Abstract
Bacillus subtilis was used for biogenic of silver nanoparticles. Characterization of the prepared silver nanoparticles was done by UV–Vis spectroscopy, Transmission Electron Microscopy (TEM), and Fourier Transform Infrared Spectroscopy (FT-IR). The particle size of the prepared nanoparticles ranges from 3 to 20 nm with spherical or roughly spherical forms. The antimicrobial efficacy of the produced nanoparticles was investigated against five strains of multidrug resistant microorganisms including: Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Klebsiella. pneumoniae,Escherichiacoli and Candida albicans tested as yeast. During this study, the minimum inhibitory concentrations (MICs) and the minimum lethal concentrations (MLCs) of synthesized silver nanoparticles were detected using selected strains of the genus Bacillus by a broth dilution method. The rate of MIC of the prepared silver nano-particles versus the investigated clinical isolates exhibit a massive anti-microbial efficacy; (230 µgml−1) for MRSA; 180 for Staphylococcus epidermidis, 200 for Escherichia coli and 100 µgml−1 for Candida albicans. On the other hand, the lowest anti-microbial efficacy (300 µgml−1) was appeared for Klebsiella pneumonia. The obtained results demonstrated the effectiveness of the biogenic nanoparticles and the possibility of using them as a new method in combating infectious diseases.
Keywords: Bacillus subtilis, AgNPs, Antimicrobial activity, Green biosynthesis
1. Introduction
In the past few years, work has increased in scientific research on the production of nanoparticles due to innovative applications in various industrial fields. Nanoparticles are the dispersion of particles from solid particles with a single dimension measured between one to one hundred nanometers. Nanoparticles contributed to opening different fronts to design new materials and assessing their properties by adjusting the size, shape, and distribution of their molecules (El-Gamal et al., 2018). Mineral nanoparticles are now widely used because of their unique properties such as being anti-bacterial and anti-cancer.
One of the most important characteristics of nanoparticles is that they have a large surface area relative to their very small size, which increases their interaction with other molecules. These remarkable properties have earned these metal nanoparticles a great interest in many applications such as biochemical sensors, electronic equipment, stimulants, biopsy, tumor imaging, drug making and pharmaceutical preparation methods (Gahlawat et al., 2016).
In fact, the production and stability of nanoparticles is done through a “top-down” or “bottom-up” strategy (Ahmed et al., 2016). In the bottom-up strategy, nanoparticles are produced by self-assembling atoms in the nucleus that develop into nanoparticles. This approach includes chemical and biological methods, while in the “top-down” strategy, large materials are broken down into small molecules by reducing their size by using different physical and chemical techniques. The physical methods used to produce nanoparticles include several methods, including grinding and thermal fusion. The chemical methods include electrochemical synthesis, chemical reduction, and optical chemical reduction technique (Gahlawat and Choudhury, 2019). A disadvantage of physical methods is that they require a large amount of energy, which makes these types of processes drain a lot of money. It also has a low yield of nanomaterials.
During the past years, the processes of synthesis of nanoparticles by chemical methods were more common because they do not require high energy during the reduction process and they also produce homogeneous particles of high accuracy in size and shape. Unfortunately, these chemical methods have serious environmental damage as a result of the use of risky chemicals, which are hydrazine and potassium betarutrate that cause cancer, genetic and cellular toxicity (Kharisov et al., 2016). The use of chemical methods for the biogenic of nano-particles used in medicine is non-existent due to the toxicity and instability of these molecules in addition to their biological bicompatibility (Shah et al., 2015). Therefore, new methods have been developed to synthesize nanoparticles that are more environmentally friendly and have an effective shape and size that are more stable (Kulkarni and Muddapur, 2015).
The biosynthesis process for nanoparticles provides a wide range of environmentally friendly specifications as the use of toxic chemical materials is low. Thus, it has been supposed to synthesize nanoparticles through a biological process using microorganisms as an alternative to chemical and physical methods (Li et al., 2016). The biogenic of nanoparticles using microbial methods is echo friendly and has advantages characteristics because it occurs at a relatively ambient temperature and pressure in addition to that they are methods that are less expensive and take a little time than those that use chemical or physical methods (Singh et al., 2016, Mohamed et al., 2017a, Mohamed et al., 2017b).
Bacteria are a prospective candidate for biogenic of nanoparticle consequent to manipulation of genetic material and the easy handling (Faramarzi and Sadighi, 2013). Also, bacteria can survive in all kinds of unfavorable conditions, for instance, high or low peaks of temperatures, variable degrees of alkalinity or acidity, and high salt concentrations. At the same time, biologically formed nanoparticles have many applications, such as being catalysts in chemical reactions (Li et al., 2016), optical receptors, an antimicrobial agent (Ranjan and Jadeja, 2017), as well as being able to precipitate nanoparticles due to their metabolic activity. The biological synthesis of nanoparticles by bacteria is facilitated by their ability to precipitate those molecules out of cells, as those nanoparticles can be obtained using cell filtration which is considered beneficial for intracellular synthesis (Natarajan and Selvaraj, 2010).
It has been known that bacteria synthesize nanoparticles in two ways, one through extracellular mechanisms and the second within cells. In this regard, Beveridge and Murray (1980) were the first to synthesize nanoparticles gold (AuNP) from the extracellular wall of B. subtilis using the gold chloride solution. In another work, Seudomonas stutzeri AG259 was used to produce AuNP nanoparticles accumulated inside cells, characterized by being of a small size (1–200 nm) using the NADH dependent enzyme. Also Srivastava et al. (2012) stated that Pseudomonas aeruginosa bacteria have the capacity to biogenic distinct types of nanoparticles within cells such as nanoparticles Pd, Ag, Rh, Ni, Fe, Co, Pt, and Li without ever having to use any type of stabilizing agent and electron, then studies continued to test many bacteria in their ability to synthesize nanoparticles such as those conducted on Escherichia coli, B.subtilis, B.megaterium, B.cereus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Alteromonas and Ochrobactrum sp.etc.
Recently a group of bacteria has developed itself to become more resistant to antibiotics, making it more dangerous for the human race. Now the nanoparticles are a newly innovative new agent with a wider surface area relative to their small size and with physical and chemical properties that make them highly capable of dealing with these pathogenic bacterial species and that can penetrate the cell wall. There are many experiments that have proven this true (Ali et al., 2018).
In this study, five strains of Bacillus were examined for the synthesis of AgNPs. Among these different Bacillus species, only Bacillus subtilis, has demonstrated greater potential for synthesis of AgNPs. Ultraviolet spectroscopy, FTIR, and TEM analyzes were used to characterize the formed AgNPs. These AgNPs are also studied for their antimicrobial activity against five different species of multidrug resistant microbes.
2. Materials and methods
2.1. Test microorganisms
Culture of five strains of Bacillus which are Bacillus subtilis, B. cereus, B. megaterium, B. pumilus, and B. circulans, were obtained from the Riyadh Military Hospital, Riyadh, Saudi Arabia to be used in the biogenic of AgNPs. The antimicrobial efficacy of the produced AgNPs were tested versus five different species of multidrug resistant microbes which also kindly provided by the Riyadh Military Hospital, Riyadh, Saudi Arabia including: Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Klebsiella pneumoniae, Escherichia coli and Candida albicans tested as yeast.
2.2. Chemicals
During my experiments, all the chemicals, media, reagents and AgNO3 were of analytical grade.
2.3. Instruments
-
•
Spectrophotometer: JASCO V-560 UV–visible spectrophotometer, Japan.
-
•
FT-IR: JASCO FT/IR-3600 infra- red spectrometer, Japan.
-
•
Transmission Electron Microscope: JEOL electron microscope JEM-100 CX, Japan.
2.4. Production of biomass
The test of the different isolates was grown aerobically. The microbial cultures were brood on an Orbital Shaker at 37 °C and continuous agitation at 200 rpm. The microbial biomass was collected after 24 h of growing and centrifuged at 10,000 rpm for ten minutes.
2.5. Biogenic of silver nanoparticles
Preparation of Ag nanoparticles was conducted according to Kalishwaralal et al., 2008 and Vaidyanathan et al. (2010). For the preparation of AgNPs, two solutions were prepared; the first one was: 100 ml of supernatant was mixed with one ml of silver nitrate solution (1 mM) and the second reaction mixture was prepared without AgNO3 that used as a control test. The designed solutions were incubated at 30 °C for 24 h. All solutions were preserved in dark to abolish any photochemical reversion during the experiment. Then, the solutions turned from yellow into brown colure. The silver nanoparticles were purified by centrifugation at 10000 rpm for five minutes twice, and collected for characterization. To screening the most potent Bacillus strains for microbiological synthesis of silver nanoparticles, the optical density of silver nanoparticles synthesized by different cell supernatants was measured.
2.6. Effect of time on AgNPs biosynthesis
To investigate the efficacy of time on the biosynthesis of silver nanoparticles, a fresh colony of the best Bacillus strain produces for silver nanoparticles was selected. The culture tubes were incubated for 60 min then one ml of the growth was inoculated in new flasks containing ten ml LB broth incubated at 37 °C with continued shaking 150 rpm for 20 h. One ml of nanoparticle suspension was added to the experimental flask. The growth of the microbe was detected by determining the O.D. of culture at regular time interval (4 h) by UV–Vis Spectroscope at 600 nm. An equal volume from each culture was outgoing and the optical density was calculated and draws the growth curves of microbial strains.
2.7. Characterization of silver nanoparticles
2.7.1. UV–vis spectroscopy
UV–vis spectrophotometer from 200 to 900 nm operated at a resolution of 1 nm was used as a function of wavelength for spectral analysis of silver nanoparticles.
2.7.2. Transmission electron Microscopy
The size and morphology of the synthesized nanoparticles were recorded by using TEM model JEOL electron microscope JEM-100 CX. TEM studies were prepared by drop coating silver nanoparticles onto carbon-coated TEM grids. The film on the TEM grids were allowed to dry, the extra solution was removed using a blotting paper.
2.7.3. FT-IR spectroscopy.
FTIR was used to recognize the conceivable biomolecules charge of the reduced Ag ions and capping of the bio-reduced silver nanoparticles produced by the microbial extract. In order to detect the functional groups and their possible partnership in the biogenic of silver nanoparticles, the freeze-dried produced nanoparticles were grinded with potassium bromide and the spectrum was detected by using FTIR spectroscopy (VERTEX 70 Spectroscopy, Japan).
2.8. Screening of the antimicrobial activity
The antimicrobial study of the silver nanoparticles synthesized including zone of inhibition, MIC, MIC50, MIC90 and minimum lethal concentration (MLC) calculations was conducted for all the investigated organisms.
2.8.1. Determination of inhibition zone by disc diffusion method
The antimicrobial efficacy of the silver nanoparticles samples was investigated by the method described by Bauer et al., (1966).
2.8.2. Determination of (MIC and MLC)
Five different strains; S. aureus (MRSA), Staphylococcus epidermidis, Klebsiella pneumoniae, Escherichia coli and Candida albicans (tested as yeast) were chosen for investigation in my study. The MICs definition were outright in Luria Bertani (LB) broth in twin using serial two-fold dilutions of silver nanoparticles in concentrations extend from 1600 to 0.049 ppm, along with positive control tube (the microorganism in LB broth) and negative control one (LB broth) (CLSI, 2009). The MIC was determined after 24 h of incubation at 37˚C with initial inoculums of 0.1 OD at 600 nm. The MIC is the lowest concentration of AgNPs that completely visually inhibits 99% growth of the tested microorganisms. The concentrations of AgNPs able to inhibit the visible growth of 50% and 90% of a population of microorganisms (MIC50 and MIC90, respectively) were also determined. After MICs determinations, aliquots of 50 ul from all tubes in which no visible growth was observed were seeded in Mueller-Hinton Agar plates (MHA) not supplemented with silver nanoparticles and were incubated for 24 h at 37 °C. The MLC was defined as the lowest concentration of AgNPs with no growth on the MHA medium (i.e. kills 100% of the initial microbial population) (Ansari et al., 2011). The bacteristatic and fungistatic effects of the silver nanoparticles versus the tested microbial strains were represented by the MIC while, the bactericidal and fungicidal activities were represented by the MLC (MBC & MFC for bacteria and yeast strains, respectively).
2.9. Statistical analysis
Testing the significance of antimicrobial activity of silver nanoparticles was carried out by standard analysis of variance (ANOVA) Version 9 by SAS Institute Inc. Cary, NC, USA. The determinations were done in triplicate and the mean values ± SD were presented.
3. Results
3.1. Screening of the activity of different Bacillus strains for AgNPs biosynthesis
On the screening of the most potent specie of the studied five Bacillus species for microbiological biogenic of silver nanoparticles, it was found that the B. subtilis strain showed the best results with the highest optical density so it was used for the rest of the study (Table 1).
Table 1.
Screening of the antimicrobial activity of five Bacillus species.
| Bacillus species | Absorbance |
|---|---|
| B. subtilis | 3.2 |
| B. cereus | 2.5 |
| B. coagulans | 2.2 |
| B. megaterium | 2 |
| B. pumilus | 1.6 |
3.2. Biogenic of AgNPs by bacterial reaction
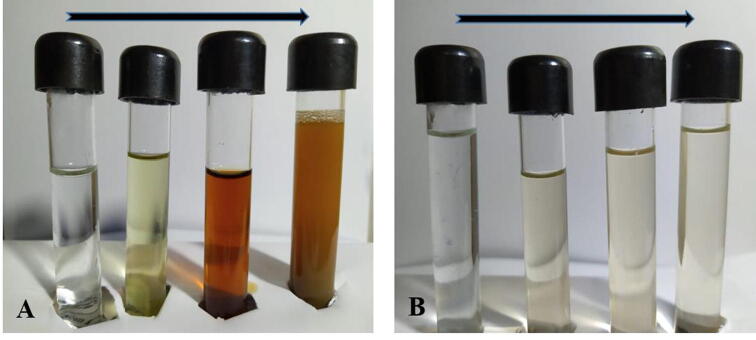
The aqueous silver ions (Ag+) were reduced to AgNPs when added to the cell-free supernatant of B. subtilis within 18 h after incubation. During the incubation period, the yellow color was changed to brown color and the control showed no change in color (Fig. 1).
Fig. 1.
Biogenic of AgNPs by bacterial reaction (A) and the negative reaction in the control test (B).
3.3. Effect of time on the biogenic AgNPs
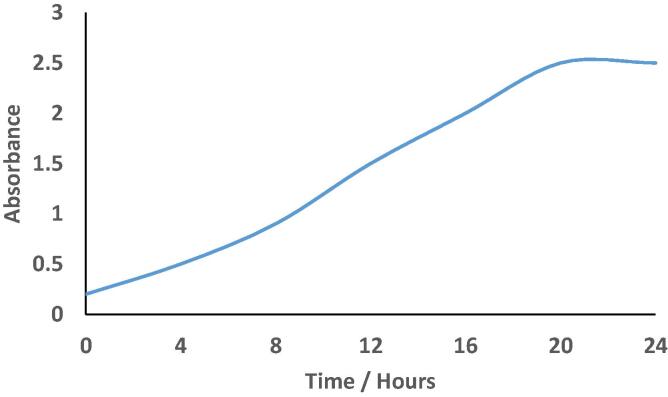
As a function of time, the UV–Vis spectra absorbance at a concentration of 1 mM silver nitrate and 5 ml of cell free supernatant, indicates that the reaction was completed during 18th hours of the incubation period. An increase in time does not affect the formation of silver nanoparticles as shown in Fig. 2.
Fig. 2.
Effect of time on the biogenic AgNPs.
3.4. Characterization of silver nanoparticles
3.4.1. UV–vis. Spectroscopy
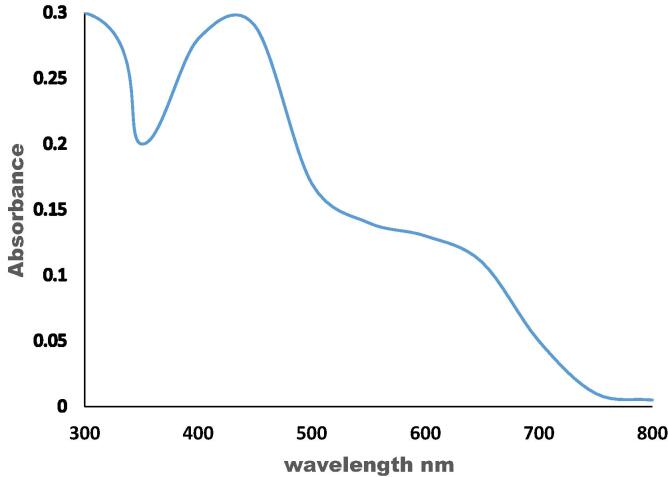
The formulation of silver nanoparticles was assured by employ a UV–visible spectral scan at 200–800 nm. The dispersed silver nanoparticles revealed an intensive color which attributed to the absorption for the surface plasmon resonance spectrum (SPR) of silver nanoparticles. Subsequently, metallic bio-nanoparticles own a distinctive visual absorption spectrum in the UV–visible region. The strong UV– visible spectrum of silver nanoparticles was a broad peak and existing between 400 and 470 nm reference the existence of round or nearly round silver nano-particles (Fig. 3).
Fig. 3.
UV Vis- Spectrum of the produced AgNPs by B.subtilis.
3.4.2. TEM analysis
TEM analysis were applied to characterize the formation type and size of produced silver nanoparticles. Low magnification TEM micrographs indicated that the particles were rounded or nearly rounded in shape and monodisperse distributed without considerable integration (Fig. 4). The NPs size ranges from 3 to 20 nm.
Fig. 4.
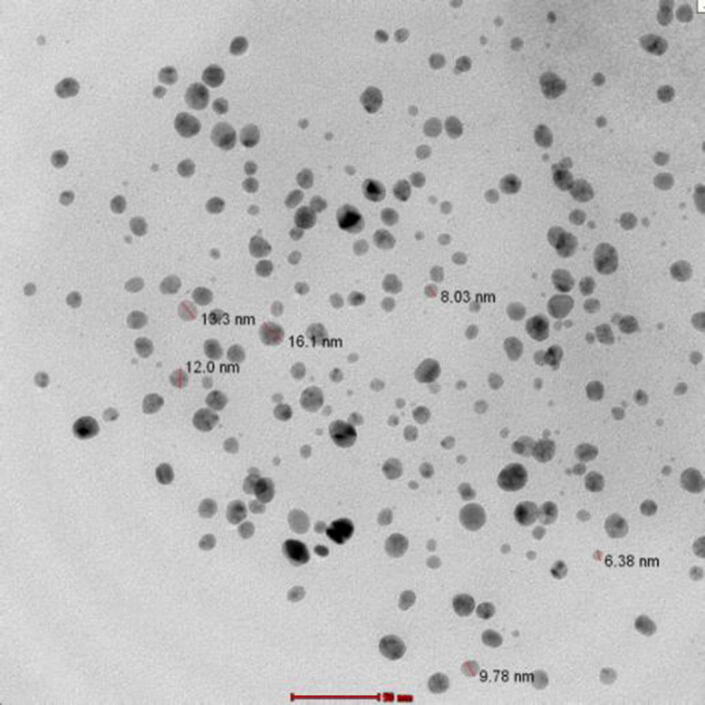
Representative TEM micrograph AgNPs synthesized by the reduction of AgNO3 ions in B.subtilis supernatant.
3.4.3. Fourier Transform Infra-Red spectroscopy (FT-IR).
FT-IR measurements were carried out in order to obtain information about chemical groups present around silver nanoparticles for their stabilization and understand the transformation of functional groups due to reduction process. The processes were carried out using JASCO FT/IR-3600 infra-red spectrometer by employing KBr pellet technique (see Fig. 5).
Fig. 5.
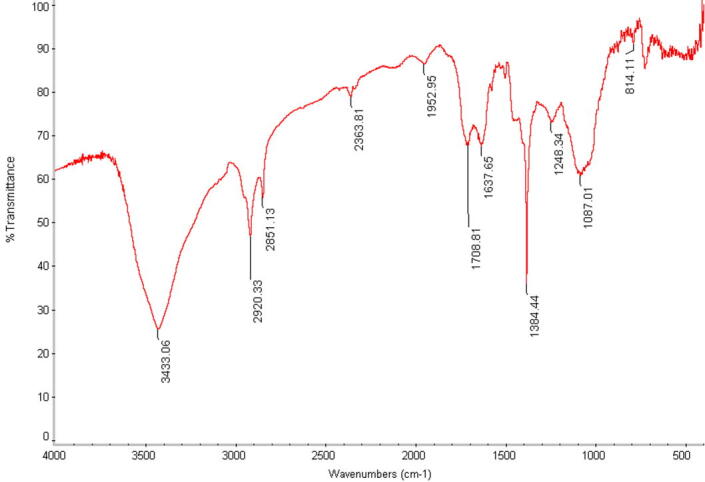
FTIR spectrum of fermented extract with silver nanoparticles.
3.5. Antimicrobial efficacy
3.5.1. AgNPs efficacy against different strains of pathogenic bacteria and yeast.
In this study, the antimicrobial efficacy of the formed silver nano-particles versus five species of highly pathogenic multidrug resistant microbes was investigated. The anti-microbial activity of silver NPs have been compared to Streptomycin and Fluconazole as antibacterial and antifungal drugs, respectively. The synthesized AgNPs were proved to have anti-microbial efficacy against all the investigated microorganisms (Table 2). The diameters of clear area (mm) determined for Staphylococcus aureus (MRSA), Klebsiella pneumoniae, Escherichia coli and Candida albicans were 30.12, 39.00, 31.04, 35.93 and 28.41, respectively. The inhibitory effect of the silver nano-particles on each test organism is particular and diverges from one to another. The obtained data clearly indicates that, the difference in the inhibitory action of silver nanoparticles on the tested microorganisms was statistically significant (P- value < 0.05) (see Table 2).
Table 2.
Diameter of inhibition zones of the synthesized AgNPs efficacy against different strains of pathogenic bacteria and yeast (Data were represented by mm as Mean of three replicates ± SD).
| Teste strain | Streptomycin (Standard antibacterial agent) | Fluconazole (Standard antifungal agent) | Mean of Inhibition zone diameter nm (Mean ± SD) | P-value |
|---|---|---|---|---|
| Staphylococcus aureus (MRSA) | 23 | ------ | 30.12 ± 0.54 | 0.0523* |
| Staphylococcus epidermidis | 32 | ------- | 39.0 ± 0.09 | 0.0276* |
| Klebsiella pneumoniae | 28 | -------- | 31.04 ± 1.52 | 0.0113* |
| Escherichia coli | 27 | ------ | 35.93 ± 0.26 | 0.0551* |
| Candida albicans | --------- | 22.01 | 28.41 ± 1.03 | 0.0917* |
The concentration of standard antibiotic was 200 µgml−1.
The concentration of AgNPs was 200 µgml−1.
p-value significant < 0.05.
3.5.2. Minimum inhibitory and minimum lethal concentration values of the silver nanoparticles synthesized for the selected strains.
Using the standard broth macro dilution method, the MIC, MIC50, MIC90 and MLC values of the silver nano-particles for the tested microbial-strains were calculated and summarized in (Table 3). The rate of MIC of the prepared silver nano-particles versus the investigated clinical isolates exhibit a massive anti-microbial efficacy; (230 µgml−1) for MRSA; 180 for Staphylococcus epidermidis, 200 for Escherichia coli and 100 µgml−1 for Candida albicans. On the other hand, the lowest anti-microbial efficacy (300 µgml−1) was appeared for Klebsiella pneumoniae.
Table 3.
Minimum inhibitory and minimum lethal concentration values of the AgNPs synthesized for the selected strains.
| Tested strains | MIC (µgml−1) | MIC50 (µgml−1) | MIC90 (µgml−1) | MLC MIC (µgml−1) |
|---|---|---|---|---|
| Staphylococcus aureus (MRSA) | 230 | 60 | 140 | 380 |
| Staphylococcus epidermidis | 180 | 70 | 100 | 220 |
| Klebsiella pneumoniae | 300 | 90 | 160 | 500 |
| Escherichia coli | 200 | 50 | 100 | 250 |
| Candida albicans | 100 | 205 | 370 | 450 |
4. Discussion
4.1. Biogenic of AgNPs by bacterial reaction
The primary confirmation of biogenic bio-nanoparticles in the microbial supernatant was described by the change in colour from yellowish-white to brown. Addition of Ag+ ions to the supernatant exhibit the data as a color-forming to brown due to the reduction of Ago (Ranganath et al., 2012). Control (culture supernatant with no silver nitrate) showed no change in color when incubated for the same duration and conditions (Fig. 1) (see Fig. 5).
4.2. Effect of time on the biogenic AgNPs
The change in color throughout the supernatant was occurred after 12 h of incubation and the maximum color was completed at 18 h depending on the stages in microbial growth. It is agreement with Gurunathan et al., 2009, Sweeney et al., 2006, Kalimuthu et al., 2008 who announces that the farthest production of bio-nanoparticles acquired during the culture in the constant (stationary) phase. However, Natarajan et al., 2010a, Natarajan et al., 2010b reported that the farthest production of bio-nanoparticles was recorded during the period with the exponential phase. It was believed the biogenic of nanoparticles due to the proteinous molecules and enzymes include nitrate- reductase enzyme which acts as a perfect streamline factor in silver nanoparticles biosynthesis (Natarajan et al., 2010a, Natarajan et al., 2010b).
4.3. UV Vis- spectrum of the produced AgNPs by B.subtilis.
The appeared color pliable to measurement the absorbance against distinct wavelength to emphasize the construction of silver nanoparticles. The conformable UV–vis absorption spectra were summarized in Fig. 3. The sample showed a broad spectrum range of around 400–470 nm. The appearance of wide resonance suggests the accumulation of silver nanoparticles. It was indicated that at 410 nm coincide with the transfer plasmon vibration in silver nanoparticles while the peak at 470 nm due to the excitement of longitudinal plasmon vibrations. The spectrum with bands in this domain has been connected with the surface-plasmon resonance of any-sized Ag metal, corroborative the appearance of silver nanoparticles in the supernatant after insinuation to ultraviolet light (Shankar et al., 2003).
4.4. TEM analysis
The amorphous nature of the silver nanoparticles is also confirmed from TEM pictures as well. The biologically prepared NPs are in round or slightly rounded shapes. The average particle size was around 3–20 nm. The biogenic silver nanoparticles can be used in different medical treatments, such as, killing of unwanted microorganisms with UV irradiation and subsequently use as an optical probe in medical diagnosis by using the visible emission (Show et al., 2015). These outcome data are comparable to the previously recorded data using culture supernatants of Bacillus subtilis (PTCC 1023) and Candida albicans (PTCC 5011) (Minaeian et al., 2008, Natarajan et al., 2010a, Natarajan et al., 2010b). The prospect to acquire bio-nanoparticle shape monitoring by employ green biogenic approaches is expectant to open up a new spectacular way for ecofriendly, large scale, and economically applicable controlled shape biogenic of bio-nano materials (Abdel-raouf et al., 2018, Abdel-raouf et al., 2019).
4.5. FTIR spectrum of fermented extract with silver nanoparticles
FTIR measurement was executed to distinguish the potential interactions between Ag and bio-active compounds, which may be accountable for the production and stability of silver nanoparticles as capping agent. The carboxylic acid derivative with an amine group among amino-acid remnant in proteinous compounds indicate the infrared region of the electromagnetic spectrum. The presence of the amino acid peaks sustenance the existence of protein in clear filtrate as spotted in UV–vis spectra. In this regard, El-Batal et al. (2013) suggest that interactions between nanoparticles and proteins can take place either through free amine groups or cysteine residues in proteins as well as through the electrical attraction processes of negatively-charged carboxyl groups in enzymes. This evidence indicates that when protein molecules are released outside the bacterial cell it can function to form and stabilize silver nanoparticles in aqueous media.
4.6. Antimicrobial activity of the biogenic silver nanoparticles
Regarding the anti-microbial efficacy of the prepared silver nanoparticles, the lethal mechanism against the tested pathogenic microbes may comprise the releasing of silver ions (Ag+) from silver nanoparticles and the consistency of crystalline carbohydrates, lipids, nucleic acids, and proteins, assembled with silver nanoparticles steady on the microbial walls, forming cavity and piercing inside the plasma membrane to the cytoplasm (Sahu et al., 2013).
Other investigations suggest that silver nanoparticles may connect to the exterior of the plasma membrane troubling the permeable process and respiratory functions of the cell or by interfering with components of the microbial electron transport system (Sharma et al., 2009). In this regard, a study was conducted that stated the antimicrobial activity of silver nanoparticles due to the positive charge on Ag+ ion which is conclusive to its antimicrobial potential through electrostatic attraction factors between the cell membranes of microbes of negative charge and positively charged NPs. (Vijayakumar et al., 2013). Furthermore, the silver bio-nanoparticles not only interface with the exterior of the cell membranes, but they also have the ability to sneak these membranes and pass into the microbial cells (Sharma et al., 2009).
On the other hand, the silver metal-free radicals increase the oxidation forces, such as the strength of the reactive oxide species (ROS), which have the power to deteriorate the microbial membranes and nucleic acids, which ultimately leads to cell death (Hajipour et al., 2012, Tamboli and Lee, 2014). Former works particular that the Ag ion liberated from silver nanoparticles was accountable for antimicrobial efficacy (Feng et al., 2000). The free Ago ion can subsequently join with the thiol series of enzymes (Zhang et al., 2013). In this respect, it was found that the silver bio-nanoparticles which synthesized at 100 °C for six hours were poisonous to both gram-positive and gram-negative bacteria. This intensity may be due to the minimal size of the silver bio-nanoparticles simulated under this status which results in a higher surface area (Cui et al., 2013). Also, from the reported results the formed nanoparticles exhibited a highly pronounced antifungal activity against yeast tested which might be probably through destruction of yeasts potential and membrane integrity resulting in the consistence of grove and cell death. Different other works suggest that, suppression of bud growth get together with membrane injury and suggest that silver nanoparticles prevent the common growth process through the demolition of membrane benignity (Nasrollahi et al., 2011).
5. Conclusion
This data suggest green and eco-friendly method to biogenic silver bio-nanoparticles by Bacillus subtilis. Silver nanoparticles was reduced in a Bacillus subtilis culture supernatant. Bacillus subtilis supernatant was used as both reducing and stabilizing factors. The antibacterial efficacy of silver bio-nanoparticles display a commitment for use as a strong agent to treat multidrug resistant microorganisms.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This publication was supported by the Deanship of Scientific Research at Prince Sattam bin Abdulaziz University, Alkharj, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-raouf N., Al-Enazi N.M., Ibraheem I.B.M., Alharbi R.M., Alkhulaifi N.M. Biosynthesis of silver nanoparticles by using of the marine brown alga Padina pavonia and their characterization. Saudi J. Biol. Sci. 2019;26(6):1207–1215. doi: 10.1016/j.sjbs.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-raouf N., Alharbi R.M., Al-Enazi N.M., Alkhulaifi N.M., Ibraheem I.B.M. Rapid biosynthesis of silver nanoparticles using the marine red alga Laurencia catarinensis and their characterization. Beni-Suef Univ. J. Basic Appl. Sci. 2018;7(1):150–157. [Google Scholar]
- Ahmed S., Ahmad M., Swami B.L., Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016;7(1):17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I., Qiang T.Y., Ilahi N., Adnan M., Sajjad W. Green synthesis of silver nanoparticles by using bacterial extract and its antimicrobial activity against pathogens. Int. J. Biosci. 2018;13(5):1–15. [Google Scholar]
- Beveridge T.J., Murray R.G. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980;141(2):876–887. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Chen P., Chen S., Yuan Z., Yu C., Ren B., Zhang K. In situ study of the antibacterial activity and mechanism of action of silver nanoparticles by surface-enhanced Raman spectroscopy. Anal. Chem. 2013;85:5436–5443. doi: 10.1021/ac400245j. [DOI] [PubMed] [Google Scholar]
- El-Batal A.I., Amin M.A., Shehata M.M.K., Hallol M.M.A. Synthesis of Silver Nanoparticles by Bacillus stearothermophilus Using Gamma Radiation and Their Antimicrobial Activity. World Appl. Sci. J. 2013;22(1):01–16. [Google Scholar]
- El-gamal M.S., Salem S.S., Abdo A.M. Biosynthesis, characterization, and antimicrobial activities of silver nanoparticles synthesized by endophytic Streptomyces sp. Egypt. J. Biotechnol. 2018;56:69–85. [Google Scholar]
- Faramarzi M.A., Sadighi A. Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv. Colloid Interface Sci. 2013;189–190:1–20. doi: 10.1016/j.cis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Feng Q., Wu J., Chen G., Cui F., Kim T., Kim J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:43.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Gahlawat G., Shikha S., Chaddha B.S., Chaudhuri S.R., Mayilraj S., Choudhury A.R. Microbial glycolipoprotein-capped silver nanoparticles as emerging antibacterial agents against cholera. Microb. Cell Fact. 2016;15(1):25. doi: 10.1186/s12934-016-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahlawat G., Choudhury A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019;9:12944–12967. doi: 10.1039/c8ra10483b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S., Kalishwaralal K., Vaidyanathan R., Venkataraman D., Pandian S.R., Muniyandi J., Hariharan N., Eom S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surfaces B: Bioniterfaces. 2009;74(1):328–335. doi: 10.1016/j.colsurfb.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Hajipour M.J., Fromm K.M., Ashkarran A.A., De-Aberasturi D.J., De-Larramendi I.R., Rojo T., Serpooshan V., Parak W.J., Mahmoudi M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Kalimuthu K., Babu S.R., Venkataraman D., Bilal M., Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf., B. 2008;65(1):150–153. doi: 10.1016/j.colsurfb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Kharisov B.I., Kharissova O.V., Ortiz-Mendez U. CRC Press; 2016. 2016CRC concise encyclopedia of nanotechnology. [Google Scholar]
- Kulkarni N., Muddapur U. Biosynthesis of Metal Nanoparticles: A Review. J. Nanotechnol. 2015;2014:1–8. [Google Scholar]
- Li J., Li Q., Ma X., Tian B., Li T., Yu J., Dai S., Weng Y., Hua Y. Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int. J. Nanomed. 2016;11:5931–5944. doi: 10.2147/IJN.S119618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minaeian S., Shahverdi A.R., Nohi A.S., Shahverdi H.R. Extracellular biosynthesis of silver nanoparticles by some bacteria. J. Sci. IAU. 2008;17:1–4. [Google Scholar]
- Mohamed A.A., Fouda A., Elgamal M.S., Hassan S., Shaheen I.T., Salem S.S. Enhancing of cotton fabric antibacterial properties by silver nanoparticles synthesized by new Egyptian strain Fusarium keratoplasticum A1–3. Egypt. J. Chem. 2017;60:63–71. [Google Scholar]
- Mohamed A.A., Hassan S., Fouda A., Elgamal M.S., Salem S.S. Extracellular biosynthesis of silver nanoparticles using Aspergillus sp. and evaluation of their antibacterial and cytotoxicity. J. Appl. Life Sci. Int. 2017;11(2):1–12. [Google Scholar]
- Nasrollahi A., Pourshamsian K.H., Mansourkiaee P. Antifungal activity of silver nanoparticles on some fungi. Int. J. Nano. 2011;1:233. [Google Scholar]
- Natarajan K., Selvaaj S., Murty V.R. Microbial production of silver nanoparticles. Digest J. Nanomater. Biostruct. 2010;5(1):135–140. [Google Scholar]
- Natarajan K., Selvaraj S. Microbial production of silver nanoparticles. Digest J. Nanomater. Biostruct. 2010;5(1):135–140. [Google Scholar]
- Natarajan K., Subbalaxmi S., Ramachandra M.V. Microbial production of silver nanoparticles. Digest J. Nanomater. Biostruct. 2010;5:135–140. [Google Scholar]
- Ranganath E., Rathod V., Banu A. Screening of Lactobacillus spp, for mediating the biosynthesis of silver nanoparticles from silver nitrate. IOSR J. Pharmacy. 2012;2(2):237–241. [Google Scholar]
- Ranjan R., Jadeja V. Isolation, characterization and chromatography-based purification of antibacterial compound isolated from rare endophytic actinomycetes Micrococcus yunnanensis. J. Pharm. Anal. 2017;7:343–347. doi: 10.1016/j.jpha.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu P.K., Giri D.D., Singh R., Pandey P., Gupta S., Shrivastava A.K., Kumar A., Pandey K.D. Therapeutic and medicinal uses of Aloe vera: a review. Pharmacol. Pharmacy. 2013;4:599–610. doi: 10.4236/pp.2013.48086. [DOI] [Google Scholar]
- Shah M., Fawcett D., Sharma S., Tripathy S.K., Poinern G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials (Basel) 2015;8(11):7278–7308. doi: 10.3390/ma8115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S.S., Ahmad A., Sastry M. Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog. 2003;19:1627–1631. doi: 10.1021/bp034070w. [DOI] [PubMed] [Google Scholar]
- Sharma V.K., Yngard R.A., Lin Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009;145:83. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Show S., Tamang A., Chowdhury T., Mandal D., Chattopadhyay B. Bacterial (BKH1) assisted silica nanoparticles from silica rich substrates: a facile and green approach for biotechnological applications. Coll Surf B. 2015;126:245–250. doi: 10.1016/j.colsurfb.2014.12.039. [DOI] [PubMed] [Google Scholar]
- Singh P., Kim Y., Zhang D., Yang D. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016;34:588–599. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Sweeney S.F., Woehrle G.H., Hutchison J.E. Rapid purification and size separation of gold nanoparticles via diafiltration. J. Am. Chem. Soc. 2006;128(10):3190–3197. doi: 10.1021/ja0558241. [DOI] [PubMed] [Google Scholar]
- Tamboli D.P., Lee D.S. Mechanistic antimicrobial approach of extracellularly synthesized silver nanoparticles against gram positive and gram negative bacteria. J. Hazard. Mater. 2014;260:878–884. doi: 10.1016/j.jhazmat.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M., Priya K., Nancy F.T., Noorlidah A., Ahmed A.B.A. Biosynthesis, characterisation and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind. Crops Prod. 2013;41:235. [Google Scholar]
- Zhang Y., Cheng X., Zhang Y., Xue X., Fu Y. Biosynthesis of silver nanoparticles at room temperature using aqueous aloe leaf extract and antibacterial properties. Colloids Surf., A. 2013;423:63–68. doi: 10.1016/j.colsurfa.2013.01.059. [DOI] [Google Scholar]
- Kalishwaralal, K., Deepak, V. Ramkumarpandian, S. Nellaiah, H., Sangiliyandi, G., 2008. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Materials Letters, 62: 4411-4413.
- Vaidyanathan, R., Gopalram, S., Kalishwaralal, K., Deepak, V., Pandian, S.R.K., Gurunathan, S. 2010. Enhanced silver nanoparticle synthesis by optimization of nitrate reductase activity Colloids and Surfaces B: Biointerfaces, 75(1): 335-341. [DOI] [PubMed]
- Ansari, M.A., H.M. Khan, A.A. Khan, A. Malik, A. Sultan, M. Shahid, F. Shujatullah and A. Azam, 2011. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MRSA on isolates from skin infections. Biology and Medicine, 3(2): 141-146.
- Srivastava, S., Bera, T., Roy, A., Singh, G., Ramachandrarao P., Dash, D., 2012. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology, 18: 225103 (9pp). [DOI] [PubMed]
- Clinical and Laboratory Standard Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 20th Informational Supplement. CLSI document M100-S19. CLSI, Wayne, PA: Clinical and Laboratory Standard Institute, 2009.