Abstract
Sugar profile and hydroxymethylfurfural (HMF) of Saudi honey were examined through high-performance liquid chromatography (HPLC) system equipped with refractive index and diode array detectors. The work was designed to assess the quality of various types of blossom honey i.e. Sider (Ziziphus spina-christi), Dhuhyana (Acacia asak), Sumra (Acacia tortilis), Qatada (Acacia hamulosa), Dhurum (Lavandula dentata), multiflora with majra (Hypoestes forskaolii), multiflora with herbs, Keena (Eucalyptus spp.) produced in the southwestern areas of the kingdom. Hierarchical cluster analysis (HCA), principal cluster analysis (PCA), and similarity and difference indices (SDI) were also applied to examine the possible grouping based on the studied quality parameters. Four main sugars (two monosaccharides i.e. fructose and glucose, two disaccharides i.e. sucrose and maltose) and HMF were investigated . The average values of fructose and glucose were in the range 33.10%–44.77% and 26.68%–37.91%, respectively. The maltose was present in all types of honey and its mean values were in the range of 0.37%–2.97%, while sucrose was absent in six types of honey, 0.25% in one unifloral honey, and 3.25% in one multi-floral honey. HMF was not detected in seven types of honey but was below the limit of quantification (0.13 mg/kg) in one type of honey. PCA displayed the accumulative variance of 79.96% for the initial two PCs suggesting that honey samples were not well distinguished by their sugar profile. Based on the sucrose and HMF contents, it was concluded that all types of blossom honey from the Asir province were of the best quality in the kingdom and met the international quality parameters.
Keywords: Sucrose, Hydroxymethylfurfural, Honey, Apis mellifera, Saudi Arabia, Analytical techniques, Multivariate analysis
1. Introduction
Honey bees forage on flowers to collect nectar and transform it to produce floral honey which is a natural aqueous supersaturated sugar solution. The monosaccharides (fructose and glucose) are the main components of honey. In addition to a concentrated solution of readily available sugars, many other minor constituents like phenolic, proteins, minerals, free amino acids, several enzymes, lipids, scent compounds, vitamins, flavonoids, organic acids, colorants, waxes, pollens, and some phytochemicals are also found in honey (Amiry et al., 2017, de Almeida-Muradian et al., 2013, Nayik and Nanda, 2015, Uran et al., 2017). Humans had used honey for thousands of years and its importance is mentioned in all religions (Al-Waili et al., 2012). It has been recognized as a valued source of energy in many traditions and has been used as a remedy for many diseases in folk medicines. It is considered a part of Apitherapy since early human and has recently been exercised in the medication of burns, gastrointestinal ailments, protracted wounds, asthma, ulcers, etc. because of its anti-microbial, anti-viral, anti-oxidant, anti-cancer, anti-inflammatory and immunosuppressive actions (Al-Ghamdi et al., 2020, Al-Ghamdi et al., 2018, Ansari et al., 2017c, Khan et al., 2019, Küçük et al., 2007, Subrahmanyam et al., 2001). Both zoological and clinical tests in several parts of the world revealed some very encouraging results regarding the remedial potential of honey (Khan et al., 2017, Küçük et al., 2007, Nayik and Nanda, 2015, Sajid et al., 2020, Subrahmanyam et al., 2001).
The composition and beneficial attributes of honey rest on the floral types, geographical origin, climatic situations, beekeeping practices, honey ripeness, processing, and storing conditions (Ansari et al., 2017b, de ALmeida et al., 2016, El Sohaimy et al., 2015, Kukurova et al., 2008). The medicinal and therapeutic attributes of honey make it of great attention among the natural foodstuff. The increased demand for honey has always a lure to adulterate it by mixing with low-cost commercial sugar syrups, hence adversely affecting the consumers' health along with the quality of honey (Cengiz et al., 2014, Jamal et al., 2020). The European Union regulation has provided a common quality standard that a honey should meet, like organoleptic features (color, flavor, consistency, aroma, etc.) and physicochemical constitutional parameters (sugars, water, and mineral contents, vitamins, acidity, organic acids, amino acids, proline, proteins, enzymes activities, electrical conductivity, and HMF content (Council-European, 2002). Although all the quality parameters are important but sucrose content and HMF are the most important indicators of quality honey. Sucrose may occur in a honey sample at a concentration of <1% but its quantity increased if the beekeepers use sugar solutions to overfeed the bees during spring (Anklam, 1998, Deifel et al., 1985). British and German honey regulations have set the maximum sucrose content in a honey sample of up to 5%. HMF is also considered a quality parameter for honey. Mostly, it does not occur in newly harvested honey but its content rises through conditioning and storage. During processing, honey is typically warmed to decrease its viscosity and to avert granulation or fermentation. The quality of honey is not affected at the temperatures of 32–40 °C; however, the application of higher temperatures tends to increase the levels of HMF in honey (Anklam, 1998, Fallico et al., 2004, Turhan et al., 2008). Codex Alimentarius (Alimentarius-Codex) ascertained that an HMF amount in honey should not exceed 80 mg/kg after processing and/or blending. While the EU (EU Directive 110/2001) proposed the HMF limit of 40 mg/kg of honey but this limit for honey produced in the countries with high temperatures is 80 mg/kg.
In Saudi Arabia, a lot of research was carried out on different aspects of honey. The effect of floral origin and altitude on physiochemical properties and concentration of vitamins was analyzed by Al-Mosa et al., 2019, Mohammed et al., 2019. Many researchers worked on antimicrobial (Abdallah and Hamed, 2019, Al-Hindi and Shehata, 2014, Ansari et al., 2017a, Ghramh et al., 2019a, Ghramh et al., 2019b, Owayss et al., 2019), antioxidant (Al-Hindi and Shehata, 2014), physicochemical properties including some quality parameters (Ahamed et al., 2017, Alqarni et al., 2014, Alqarni et al., 2016, Mesallam and El-Shaarawy, 1987, Osman et al., 2007), mineral contents (Al-Hindi and Shehata, 2014, Alqarni et al., 2014, Arida et al., 2012, Osman et al., 2007, Taha et al., 2018), floral pigments (Alqarni et al., 2016), heavy metals (Bazeyad et al., 2019), validation of botanical origins and geographical sources through ultraviolet spectroscopy and chemo metric analysis (Ansari et al., 2018), and formation and biological activities of nanoparticles by using honey samples (Al-Brahim and Mohammed, 2020, Ghramh et al., 2019b). Most of the previous research on honey deals with the above-mentioned parameters rather than quantifying the two most important quality parameters (sucrose and HMF) present in honey samples. Only a few studies in Saudi Arabia focused on sucrose and HMF contents. To illuminate this uncharted area, this study examined the sugar profile and HMF content of various honey samples collected from the Asir province (one the most suitable areas for beekeeping in the kingdom) through modern analytical techniques by using HPLC.
2. Materials and methods
2.1. Chemicals and reagents
Chemicals and reagents: All the chemicals and reagents employed were of high-performance liquid chromatography (HPLC) ranking. Fructose, glucose, sucrose, and maltose were acquired from Loba Chemie Pvt. Ltd. Mumbai, India; Chem-Lab NV, Zedelgem, Belgium; Central Drug House (P) Ltd, New Delhi, India; and Techno Pharmchem, New Delhi, India respectively. 5-(hydroxymethyl) furfural were obtained from Sigma-Aldrich (St. Louis, MO, USA). Water and Acetonitrile, HPLC grade were obtained from Chemie Pvt. Ltd. Mumbai, India.
2.2. Honey samples
Honey samples: Eight types of blossom honey were collected from the apiaries of the Unit of Bee Research and Honey Production, King Khalid University Abha, Saudi Arabia and from local beekeepers placed at different locations between the years 2018–2019. The details of honey types are given in Table 1. The botanical origin of the honey was investigated by following the method of Louveaux et al. (1978). Pollen analysis showed two multifloral and six unifloral honeys.
Table 1.
Details of honey samples of different locations of the Asir province of Saudi Arabia.
| S.No | Type of Honey |
Harvesting Season | Location | ||
|---|---|---|---|---|---|
| Local name | Abbreviation | Scientific name | |||
| 01 | Sider | SDR | Ziziphus spina-christi | Oct-Nov 2019 |
Rijal Alma |
| 02 | Dhuhyana | DHY | Acacia asak | Oct-2019 | Billahmar-Albatna |
| 03 | Sumra | SMR | Acacia tortilis | Oct-Nov 2019 | Bisha |
| 04 | Qatada | QTD | Acacia hamulosa | Oct-2019 | Billahmar-Touma |
| 05 | Dhurum | DRM | Lavandula dentata | Oct-2019 | Billasmar-Alhijaz |
| 06 | Multiflora | MFJ | Herbs and Hypoestes forskaolii | Oct-2019 | Sarat Abeedah-Aljow |
| 07 | Multiflora | MLF | Herbs | 2018 | Al-Huridha |
| 08 | Keena | KNA | Eucalyptus spp. | 2018 | Abha |
2.3. Sample preparation and sugars analysis using HPLC-RID
Two hundred milligram of the honey sample was weighed through an electric balance (Shimadzu, Kyoto, Japan) in a 15 mL falcon tube (Jet Biofil, Guangzhou, China) and HPLC grade water were mixed to create a total volume of 10 mL. Honey was dissolved thoroughly by vortex and filtered using 0.22 µm syringe filters (Isolab, Laborgeräte GmbH). Fructose, glucose, sucrose, and maltose were analyzed using an HPLC system (Agilent 1260 Infinity II, Agilent Technologies, California, USA) fitted with pump (1260 Quat Pump VL, Agilent Technologies, California, USA), vial sampler (1260 vial sampler, Agilent Technologies, California, USA), and a refractive index detector (1260 RID, Agilent Technologies, California, USA) by using a ZORBAX Carbohydrate analyzing column (4.6 × 150 mm, 5 μm; Agilent Technologies, California, USA), with an isocratic mobile phase of Acetonitrile: water (75:25, v/v), retained at a flow rate 1.0 mL/min. The sample injection volume was 10 μL, and analyses were performed at 35 °C. Standard curves for fructose and glucose were primed in HPLC grade water at concentrations range of 0.0625−2.00% (w/v) and for sucrose and maltose from 0.03125−1.00% (w/v). All the honey samples and standards were analyzed in triplicate. The chromatographic peaks matching to each sugar were coordinated with the retention time of the standard. A calibration curve fitted by linear regression analysis was prepared using serial dilutions of standards to define the correlation in peak area and concentration. The sugar results are expressed in percentages.
2.4. Sample preparation and hydroxymethylfurfural (HMF) analysis using HPLC-DAD
Honey solutions (2%) were prepared as previously mentioned in Section 2.3. HMF was analyzed using an HPLC system (Agilent 1260 Infinity II, Agilent Technologies, California, USA) equipped with pump (1260 Quat Pump VL, Agilent Technologies, California, USA), vial sampler (1260 vial sampler, Agilent Technologies, California, USA), and a Diode Array HPLC Detector (1260 DAD WR, Agilent Technologies, California, USA) by using an Infinity lab Poroshell 120 EC-C18 analyzing column (3.0 × 150 mm, 2.7 μm; Agilent Technologies, California, USA), with a gradient mobile phase i.e. 90% water at 1% of acetic acid and 10% methanol kept at a flow rate 0.300 mL /min. The sample injection volume was 5 μL, and the examination was performed at 35 °C. The wavelength was adjusted at 284 nm and the chromatograms were observed at 284 nm. The standard curve for HMF was prepared in HPLC grade water at concentrations ranging from 0.004 to 0.645 mg/mL (w/v). All the samples and standards were analyzed in triplicate. The obtained peaks relating to each sample were linked with the retention time of the standard. A calibration curve fitted by linear regression analysis was prepared using serial dilutions of HMF standard to define the correlation in peak area and concentration. The HMF results are mentioned in mg/kg.
2.5. Statistical analysis
All results related to sugar profile and HMF contents were presented as the means of three replicates ± standard deviation (SD). The statistical analyses were made using Statistix 8.1 software. All pairwise comparison of means was performed via Tukey’s Honest Significant Difference (HSD) test. Variances between means at p ≤ 0.05 were considered as statistically significant. Multivariate statistical treatments i.e. Hierarchical Cluster Analysis (HCA) and Principal Cluster Analysis (PCA), Similarity and difference indices (SDI) were carried out using computer software, Past3. These multivariate statistical methods allowed the attaining of graphic and tabular demonstrations which found the finest possible abstract of the information present in a large set of data. These analyses also enabled to characterize variables on a graph to study the closeness of entities and to categorize them.
3. Results
3.1. Preparation of calibration curves
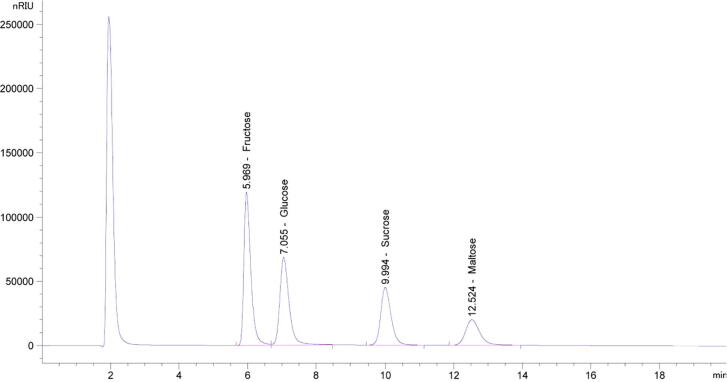
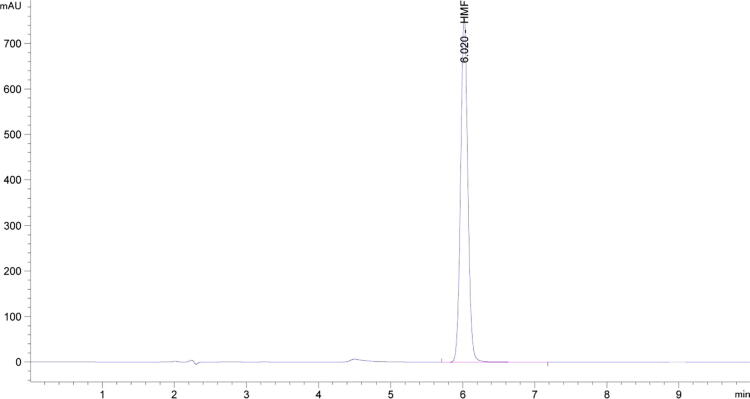
Chromatographic measurements of sugars and HMF were standardized using the absolute calibration method. HPLC chromatograms of sugar standards (fructose, glucose, sucrose, and maltose) and their respective retention times (RT) are presented in Fig. 1 when the isocratic mobile phase containing 75% Acetonitrile and 25% water were used. Calibration curves were prepared using a series of standard solutions of fructose and sucrose at concentrations ranging from 0.0625% to 2.00% (w/v) and for sucrose and maltose from 0.03125% to 1.00% (w/v). Similarly, HPLC chromatograms of HMF standard and its respective retention time (RT) are shown in Fig. 2 when the gradient mobile phases i.e. 90% water with 1% of acetic acid and 10% methanol were used. A calibration curve was made using a series of standard solutions of HMF at the concentrations ranging from 0.004 to 0.645 mg/mL (w/v).
Fig. 1.
The high performance liquid chromatography (HPLC) chromatograms of standards fructose (2%), glucose (2%), sucrose (1%), and maltose (1%) using 75% Acetonitrile: 25% Water. Retention times of fructose, glucose, sucrose, and maltose were 5.969, 7.055, 9.994, and 12.524 min respectively.
Fig. 2.
The high performance liquid chromatography (HPLC) Chromatograms of 5-hydroxymethylfurfural (HMF) standard 0.0645 mg/gm using gradient mobile phase i.e. 90% water with 1% of acetic acid and 10% methanol maintained at a flow rate 0.300 mL /min.
3.2. Sugar profiling of Saudi honey samples
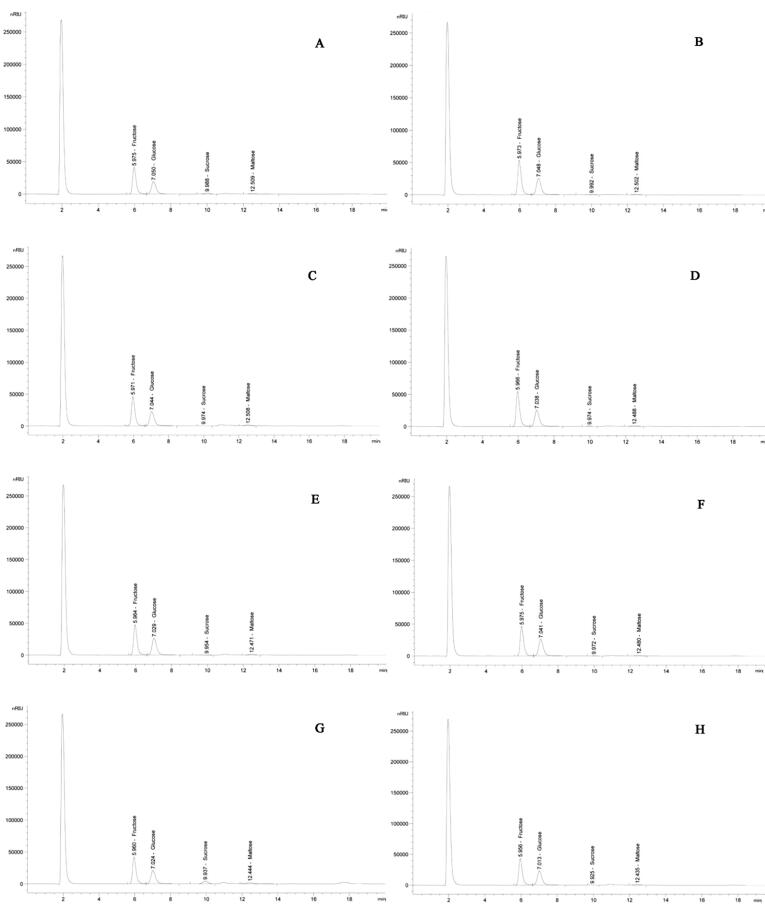
All sugar profiles of honey samples are reported in Table 2 and the peaks obtained by chromatography are shown in Fig. 3. Fructose, glucose, and maltose were present in all types of honey. Fructose and glucose were found to be the major sugars in all of the tested samples. The amounts of fructose and glucose in the honey samples of the Asir province ranged from 33.10% to 44.77% and from 26.68% to 37.91% respectively. Comparison of fructose contents showed that QTD honey had the highest content whereas in SDR and MLF honey samples were present at lower levels. It was also observed that glucose contents were significantly (p ≤ 0.05) high in DRM honey samples, low in SDR, and significantly (p ≤ 0.05) different among other honey types. Fructose is quantitatively the main sugar next to glucose. In this study, glucose was lower than fructose in all types of honey samples. Maltose was present in all the tested honey samples but its value was less than 1% in three honey samples i.e. DHY, MFJ, and KNA and its overall value ranged from 0.37% to 2.97%. Sucrose was less than the limit of quantification (0.002%) in six honey samples and detected only in two honey sample i.e. SDR (0.25%) and MLF (3.25%). No honey sample had the same level of total sugars and a significant difference (p ≤ 0.05) was observed among them. The total sugar content of the tested honey types ranged from 61.07% to 82.28%. The fructose/glucose (F/G) ratio and the reducing sugars (fructose + glucose) in all types of honey ranged from 1.01 to 1.24 and from 59.78% to 81.24% respectively.
Table 2.
Mean sugars content (expressed in percentage) and standard deviations of different honey samples of the Asir province of Saudi Arabia.
| Honey Type | Different kinds of sugars and their related variables |
HMF mg/kg |
||||||
|---|---|---|---|---|---|---|---|---|
| Fructose (%) | Glucose (%) | Sucrose (%) | Maltose (%) | Reducing sugars (%) | Total sugars (%) | F/G ratio | ||
| SDR | 33.10 ± 0.04h | 26.68 ± 0.07g | 0.25 ± 0.11b | 1.04 ± 0.06c | 59.78 ± 0.03h | 61.07 ± 0.15h | 1.24 ± 0a | ND |
| DHY | 43.62 ± 0.07b | 36.38 ± 0.07c | <LOQ | 0.46 ± 0.04d | 80.00 ± 0.14b | 80.46 ± 0.17b | 1.20 ± 0bc | ND |
| SMR | 37.98 ± 0.07d | 32.06 ± 0.05e | <LOQ | 1.39 ± 0.04b | 70.04 ± 0.11e | 71.43 ± 0.15e | 1.18 ± 0c | ND |
| QTD | 44.77 ± 0.19a | 36.49 ± 0.28c | <LOQ | 1.05 ± 007c | 81.24 ± 0.16a | 82.28 ± 0.16a | 1.23 ± 0.01ab | ND |
| DRM | 38.77 ± 0.32c | 37.91 ± 0.21a | <LOQ | 1.08 ± 0.05c | 76.68 ± 0.31c | 77.76 ± 0.29c | 1.02 ± 0.01f | ND |
| MFJ | 37.44 ± 0.23e | 37.20 ± 0.14b | <LOQ | 0.98 ± 0.06c | 74.63 ± 0.11d | 75.61 ± 0.10d | 1.01 ± 0.01f | ND |
| MLF | 33.97 ± 0.05g | 29.51 ± 0.23f | 3.25 ± 0.13a | 2.97 ± 0.09a | 63.42 ± 0.32g | 69.64 ± 0.27f | 1.15 ± 0.01d | <LOQ |
| KNA | 35.15 ± 0.08f | 32.77 ± 0.13d | <LOQ | 0.37 ± 0.10d | 67.86 ± 0.17f | 68.23 ± 0.18g | 1.07 ± 0.01e | ND |
All analyses were performed in triplicate and the mean value ± standard deviation (SD) is reported. Mean values in the same column but with different superscript letters vary significantly (p ˃ 0.05). F/G = Fructose/Glucose; ND = Not detected; LOQ = Limit of quantification; LOD = Limit of Detection; for sucrose (LOQ = 0.002% & LOD = 0.0006%); for HMF (LOQ = 1.33 mg/kg & LOD = 0.44 mg/kg). SDR = Sider (Ziziphus spina-christi), DHY = Dhuhyana (Acacia asak), SMR = Sumra (Acacia tortilis), QTD = Qatada (Acacia hamulosa), DRM = Dhurum (Lavandula dentata), MFJ = Multiflora with majra (Hypoestes forskaolii), MLF = multiflora with herbs, KNA = Keena (Eucalyptus spp.).
Fig. 3.
High performance liquid chromatography (HPLC) chromatograms four sugars (fructose, glucose, sucrose, and maltose) found in Saudi honey samples: (A) Sider, Ziziphus spina-christi; (B) Dhuhyana, Acacia asak; (C) Sumra, Acacia tortilis; (D) Qatada, Acacia hamulosa; (E) Dhurum, Lavandula dentata; (F) Multiflora containing herb and majra, Hypoestes forskaolii; (G) Multiflora with herbs; (H) Keena, Eucalyptus spp. using an isocratic mobile phase containing 75% of Acetonitrile and 25% of Water maintained at 1 mL/min.
3.3. HMF contents in Saudi honey samples
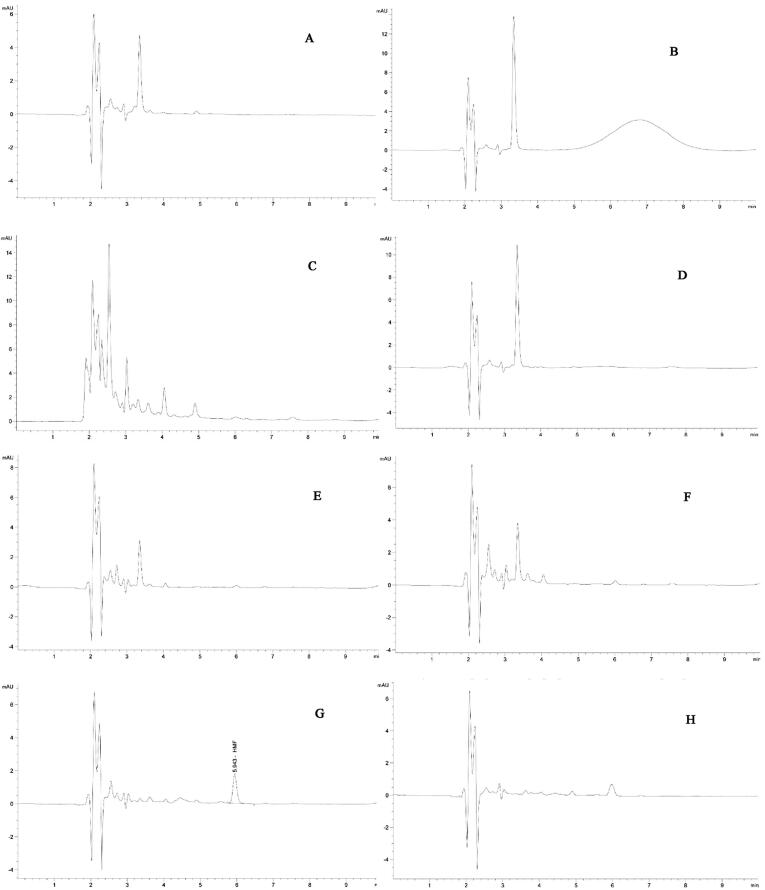
HMF contents of the studied honey samples are summarized in Table 2 and the peaks obtained by chromatography are shown in Fig. 4. It can be seen, that HMF was not detected in seven honey samples. It was only found in one honey sample but its value was below the limit of quantification (LOQ) i.e. 1.33 mg/kg.
Fig. 4.
High performance liquid chromatography (HPLC) chromatogram of hydroxymethylfurfural (HMF) found in Saudi honey samples: (A) Sider, Ziziphus spina-christi; (B) Dhuhyana, Acacia asak; (C) Sumra, Acacia tortilis; (D) Qatada, Acacia hamulosa; (E) Dhurum, Lavandula dentata; (F) Multiflora containing herb and majra, Hypoestes forskaolii; (G) Multiflora with herbs; (H) Keena, Eucalyptus spp. using a gradient mobile phase i.e. 90% water with 1% of acetic acid and 10% methanol maintained at a flow rate 0.300 mL /min.
3.4. Multivariate analysis
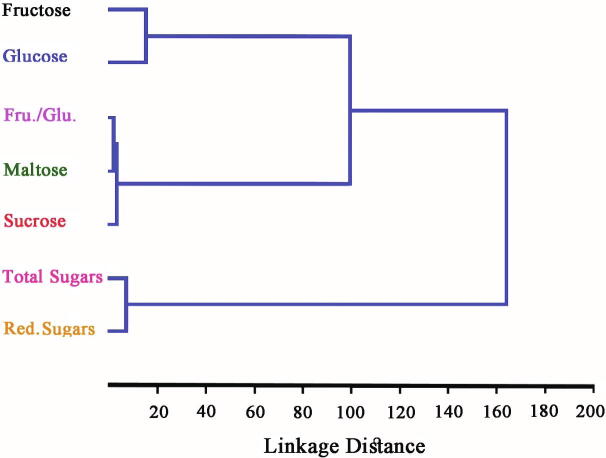
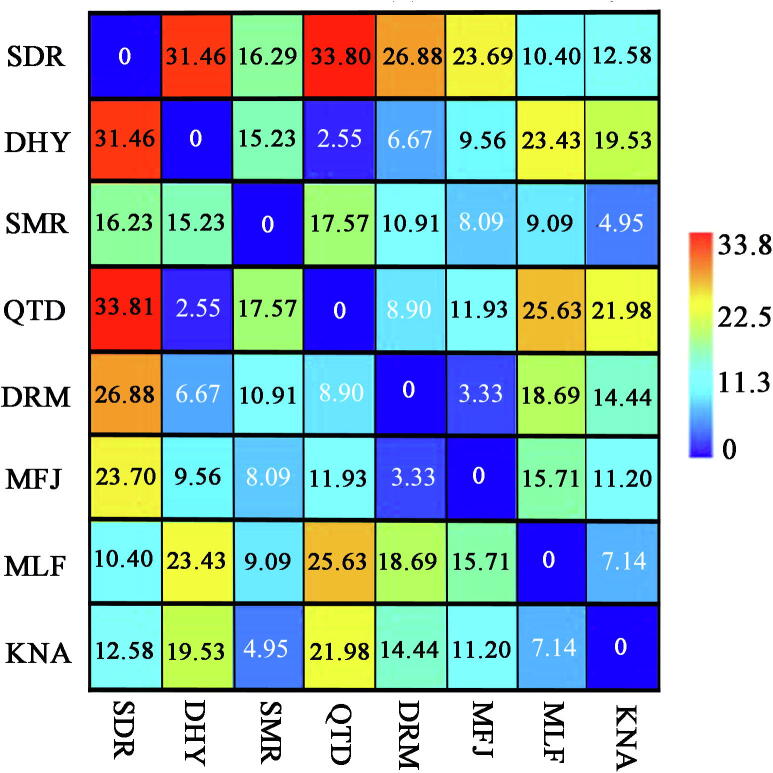
HCA of sugars present in honey samples acquired through Euclid’s distances using paired group (UPGMA) algorithm (Fig. 5) showed that the fructose and glucose formed one cluster since there was not a conspicuous variance in the levels of fructose and glucose while, in disaccharides, the sucrose was present in very low levels and constituted a separate sub-group in another group of maltose and fructose/glucose ratio. The principal component analysis was tested to the sugar results to define the variances among the Saudi honey types (Fig. 6). The 1st principal component contained 40.74% of the variance, the 2nd for 39.22% and the 3rd for 13.94%. The collective variance was about 93.9%, which demonstrated that the honey samples were not properly separated by their respective sugar data. The similarity and distance index (Euclidean method) of the eight Saudi honey samples was measured which is shown in Fig. 7. The index ranged between high similarities (0) to more distant (33.8). There was much similarity between some honey samples i.e. DHY-QTD, DRM-MFJ, and KNA-SMR while DTD-SDR and DRM-SDR were more distant from each other.
Fig. 5.
The hierarchical cluster analysis of sugars found in various types of Saudi honey acquired through Euclid's distance using a paired group (UPGMA) algorithm.
Fig. 6.
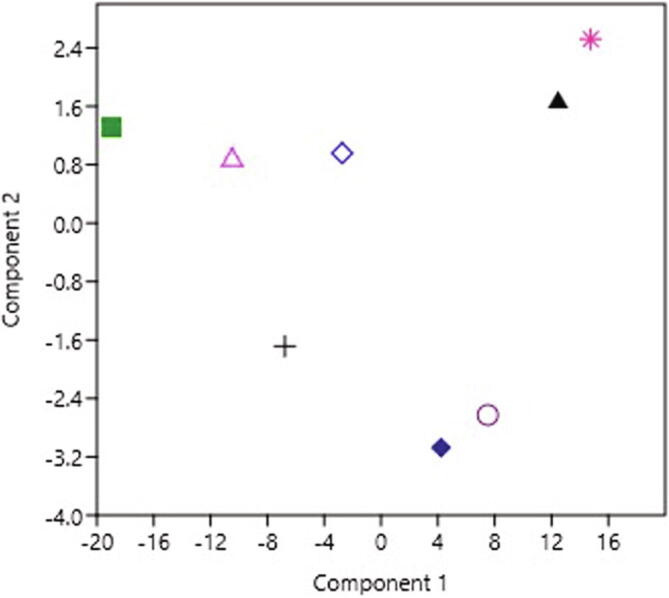
Principal component loadings plot, first component set against the second component, for the grouping of Saudi honey according to their botanical origin. (*) Qatada (Acacia hamulosa), (▲) Dhuhyana (Acacia asak), (◊) Sumra (Acacia tortilis), (Δ) multiflora of herbs, (■) Sider (Ziziphus spina-christi), (+) Keena (Eucalyptus spp.). (Ο) Dhurum (Lavandula dentata), (♦) Multiflora with majra (Hypoestes forskaolii).
Fig. 7.
Similarity and distance index (Euclidean method) of Saudi honey samples based on their sugar profile. Whereas, SDR = Sider (Ziziphus spina-christi), DHY = Dhuhyana (Acacia asak), SMR = Sumra (Acacia tortilis), QTD = Qatada (Acacia hamulosa), DRM = Dhurum (Lavandula dentata), MFJ = Multiflora with majra (Hypoestes forskaolii), MLF = multiflora with herbs, KNA = Keena (Eucalyptus spp.).
4. Discussion
Honey is primarily composed of carbohydrates (Rodríguez Flores et al., 2014) and among these monosaccharides (fructose and glucose) are important components while fructose is the primary sugar all the time after glucose (Habib et al., 2014). In this study, it was observed that the monosaccharides were the main sugars and fructose contents exceeded quantitatively by glucose in all the examined honey samples. These results were in accordance with that of Habib et al., 2014, Khan et al., 2016, and Mesallam and El-Shaarawy (1987). Sugar constituents in honey are determined by the nectar sources (flowers or plant secretions) consumed by the bees (Sobrino-Gregorio et al., 2017) regional and climatic conditions (Mateo and Bosch-Reig, 1998), and storage circumstances (Dobre et al., 2012). Glucose is comparatively less soluble in water than fructose hence the fructose/glucose ratio can most likely be used to evaluate the crystallization of honey. It is found that DRM, MFJ, and KNA honey samples had the F/G ratio almost 1 and all of these three honey samples granulated by the passage of time which is the confirmatory of Boussaid et al., 2015, El Sohaimy et al., 2015, Karabagias, 2019 who reported that the ratio below 1 has tendency a tendency for crystallization rather than to remain in liquid form for longer period. So, it is confirmed that the crystallization of honey is not due to any adulteration but is a natural process. The reducing sugars of all of the tested honey samples were higher than 60%, except for the SDR honey. The results of the current study were in line with the international quality regulations of the Codex-Alimentarius-Commission (2001). The reducing sugars less than 60% in few honey samples was also reported by Geană et al. (2020). The results of total sugars contents of Saudi honey types were in agreement with those reported by Geană et al., 2020, Khan et al., 2016, Mesallam and El-Shaarawy, 1987, Ouchemoukh et al., 2010. Maltose was reported as the main disaccharide sugar in Morocco honey (mean 3.47%) (Aazza et al., 2014), Saudi local honey (mean 4.6%) (Mesallam and El-Shaarawy, 1987), and Spanish honey samples (mean 3.96%) (Mateo and Bosch-Reig, 1997). While on the other hand the mean percentages of maltose in Algerian honey, Pakistani honey, and Romanian honey were reported as 1.79%, 2.11%., and 1.62% respectively (Geană et al., 2020, Khan et al., 2016, Ouchemoukh et al., 2010). The values of maltose found in the current study are in agreement with Geană et al., 2020, Khan et al., 2016, Ouchemoukh et al., 2010 but contrary to the results of Aazza et al., 2014, Mateo and Bosch-Reig, 1997, Mesallam and El-Shaarawy, 1987. As discussed earlier that the sugar contents in honey vary with nectar sources, regional and climatic conditions, and storage conditions therefore these could be possible reasons for the contradiction of the maltose results. Anklam (1998) also reported that honey from the same floral origin can even be different because of seasonal and climatic differences or to a diverse geographical origin.
Sucrose (saccharose) is one of the quality parameters considered to identify the adulteration in honey samples. The small supply and the high price of honey have offered key inducements for falsification. Some common practices of honey adulteration are the addition sucrose, over-feeding of bees through sucrose solution, or premature honey harvesting. Sucrose should not exceed 1% of the dried mass in natural honey (Cotte et al., 2003, Guler et al., 2007, Saxena et al., 2010). In the present study sucrose contents were not quantifiable in six types of honey, traceable in SDR honey, and below the 5% in MLF honey samples. The results ensure the high quality of Saudi honey samples, free from sucrose adulteration, at a superior ripening stage, and are reliable since the sucrose is the key sugar from a legislative viewpoint. It also depicts the good beekeeping practices adopted by the local beekeepers by harvesting the mature honey. The results are in agreement with that of Geană et al. (2020) who did not detect sucrose contents in the Romanian honey samples and Habib et al. (2014) who did not detect sucrose contents in six and detected <1% in honey samples from arid regions i.e. United Arab Emirates, Oman, Yemen, and Pakistan. But are far less the number of sucrose contents (2.8–11.7%) of Saudi honey samples reported by Mesallam and El-Shaarawy (1987). The difference is might be due to the improvement of beekeeping practices adopted by local beekeepers as compared to those were practiced in 1987.
HMF is also an important quality element and employed to gauge honey freshness and/or overheating. Fresh honey samples have usually no HMF content, but its presence rises while long time storage, reliant on pH and storage temperature (Lee and Nagy, 1990). HMF can even be formed at low temperatures in acidic conditions. Several factors like temperature, heating intervals, storage conditions, pH, and nectar source of a honey influence the levels of HMF. In the present study, the HMF has not detected in seven honey samples and one honey sample was not quantifiable. These results are in accordance with Boussaid et al., 2015, Khan et al., 2016, Özcan and Ölmez, 2014, Truzzi et al., 2014 who reported the HMF contents ≤ 20 mg/kg in their analyzed honey samples from Tunisia, Italy, Turkey, and Pakistan respectively. These results are also in compliance with an earlier study conducted in Saudi Arabia (Mesallam and El-Shaarawy, 1987). These results are also in compliance with the past study on honey samples from arid regions of United Arab Emirates, Oman, Yemen, and Pakistan by Habib et al. (2014) who reported HMF content < 16 mg/kg in most of the samples except three. Arida et al. (2012) reported the HMF contents of 41, 140, and 34 mg/kg in Blossoms, Samra (Elshoka), and multi-floral Saudi honey samples respectively and below the limit of quantification in other four Saudi honey samples (Acacia, Orange, Seder, and Dorm). Our results are in agreement with those honey samples analyzed by Arida et al. (2012) in which HMF was not detected/quantified but contradictory to those samples in which the HMF range was above the accepted limits. The results of this study again confirm the high quality, freshness, and/or proper storage of Saudi honey samples collected from the Asir province of Saudi Arabia. The moderate mean annual temperature (summer = 20° – 28 °C; winter = 9°–14 °C) of this regions could be one of the reasons for very low HMF contests in the honey samples collected from this area.
HCA analysis applied to sugar profile data of the honey samples showed a clear grouping, suggesting that the monosaccharides, disaccharides, and their related data were able to differentiate among the honey samples. The cluster formation indicated that the data related to sugar analysis of honey samples contained useful information in achieving three categories of classification. The results in this study are in agreement with Terrab et al. (2003) who characterized the Moroccan monofloral honey samples through multivariate analysis of various physicochemical data including HMF and sugar contents. PCA was executed to present a data structure in a summarized way, covering the maximum quantity of information existing in the data. PCA denotes the actual data matrix to the projection of the data matrix onto a few-dimensional space. The cumulative variance of the two first PCs was 79.96%, which showed that the six unifloral and two multi floral kinds of honey were not well distinguished by studied parameters. SDI was performed to know how the tested honey samples are near and far from each other based on HMF and sugar contents. The honey samples which were close to each other exhibited some common attributes. QTD and DHY were close with each other and both became granulated with time. The same observation was also noted for DRM and MFJ.
5. Conclusions
In this study, two very important quality parameters i.e. sucrose and HMF contents in honey samples from the Asir province of Saudi Arabia were evaluated to measure the honey quality. This province is located in the southwestern part of the country, has mountainous range and bestowed with plenty of bee forage plants and considered the best suited area for beekeeping in the kingdom. Total eight types of honey samples were analyzed and two, Dhuhyana (Acacia asak) & Qatada (Acacia hamulosa) were first time investigated in the Kingdom. The results indicated that sucrose contents were not present/quantifiable in six honey samples, <0.5% in one sample, and 3.35% in another sample. Similarly, HMF contents were not detected in seven honey samples and not quantifiable in one honey samples. These results confirm the high quality of honey and best beekeeping practices in the region. HCA analysis showed a clear grouping of monosaccharides and disaccharides suggesting that the data were able to differentiate among the honey samples. PCA analysis showed that the cumulative variance of the two first PCs was 96.22%, which indicated that studied parameters were not able to distinguish the honey samples. SDI of the honey samples showed that the samples which were close to each other exhibited similar characteristics e.g. Dhuhyana and Qatada were near to each other and both exhibited the granulation. Future investigations are recommended by considering more physico-chemical and quality parameters of honey to validate the kinds of conclusions that can be drawn from the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge the support of the King Khalid University through a grant RCAMS/KKU/02-20 under the Research Center for Advanced Materials Science (RCAMS) at King Khalid University, Abha, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aazza S., Lyoussi B., Antunes D., Miguel M.G. Physicochemical characterization and antioxidant activity of 17 commercial Moroccan honeys. Int. J. Food Sci. Nutr. 2014;65:449–457. doi: 10.3109/09637486.2013.873888. [DOI] [PubMed] [Google Scholar]
- Abdallah E.M., Hamed A.I. Screening for antibacterial activity of two jujube honey samples collected from Saudi Arabia. J. Apitherapy. 2019;5:6–9. [Google Scholar]
- Ahamed, M.M.E., Abdallah, A., Abdalaziz, A., Serag, E., Atallah, A.-b.E.H., 2017. Some physiochemical properties of Acacia honey from different altitudes of the Asir Region in Southern Saudi Arabia. Czech J. Food Sci. 35, 321–327.
- Al-Brahim J.S., Mohammed A.E. Antioxidant, cytotoxic and antibacterial potentials of biosynthesized silver nanoparticles using bee’s honey from two different floral sources in Saudi Arabia. Saudi J. Biol. Sci. 2020;27:363–373. doi: 10.1016/j.sjbs.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghamdi A., Al-Abbadi A.A., Khan K.A., Ghramh H.A., Ahmed A.M., Ansari M.J. In vitro antagonistic potential of gut bacteria isolated from indigenous honey bee race of Saudi Arabia against Paenibacillus larvae. J. Apic. Res. 2020:1–9. [Google Scholar]
- Al-Ghamdi A., Khan K.A., Ansari M.J., Almasaudi S.B., Al-Kahtani S. Effect of gut bacterial isolates from Apis mellifera jemenitica on Paenibacillus larvae infected bee larvae. Saudi J. Biol. Sci. 2018;25:383–387. doi: 10.1016/j.sjbs.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hindi R.R., Shehata A. Evaluation of antioxidant and antibacterial activities and essential elements content of locally produced honey in Saudi Arabia. Life Sci. J. 2014;11 [Google Scholar]
- Al-Mosa A., Brima E.I., Fawy K.F., Ghrama A., Hamed A., Mohammed M.E. Antioxidant vitamins in honey samples from different floral origins and altitudes in Asir Region at the South-Western Part of Saudi Arabia. Curr. Nutr. & Food Sci. 2019;15:296–304. [Google Scholar]
- Al-Waili N., Salom K., Al-Ghamdi A., Ansari M.J. Antibiotic, pesticide, and microbial contaminants of honey: human health hazards. Sci. World J. 2012;2012 doi: 10.1100/2012/930849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimentarius-Codex, Alinorm 01/25, 2000. Draft revised standard for honey at step 8.
- Alqarni A.S., Owayss A.A., Mahmoud A.A. Physicochemical characteristics, total phenols and pigments of national and international honeys in Saudi Arabia. Arabian J. Chem. 2016;9:114–120. [Google Scholar]
- Alqarni A.S., Owayss A.A., Mahmoud A.A., Hannan M.A. Mineral content and physical properties of local and imported honeys in Saudi Arabia. J. Saudi Chem. Soc. 2014;18:618–625. [Google Scholar]
- Amiry S., Esmaiili M., Alizadeh M. Classification of adulterated honeys by multivariate analysis. Food Chem. 2017;224:390–397. doi: 10.1016/j.foodchem.2016.12.025. [DOI] [PubMed] [Google Scholar]
- Anklam E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998;63:549–562. [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Khan K.A., Adgaba N., El-Ahmady S.H., Gad H.A., Roshan A., Meo S.A., Kolyali S. Validation of botanical origins and geographical sources of some Saudi honeys using ultraviolet spectroscopy and chemometric analysis. Saudi J. Biol. Sci. 2018;25:377–382. doi: 10.1016/j.sjbs.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Nuru A., Ahmed A.M., Ayaad T.H., Khan K.A., Al-Waili N. Diagnosis and molecular detection of Paenibacillus larvae, the causative agent of American foulbrood in honey bees in Saudi Arabia. Int. J. Trop. Insect Sci. 2017;37:137–148. [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Nuru A., Khan K.A., Alattal Y. Geographical distribution and molecular detection of Nosema ceranae from indigenous honey bees of Saudi Arabia. Saudi J. Biol. Sci. 2017;24:983–991. doi: 10.1016/j.sjbs.2017.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Usmani S., Khan K.A., Alqarni A.S., Kaur M., Al-Waili N. In vitro evaluation of the effects of some plant essential oils on Ascosphaera apis, the causative agent of Chalkbrood disease. Saudi J. Biol. Sci. 2017;24:1001–1006. doi: 10.1016/j.sjbs.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arida H., Hassan R., El-Naggar A. Quality assessment of honey using modern analytical tools. Anal. Lett. 2012;45:1526–1536. [Google Scholar]
- Bazeyad A.Y., Al-Sarar A.S., Rushdi A.I., Hassanin A.S., Abobakr Y. Levels of heavy metals in a multifloral Saudi honey. Environ. Sci. Pollut. Res. 2019;26:3946–3953. doi: 10.1007/s11356-018-3909-7. [DOI] [PubMed] [Google Scholar]
- Boussaid A., Chouaibi M., Rezig L., Missaoui R., Donsí F., Ferrari G., Hamdi S. Physicochemical, rheological, and thermal properties of six types of honey from various floral origins in Tunisia. Int. J. Food Prop. 2015;18:2624–2637. [Google Scholar]
- Cengiz M.F., Durak M.Z., Ozturk M. In-house validation for the determination of honey adulteration with plant sugars (C4) by Isotope Ratio Mass Spectrometry (IR-MS) LWT-Food Sci. Technol. 2014;57:9–15. [Google Scholar]
- Codex-Alimentarius-Commission, 2001. Codex STAN, 12-1981, Rev. 1 (1987), Rev. 2, Revised codex standard for honey. Codex Alimentarius Commission, 2001, 1–8.
- Cotte J.-F., Casabianca H., Chardon S., Lheritier J., Grenier-Loustalot M.-F. Application of carbohydrate analysis to verify honey authenticity. J. Chromatogr. A. 2003;1021:145–155. doi: 10.1016/j.chroma.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Council-European, 2002. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Official Journal of the European Communities L 10, 47–52.
- de Almeida‐Muradian, L.B., Stramm, K.M., Horita, A., Barth, O.M., da Silva de Freitas, A., Estevinho, L.M., 2013. Comparative study of the physicochemical and palynological characteristics of honey from Melipona subnitida and Apis mellifera. Int. J. Food Sci. Technol. 48, 1698–1706.
- de ALmeida, A.M.M., Oliveira, M., da Costa, J.G., Valentim, I.B., Goulart, M.O., 2016. Antioxidant capacity, physicochemical and floral characterization of honeys from the northeast of Brazil. Embrapa Tabuleiros Costeiros-Artigo em periódico indexado (ALICE).
- Deifel A., Gierschner K., Vorwohl G. Saccharose in honey. Saccharose and its transglycosidification products in natural and in sugar-feeding honeys. Deutsche Lebensmittel-Rundschau (Germany, FR) 1985;81:356–362. [Google Scholar]
- Dobre I., Georgescu L.A., Alexe P., Escuredo O., Seijo M.C. Rheological behavior of different honey types from Romania. Food Res. Int. 2012;49:126–132. [Google Scholar]
- El Sohaimy S., Masry S., Shehata M. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015;60:279–287. [Google Scholar]
- Fallico B., Zappala M., Arena E., Verzera A. Effects of conditioning on HMF content in unifloral honeys. Food Chem. 2004;85:305–313. [Google Scholar]
- Geană E.-I., Ciucure C.T., Costinel D., Ionete R.E. Evaluation of honey in terms of quality and authenticity based on the general physicochemical pattern, major sugar composition and δ13C signature. Food Control. 2020;109 [Google Scholar]
- Ghramh H.A., Ibrahim E.H., Kilany M. Study of anticancer, antimicrobial, immunomodulatory, and silver nanoparticles production by Sidr honey from three different sources. Food Sci. Nutr. 2019 doi: 10.1002/fsn1003.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghramh H.A., Khan K.A., Alshehri A.M.A. Antibacterial potential of some Saudi honeys from Asir region against selected pathogenic bacteria. Saudi J. Biol. Sci. 2019;26:1278–1284. doi: 10.1016/j.sjbs.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler A., Bakan A., Nisbet C., Yavuz O. Determination of important biochemical properties of honey to discriminate pure and adulterated honey with sucrose (Saccharum officinarum L.) syrup. Food Chem. 2007;105:1119–1125. [Google Scholar]
- Habib H.M., Al Meqbali F.T., Kamal H., Souka U.D., Ibrahim W.H. Physicochemical and biochemical properties of honeys from arid regions. Food Chem. 2014;153:35–43. doi: 10.1016/j.foodchem.2013.12.048. [DOI] [PubMed] [Google Scholar]
- Jamal M., Aziz M.A., Naeem M., Iqbal Z., Khalid A., Siddique F., Khan K.A., Ghramh H.A. Detection of flumethrin acaricide residues from honey and beeswax using high performance liquid chromatography (HPLC) technique. J. King Saud Univ.-Sci. 2020;32(3):2229–2235. [Google Scholar]
- Karabagias I.K. Seeking of reliable markers related to Greek nectar honey geographical and botanical origin identification based on sugar profile by HPLC-RI and electro-chemical parameters using multivariate statistics. Eur. Food Res. Technol. 2019;245:805–816. [Google Scholar]
- Khan K.A., Al-Ghamdi A.A., Ansari M.J. The characterization of blossom honeys from two provinces of Pakistan. Ital. J. Food Sci. 2016;28:625–638. [Google Scholar]
- Khan K.A., Al-Ghamdi A.A., Ghramh H.A., Ansari M.J., Ali H., Alamri S.A., Al-Kahtani S.N., Adgaba N., Qasim M., Hafeez M. Structural diversity and functional variability of gut microbial communities associated with honey bee. Microb. Pathog. 2019;103793 doi: 10.1016/j.micpath.2019.103793. [DOI] [PubMed] [Google Scholar]
- Khan K.A., Ansari M.J., Al-Ghamdi A., Nuru A., Harakeh S., Iqbal J. Investigation of gut microbial communities associated with indigenous honey bee (Apis mellifera jemenitica) from two different eco-regions of Saudi Arabia. Saudi J. Biol. Sci. 2017;24:1061–1068. doi: 10.1016/j.sjbs.2017.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küçük M., Kolaylı S., Karaoğlu Ş., Ulusoy E., Baltacı C., Candan F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007;100:526–534. [Google Scholar]
- Kukurova, K., Karovièová, J., Kohajdova, Z., Bilikova, K., 2008. Authentication of honey by multivariate analysis of its physico--chemical parameters. J. Food Nutr. Res. 47.
- Lee H., Nagy S. Relative reactivities of sugars in the formation of 5-hydroxymethylfurfural in sugar-catalyst model systems 1. J. Food Process. Preserv. 1990;14:171–178. [Google Scholar]
- Louveaux J., Maurizio A., Vorwohl G. Methods of melissopalynology. Bee World. 1978;59:139–157. [Google Scholar]
- Mateo R., Bosch-Reig F. Sugar profiles of Spanish unifloral honeys. Food Chem. 1997;60:33–41. doi: 10.1021/jf970574w. [DOI] [PubMed] [Google Scholar]
- Mateo R., Bosch-Reig F. Classification of Spanish unifloral honeys by discriminant analysis of electrical conductivity, color, water content, sugars, and pH. J. Agric. Food. Chem. 1998;46:393–400. doi: 10.1021/jf970574w. [DOI] [PubMed] [Google Scholar]
- Mesallam A.S., El-Shaarawy M.I. Quality attributes of honey in Saudi Arabia. Food Chem. 1987;25:1–11. [Google Scholar]
- Mohammed M.E.A., Alaergani W., Suleiman M.H., Al-Gramah H.A. Hydrogen peroxide and dicarbonyl compounds concentration in honey samples from different botanical origins and altitudes in the South of Saudi Arabia. Curr. Res. Nutr. Food Sci. J. 2019;7:150–160. [Google Scholar]
- Nayik G.A., Nanda V. Physico-chemical, enzymatic, mineral and colour characterization of three different varieties of honeys from Kashmir valley of India with a multivariate approach. Polish J. Food Nutr. Sci. 2015;65:101–108. [Google Scholar]
- Osman K.A., Al-Doghairi M.A., Al-Rehiayani S., Helal M.I. Mineral contents and physicochemical properties of natural honey produced in Al-Qassim region, Saudi Arabia. J. Food Agric. Environ. 2007;5:142. [Google Scholar]
- Ouchemoukh S., Schweitzer P., Bey M.B., Djoudad-Kadji H., Louaileche H. HPLC sugar profiles of Algerian honeys. Food Chem. 2010;121:561–568. [Google Scholar]
- Owayss A.A., Elbanna K., Iqbal J., Abulreesh H.H., Organji S.R., Raweh H.S., Alqarni A.S. In vitro antimicrobial activities of Saudi honeys originating from Ziziphus spina-christi L. and Acacia gerrardii Benth. trees. Food Sci. Nutr. 2019 doi: 10.1002/fsn3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan M.M., Ölmez Ç. Some qualitative properties of different monofloral honeys. Food Chem. 2014;163:212–218. doi: 10.1016/j.foodchem.2014.04.072. [DOI] [PubMed] [Google Scholar]
- Rodríguez Flores M.S., Escuredo Pérez O., Seijo Coello M.C. Characterization of Eucalyptus globulus honeys produced in the eurosiberian area of the Iberian Peninsula. Int. J. Food Prop. 2014;17:2177–2191. [Google Scholar]
- Sajid Z.N., Aziz M.A., Bodlah I., Rana R.M., Ghramh H.A., Khan K.A. Efficacy assessment of soft and hard acaricides against Varroa destructor mite infesting honey bee (Apis mellifera) colonies, through sugar roll method. Saudi J. Biol. Sci. 2020;27:53–59. doi: 10.1016/j.sjbs.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S., Gautam S., Sharma A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010;118:391–397. [Google Scholar]
- Sobrino-Gregorio L., Vargas M., Chiralt A., Escriche I. Thermal properties of honey as affected by the addition of sugar syrup. J. Food Eng. 2017;213:69–75. [Google Scholar]
- Subrahmanyam M., Sahapure A., Nagane N. Effects of topical application of honey on burn wound healing. Ann. Burns Fire Disasters. 2001;14:143–145. [Google Scholar]
- Taha E.-K.A., Al-Kahtani S., Taha R. Comparison of pollen spectra and amount of mineral content in honey produced by Apis florea F. and Apis mellifera L. J. Kansas Entomol. Soc. 2018;91:51–57. [Google Scholar]
- Terrab A., González A.G., Díez M.J., Heredia F.J. Characterisation of Moroccan unifloral honeys using multivariate analysis. Eur. Food Res. Technol. 2003;218:88–95. [Google Scholar]
- Truzzi, C., Illuminati, S., Annibaldi, A., Finale, C., Rossetti, M., Scarponi, G., 2014. Physicochemical properties of honey from Marche, Central Italy: classification of unifloral and multifloral honeys by multivariate analysis. Nat. Prod. Commun. 9, 1934578X1400901117. [PubMed]
- Turhan I., Tetik N., Karhan M., Gurel F., Tavukcuoglu H.R. Quality of honeys influenced by thermal treatment. LWT-Food Sci. Technol. 2008;41:1396–1399. [Google Scholar]
- Uran, H., Aksu, F., Dülger ALTiner, D., 2017. A research on the chemical and microbiological qualities of honeys sold in Istanbul. Food Sci. Technol. 37, 30–33.