrauterine infection group are intraperitoneally injected with lipopolysaccharide (LPS) at a dose of 0.3 mg/kg. The control group mice are injected with the same amount of sterile saline. After the injection, it is placed in a clean cage and fed regularly. After delivery, the placenta is taken and sliced and stained with hematoxylin-eosin (HE). Vascular congestion, edema and neutrophil proliferation are the criteria for intrauterine infection.
After delivery, 16 newborn mice in the control group are randomly selected as the blank control group. Thirty-two neonatal mice in the intrauterine infection group are selected as model group and subdivided into infection control group (16 mice) and EPO treatment group (16 mice). The specific intervention measures of the three groups of mice are as follows. After birth, mice in the blank control group are intraperitoneally injected with 0.2 mL saline daily. After birth, the infected mice are intraperitoneally injected with 0.2 mL saline daily. After birth, mice in the EPO treatment group are intraperitoneally injected with rhEPO 5000 IU/kg daily.
3.2. Sample collection and RNA extraction from brain tissue
Four mice in each group are randomly selected and executed at postnatal time and at three, seven and fourteen days after birth. Firstly, mice are given chloral hydrate orally at a dose of 2.5 mL/kg. After about 1 min of complete anesthesia, the mice are fixed on the operating board, cut the surrounding tissue along the median line, and cut the sternum of the mice, so that the heart is completely exposed to the field of vision. Needle No. 4 is inserted into the left ventricular aorta of mice and ligated. When the mice are stable, 4% polyformic acid is injected. At the same time, the right atrium of mice is cut and perfused. When the fluid from the right atrium becomes clear gradually, it is necessary to stop perfusion. When the perfusion fluid increases in mice, the limbs become stiff and pale. It indicates that the perfusion model is successfully established. After the mice are decapitated, the brain tissue is quickly removed and washed with cold saline. Brain is divided into left and right brain by sagittal suture, suggesting successful perfusion. Coronal section of hypothalamus is selected for serial section. After fixed in 10% neutral formaldehyde, paraffin embedding and HE staining are needed to observe the pathological changes of periventricular white matter. The right brain tissue is placed in a refrigerator at –80 °C for the detection of EPOR protein levels by ELISA.
ELISA is used to detect EPOR protein in brain tissue of mice. First, blank pore, standard pore and sample pore are set up. Among them, the blank pore is added with sample dilution liquid standard 100uL, and the rest is added with equivalent standard or test sample 100uL, respectively. Samples are added to the bottom of the pore of the enzyme label plate, shaken to uniformity, covered and reacted for 2 h. The second is to discard the liquid and dry it. A working fluid is added into each hole to test for 1 h. The third is to discard the liquid and dry it. The plate is washed 3 times, soaked for 2 min each time. Fourthly, 100uL B working fluid is added into each pore to react for 1 h. The fifth is to discard the liquid and dry it. The plate is washed 5 times, soaked for 2 min each time. The sixth step is to add 90uL substrate liquid, 0.5 h of light avoidance, and finally 50uL termination liquid is added. The seventh step is to measure the optical density (OD) of each pore at 450 nm.
RNA extraction: Brain tissue of newborn mice is ground into pulp. 100 mg brain tissue is added with 1 mL lysis solution, then mixed and incubated at 20 °C for 5 min. 0.2 mL chloroform is added. The EP tube wound with gauze is placed in oscillator for 10 s and incubated at room temperature for 3 min. The samples are centrifuged at 4 °C for 10 min. After stationary, the supernatant is transferred into the new EP tube, and 70% ethanol is added to mix. The mixture is transferred to the sterile adsorption column and centrifuged in a centrifuge for 40 s before discarding the waste liquid. 500 μL deproteinized solution is added. After centrifugation for 40 s, the waste liquid needs to be discarded. 500 μL rinse solution is added. After centrifugation for 60 s, the waste liquid is discarded. The adsorption column is placed in the collection tube and centrifuged for 3 min to remove the rinse solution. At the center of the adsorbent membrane, 30 μL of water for deRNAase is added. After 3 min at room temperature, centrifugation is needed for 1 min.
3.3. Statistical methods
In this experiment, SPSS26.0 statistical software is used for data analysis. Firstly, it is tested whether the original data satisfies the normal distribution and the homogeneity of variance. If satisfied, the results of measurement data are expressed as mean ± standard deviation, and single factor analysis of variance is used for multigroup significance test. LSD method is used to compare the two, and P < 0.05 is used to show the statistical difference.
4. Results
4.1. HE staining results of mouse placenta and brain tissue
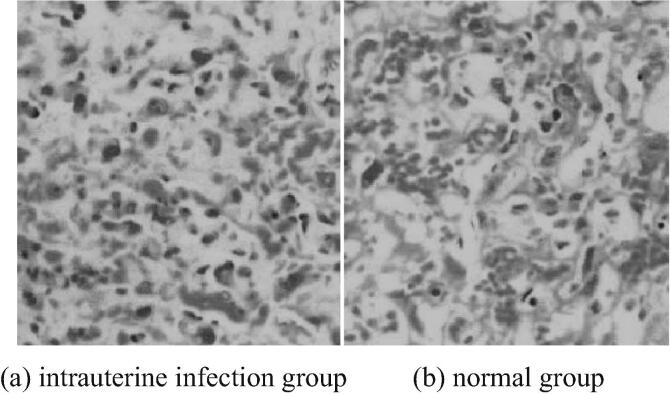
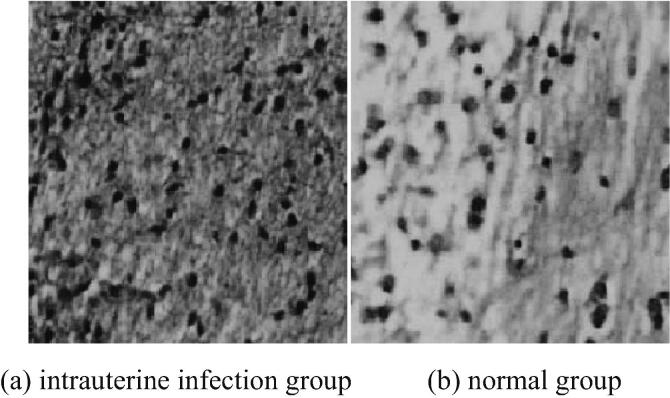
The placentas of mice in the intrauterine infection group and the normal group are stained with HE. The HE staining of placenta pathology is shown in Fig. 1. The brain tissues of neonatal mice are stained with HE. Pathological examination shows HE staining as shown in Fig. 2. As can be seen from Fig. 1, typical pathological changes are observed in mice in the intrauterine infection group. Vascular hyperemia, edema and neutrophil infiltration increase. As can be seen from Fig. 2, the white matter structure of neonatal mice in intrauterine infection group is loose and stained lightly. The nerve fibers in the brain are different in thickness, disordered in arrangement and small, dark-stained nuclei. It can be seen that nuclear pyknosis or nucleolysis, the number of glial cells in brain tissue increase significantly.
Fig. 1.
HE staining in pathological examination of placenta of mice in intrauterine infection group and normal group (* 400).
Fig. 2.
HE staining in brain tissue of neonatal mice in intrauterine infection group and normal group (* 400).
4.2. Effect of EPO on expression of EPOR protein in brain tissue of neonatal mice infected with intrauterine infection
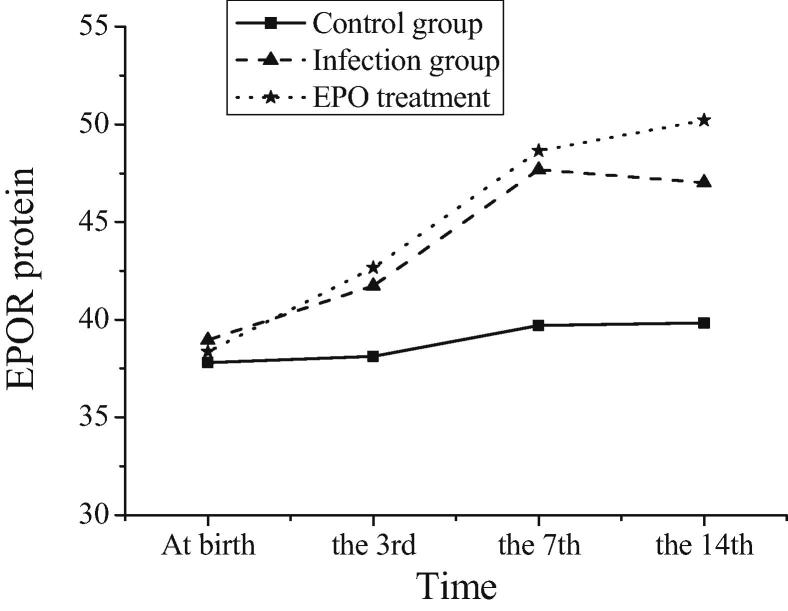
The EPOR protein levels of three groups of mice at postnatal and three, seven and fourteen days after birth are compared in Table 1, and the trend is shown in Fig. 3. As can be seen from Table 1, there is no significant change in EPOR protein concentration in brain tissues of newborn mice in blank control group at different time points. It can be seen that EPOR protein is expressed at a low level in brain tissue at normal physiological level. EPOR protein expression and physiological level of neonatal mice in intrauterine infection group increase significantly at 3, 7 and 14 days after birth. Compared with the blank control group, the difference is statistically significant (P < 0.05). It is concluded that the expression of EPOR protein in the inflammatory state of intrauterine infection is significantly higher than that in normal physiological level. The inflammatory injury could last until 14 days after birth. The body can protect brain tissue by increasing EPOR concentration. On the 3rd day after birth, the expression level of EPOR protein in neonatal mice in EPO treatment group is significantly higher than that in intrauterine infection group (P < 0.05), and the high expression of EPOR protein continues at the 14th day after birth. Thus, EPO can reduce the damage of brain tissue by up-regulating the expression of EPOR protein, and the protective effect of EPO on brain lasts until the 14th day after birth (see Table 2).
Table 1.
Comparison of EPOR protein levels at different time points in three groups of mice.
| Group | Time |
|||
|---|---|---|---|---|
| At birth | the 3rd | the 7th | the 14th | |
| Control group | 37.81 ± 3.03 | 38.14 ± 3.37 | 39.72 ± 5.77 | 39.85 ± 2.70 |
| Infection group | 38.97 ± 5.77 | 41.74 ± 0.65 | 47.69 ± 3.10 | 47.03 ± 3.37 |
| EPO treatment group | 38.37 ± 4.53 | 42.67 ± 2.98* | 48.65 ± 1.77* | 50.21 ± 2.08* |
Note: * Compared with the mice in the intrauterine infection group at the same period, P < 0.05.
Fig. 3.
Trends of EPOR protein levels after birth in three groups of mice.
Table 2.
Expression of NMDAR1 in three groups of mice at different time points.
| Group | Time |
|||
|---|---|---|---|---|
| At birth | the 3rd | the 7th | the 14th | |
| Control group | 1.00 ± 0.00 | 1.01 ± 0.03 | 1.01 ± 0.02 | 1.00 ± 0.04 |
| Infection group | 1.34 ± 0.02 | 2.01 ± 0.02 | 2.29 ± 0.13 | 1.94 ± 0.14 |
| EPO treatment group | 1.21 ± 0.02 | 1.68 ± 0.04* | 1.77 ± 0.02* | 1.51 ± 0.02* |
Note: * Compared with the mice in the intrauterine infection group at the same period, P < 0.05.
4.3. Expression of NMDAR1 in brain tissue of newborn mice after birth
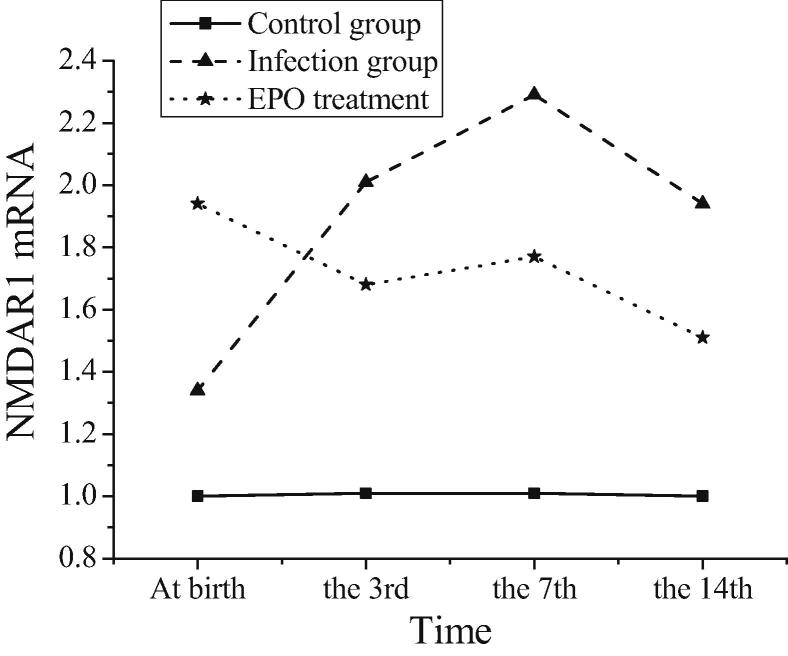
The expression of NMDAR1 in three groups of mice at postnatal time and at three, seven and fourteen days after birth is shown in Table 1, and the trend is shown in Fig. 4. The expression of NMDAR1 is detected at all time points after birth in the three groups of neonatal mice, but there is no significant difference in the expression level of NMDAR1 at different time points in the blank control group (P > 0.05). The expression level of NMDA R1mRNA in mice with intrauterine infection increases significantly after birth, peaked on the 7th day after birth, and then declines until the 14th day after birth. The expression level of NMDA R1mRNA in each period is significantly different from that in the blank control group (P < 0.05). The expression level of NMDA R1mRNA in brain tissue of neonatal mice in EPO treatment group is significantly lower than that in intrauterine infection group at birth, on the 3rd and 7th day after birth (P < 0.05). It can be concluded that the induced intrauterine infection can lead to the over-activation of amino acid receptor NMDA, and then mediate the excitotoxicity of amino acids, leading to neuronal apoptosis. After treatment with rhEPO, NMDA overactivation can be inhibited by inhibiting the excitatory toxicity of amino acids, and the effect lasts until the 7th day after birth.
Fig. 4.
Trends of NMDAR1 expression in three groups of mice after birth.
5. Discussion
The incidence of brain injury after intrauterine infection is higher, especially in WMD. Its pathological mechanism is neuropathological changes, including focal necrosis of deep white matter around ventricle and diffuse WMD. EPO and its EPOR are endocrine hormones that promote the proliferation, differentiation and maturation of erythroid progenitor cells in mammals, so they are widely used in clinical treatment of anemia caused by various reasons. At present, it has been found that EPO can promote the proliferation and differentiation of brain endogenous neural stem cells in addition to its hematopoietic function, and play a significant protective role in the injury caused by cerebral ischemia and hypoxia (Jantzie et al., 2018).
To explore the protective effect of EPO on brain injury caused by intrauterine infection of premature infants and its related mechanism, the preterm mice are treated with erythropoietin. Firstly, the intrauterine infection model is established by injecting LPS into pregnant mice, and the intrauterine infection is judged by HE staining of mouse placenta. In the intrauterine infection group, typical pathological changes are observed, blood vessels are markedly congested and edema, and neutrophil infiltration is increased. HE staining is performed on the brain tissue of neonatal mice in the intrauterine infection group. It is found that the white matter structure of neonatal mice is loose and stained lightly. The nerve fibers in the brain are different in thickness and disordered in arrangement. The nucleus is small and dark stained. Nuclear condensation or dissolution can be seen. The number of glial cells in brain tissue increases significantly. It can be concluded that intrauterine infection has obvious damage to brain tissue of neonatal mice.
rhEPO is produced by recombinant DNA technology. The amino acid sequence of rhEPO is identical with that of naturally isolated EPO, and its biological activity is the same. Therefore, rhEPO is selected as an intervention drug in mice treated with EPO in this experiment. The mice are decapitated and executed at birth and 3, 7 and 14 days after birth. ELISA is used to detect EPOR protein in brain tissue of mice. It is found that the expression of EPOR protein in neonatal mice is significantly higher than that in normal physiological level in the inflammatory state of intrauterine infection, and the inflammatory damage lasts until 14 days after birth. EPO can reduce the damage of brain tissue caused by inflammation of intrauterine infection by up-regulating the expression of EPOR protein, and the protective effect of EPO on brain lasts until the 14th day after birth. The expression level of NMDA R1mRNA in brain tissue of neonatal mice at birth, on the 3rd and 7th day after EPO treatment is significantly lower than that of mice in intrauterine infection group (P < 0.05).
It can be concluded that the induced intrauterine infection can lead to the over-activation of amino acid receptor NMDA, and then mediate the excitotoxicity of amino acids, leading to neuronal apoptosis. EPO has a certain therapeutic effect on brain injury of premature mice caused by intrauterine infection. The results of this experiment are almost consistent with the results of Ponce et al., confirming the reliability of this study. Due to time constraints, dynamic evaluation and analysis could not be performed during this experimental study. There are certain limitations. In future experiments, it is necessary to consider taking samples at each time node for testing, such as continuous sampling at 12 h, 24 h, 48 h, 72 h, and 96 h after modeling to dynamically evaluate the effect of rhEPO on nerve cell proliferation and apoptosis. It is hoped to guide the clinical treatment of ischemic and hypoxic brain injury in children with drug administration time and treatment course, thereby obtaining greater clinical application value.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Asada N., Tsukahara T., Furuhata M. Polycythemia, capillary rarefaction, and focal glomerulosclerosis in two adolescents born extremely low birth weight and premature. Pediatric Nephrol. 2017;32(7):1–4. doi: 10.1007/s00467-017-3654-z. [DOI] [PubMed] [Google Scholar]
- Bo H., Dan X., Chong Z. Prenatal food restriction induces neurobehavioral abnormalities in adult female offspring rats and alters intrauterine programming. Toxicol. Res. 2018;7(2):293–306. doi: 10.1039/c7tx00133a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty M.S., Salafia C., Shenschwarz S. Histologic funisitis and likelihood of intrauterine inflammation or infection: a case-control study. Am. J. Perinatol. 2018;35(09):858–864. doi: 10.1055/s-0037-1620232. [DOI] [PubMed] [Google Scholar]
- Jantzie L.L., Oppong A.Y., Conteh F.S. Repetitive neonatal erythropoietin and melatonin combinatorial treatment provides sustained repair of functional deficits in a rat model of cerebral palsy. Front. Neurol. 2018;9:233. doi: 10.3389/fneur.2018.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul S. Erythropoietin in the central nervous system, and its use to prevent hypoxic-ischemic brain damage. Acta Paediatr. 2002;91:36–42. doi: 10.1111/j.1651-2227.2002.tb02904.x. [DOI] [PubMed] [Google Scholar]
- Mcdougall A.R.A., Hale N., Rees S. Erythropoietin protects against lipopolysaccharide-induced microgliosis and abnormal granule cell development in the ovine fetal cerebellum. Front. Cell. Neurosci. 2017;11:224. doi: 10.3389/fncel.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S., Wan Z., Liu T. Incidence and risk factors of intrauterine transmission among pregnant women with chronic hepatitis B virus infection. J. Clin. Gastroenterol. 2019;53(1):51–57. doi: 10.1097/MCG.0000000000001001. [DOI] [PubMed] [Google Scholar]
- Perreault A.A., Venters B.J. Integrative view on how erythropoietin signaling controls transcription patterns in erythroid cells. Curr. Opin. Hematol. 2018;25(5):1. doi: 10.1097/MOH.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping T., Guang H., Ling H. The effects of rhEPO intervention for perinatal intrauterine herpes virus infection on preventing brain injury in preterm infants. Exp. Therap. Med. 2018;15(1):271–275. doi: 10.3892/etm.2017.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidu A.D., Tunau K.A., Panti A.A. Comparison of genital microbial isolates between intrauterine contraceptive device users and nonusers in Sokoto Nigeria. Tropical J. Obstet. Gynaecol. 2017;34(3):234. [Google Scholar]
- Schwartz D.A. Autopsy and postmortem studies are concordant: pathology of zika virus infection is neurotropic in fetuses and infants with microcephaly following transplacental transmission. Arch. Pathol. Lab. Med. 2017;141(1):68–72. doi: 10.5858/arpa.2016-0343-OA. [DOI] [PubMed] [Google Scholar]
- Sukenik-Halevy R., Katz A., Regev R. The yield of the prenatal work-up in intrauterine growth restriction and the spectrum of fetal abnormalities detected postnatally. J. Matern Fetal Neonatal Med. 2017;32(5):1–181. doi: 10.1080/14767058.2017.1391776. [DOI] [PubMed] [Google Scholar]
- Sun J., Boado R.J., Pardridge W.M. Plasma pharmacokinetics of high-affinity transferrin receptor antibody-erythropoietin fusion protein is a function of effector attenuation in mice. Mol. Pharm. 2019;16(8):3534–3543. doi: 10.1021/acs.molpharmaceut.9b00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.J., Cho B., Lee D. The erythropoietin-derived peptide MK-X and erythropoietin have neuroprotective effects against ischemic brain damage. Cell Death Dis. 2017;8(8) doi: 10.1038/cddis.2017.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang L., Ning F.B. Erythropoietin reduces hippocampus injury in neonatal rats with hypoxic ischemic brain damage via targeting matrix metalloprotein-2. Eur. Rev. Med. Pharmacol. Sci. 2017;21(19):4327–4333. [PubMed] [Google Scholar]