Abstract
Cancer is still remain as a global burden with the 18.1 million and 9.6 million new cases and mortlities, respectively estimated globally. Leukemia may arise at all ages varied from the infants to elders. In this exploration, we planned to evaluate the antiproliferative effect of D-pinitol on human leukemia MOLT-4 cells. Anticancer potential of D-pinitol was examined using MTT assay. Reactive oxygen species (ROS) generation was studied by fluorescence microscopic method using DCFH-DA staining. Apoptotic morphological alterations were determined by dual staining (acridine orange and ethidium bromide). Western blot and ELISA methods were employed to study apoptotic protein expression. D-pinitol treatment significantly induced cytotoxicity in human leukemia MOLT-4 cells. We observed that D-pinitol induces the generation of ROS in MOLT-4 cells. Further, we noticed that D-pinitol significantly induced apoptosis in a dosage dependent manner. Moreover, western blot and ELISA based analysis revealed that D-pinitol elevated the Bax, Caspase-3, Caspase-9 and attenuated the Bcl-2 expression in leukemic cancer cell. Our findings suggest that D-pinitol treatment induces the apoptosis in human leukemic cells by generating intracellular ROS and modulating apoptotic protein expression.
Keywords: Apoptosis, Leukemia, D-pinitol, Caspases, MOLT-4 cells, Reactive oxygen species
1. Introduction
Cancer is distinguished by the abnormal growth and multiplication of cells and remains a global burden. According to statistics from GLOBOCAN, 2018, there were about 18.1 million and 9.6 million new cases and mortlities, respectively estimated globally. Among them, 437,033 number of new leukemia cases and 309,006 numbers of leukemia death was recorded (Bray et al., 2018a, Bray et al., 2018b). Several malignant disorders with a characteristic of increased numbers of leucocytes in blood and/or bone marrow collectively called as leukemia. Leukemias may arise at all ages varied from the infants to elders. Acute lymphoblastic leukemia is common in infancy; acute myeloid leukemia is frequent in older peoples; chronic myeloid leukemia is infrequent in young children; chronic lymphocytic leukemia is general form of leukemia in Western world (Juliusson et al., 2016).
Childhood leukemia is the most common diagnosed among all other leukemia worldwide. The excessive level of radiation and contact to the environmental radiation are identified as a influenced risk factor for leukemia (Belson et al., 2006). Whereas, DNA damaging therapy and previously diagnosed hematologic malignancy is more prone to develop AML. Therapy-linked ailments is primarily due to the chemotherapy, like topoisomerase II-inhibitors, alkylating agents and radiation, which is employed to treat the breast cancer and lymphoma, and is thus more frequent in elder peoples (Sill et al., 2011). Topoisomerase II-inhibitors and alkylating agents like chemotherapeutic agent ends up with therapy related disease. About 8% of adult AML patient has been reported for therapy related disease (Sasaki et al.,2016). Apart from these some of genetic factors such as Down’s syndrome, germline mutations and ETV6 have also been documented to be linked with an augmented risk.
The intrinsic apoptotic pathway is activated in reaction to cellular stimuli, including failure in a repair of DNA damage, hypoxia, oxidative stress, high intracellular calcium (Elmore et al., 2007). Mitochondria play a crucial function in orchestrating intrinsic apoptosis, by regulating the stability between pro-apoptotic and anti-apoptotic family of proteins. When the cells receive intracellular apoptotic signals, the pro-apoptotic overwhelm the anti-apoptotic proteins, resulting in augmented permeability of the mitochondrial membrane that finally results the cytochrome-c release into the cytoplasm. Later, it forms the multi-protein complex called as apoptosome. This apoptosome complex cleaves the pro-caspases into its active caspases, which are responsible for the morphological and biochemical changes during the apoptotic process (Wang et al., 2009). The extrinsic death receptor pathway is stimulated in response to extracellular stimuli-binding of death ligands such as TRAIL (TNF-related apoptosis inducing ligand), FasL, TNF (Tumor Necrosis Factor). Once the death ligands binds with their corresponding receptors, it forms DISC (Death Inducing Signaling Complex). This DISC has an ability to induce apoptosis through activating caspases-8 (Guicciardi et al., 2009).
Scientific and research interest in identification of novel agents from naturally derived drugs because of their less toxic side effects and health promoting activities (Cragg et al., 2013). Plants have been a prehistoric narrations of their usage in folk medicines. The flavonoids are secondary metabolite most commonly found in plants. It is well known for its versatile health benefit for example antioxidant activity, anti viral and anti bacterial infection, antinflammatory activity (Kumar et al., 2013). The vegetables and fruits containing rich flavonoids consumption are reported to prevent various types of human cancer in experimental models. D-pinitol is an active componenet of soybean. In soybean meal, found about 1% of dry weight (Smith et al., 1982). It is also found in cotyledon, embryo axis, and the seed coat of soybean (Kuo et al., 1997). Moreover, it received greater attention attention for their diverse range of biological activities such as antiinflammatory, antiviral, and antioxidant (Sethi et al., 2008, Sivakumar et al., 2010). In this investigation, we aimed to inspect the anticancer effect of D-pinitol in human leukemic cancer cell through the stimulation of apoptotic pathway.
2. Materials and methods
2.1. Chemicals
D-pinitol, dulbecco’s modified eagle medium (DMEM), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and all other chemicals, fluorescent stains, and reagents were procured in Sigma-Aldrich, USA. The kit and antibodies for western blotting were procured from the Biorad, Germany and R&D Biosystem, China, respectively.
2.2. Cell culture and condition
The anticancer potential of D-pinitol was inspected against the human leukemia (MOLT-4) cells. The cells were received from Cell Bank of Chinese Academy of Sciences (Shanghai, China) and grown as monolayer and at 37 °C in 5% CO2 atmosphere. DMEM medium along with the supplementation of 10% FBS, 1% glutamine, and 100 U/ml penicillin–streptomycin were used to maintain the cells. The stocks were sustained in T75 cm2 tissue culture flasks.
2.3. Cell viability assay
The cytotoxic level of D-pinitol against the human leukemia MOLT-4 cells was evaluated using 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltet-razolium bromide (MTT) based colorimetric assay (Liu et al., 1997). Cells were harvested from the exponentially grown stock culture and 10,000 numbers of cells per well loaded in 96 well plate. The cells were allowed for 24 h incubation at 5% CO2 chamber at 37 °C. After 24 h of incubation, cells undergone with various concentration of D-pinitol (from 5 to 100 µM) treatment and incubated for additional 24 h. After the treatment procedure, 100 µl of MTT solution was amalgamated and the incubation was continued for further 4 h. Finally, the DMSO (100 µl) was mixed to dissolve the MTT crystal and to stop the reaction. The absorbance were made at 570 nm.
2.4. Detection of intracellular ROS
Qualitative analysis of intracellular ROS were determined by using a fluorescent probe named 2, 7-diacetyldichlorofluorescein (DCFH-DA) in control and D-pinitol treated human leukemia (MOLT-4) cells as described previously (Lu et al., 2018). In brief, from the parental flask, cells were obtained by trypsinization and 1 × 106 cells per well were loaded in 6 well plates. Then, cells were supplemented with 25 and 50 µM of D-pinitol then maintained for 24 h. After that cells were thoroughly washed thrice with PBS at 5 min interval. Then, 5 μl of DCFH-DA (1 mg/ml) was mixed and incubated under dark for 30 min at 37 °C. Excessive dyes were removed by repeated washing with PBS. Atlast, cells were examined with the aid of the fluorescence microscope and images were photographed.
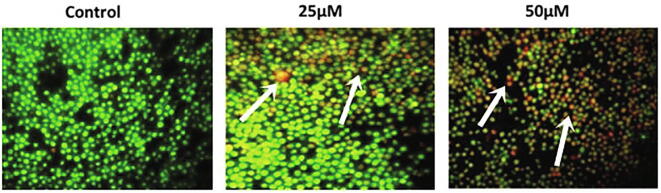
2.5. Detection of apoptotic morphological changes by acridine orange and ethidium bromide (Ao/EtBr) staining
D-pinitol stimulated apoptotic morphological alterations were studied in human leukemia MOLT-4 cells by employing AO/EtBr dual staining followed by the earlier methods Aithal et al., (2009). Briefly, from the parental flask, cells were collected by trypsinization and 1 × 106 cells per well were loaded in 6 well plates. Then, cells were supplemented with D-pinitol for 24 h. After that cells were thoroughly washed thrice with PBS at 5 min interval. Then, 1 μl of AO/EtBr (5 mg/ml concentration) was amalgamated and incubated under dark for 30 min at 37 °C. Excessive dyes were removed by repeated washing with PBS. Finally, cells were inspected under the fluorescence microscope and images were taken.
2.6. Western blot analysis
Proteins from the untreated normal and D-pinitol supplemented human leukemia MOLT-4 cells were separated with the aid of RIPA buffer, which consists of protease inhibitor cocktail (Sigma-Aldrich, Saint Louis, USA) in accordance with the instructions of manufacturer. The samples containing 50 µg of protein were boiled for 5 min with loading buffer and size-separated through 10% SDS-PAGE gel. Then gel was blotted onto a nitrocellulose membrane via the semi-dry equipment (Biorad, Germany). The membrane was incubated with 5% BSA at 4 °C for overnight. Later than washed with TBST and relevant primary monoclonal antibodies for Bcl-2, Bax, Cas-9, and Cas-3 were added (dilution 1:1000) and sustained for 5–6 h at 37 °C. Then secondary antibodies coupled with horseradish peroxidase (dilution 1:2000) was added for 2 h at 37 °C. Lastly, enhanced chemiluminescence method were employed to detect the bands (Biorad, Germany). The images were attained through Image Studio software (LI-COR) (Dalmau et al., 1990).
2.7. ELISA assay for apoptosis
The expression status of apoptotic proteins were examined through the respective ELISA test kits (R&D Biosystem, China) as mentioned previously by Chen et al. (2014).
2.8. Statistical analysis
Statistical assessments were executed in the SPSS (ver.16) statistical tool. Data were illustrated as mean ± standard deviation (SD). The one way analysis of variance (ANOVA) subsequently Duncan Multiple Range Test (DMRT) comparison test was executed to compare the statistical variations between the test groups. Data was regarded as significant if p value are less than 0.05.
3. Result and discussion
Cancer in humans has been recognized for age back in prehistoric periods (David et al., 2010). Currently, it accounts for about 18.1 million new incidences and 9.6 million mortalities estimated worldwide (Bray et al., 2018a, Bray et al., 2018b). Defeating cancer is being continuously failed, because cancer is a complex disease arise from different factors including environmental and genetic. However, still the treatment for is largely based on chemotherapeutic drugs to eliminate, reduce and also to alleviate pain (Tian et al., 2018). Chemotherapeutic agents are targeted to induce cell death in cancer cells by inducing DNA damage. The major limitation of these anticancer agents is often leads to disease recurrence (Sawicka et al., 2016). The next and most important limitation is an adverse toxicity of non-targeted organs or tissues.
Most of the clinical anticancer drugs work by damaging DNA. Such a DNA damaging chemotherapeutics like alkylating agent topoisomerase II-inhibitors, platinum derived drugs and radiation therapy or combination of these modalities are considered as complication and risk factors for different types of leukemia. Therapy-linked acute myeloid leukemia and myelodysplastic syndrome are well recognized clinical syndrome turn out into a late hurdle following the cytotoxic treatments (Goldstein et al., 2015). The distinctiveness of therapy induced leukemia primarily depends on the particular agents in addition to the collective dosage and strength of the radiotherapy. With a view to limiting these adverse effects of existing anticancer agent like cisplatin, new platinum derived anticancer drugs were developed (Hambley, 1997). Though they were successful in terms stability, unfortunately requires a higher dosage compared to cisplatin, ultimately ends up with myelosuppression (Kelland et al., 2007). The same kind of observation can also be seen in radiotherapy which stimulates rapid repopulation in cancer in in vitro and in vivo models.
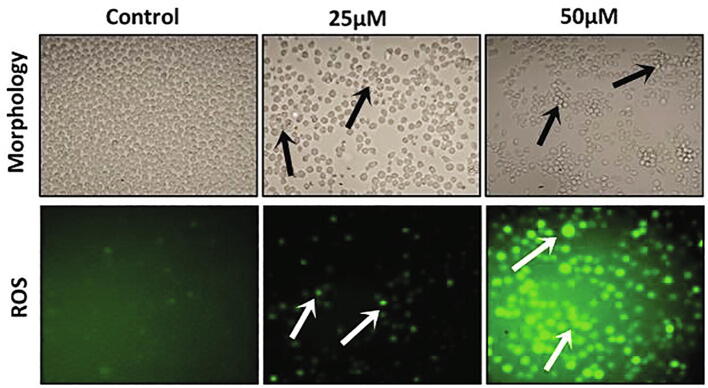
Over the four decades of time, there has been the continuous search for the small molecules from naturally occurring sources like plant or microbes chemotherapeutic drugs. Natural products displayed an significant benefits in establishing the cancer chemotherapeutic agents, either as its original/unmodified or synthetically modified forms. More than 50% FDA approved anticancer agents derived from natural products (Clark et al., 1996). In this current exploration, we inspected the anticancer activity of D-pinitol, a naturally occurring flavonoid against human leukemic cancer MOLT-4 cells. The level of the cytotoxicity of D-pinitol against the MOLT-4 cells investigated by the MTT assay (Fig. 1). D-pinitol at various concentrations (5–100 µM) for 24 hr treatment showed gradual decrease in cell proliferation. Further, we observed that D-pinitol inhibited over 50% of the cell growth at 25 µM dosage for 24 h. Further, we evaluated the ROS inducing ability of D-pinitol using fluorescent microscopic methods (Fig. 2). We noticed that D-pinitol effectively induces ROS generation in human leukemia MOLT-4 cells in a dosage relient mode. It is well documented that flavonoids undergo autoxidation thereby produces superoxide anions. Moreover, peroxidases can metabolize the phenol ring resulting in production of phenoxyl radicals (Nimse et al., 2015). Hence, we conclude that this might be the reason for D-pinitol mediated ROS and cytotoxicity in human leukemia MOLT-4. We examined the apoptosis inducing effect of D-pinitol on human leukemia MOLT-4 cells using AO/EtBr staining. The AO/EtBr staining results showed that D-pinitol treatment significantly induces the apoptosis in human leukemia MOLT-4 cells (Fig. 3). Further, we revealed that the D-pinitol mediated apoptotic effect was concentration dependent manner. The fluorescent microscopic images clearly shows that there were no apoptotic changes in untreated control cell (Fig. 3).
Fig. 1.
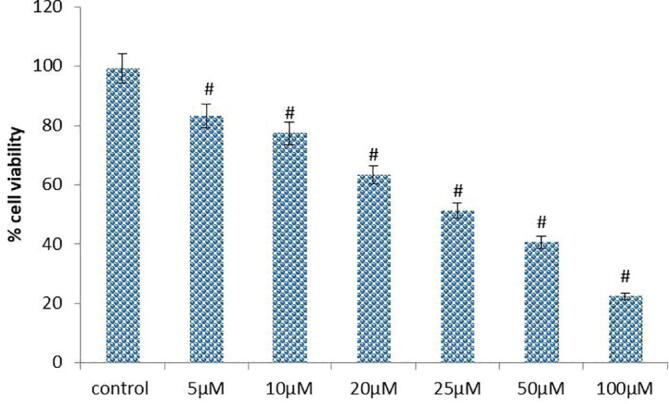
Effect of D-Pinitol induced cytotoxicity on human leukemic MOLT-4 cells. Cells (1X105 cells/well) were treated with increasing concentrations of D-Pinitol ranging from 5 to 100 µM for 24 h and then assessed for cell viability using MTT assay. Values are given as means ± S.D. of three experiments in each group. significantly at #P ≤ 0.05(DMRT).
Fig. 2.
Effect of D-Pinitol on intracellular ROS generation using DCFH-DA staining. Photomicrograph of D-Pinitol treated MOLT-4 cells show enhanced green fluorescence under a green lamp (20×).
Fig. 3.
Effect of D-Pinitol induced apoptosis using AO/EtBr staining. Photomicrograph of D-Pinitol treated MOLT-4 cells showed typical morphological changes and fragmented DNA. Images were taken under fluorescence microscope (20×).
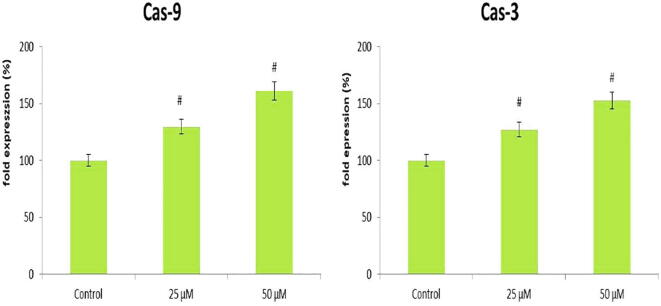
The Bcl-2 family of proteins performs a critical function in regulating the intrinsic mitochondrial apoptotic pathway. The modulation of Bcl-2 family proteins is frequently reported in human cancer. Recent progress made in understanding Bcl-2 family proteins or perturb mitochondrial integrity leads to development novel anticancer therapies that target Bcl-2. Many anticancer agents including phytochemicals mostly triggers the mitochondria mediated cell death through inhibiting Bcl-2 and activating Bax (Guo et al., 2003). In our finding, we noticed that D-pinitol increased the Bax and diminished the Bcl-2 activity in a dose relient mode (Fig. 4). Our outcomes were coincident with previous findings reported (Rengarajan et al., 2014). Hence, we conclude that increasing free radical generation and direct activation Bax might be responsible for this reported activity. In this investigation, further, we inspected the potential of D-pinitol induced apoptosis associated caspase mediated responses in MOLT-4 cells. Because, the caspase-3 is known to execute the apoptotic process. Results from the western blot analysis revealed that D-pinitol effectively induced caspases – 3 & 9 in a dosage dependent mode in MOLT-4 cells (Fig. 5). Similarly, it is important to observe that D-pinitol is already reported for inducing caspases and proapoptotic proteins like Bax in a concentration dependent fashion in breast cancer cells. This was further confirmed by fluorescent based apoptotic staining studies. In conclusion, our studies suggest that D-pinitol induces apoptosis via modulating proapoptotic protein expression through generating ROS in human leukemia MOLT-4 cells. However the further studies were still needed in the future to understand the exact therapeutic mechanisms of the D-pinitol against the leukemia.
Fig. 4.
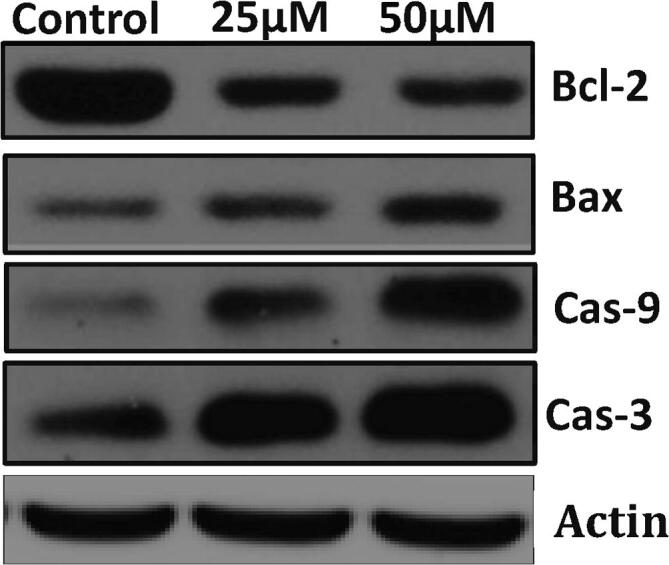
D-Pinitol induced apoptosis in MOLT-4cells. Cells treated with D-Pinitol for 24 h with or without were collected. Immunoblots were performed with cell lysate to determine the apoptotic protein expression such as Bax, Bcl-2, caspase-9 and caspase-3 by western blot analysis.
Fig. 5.
D-Pinitol induced Caspase-3 & 9 expression using ELISA method in human leukemiaMOLT-4 cells. Values are depicted as mean ± S.D. of three individual experiments in each group. Statistical significance at #P ≤ 0.05(DMRT).
Funding
Yunnan Applied Basic Research Projects (Joint fund for applied basic research of Kunming Medical University, Yunnan Provincial Department of science and technology): (A fundamental research on the effectiveness of EBNA1-CAR-T on EB virus and EBV related B cell lymphoma), NO. 2017FE468-105).
Footnotes
Peer review under responsibility of King Saud University.
References
- Aithal K.B., Kumar S.M., Rao N.B., Udupa N., Rao S.B. Juglone, a naphthoquinone from walnut, exerts cytotoxic and genotoxic effects against cultured melanoma tumor cells. Cell Biol. Int. 2009;33(10):1039–1049. doi: 10.1016/j.cellbi.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Belson M., Kingsley B., Holmes A. Risk factors for acute leukemia in children: a review. Environ. Health Perspect. 2006;115(1):138–145. doi: 10.1289/ehp.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer tatistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhang W., Lin J., Wang F., Wu M., Chen C., Zheng Y., Peng X., Li J., Yuan Z. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol. Ther. 2014;22(2):303–311. doi: 10.1038/mt.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.M. Natural products as a resource for new drugs. Pharm. Res. 1996;13(8):1133–1141. doi: 10.1023/a:1016091631721. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta. 2013;1830(6):3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J., Furneaux H.M., Gralla R.J., Kris M.G., Posner J.B. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer—a quantitative western blot analysis. Ann. Neurol.: Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1990;27(5):544–552. doi: 10.1002/ana.410270515. [DOI] [PubMed] [Google Scholar]
- David A.R., Zimmerman M.R. Cancer: an old disease, a new disease or something in between? Nat. Rev. Cancer. 2010;10(10):728. doi: 10.1038/nrc2914. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M., Kastan M.B. The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu. Rev. Med. 2015;66:129–143. doi: 10.1146/annurev-med-081313-121208. [DOI] [PubMed] [Google Scholar]
- Guicciardi M.E., Gore G.J. Life and death by death receptors. FASEB J. 2009;23(6):1625–1637. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P., Hu B., Gu W., Xu L., Wang D., Huang H.J., Cavenee W.K., Cheng S.Y. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am. J. Pathol. 2003;162(4):1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambley T.W. The influence of structure on the activity and toxicity of Pt anti-cancer drugs. Coord. Chem. Rev. 1997;166:181–223. [Google Scholar]
- Juliusson G., Hough R. Leukemia. Prog. Tumor Res. 2016;43:87–100. doi: 10.1159/000447076. [DOI] [PubMed] [Google Scholar]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7(8):573. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013;29:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T.M., Lowell C.A., Nelsen T.C. Occurrence of pinitol in developing soybean seed tissues. Phytochem. 1997;45(1):29–35. [Google Scholar]
- Liu Y., Peterson D.A., Kimura H., Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 1997;69(2):581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- Lu F., Sun J., Sun T., Cheng H., Yang S. Fluorescence-based measurements of store-operated Ca (2+) entry in cancer cells using fluo-4 and confocal live-cell imaging. Methods Mol. Biol. 2018;1843:63–68. doi: 10.1007/978-1-4939-8704-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Adv. 2015;5(35):27986–28006. [Google Scholar]
- Rengarajan T., Nandakumar N., Rajendran P., Haribabu L., Nishigaki I. Balasubramanian MP. D-pinitol promotes apoptosis in MCF-7 cells via induction of p53 and Bax and inhibition of Bcl-2 and NF-κB. Asian Pac. J. Cancer Prev. 2014;15(4):1757–1762. doi: 10.7314/apjcp.2014.15.4.1757. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Jabbour E., Cortes J. Outcome of patients with therapy-related acute myeloid leukemia with or without a history of myelodysplasia. Clin. Lymphoma Myeloma Leuk. 2016;16(11):616–624. doi: 10.1016/j.clml.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicka E., Mirończuk A., Wojtukiewicz M.Z., Sierko E. Chemoradiotherapy for locally advanced pancreatic cancer patients: is it still an open question? Contemp. Oncol. (Pozn). 2016;20(2):102–108. doi: 10.5114/wo.2016.60066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi G., Ahn K.S., Sung B., Aggarwal B.B. Pinitol targets nuclear factor-κB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol. Cancer Ther. 2008;7(6):1604–1614. doi: 10.1158/1535-7163.MCT-07-2424. [DOI] [PubMed] [Google Scholar]
- Sill H., Olipitz W., Zebisch A., Schulz E., Wölfler A. Therapy-related myeloid neoplasms: pathobiology and clinical characteristics. Br. J. Pharmacol. 2011;162(4):792–805. doi: 10.1111/j.1476-5381.2010.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar S., Palsamy P., Subramanian S.P. Attenuation of oxidative stress and alteration of hepatic tissue ultrastructure by D-pinitol in streptozotocin-induced diabetic rats. Free Radic. Res. 2010;44(6):668–678. doi: 10.3109/10715761003733901. [DOI] [PubMed] [Google Scholar]
- Smith A.E., Phillips D.V. Influence of sequential prolonged periods of dark and light on pinitol concentration in clover and soybean tissue. Physiolplantarum. 1982;54(1):31–33. [Google Scholar]
- Tian L., Kewei X., Yifan L. Anticancer effect of salidroside reduces viability through autophagy/PI3K/Akt and MMP-9 signaling pathways in human bladder cancer cells. Oncol. Lett. 2018;16:3162–3168. doi: 10.3892/ol.2018.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Youle R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]