Abstract
The objective of the present work was to observe and profile various antibiotic resistant strains of Staphylococcus aureus and highlight the need for continuous surveillance. Data regarding antibiotic-resistant S. aureus strains isolated and identified at the Medical Microbiology Department, King Khalid Hospital, Riyadh was obtained. Bacterial isolates were collected from several sites of infections in patients and an evaluation of susceptibility were carried out using a fully automated Vitek2 system. Relative frequency (%), odds ratios and Ward's minimum variance were calculated. The results showed that wounds were a source of more than 40% of the S. aureus (MRSA) strains that have ability to resist methicillin, and more than 45% of the methicillin-susceptible S. aureus (non-MRSA) strains. 40% of the isolates were MRSA (N = 251), and all MRSA strains were sensitive to vancomycin, daptomycin, teicoplanin, tigecycline, nitrofurantoin, and itraconazole while all non-MRSA (N = 338) strains were sensitive to vancomycin, cefoxitin, daptomycin, gentamicin, oxacillin, teicoplanin, tigecycline, and mupirocin. Strength of association between antibiotic-resistant S. aureus strains and source of samples (site of infection) was established. The study concluded that S. aureus strains had developed resistance towards 20 (for non-MRSA) and 22 (for MRSA) of the antibiotics tested. All MRSA strains were non-sensitive to amoxicillin/clavulanate, ampicillin cefoxitin, cefazolin, imipenem, oxacillin, and penicillin.
Keywords: Staphylococcus aureus, Retrospective, MRSA, Non-MRSA, Vitek2 system
1. Background
Staphylococcus aureus is an opportunistic gram-positive bacterium, being increasingly isolated from hospitals and environmental fields. This pathogen is ubiquitous in the air and is commonly found on human skin, causing infections. Approximately 33% of the human population hosts strains of this bacterium in the nasal cavity as normal flora as stated by to data by the Centre for Disease Control (CDC) (Sodhi et al., 2019). In the last few decades, strains of S. aureus have shown capability to resist an enormous range of antibiotics (Feng et al., 2008, Delorme et al., 2017) that were previously effective against their infections. By the early 1990s, the infections caused by methicillin-resistant S. aureus in hospitals (HA-MRSA) were the most prevalent nosocomial infections worldwide, while new strains known as community-associated MRSA (CA-MRSA) among individuals were identified some years later (Charlebois et al., 2004, Delorme et al., 2017). Although, CA-MRSA has no apparent risk factor, its emergence is an evidence of epidemiological change in the distribution of MRSA among individuals. Epidemiologically, understanding the microbial infections and the antibiotic-resistance manners of MRSA are substantial issues for the successful treatment of infected patients. The diversity and geographical variation in antimicrobial susceptibility patterns and bacterial spectra necessitate local and regional surveillance. In industrialized nations, these informative data are available at different levels (regional, national and international characteristics); an example of that, as accessible on the European Antimicrobial Resistance Surveillance System (EARSS) database (De Kraker et al., 2013).
The emergence of antimicrobial resistance is becoming a global challenge and might will be a destructive hazard taking into consideration the restricted access to second-line antimicrobial drugs (e. g. vancomycin, carbapenems) in some parts of the world. Previous studies have shown great variation in the epidemiology of MRSA and non-MRSA incidences among patients of different ages and sex. In 2010, while examining pediatric patients in the emergency units of hospitals in the USA between 1996 and 2006, Frei et al. identified 616,375 infection cases of MRSA from clinical infections in skin and soft tissue. They observed seasonal diversity with increased incidence of HA-MRSA from 1 case to about 25.5 cases per 100,000 per year. During this period, seasonal variation was observed across all regions of the US with peak incidence occurring between May to December (Frei et al., 2010). In another research by Abraham et al. in Gabon (2013), S. aureus was the predominant cause of bacterial infections in children (33.7%) and adults (23.5%) with 5.8% of S. aureus strains being resistant to methicillin (Alabi et al., 2013). A recent study in Saudi Arabia reported that consistent monitoring of MRSA and non-MRSA is imperative (Senok et al., 2019). In Saudi Arabia, the medical malpractices of use of antibiotics could lead to evolution of new MRSA strains, which would be potential carriers of resistance genes. Fusidic acid misuse has been related to a high rate of transfer of the fusC gene in MRSA strains (Senok et al., 2019). Also, mass migration aids in rapid global distribution of MRSA and non-MRSA strains (Al-Zahrani et al., 2019). In 2001, it was reported that new approaches were needed to control hospital infections caused by MRSA (Bukharie et al., 2001). We believe that all countries must continuously assess S. aureus prevalence in hospitals in order to devise protocols to deal with MRSA-related infections. This study was aimed at evaluating the prevalence of antibiotic-resistant S. aureus strains at the King Khalid University Hospital (KKUH) in University Medical City (UMC). KKUH is the teaching hospital of King Saud University, Riyadh, Saudi Arabia.
2. Materials and methods
This is a retrospective cohort study regarding the prevalence of MRSA and non– MRSA in samples obtained from various sites of infections in patients (male and female) that visited the Medical Microbiology Department, King Khalid Hospital, Riyadh, Saudi Arabia between 2nd October 2016 to 2nd June 2017. The study was conducted according to the directions of the Ethics Committee (17/0449/IRB, Institutional Review Board of College of Medicine, KSU, Saudi Arabia). The hospital medical microbiology laboratory provided clinical samples from outpatient as well as patients admitted to the hospital. A total of 251 MRSA and 338 non-MRSA cases were included in the study. The diagnosis of MRSA versus non-MRSA sources of infection was determined based on clinical laboratory culture findings for identification and antibiotic resistance screening. All samples with positive MRSA and non-MRSAwere analysed without inclusion or exclusion criteria. Fully automated Vitek2 system (Biomerieux, France) was used for microbial identification and antibiotic susceptibility testing, in accordance with the manufacturer’s instructions. The Vitek 2 ID-GPC card, Gram-positive bacteria cefoxitin test, and AST for oxacillin were applied in this system. The identification has been based upon phenotypic features of MRSA and MRSA strains where the card consists of 43 biochemical resections, including 17 enzymatic activity tests.
2.1. Statistical analysis
Odds ratios were calculated using the SPSS software (Version 25, IBM Corporation, USA) and hierarchical cluster analyses were done using Ward's method. OriginPro 9.1 (2018) software was used to determine relative frequency (%).
3. Results
3.1. The sources of the isolates
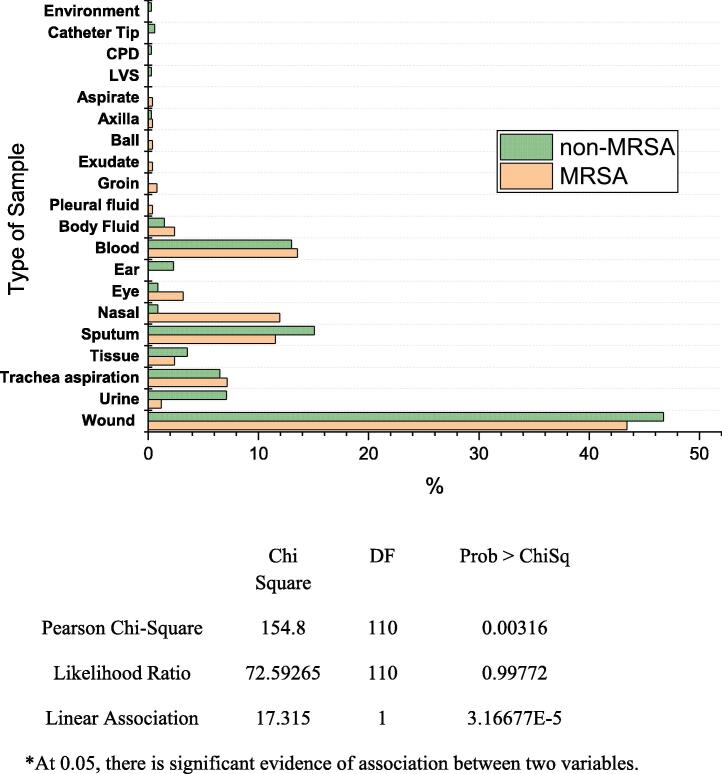
A total of 589 MRSA + non-MRSA S. aureus samples were examined in this study. MRSA and non-MRSA cases were assessed separately, while types of sample sources were categorized as aspirate, axilla, ball, blood, body fluids, exudate, eye, ear, groin, nasal, sputum, tissue, trachea, urine, and wound. The results showed that 43.42, 13.54, 11.95, 11.55, 7.17, 3.18, 2.39, 2.39, and 1.19% of the MRSA strains were isolated from wound, blood, nasal, sputum, tracheal aspirate, eye, tissue, body fluids, and urine samples respectively. On the other hand, 46.74, 15.08, 13.01, 7.10, 6.50, 3.55, 2.07, and 1.47% of non-MRSA strains were isolated from wound, sputum, blood, urine, tracheal aspirate, tissue, ear, and body fluids respectively (Fig. 1). The results revealed that MRSA strains were present in more than 74% of the isolates and more than 40% of them were isolated from wounds.
Fig. 1.
Relative frequency (%)* of MRSA and non-MRSA strains isolated from different body sites from patients attending the King Khalid University Hospital, Riyadh (N = 589) using OriginPro (9.1, 2018). CPD = continuous peritoneal dialysis, LVS = Low vaginal swab.
3.2. Antibiotic susceptibility
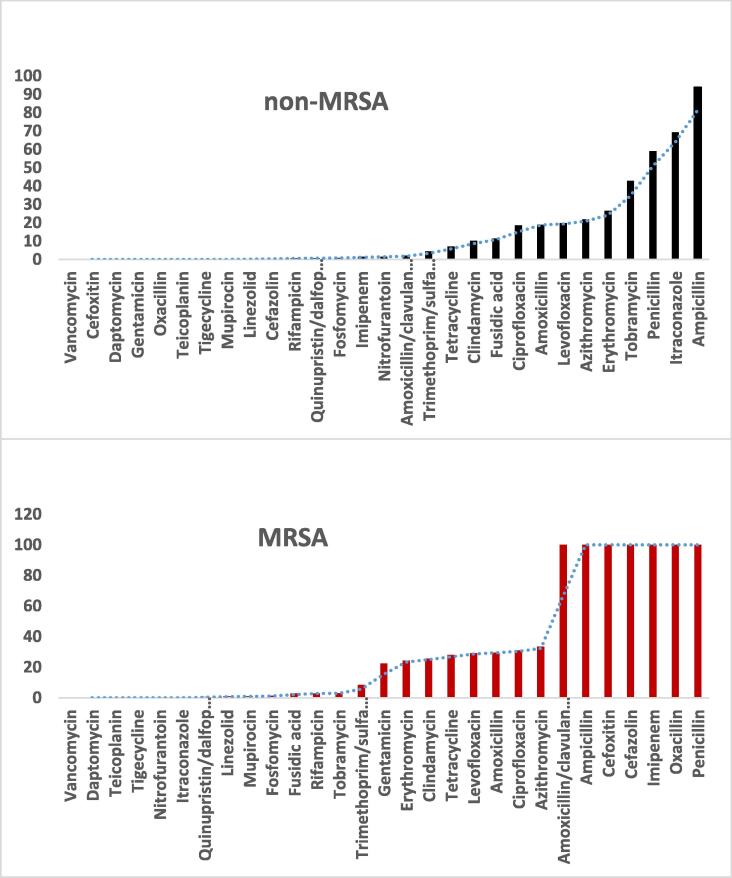
The data obtained from antibiotic susceptibility tests showed that more than 80% of the non-MRSA isolates contained strains resistant to ampicillin while all MRSA isolates contained strains resistant to amoxicillin/clavulanate, ampicillin, cefoxitin, cefazolin, imipenem, oxacillin, and penicillin (Fig. 2). Additionally, it was observed that all isolates (MRSA and non-MRSA) contained strains that were susceptible to vancomycin, daptomycin, teicoplanin, and tigecycline. All non-MRSA isolates contained strains susceptible to gentamicin and more than 50% of non-MRSA isolates contained strains resistant to penicillin and itraconazole while 22.3% of the MRSA isolates contained gentamicin-resistant strains.
Fig. 2.
Percentage of resistance of antibiotic-resistant MRSA (N = 251) and non-MRSA strains (N = 338) isolated to standard antibiotics among patients attending the King Khalid University Hospital, Riyadh.
3.3. Ward's minimum variance criterion
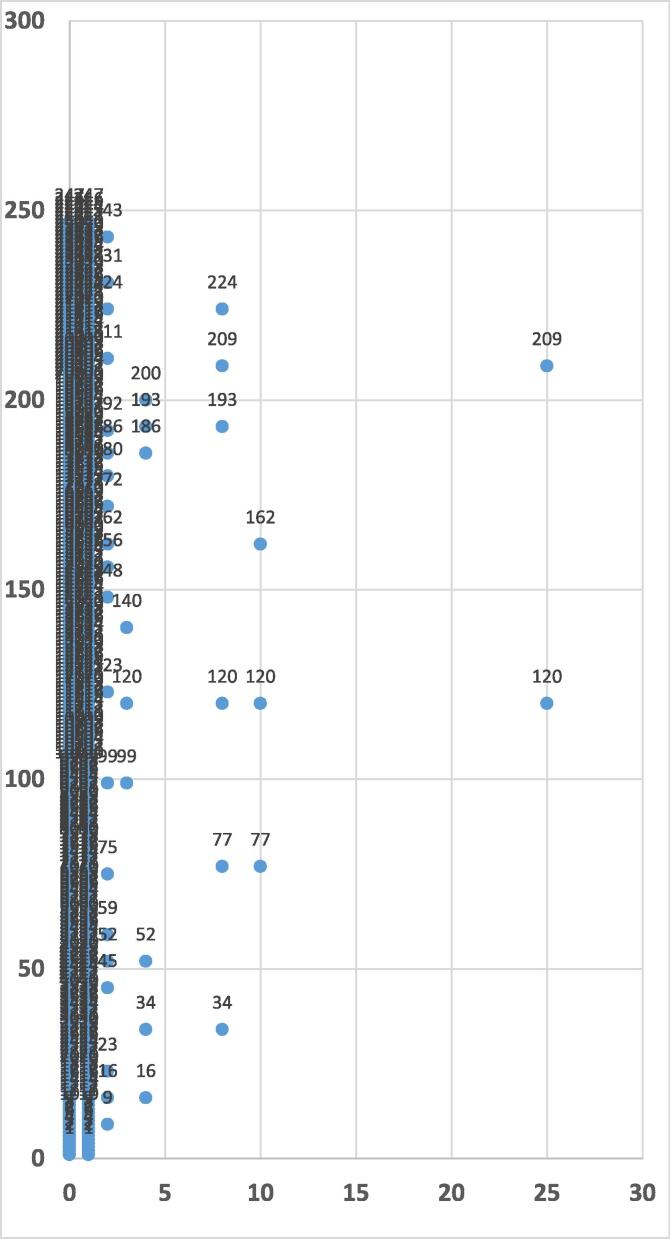
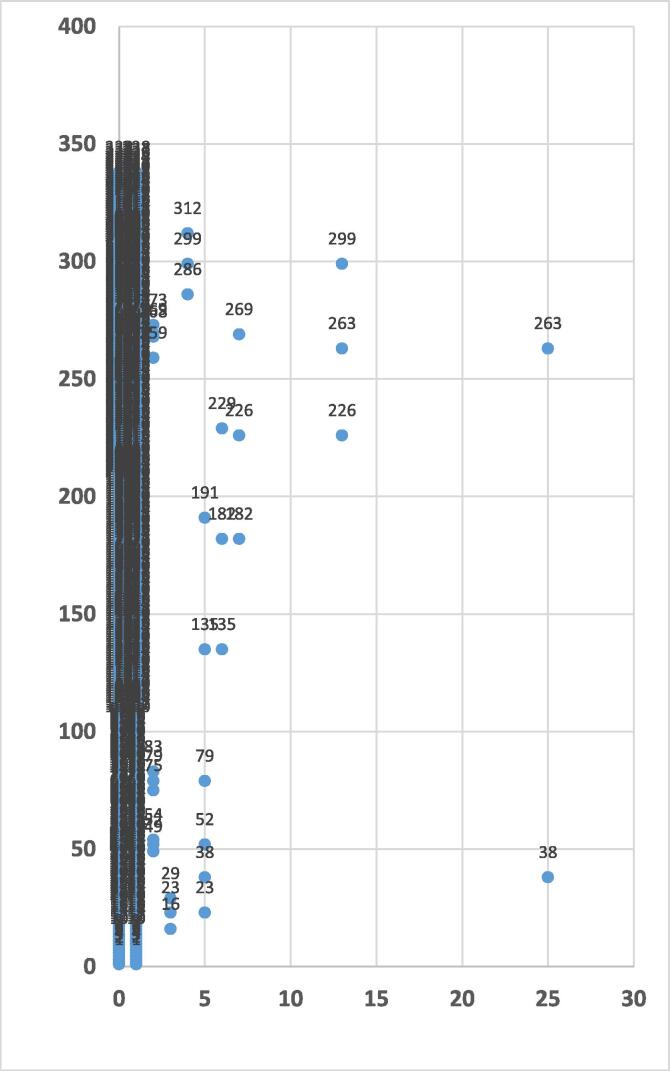
The results obtained indicate that the MRSA isolates can be grouped into five groups when dissimilarity is less than 5 (Fig. 3). Two isolates have been grouped together where the dissimilarity is 25, whereas 6 isolates have been grouped into one with the dissimilarity between 5 and 10. The dissimilarity between the isolates has an obvious association with the source of the isolates. For example, for strains numbered 120 and 209 isolated from the trachea and wound respectively, the dissimilarity was 25 (Fig. 3). Fig. 4 shows that the non-MRSA isolates can be classified into four groups at dissimilarity less than 5, while when the dissimilarity is 25 the isolates are grouped into one cluster containing two isolates (strains numbered 38 and 263 isolated from body fluids and wound respectively). The results indicate that the dissimilarity of antibiotic-resistance patterns has a clear correlation with the source of MRSA and non-MRSA isolates.
Fig. 3.
Dendrogram of MRSA isolated from patients with Ward Linkage and Euclidean distance using SPSS (N = 247). The Y-axis indicates number of isolates and the X-axis indicates the distance or dissimilarity between clusters.
Fig. 4.
Dendrogram of non-MRSA strains isolated from patients with Ward linkage and Euclidean distance using SPSS (N = 338). The Y-axis indicates number of isolates and the X-axis indicates the distance or dissimilarity between clusters.
3.4. Risk estimate of antibiotic-resistant strains
The risk of infection by the S. aureus strains resistant to antibiotics was estimated. The odds ratios of infection by trimethoprim/sulfamethoxazole resistant S. aureus strains found in blood, body fluid, nasal, eye, and wound samples were 7.8, 5.8, 2.5, 1.5, and 0.1 respectively (Table 1, Table 2, Table 3). Trimethoprim/sulfamethoxazole resistant S. aureus strains were not recorded in urine, ear, tissue, and trachea infection under this study. For S. aureus strains resistant to amoxicillin, the odds ratios of infection in blood, ear, eye, and wound samples were 0.001, 27.1, 1.1, and 16.9 respectively, whereas amoxicillin-resistant S. aureus strains were not isolated from other sources. The results showed that the amoxicillin-resistant S. aureus strains were much more likely to infect ears and wounds as compared to blood. For S. aureus strains resistant to ampicillin, the odds ratios of infection in blood, nasal, eye, and wound samples were 0.001, 5.4, 1.1, and 16.8 respectively, implying that wounds are more susceptible to these strains than other sources. The data showed that the odds ratio of infection by cefoxitin-resistant S. aureus strains isolated from the trachea was the highest among all the infections followed by the odds ratio of gentamicin-resistant S. aureus strains isolated from tissue.
Table 1.
Risk evaluation of antibiotic-resistant S. aureus isolates obtained from blood, body fluids and urine.
| Source | Antibiotic | 95.00% | P-value | ||
|---|---|---|---|---|---|
| Odds ratio | Low | Upper | |||
| Blood | Amoxicillin | 0.001 | 0.00 | 0.005 | |
| Ampicillin | 0.001 | 0 | 0.007 | 0.04 | |
| Ciprofloxacin | 0.069 | 0.009 | 0.518 | 0.001 | |
| Clindamycin | 0.247 | 0.073 | 0.84 | 0.016 | |
| Cloxacillin | 1.125 | 0.462 | 2.74 | 0.795 | |
| Erythromycin | 0.496 | 0.183 | 1.343 | 0.161 | |
| Fusidic acid | 1.066 | 0.124 | 9.135 | 0.954 | |
| Gentamicin | 0.299 | 0.088 | 1.019 | 0.042 | |
| Imipenem | 0.001 | 0 | 0.008 | 0 | |
| Levofloxacin | 0.378 | 0.14 | 1.018 | 0.047 | |
| Moxifloxacin | 0.28 | 0.095 | 0.826 | 0.015 | |
| Rifampicin | 1.285 | 0.146 | 11.345 | 0.821 | |
| Trimethoprim/sulfamethoxazole | 7.803 | 3.003 | 20.278 | 0 | |
| Tetracycline | 0.512 | 0.202 | 1.297 | 0.152 | |
| Body fluids | Azithromycin | 2.594 | 0.511 | 13.164 | 0.234 |
| Cefazolin | 6.321 | 0.732 | 54.556 | 0.055 | |
| Ciprofloxacin | 1.254 | 0.224 | 7.013 | 0.796 | |
| Clindamycin | 1.46 | 0.261 | 8.166 | 0.665 | |
| Cloxacillin | 0.142 | 0.025 | 0.801 | 0.011 | |
| Erythromycin | 1.559 | 0.279 | 8.73 | 0.61 | |
| Fusidic acid | 0.632 | 0.073 | 5.495 | 0.675 | |
| Gentamicin | 1.741 | 0.31 | 9.762 | 0.524 | |
| Levofloxacin | 1.211 | 0.217 | 6.763 | 0.827 | |
| Moxifloxacin | 1.181 | 0.211 | 6.59 | 0.85 | |
| Oxacillin | 0.017 | 0.002 | 0.149 | 0 | |
| Rifampicin | 9.48 | 0.929 | 96,712 | 0.021 | |
| Trimethoprim/sulfamethoxazole | 5.895 | 1.093 | 34.29 | 0.026 | |
| Tetracycline | 1.313 | 0.253 | 7.339 | 0.755 | |
| Urine | Azithromycin | 1.246 | 0.111 | 13.964 | 0.858 |
| Ciprofloxacin | 1.379 | 0.123 | 15.458 | 0.794 | |
| Erythromycin | 1.567 | 0.14 | 17.583 | 0.714 | |
| Levofloxacin | 61.5 | 3.836 | 986.004 | 0 | |
| Moxifloxacin | 4.889 | 0.436 | 54.764 | 0.155 | |
| Oxacillin | 0.008 | 0 | 0.18 | 0 |
Table 2.
Risk evaluation of antibiotic-resistant S. aureus isolates obtained from ear, nasal cavity and eye.
| Source | Antibiotic | 95.00% | P-value | ||
|---|---|---|---|---|---|
| Odds ratio | Low | Upper | |||
| Ear | Amoxicillin | 27.167 | 4.15 | 177.98 | 0 |
| Azithromycin | 1.82 | 0.36 | 9.28 | 0.46 | |
| Ciprofloxacin | 0.75 | 0.09 | 6.19 | 0.79 | |
| Erythromycin | 0.92 | 0.18 | 4.62 | 0.92 | |
| Levofloxacin | 0.57 | 0.07 | 4.73 | 0.6 | |
| Moxifloxacin | 0.61 | 0.07 | 5.01 | 0.64 | |
| Tetracycline | 1.91 | 0.23 | 16.17 | 0.55 | |
| Nasal | Ampicillin | 5.486 | 0.724 | 41.605 | 0.066 |
| Azithromycin | 1.159 | 0.683 | 3.378 | 0.303 | |
| Cefazolin | 2.497 | 0.726 | 8.585 | 0.134 | |
| Ciprofloxacin | 2.774 | 1.27 | 6.056 | 0.008 | |
| Clindamycin | 1.294 | 0.559 | 2.991 | 0.547 | |
| Cloxacillin | 2.839 | 0.828 | 9.743 | 0.084 | |
| Erythromycin | 1.98 | 0.884 | 4.436 | 0.092 | |
| Fosfomycin | 3.776 | 0.332 | 42.954 | 0.251 | |
| Fusidic acid | 3.086 | 0.571 | 16.663 | 0.169 | |
| Gentamicin | 0.855 | 0.331 | 2.207 | 0.746 | |
| Imipenem | 5.643 | 0.745 | 42.762 | 0.06 | |
| Levofloxacin | 1.749 | 0.795 | 3.844 | 0.161 | |
| Moxifloxacin | 3.212 | 1.476 | 6.988 | 0.002 | |
| Mupirocin | 7.586 | 0.462 | 124.593 | 0.096 | |
| Oxacillin | 0.132 | 0.008 | 2.165 | 0.096 | |
| Trimethoprim/sulfamethoxazole | 2.563 | 0.864 | 7.596 | 0.08 | |
| Tetracycline | 1.59 | 0.714 | 3.538 | 0.253 | |
| Eye | Amoxicillin | 1.139 | 0.136 | 9.548 | 0.905 |
| Ampicillin | 1.178 | 0.141 | 9.869 | 0.88 | |
| Azithromycin | 0.348 | 0.042 | 2.881 | 0.307 | |
| Cefazolin | 1.813 | 0.218 | 15.082 | 0.576 | |
| Ciprofloxacin | 0.383 | 0.046 | 3.174 | 0.356 | |
| Clindamycin | 0.408 | 0.049 | 3.383 | 0.391 | |
| Cloxacillin | 0.857 | 0.168 | 4.369 | 0.853 | |
| Erythromycin | 0.436 | 0.053 | 3.614 | 0.429 | |
| Gentamicin | 0.488 | 0.059 | 4.055 | 0.498 | |
| Imipenem | 2.217 | 0.145 | 10.193 | 0.856 | |
| Levofloxacin | 0.975 | 0.185 | 5.141 | 0.976 | |
| Moxifloxacin | 0.792 | 0.156 | 4.016 | 0.778 | |
| Rifampin | 5.643 | 0.597 | 53.35 | 0.09 | |
| Trimethoprim/sulfamethoxazole | 1.521 | 0.178 | 12.994 | 0.699 | |
| Tetracycline | 0.858 | 0.169 | 4.355 | 0.853 |
Table 3.
Risk evaluation of antibiotic-resistant S. aureus isolates obtained from wounds, trachea and tissue.
| Source | Antibiotic | 95.00% | P-value | ||
|---|---|---|---|---|---|
| Odds Ratio | Low | Upper | |||
| Wounds | Amoxicillin | 16.97 | 3.792 | 69.188 | 0 |
| Ampicillin | 16.843 | 3.947 | 71.868 | 0 | |
| Azithromycin | 0.654 | 0.372 | 1.149 | 0.138 | |
| Cefazolin | 2.698 | 1.356 | 5.37 | 0.004 | |
| Ciprofloxacin | 0.023 | 0.008 | 0.062 | 0 | |
| Clindamycin | 0.72 | 0.402 | 1.289 | 0.268 | |
| Cloxacillin | 2.544 | 1.322 | 4.896 | 0.004 | |
| Erythromycin | 0.455 | 0.245 | 0.846 | 0.012 | |
| Fosfomycin | 0.648 | 0.058 | 7.242 | 0.723 | |
| Fusidic acid | 0.976 | 0.214 | 4.456 | 0.975 | |
| Gentamicin | 1.283 | 0.707 | 2.329 | 0.412 | |
| Imipenem | 17.5 | 4.105 | 74.599 | 0 | |
| Levofloxacin | 0.946 | 0.546 | 1.641 | 0.844 | |
| linezolid | 1.306 | 0.081 | 21.11 | 0.851 | |
| Moxifloxacin | 0.846 | 0.488 | 1.467 | 0.551 | |
| Mupirocin | 1.306 | 0.081 | 21.11 | 0.851 | |
| Rifampin | 0.976 | 0.214 | 4.456 | 0.975 | |
| Trimethoprim/sulfamethoxazole | 0.121 | 0.028 | 0.531 | 0.001 | |
| Tetracycline | 1.446 | 0.831 | 2.517 | 0.191 | |
| Tissue | Azithromycin | 1.902 | 0.415 | 8.72 | 0.4 |
| Ciprofloxacin | 0.449 | 0.053 | 3.804 | 0.452 | |
| Clindamycin | 1.174 | 0.222 | 6.206 | 0.85 | |
| Erythromycin | 2.405 | 0.523 | 11.06 | 0.246 | |
| Gentamicin | 23.28 | 2.74 | 197.808 | 0 | |
| Imipenem | 1.038 | 0.121 | 8.883 | 0.973 | |
| Levofloxacin | 0.398 | 0.047 | 3.366 | 0.382 | |
| Moxifloxacin | 0.39 | 0.046 | 3.301 | 0.371 | |
| Tetracycline | 1.035 | 0.196 | 5.464 | 0.967 | |
| Trachea | Azithromycin | 5.767 | 2.073 | 16.041 | 0 |
| Cefazolin | 0.171 | 0.064 | 0.459 | 0 | |
| Cefoxitin | 46.4 | 4.548 | 473.394 | 0 | |
| Ciprofloxacin | 1.061 | 0.393 | 3.097 | 0.914 | |
| Clindamycin | 5.337 | 1.971 | 14.448 | 0 | |
| Cloxacillin | 1.577 | 0.586 | 4.24 | 0.364 | |
| Erythromycin | 5.751 | 2.12 | 15.601 | 0 | |
| Gentamicin | 0.995 | 0.314 | 3.151 | 0.993 | |
| Levofloxacin | 0.933 | 0.32 | 2.719 | 0.899 | |
| Moxifloxacin | 1.577 | 0.586 | 4.24 | 0.364 |
4. Discussion
In the present study, a retrospective analysis has been performed for 589 S. aureus strains isolated from patients at the King Khalid University Hospital (KKUH), Riyadh, Saudi Arabia. Varying sources of samples and the patterns of resistance to antibiotics have been evaluated to determine the sources of antibiotic-resistant S. aureus strains. These findings confirmed that the major sources of MRSA and non-MRSA isolates in the patients were wound, blood, and sputum. Association between Staphylococcus spp. with wounds in hospitalized patients has been reported by Almeida et al. (2014). There are several potential sources of S. aureus infected wounds, with nasal carriage (Wenzel and Perl, 1995) and skin (Hauser et al., 1985) being the most important ones. Several studies have mentioned S. aureus strains isolated from wound infections; for example, 23% of the isolates from pediatric wound infections in China were S. aureus strains (Ran et al., 2010), whereas 20% and 32% of the isolates from wounded patients in Brazil were non-MRSA and MRSA strains respectively (Almeida et al., 2014). Infection of wounds by S. aureus strains is a significant risk factor because of their ability to develop antibiotic resistance (Livermore, 2000, Pantosti et al., 2007). The present study found that MRSA and non-MRSA strains could be isolated from almost all known biological samples in patients. MRSA and non-MRSA strains have been isolated from blood and cerebrospinal fluid (Speller et al., 1997), sputum (Shah et al., 1999), eye (Freidlin et al., 2007), ear (Hwang et al., 2002), nasal cavity (Wu et al., 2019), skin and soft tissues (Jorgensen et al., 2019), among other sites in the human body. The results suggest that a group of antibiotics (vancomycin, daptomycin and tigecycline) has the ability to treat infections caused by non-MRSA and MRSA since none of the strains showed resistance to these antibiotics in this study. In these findings, eight antibiotics (vancomycin, cefoxitin, daptomycin, gentamicin, oxacillin, teicoplanin, tigecycline, and mupirocin) displayed the potential to treat the non-MRSA infections while six antibiotics (vancomycin, daptomycin, teicoplanin, tigecycline, nitrofurantoin, and itraconazole) showed promise against MRSA infections. With regard to other antibiotics, it would be premature to suggest using them for treatment (MRSA or non-MRSA infections) without conducting susceptibility tests to choose suitable antibiotics.
Rising resistance to antibiotics and the emergence of new strains of S. aureus is a global health problem, with many scientific reports raising concern over this worsening problem. For example, although linezolid is largely effective against pathogenic gram-positive bacteria including MRSA and non-MRSA strains (Dennis et al., 2002), the present study, in line with previous studies (Tsiodras et al., 2001, García et al., 2010, Morales et al., 2010), reported that there are now linezolid-resistant MRSA and non-MRSA strains. Even though the resistance of pathogenic S. aureus strains to vancomycin has been reported (Smith et al., 1999, de Albuquerque et al., 2019), in the present work, all strains were susceptible to vancomycin. Cephalosporins, nafcillin, sulfa drugs, and vancomycin could be prescribed for treatment of Staphylococcus infections. According to our findings, there are non-MRSA strains that are resistant to cefazolin (a first-generation cephalosporin) whereas there is no non-MRSA strain with resistance to cefoxitin (a second-generation cephalosporin). It has been confirmed that methicillin-susceptible Staphylococcus aureus (MSSA) can synthesize β-lactamases and may be resistant to first-generation cephalosporins (McNeil et al., 2019). Staphylococcus spp. (MRSA and non-MRSA) are considered susceptible to trimethoprim/sulfamethoxazole; however, several studies, including ours, have reported the isolation of trimethoprim/sulfamethoxazole-resistant MRSA and non-MRSA strains from patients (Harris et al., 2018, Sato et al., 2018, Coombs et al., 2019, Tissot-Dupont et al., 2019). In fact, mutability of the Staphylococcus strains, including MRSA strains is a very perplexing issue.
Many environmental and genetic factors–infection type, location of study, management of treatment, natural and non-natural mutations, etc. – are responsible for the increasing resistance to antibiotics. Almost all studies investigating the interaction between antibiotics and Staphylococcus strains indicate the emergence of one or multiple drug resistance. Most of the antibiotics included in this study proved to be ineffective towards one or more bacterial strains isolated from patients at the King Khalid University Hospital. In the present study, analysis of the results confirmed that the dissimilarity of susceptibility to antibiotics in the bacterial isolates depends on the type of sample obtained from patients, implying that the resistance of isolated strains to antibiotics differs depending on the site of infection. Source of infection and the management of treatment can vary as per the type of infection, which could explain the emergence of different bacterial strains depending on the site of infection. Incorrect management of treatment is one of the most important factors leading to the development of antibiotic resistance (Heisner and Skelton, 2019). For example, in our study, clindamycin-resistant non-MRSA strains comprised of 5.7% of the isolates obtained from wounds whereas they comprised of 22.2% of the isolates obtained from blood. Trimethoprim/sulfamethoxazole-resistant non-MRSA strains (27.2% of the isolates) were isolated from the blood whereas none were isolated from wounds. We quantified the association between antibiotic-resistant S. aureus isolates and the source of samples (site of infection) using odds ratio. Our findings suggest that there is a clear association between the source of samples and the infection caused by antibiotic-resistant strains. For example, amoxicillin – a broad-spectrum antibiotic that has the ability to destroy bacterial cells – is primarily and frequently prescribed for the treatment of aural bacterial infections (Sodhi et al., 2019). The present study showed that the strength of the association between amoxicillin-resistant S. aureus strains and ear and wound infections was high compared to that of amoxicillin-susceptible S. aureus. A high strength of association between S. aureus strains resistant to trimethoprim/sulfamethoxazole and infections in blood, body fluids, nasal cavity, and eye samples has been recorded while the strength of association between trimethoprim/sulfamethoxazole-resistant S. aureus strains and wound samples was very low. These findings state that some antibiotic-resistant S. aureus strains tend to infect particular sites in the body and sometimes the probability of infection caused by these strains is more than that caused by antibiotic-susceptible strains due to site of the infection.
5. Conclusion
The present study inferred that wounds, blood, and sputum are the primary sources of MRSA and non-MRSA strains isolated at University Medical City’s (UMC) King Khalid University Hospital (KKUH) in Riyadh, Saudi Arabia. More than 40% of the S. aureus strains were MRSA strains. All MRSA isolates were resistant to amoxicillin/clavulanate, ampicillin, cefoxitin, cefazolin, imipenem, oxacillin, and penicillin. All non-MRSA strains were susceptible to vancomycin, cefoxitin, daptomycin, gentamicin, oxacillin, teicoplanin, tigecycline, and mupirocin. The emergence of new strains with the ability to resist one or more antibiotics has been reported in this work. With respect to the antimicrobial susceptibility patterns, the dissimilarity between the strains depends upon the type of sample obtained from sites of infections. Some antibiotic-resistant strains were found to infect certain sites more than some antibiotic-susceptible strains.
Acknowledgments
Acknowledgements
The authors extend their appreciation to the Research Supporting Project number RSP-2019/70, King Saud University, Riyadh, Saudi Arabia.
Authors' contributions
The collected of data, analysis, writing have been done by all authors, also all authors read and approved the manuscript before the submitting.
Availability of data and materials
The data and materials applied during our work have been included.
Ethics statement
The procedures followed by the present work was performed in accordance with guidelines of Institutional Review Board of College of Medicine, KSU, Saudi Arabia (17/0449/IRB).
Consent for publication
Not applicable.
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Jamal M. Khaled, Email: gkhaled@ksu.edu.sa.
Muhammed R. Shehu, Email: shehuroj@gmail.com.
References
- Al-Zahrani I.A., Azhar E.I., Jiman-Fatani A.A., Siddig L.A., Yasir M., Al-Ghamdi A.K., Harwood C.R. Impact of mass migrations on the clonal variation of clinical staphylococcus aureus strains isolated from the western region of saudi arabia. J. Infect. Public Health. 2019;12(3):317–322. doi: 10.1016/j.jiph.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Alabi A.S., Frielinghaus L., Kaba H., Kösters K., Huson M.A., Kahl B.C., Peters G., Grobusch M.P., Issifou S., Kremsner P.G. Retrospective analysis of antimicrobial resistance and bacterial spectrum of infection in gabon, central africa. BMC Infect. Dis. 2013;13(1):455. doi: 10.1186/1471-2334-13-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida G.C.M., dos Santos M.M., Lima N.G.M., Cidral T.A., Melo M.C.N., Lima K.C. Prevalence and factors associated with wound colonization by staphylococcus spp. and staphylococcus aureus in hospitalized patients in inland northeastern Brazil: a cross-sectional study. BMC Infect. Dis. 2014;14(1):328. doi: 10.1186/1471-2334-14-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukharie H.A., Abdelhadi M.S., Saeed I.A., Rubaish A.M., Larbi E.B. Emergence of methicillin-resistant staphylococcus aureus as a community pathogen. Diagn. Microbiol. Infect. Dis. 2001;40(1–2):1–4. doi: 10.1016/s0732-8893(01)00242-5. [DOI] [PubMed] [Google Scholar]
- Charlebois E.D., Perdreau-Remington F., Kreiswirth B., Bangsberg D.R., Ciccarone D., Diep B.A., Ng V.L., Chansky K., Edlin B., Chambers H.F. Origins of community strains of methicillin-resistant staphylococcus aureus. Clin. Infect. Dis. 2004;39(1):47–54. doi: 10.1086/421090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs G.W., Mowlaboccus S., Daley D., Lee T., Pearson J., Pang S., Robinson J.O. Sulfamethoxazole/trimethoprim resistance overcall by vitek® 2 and bd phoenix™ in community-associated mrsa and mssa. J. Antimicrobial Chemotherapy. 2019 doi: 10.1093/jac/dkz361. [DOI] [PubMed] [Google Scholar]
- de Albuquerque V.V.C., Flores V.C.J., Zeron R.M.C., Godoi B.B., Eulálio Filho W.M.N., dos Santos B.A. Study of vancomycin resistance among staphylococcus aureus. Amadeus Int. Multidiscipl. J. 2019;3(6):31–37. [Google Scholar]
- De Kraker M., Jarlier V., Monen J., Heuer O., Van De Sande N., Grundmann H. The changing epidemiology of bacteraemias in europe: Trends from the European antimicrobial resistance surveillance system. Clin. Microbiol. Infect. 2013;19(9):860–868. doi: 10.1111/1469-0691.12028. [DOI] [PubMed] [Google Scholar]
- Delorme T., Garcia A., Nasr P. A longitudinal analysis of methicillin-resistant and sensitive staphylococcus aureus incidence in respect to specimen source, patient location, and temperature variation. Int. J. Infect. Diseases. 2017;54:50–57. doi: 10.1016/j.ijid.2016.11.405. [DOI] [PubMed] [Google Scholar]
- Dennis, L.S., H. *Daniel, L. Harry, L.H. John, H.B. Donald, H. Barry and L.M.S. Group, 2002. Linezolid versus vancomycin for the treatment of methicillin-resistant staphylococcus aureus infections. Clinical Infectious Diseases, 34(11): 1481–1490. [DOI] [PubMed]
- Feng Y., Chen C.-J., Su L.-H., Hu S., Yu J., Chiu C.-H. Evolution and pathogenesis of staphylococcus aureus: lessons learned from genotyping and comparative genomics. FEMS Microbiol. Rev. 2008;32(1):23–37. doi: 10.1111/j.1574-6976.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- Frei C.R., Makos B.R., Daniels K.R., Oramasionwu C.U. Emergence of community-acquired methicillin-resistant staphylococcus aureus skin and soft tissue infections as a common cause of hospitalization in united states children. J. Pediatr. Surg. 2010;45(10):1967–1974. doi: 10.1016/j.jpedsurg.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Freidlin J., Acharya N., Lietman T.M., Cevallos V., Whitcher J.P., Margolis T.P. Spectrum of eye disease caused by methicillin-resistant staphylococcus aureus. Am. J. Ophthalmol. 2007;144(2):313–315. doi: 10.1016/j.ajo.2007.03.032. [DOI] [PubMed] [Google Scholar]
- García M.S., De la Torre M.Á., Morales G., Peláez B., Tolón M.J., Domingo S., Candel F.J., Andrade R., Arribi A., García N. Clinical outbreak of linezolid-resistant staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260–2264. doi: 10.1001/jama.2010.757. [DOI] [PubMed] [Google Scholar]
- Harris T., Bowen A., Holt D., Sarovich D., Stevens K., Currie B., Howden B., Carapetis J., Giffard P., Tong S. Investigation of trimethoprim/sulfamethoxazole resistance in an emerging sequence type 5 methicillin-resistant staphylococcus aureus clone reveals discrepant resistance reporting. Clin. Microbiol. Infect. 2018;24(9):1027–1029. doi: 10.1016/j.cmi.2018.04.020. [DOI] [PubMed] [Google Scholar]
- Hauser C., Wuethrich B., Matter L., Wilhelm J., Sonnabend W., Schopfer K. Staphylococcus aureus skin colonization in atopic dermatitis patients. Dermatology. 1985;170(1):35–39. [PubMed] [Google Scholar]
- Heisner K., Skelton L. Antimicrobial stewardship: Analysis of provider management of drug-bug mismatch in multi-drug resistant organisms in a large medical group. Am. J. Infect. Control. 2019;47(6):S15. [Google Scholar]
- Hwang J.-H., Tsai H.-Y., Liu T.-C. Community-acquired methicillin-resistant staphylococcus aureus infections in discharging ears. Acta Otolaryngol. 2002;122(8):827–830. doi: 10.1080/0036554021000028076. [DOI] [PubMed] [Google Scholar]
- Jorgensen S.C., Lagnf A.M., Bhatia S., Singh N.B., Shammout L.K., Davis S.L., Rybak M.J. Diagnostic stewardship: A clinical decision rule for blood cultures in community-onset methicillin-resistant staphylococcus aureus (mrsa) skin and soft tissue infections. Infect. Diseases Therapy. 2019;8(2):229–242. doi: 10.1007/s40121-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore D.M. Antibiotic resistance in staphylococci. Int. J. Antimicrob. Agents. 2000;16:3–10. doi: 10.1016/s0924-8579(00)00299-5. [DOI] [PubMed] [Google Scholar]
- McNeil, J.C., L. Sommer, M.G. Boyle, P. G. Hogan, J. G. Vallejo, K.G. Hulten, S.L. Kaplan and S. Fritz, 2019. 852. The cefazolin inoculum effect and methicillin-susceptible staphylococcus aureus osteoarticular infections in children: Does it matter? In: Open Forum Infectious Diseases. Oxford University Press US: pp: S17–S18. [DOI] [PMC free article] [PubMed]
- Morales G., Picazo J.J., Baos E., Candel F.J., Arribi A., Peláez B., Andrade R., de la Torre M.-Á., Fereres J., Sánchez-García M. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant staphylococcus aureus. Clin. Infect. Dis. 2010;50(6):821–825. doi: 10.1086/650574. [DOI] [PubMed] [Google Scholar]
- Pantosti, A., Sanchini, A., Monaco, M., 2007. Mechanisms of antibiotic resistance in staphylococcus aureus. [DOI] [PubMed]
- Ran Y.-C., Ao X.-X., Liu L., Fu Y.-L., Tuo H., Xu F. Microbiological study of pathogenic bacteria isolated from paediatric wound infections following the 2008 wenchuan earthquake. Scand. J. Infect. Dis. 2010;42(5):347–350. doi: 10.3109/00365540903510682. [DOI] [PubMed] [Google Scholar]
- Sato T., Kawamura M., Furukawa E., Fujimura S. Screening method for trimethoprim/sulfamethoxazole-resistant small colony variants of staphylococcus aureus. J. Glob. Antimicrobial Resist. 2018;15:1–5. doi: 10.1016/j.jgar.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Senok A., Somily A.M., Nassar R., Garaween G., Sing G.K., Müller E., Reissig A., Gawlik D., Ehricht R., Monecke S. Emergence of novel methicillin-resistant staphylococcus aureus strains in a tertiary care facility in riyadh, saudi arabia. Infect. Drug Resist. 2019;12:2739. doi: 10.2147/IDR.S218870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P., Mawdsley S., Nash K., Cullinan P., Cole P., Wilson R. Determinants of chronic infection with staphylococcus aureus in patients with bronchiectasis. Eur. Respir. J. 1999;14(6):1340–1344. doi: 10.1183/09031936.99.14613409. [DOI] [PubMed] [Google Scholar]
- Smith T.L., Pearson M.L., Wilcox K.R., Cruz C., Lancaster M.V., Robinson-Dunn B., Tenover F.C., Zervos M.J., Band J.D., White E. Emergence of vancomycin resistance in staphylococcus aureus. N. Engl. J. Med. 1999;340(7):493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- Sodhi K.K., Kumar M., Balan B., Dhaulaniya A.S., Singh D.K. Isolation and characterization of amoxicillin-resistant bacteria and amoxicillin-induced alteration in its protein profiling and rna yield. Arch. Microbiol. 2019:1–8. doi: 10.1007/s00203-019-01737-6. [DOI] [PubMed] [Google Scholar]
- Speller D., Johnson A., James D., Marples R., Charlett A., George R. Resistance to methicillin and other antibiotics in isolates of staphylococcus aureus from blood and cerebrospinal fluid, england and wales, 1989–95. The Lancet. 1997;350(9074):323–325. doi: 10.1016/s0140-6736(97)12148-1. [DOI] [PubMed] [Google Scholar]
- Tissot-Dupont H., Gouriet F., Oliver L., Jamme M., Casalta J.-P., Jimeno M.-T., Arregle F., Lavoute C., Hubert S., Philip M. High dose trimethoprim-sulfamethoxazole and clindamycin for staphylococcus aureus endocarditis. Int. J. Antimicrob. Agents. 2019 doi: 10.1016/j.ijantimicag.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Tsiodras S., Gold H.S., Sakoulas G., Eliopoulos G.M., Wennersten C., Venkataraman L., Moellering R.C., Jr, Ferraro M.J. Linezolid resistance in a clinical isolate of staphylococcus aureus. The Lancet. 2001;358(9277):207–208. doi: 10.1016/S0140-6736(01)05410-1. [DOI] [PubMed] [Google Scholar]
- Wenzel R., Perl T. The significance of nasal carriage of staphylococcus aureus and the incidence of postoperative wound infection. J. Hosp. Infect. 1995;31(1):13–24. doi: 10.1016/0195-6701(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Wu T.-H., Lee C.-Y., Yang H.-J., Fang Y.-P., Chang Y.-F., Tzeng S.-L., Lu M.-C. Prevalence and molecular characteristics of methicillin-resistant staphylococcus aureus among nasal carriage strains isolated from emergency department patients and healthcare workers in central taiwan. J. Microbiol. Immunol. Infect. 2019;52(2):248–254. doi: 10.1016/j.jmii.2018.08.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials applied during our work have been included.