Abstract
Recent advances in pharmacological agents have led to successful treatment of a variety of retinal diseases such as neovascular age-related macular degeneration (AMD), diabetic macular oedema (DMO), and retinal vascular occlusions (RVO). These treatments often require repeated drug injections for an extended period of time. To reduce these repeated treatment burdens, minimally invasive drug delivery systems are needed. An ideal therapy should maintain effective levels of drug for the intended duration of treatment following a single application, recognising that a significant number of months of therapy may be required. There are numerous approaches under investigation to improve treatment options. This review will highlight the advantages and limitations of selected drug delivery systems of novel biomaterial implants and depots. The main emphasis will be placed on less invasive, longer acting, sustained release formulations for the treatment of retinal disorders.
Subject terms: Retinal diseases, Eye diseases
摘要
近年来, 药理学方面的进步改善了多种视网膜疾病的预后, 例如新生血管性年龄相关性黄斑变性 (AMD), 糖尿病性黄斑水肿 (DMO) 和视网膜血管阻塞 (RVO)。此类疾病的治疗通常需要长期反复注射给药, 为了减少重复性治疗的负担, 尽可能使用无创的药物传输系统。考虑到可能需要数月的治疗周期, 理想的治疗方式是在单次给药后较长时间内能保持有效的药物浓度。目前多种药物正在研发之中以改善药物治疗方案。本篇综述概括了几种特定的新型生物材料植入物和缓释库给药途径的优势和局限性。重点介绍了治疗视网膜疾病的侵入性小, 作用时间长, 持续性释放的几种制剂。
Introduction
Retinal diseases such as choroidal neovascularisation (CNV) secondary to age-related macular degeneration (AMD), diabetic macular oedema (DMO), and retinal vein occlusions (RVO) can lead to severe visual complications and even blindness. Treatments for these diseases often require repeated intravitreal drug injection, where the frequency and duration vary depending on the specifics of the disease. Though the treatment provides benefits to the patients, repeated treatment becomes a significant burden on the patients, their families, physicians, and healthcare system.
The anatomy of the eye makes it a challenge to deliver therapeutic agents. Due to the blood-retinal barrier (BRB), the eye is resistant to exposure of foreign substances, and pharmaceutical agents trying to reach the intended ocular tissues [1, 2]. The BRB is composed of an inner and an outer barrier. The BRB is maintained by tight junctions at the retinal vascular endothelium, the iris vascular epithelium, and the non-pigmented ciliary epithelium and the barrier is essential in maintaining retinal homoeostasis [3]. The outer component consists of junctional complexes of retinal pigmented epithelium (RPE) and the pigment epithelial cells of the pars plana. The inner segment consists of tight junctions between the endothelial cells of the retinal capillaries. Due to the blood-retinal barrier, there is little convection of molecules since it has no cellular components and is selectively permeable to more lipophilic molecules [4].
The eye offers multiple entry routes through which ocular drugs may be delivered (for review Kang-Mieler et al. [5].). Delivery to the anterior segment of the eye may be achieved through topical and subconjunctival routes, or injected intracamerally. Posterior segment delivery can be achieved topically, systemically, and periocularly (i.e., through sub-Tenon’s), via the suprachoroidal space, and via intraocular (i.e., intravitreal) injections. Success of therapeutic drug delivery depends on the delivery site, tissue barriers, and the type of pharmacological agents involved [6].
The U.S. Food and Drug Administration (FDA) has approved several anti-vascular endothelial growth factor (anti-VEGF) therapeutics, including pegaptanib, ranibizumab, aflibercept, and most recently brolucizumab (along with the previous off-label use of bevacizumab) to treat neovascular eye diseases. All of these therapies involve treatment via intravitreal injections every month or two (or perhaps up to 3 months with brolucizumab) for an extended period of time. Since the introduction of anti-VEGF therapy, it has become the gold standard treatment of CNV secondary to AMD, RVO, and DMO as well. These agents are also employed in the settings of a variety of other inflammatory and/or degenerative causes of CNV. Though the effective treatment efficacy, the repeated treatment burden cannot be ignored and extended release drug delivery systems (DDSs) are an attractive alternative to the current therapies, especially in cases where a repeated dosing or injection is required.
Advances in biomaterials and nanotechnology have led to major growth in research of biodegradable microparticles and nanoparticles, hydrogels and ocular implants, all of which may contain ocular pharmacologic agents thereby providing improved delivery of a variety of medications. Furthermore, sustained release drug therapies may improve the side effects associated with current clinical treatments and lower the overall socio-economic impact of ocular diseases [5]. With ever evolving approaches to targeted delivery, ocular drug delivery is progressing at a rapid pace. In the following review, we present recent advances in ocular drug delivery with a focus on the posterior segment.
Microneedles
Due to the fibrous composition and large surface area, the sclera offers less resistance to drug diffusion which makes it an attractive delivery site. The large surface area (~95% of the total ocular surface area of the eye) offers the possibility of delivering neuroprotective agents, antioxidants, or anti-angiogenic agents to specific locations of the retina via transcleral absorption [7]. Studies have shown that molecules up to 70 kDa can readily penetrate the sclera, whereas through the cornea it is less than 1 kDa [8]. A major challenge of transcleral delivery is that with high drug clearance mechanisms and static, dynamic, and metabolic barriers, an effective drug concentration within the eye may not be readily achieved [9].
Microneedles enable minimally invasive delivery of free or encapsulated drug. Clearside Biomedical (Alpharetta, GA, USA) developed a microneedle and injector that administers a suprachoroidal injection of corticosteroid triamcinolone acetonide (CLS-TA), which is Clearside Biomedical’s proprietary suspension of TA. The injector allows for consistent insertion of microneedle into the suprachoroidal space. Thus, this method reduces the risks commonly associated with intravitreal injections, including potential for retinal damage [10]. Due to the small surface area of the microneedle, this system is limited to small molecules and microneedles cannot always deliver a therapeutic dose. Clearside Biomedical recently presented the Phase 3 PEACHTREE trial of CLS-TA in patients with macular oedema associated with noninfectious uveitis [10, 11]. The study met the primary endpoint, where 46.9% of patients-receiving treatment had an increase in visual acuity from baseline as compared to only 15.6% of the control patients [10, 11]. As for safety, 11.5% of treated patients had increased intraocular pressure (IOP) but the control patients did not have any increases [10]. CLS-TA improved macular oedema in uveitis patients and vast majority of patients did not require rescue therapy.
Clearside Biomedical also conducted a Phase 2 clinical trial (TYBEE) for a combination therapy of suprachoroidal CLS-TA with intravitreal injections of aflibercept with patients with macular oedema (DME) over a 6-months evaluation period [12]. The goal was to deliver a combination of TA and anti-VEGF to reduce the number of re-treatments. Patients received either quarterly treatment of CLS-TA and intravitreal aflibercept (months 0 and 3) or four monthly treatment of intravitreal aflibercept with a sham suprachoroidal procedure (months 0, 1, 2, and 3). If needed, either group received intravitreal aflibercept at months 4 and 5. The trial met its primary endpoint of mean improvement in best corrected visual acuity (BCVA) from baseline to 6 months using the Early Treatment of Diabetic Retinopathy Trial (ETDRS) scale. Patients gained on average of 12.3 ETDRS letters compared to 13.5 ETDRS letters in the control group [12]. The study also met its secondary endpoint with a mean reduction from baseline of 208 µm in central subfield thickness at 6 months [12]. Based on the success of Phase 2 study, they are undertaking a Phase 3 study of the combination therapy.
In addition, Clearside Biomedical recently announced that they have granted exclusive worldwide option and licence agreement to REGENXBIO (Rockville, MD, USA) for in-office delivery of RGX-314 gene therapy to suppress VEGF activity in patients with wet AMD and diabetic retinopathy [13].
Microcannulation or microcatheter
Microcannulation or microcatheter was originally designed for canaloplasty [14, 15], but the technology is now being investigated for ocular drug delivery system to the suprachoroidal space. The iTrack microcathether (iScience Interventional, Menlo Park, CA, USA) includes an optical fibre for light illumination for easy insertion guidance. Olsen and colleagues demonstrated in their pharmacokinetic study that triamcinolone acetonide (TA) remained in the pig ocular tissue for at least 120 days with low systemic levels [16]. Histopathology showed maintenance of normal anatomy. They concluded that the suprachoroidal space accessed by the microcannulation is safe and reproducible. They also examined the pharmacokinetics of bevacizumab between intravitreal and suprachoroidal injection in pigs [17]. They reported that intravitreal injection of bevacizumab distributed more to the inner retina, where suprachoroidal injection distributed to the choroid, RPE, and photoreceptor outer segments. Rizzo and colleagues evaluated the safety and efficacy of suprachoroidal delivery of a combination of bevacizumab and TA via microcatheter in chronic macular oedema [18]. BCVA improved by two lines or greater in four eyes and remained stable in two eyes [18]. At 1–2 months after administration, hard exudates were almost completely resolved in all eyes, and macular oedema was significantly reduced. There were no surgical or postoperative complications during 12 months of follow-up.
Though the early studies were encouraging, use of a microcatheter to deliver anti-VEGF alone is not a viable option due to high clearance of agent in the choroidal circulation and limited ability to penetrate the retina. Recently, a microcatheter was used for the delivery of stem cell transplantation into the subretinal space. Jassen Biotech Inc. (Horsham, PA, USA) conducted Phase 1/2 clinical cell therapy of human umbilical tissue-derived cells (CNTO 2476) in geographic atrophy secondary to AMD [19]. A suspension of CNTO 2476 was delivered into the subretinal space near the macular geographic atrophy through a sclerotomy and choroidotomy [20]. They reported that the eyes that received the treatment improved 4–5 letters while the untreated eyes lost two letters. They reported a 15% rate of retinal detachment but no other negative side effects were noted [20]. Recently, Ehab El Rayes used a microcatheter to deliver a fluocinolone acetonide (Iluvien®) implant into the subretinal space for chronic DMO treatment [21, 22]. Using a flexible-tipped disposable cannula (Olive Tip (El Rayes) SC Cannula, MedOne Surgical, Sarasota, FL, USA), they investigated optimal location and efficacy with the injection technique. With 8 months follow-up, they noted no injection complications, no increase in IOP, and no lens changes in the phakic patients [22]. The study is still ongoing. The use of microcathether and suprachoroidal space is still evolving.
Intravitreal implants
Intravitreal injection provides the most direct mode for delivering therapeutic levels of drug into the eye. The intravitreal injection is an office-based procedure in the United States though does have a finite number of injection-related complications such as endophthalmitis, retinal detachment, uveitis, cataract, and glaucoma [23, 24]. Intraocular implants have many advantages over more traditional methods of drug administration to the eye, including delivering a known dose of sustained therapeutic agent directly to the target site and bypassing the blood-ocular barrier. Sustained-release intravitreal implants may also decrease the risk of infection or retinal detachment and potentially localise the therapy to the vitreous, with low systemic exposure [25–27].
The ganciclovir implant (Vitrasert®, Bausch & Lomb, Rochester, NY, USA) was the first FDA approved implantable device used for the treatment of cytomegalovirus (CMV) retinitis. Due to controlled release rates, active drug concentrations remain well below toxic levels and higher concentrations of the drug may be achieved with limited systemic side effects [4, 28]. With the original success of the ganciclovir implant, numerous biodegradable and non-biodegradable implants are either clinically in use or in development.
Encapsulated cell technology
Neurotech Pharmaceuticals, Inc. (Cumberland, RI, USA) has been developing NT-501 (Renexus®), an encapsulated cell technology that allows for delivery of ciliary neurotrophic factor (CNTF) [29]. NT-501 is an implantable polymeric device containing a genetically modified cell line that secretes CNTF for the potential treatment of retinitis pigmentosa (RP) and dry AMD [29]. Genetically modified human RPE cells secrete recombinant human CNTF and are packaged in a hollow tube capsule consisting of a semipermeable membrane surrounding a scaffold of six strands of polyethylene terephthalate yarn, which can be loaded with cells. The capsule prevents immune cell entry yet allows nutrients and therapeutic molecules to diffuse freely across the membrane [30, 31]. Two ends of the polymer section are sealed, and a titanium loop is placed on the anchoring end, which is implanted at the pars plana and anchored to the sclera. Despite its invasive implantation and surgical removal procedure, this system is theoretically versatile for different cell lines and protein therapeutics with controlled, continuous, and sustained release [32]. Neurotech’s original study of dry AMD and RP did not meet the primary endpoint and they are no longer actively investigating these indications. However, they redirected the use of NT-501 for Macular Telangiectasia and glaucoma [33, 34]. The recent Phase 2 study of MacTel type 2 showed that NT-501 treatment slowed the progression of retinal degeneration compared to the sham group [35]. Patients were randomised 1:1 to surgical implantation or sham procedure. In addition to retinal functional benefit, reading speed was stabilised in patients receiving the implant [35]. They are currently enroling patients in a Phase 3 clinical trial. Based on their positive results, the FDA granted Fast Track designation for the treatment of MacTel 2.
Solid corticosteroid implants
Solid ocular implants eliminate the need for repetitive treatments by administering drug for prolonged periods of time. EyePoint Pharmaceuticals (Watertown, MA, USA) developed a solid polymer implant called Durasert™. This implant can release small molecules for up to three years. The implant is only 3.5 mm in length and 0.37 mm in diameter, allowing it to be injected through a small gauge needle. The Durasert™ technology has been approved by the FDA for the following products: Iluvien® (fluocinolone acetonide intravitreal implant), Retisert® (fluocinolone acetonide intravitreal implant), and Vitrasert® (ganciclovir). The properties of this system allow for the customisation of release duration, linear release kinetics, and high drug loading [36, 37].
Fluocinolone acetonide intravitreal implant (Retisert®, Bausch & Lomb, Inc., Rochester, NY, USA) has been previously approved by the FDA for the treatment of chronic non-infectious posterior uveitis [38]. It is a non-biodegradable delivery system composed of 0.59 mg fluocinolone acetonide core compressed into a 1.5 mm tablet encased in a silicone elastomer cup containing a release orifice. A semi-permeable layer of polyvinyl alcohol (PVA) coats the tablet inside the cup reservoir near the release orifice creating a membrane between the tablet and the orifice that serves as an additional barrier to drug release. Retisert is capable of release for up to 2.5 years with an initial rate of 0.6 μg/day, decreasing over the first month to a steady-state release of 0.3–0.4 μg/day [39].
Iluvien® (Alimera Sciences, Alpharetta, GA, USA) is also a fluocinolone acetonide intravitreal insert designed for the treatment of DMO. It is injectable through a 25-gauge inserter in an office setting [40]. Iluvien is a small cylindrical (3.5 mm × 0.37 mm) polyimide tube that releases a low dose of fluocinolone acetonide (0.23–0.45 μg/day) for 18–36 months after injection. Like Retisert, Iluvien does not degrade and remains in the vitreous cavity after its drug release has been exhausted [40]. Therefore, patients requiring repeat injections may end up with multiple devices within the vitreous cavity.
Ozurdex®, which releases dexamethasone (Allergan, Inc., Irvine, CA, USA) is FDA-approved to treat macular oedema following branch and central RVOs, macular oedema secondary to noninfectious posterior segment uveitis, and chronic pseudophakic DME as well. The implant contains 0.7 mg dexamethasone in a biodegradable, solid poly lactic-co-glycolic acid (PLGA) drug delivery system and is preloaded into a single-use, specially designed 22 gauge applicator to facilitate injection of the rod-shaped implant directly into the vitreous cavity [41]. Although it has a relatively short duration of action of about 1 month, the therapeutic effect seems to last much longer, as seen in a Phase 2 clinical study where therapeutic effects persisted at 180 days in some eyes [41]. Currently, these solid corticosteroid implants are FDA approved in USA.
Polymeric nanoparticles and microparticles
Nanoparticle (ranging in size from 10 nm to 1 µm) and microparticle (ranging from 1 to 1000 µm) systems are attractive drug delivery platforms due to the wide availability of different materials (natural or synthetic) that can be tailored for specific drugs and applications. Synthetic, biodegradable polymers are commonly used to synthesise particles because they are biocompatible and hydrolytically degradable with byproducts that can be metabolised by the human body [42]. Since polymers are fully biodegradable, no removal surgery is required once drug release is complete [43]. Nanoparticles and microparticles have the ability to encapsulate both hydrophilic and hydrophobic molecules, proteins, peptides, vaccines, and biological macromolecules. Their characteristics can be tailored for specific use through modification of polymer composition and ratios, polysaccharide blending, and surface modifications [44]. However, major challenges for particle systems are low protein encapsulation efficiency (usually <30% for nanoparticles and <60% for microparticles), high initial bursts (20–50% of encapsulated protein in first 24 h), incomplete release of the entrapped proteins, and loss of protein drug bioactivity during release [45–47].
Varshochian et al. developed albuminated PLGA nanoparticles for sustained delivery of bevacizumab [48, 49]. The nanoparticles (~200 nm) were fabricated by water-in-oil-in-water (w/o/w) double emulsion using albumin as a stabiliser. Their recent studies in rabbits showed that vitreous concentration of bioactive bevacizumab was maintained above 500 ng/ml for about 8 weeks after single intravitreal injection [48, 49]. Bevacizumab-loaded PLGA microparticles (2–7 µm in diameter) were also fabricated using solid-in-oil-in-water (s/o/w) method by Ye et al. [50]. A significantly prolonged half-life of bevacizumab in the vitreous humour (9.6 days) and aqueous humour (10.2 days) has been achieved in New Zealand albino-rabbits, compared to 3.91 days in the vitreous humour and 4.1 days in the aqueous humour for free drug [50].
Graybug Vision, Inc (Redwood City, CA, USA) has developed injectable PLGA microparticles for applications involving both the posterior and anterior segments. Their microparticles platform allows drug release profiles without initial burst of drug and can be modified to deliver drug for up to several months [51]. GB-102 is a microparticle depot formulation of sunitinib malate, a small molecule receptor tyrosine kinase inhibitor (TKI). Their recent Phase 1/2a study investigated 32 patients from eight centres in US [52]. Patients were previously treated with at least three prior intravitreal injection of an anti-VEGF agent. They received a single intravitreal injection of GB-102 (0.25, 0.5, 1, or 2 mg). They reported that 50–88% of patients (depend on dose cohorts) required no additional injections of anti-VEGF agent for 6 months. They are initiating a Phase 2 wet AMD study where two doses of GB-102 (1 and 2 mg) administered every six months compared to aflibercept administered every two months [53].
Hydrogel delivery systems
Ocular Therapeutix (Bedford, MA, USA) is developing an injectable and bioresorbable hydrogel technology for sustained anti-VEGF delivery in collaboration with Regeneron (Tarrytown, NY, USA). They are investigating the feasibility of OTX-IVT for sustained delivery of aflibercept for 4–6 months targeting wet AMD and other retinal neovascular diseases [54]. Since the announcement of collaboration, no study updates have been released.
They are also developing a sustained-release tyrosine kinase inhibitor (TKI) implant based on their bioresorbable hydrogel (OTX-TKI). The implant is designed to deliver TKI for a duration of up to 12 months [55]. Preclinical data demonstrated that OTX-TKI can deliver TKI to the posterior segment with sustained pharmacokinetic effects for the treatment of VEGF-induced retinal leakage for a duration of up to 12 months. Ocular Therapeutix initiated an external US Phase 1 study in Sydney, Australia [56]. The Phase 1 trial is a multicenter, open-label study testing the safety, durability, and tolerability of OTX-TKI. They study will evaluate the retinal thickness and visual acuity.
Thermoresponsive hydrogels are a particularly attractive means of extended drug delivery, as it only requires a minimally invasive intravitreal injection, which then employs temperature change as a trigger for gelation and swelling. At room temperature, these hydrogels are designed as a solution or have a fluid-like consistency. After injection into the eye, they solidify into a solid form upon reaching body temperature [57, 58]. These hydrogels usually have a sharp volume phase transition temperature at ~30–33 °C, which makes them ideal candidates for localised and extended drug delivery [59]. Kang-Mieler at al. have developed a poly(N-isopropylacrylamide) (PNIPAAm) based thermoresponsive hydrogel by crosslinking PNIPAAm with either poly(ethylene glycol) diacrylate (PEG-DA) or poly(ethylene glycol)-co-(l-lactic acid) diacrylate (PEG-PLLA-DA) through free radical polymerisation [59, 60]. Their thermoresponsive system has been shown to be capable of localised release of bevacizumab or ranibizumab for about a month and induced no long-term effects on retinal function [60, 61]. Additionally, controlled degradation and complete release from theses hydrogels was achieved by incorporating biodegradable copolymer and other additives [60].
Wang et al. used copolymer poly (2-ethyl-2-oxazoline)-b-poly (ε-caprolactone)-b-poly (2-ethyl-2-oxazoline) (PEOz-PCL-PEOz) to make biodegradable thermoresponsive hydrogels for extended release of bevacizumab [62]. They demonstrated biocompatibility in vitro and in vivo with a human retinal pigment epithelial cell line in a rabbit model for two months, respectively [62]. Bioactive bevacizumab from hydrogels for one month in vitro was established, although no in vivo efficacy data on animal models have been reported [62].
Instead of using stimuli to initiate gelation after injection, in situ hydrogels can be used to achieve a sustained delivery. These in situ hydrogels spontaneously form through coupling of reactive species at the injection site [57]. These systems are typically formulated such that the hydrogel precursor is mixed immediately prior to use and the injected pre-gel reacts to form a cohesive network. Loading with adjustable drug dosage can be achieved for these hydrogels since most protein drugs are hydrophilic and can be completely dissolved into the aqueous precursor at desirable concentrations. In addition, since the procedure does not use initiator, better protein stability and biocompatibility can be anticipated [57].
Recently, vinyl sulphone functionalised hyaluronic acid (HA-VS)-thiolated dextran (Dex-SH) in situ forming hydrogels have been developed by Yu et al. for controlled delivery of bevacizumab [63]. The bevacizumab-containing polymer solutions were injected into rabbit eyes and then chemically cross-linked into transparent hydrogels at physiological conditions. Binocular indirect ophthalmoscope (BIO) images, full-field electroretinogram (ERG), and histology showed that the hydrogels were safe for rabbit eyes after injections. They also reported that the concentration of bevacizumab was ~107 times higher than bolus injection [63].
Composite drug delivery systems (DDS)
Although injectable polymeric nanoparticles and microparticles provide controllable and sustained drug release, one of the challenges is to localise them to the injection site in the eye. It has been shown that normal eyes can clear microparticles within 50 days and vitrectomised eyes can clear microparticles within 14 days [64]. To limit particle movement in the eye, injectable hydrogels can be good candidates as second carrier for nano and microparticles to provide localised and extended drug release after injection [65]. This composite DDS, a mixture of micro-/nano-particles and hydrogel, also offer advantages over both particles and hydrogels alone by further extending release and reducing initial burst [66, 67]. In addition, both proteins and small molecules can be encapsulated into particles and hydrogels in a variety of ways to enhance delivery potential.
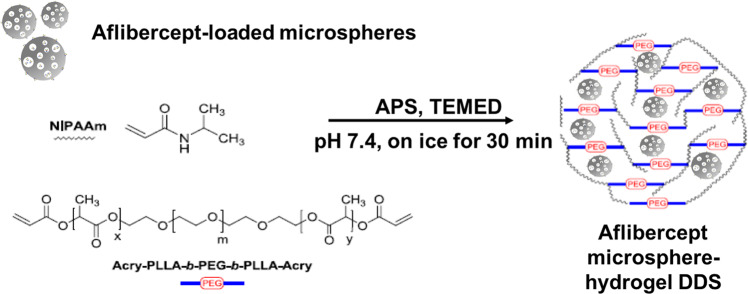
Recently, this strategy has been validated by Kang-Mieler et al. who have combined their injectable PNIPAAm-based thermo-responsive hydrogel with PLGA microspheres to create a microsphere-hydrogel composite DDS [66–68]. Figure 1 shows a schematic demonstrating their composite DDS, where drug-loaded microspheres are suspended in their injectable thermoresponsive hydrogel. Their DDS was able to encapsulate ranibizumab or aflibercept and release them in a controlled manner for ~200 days [67, 68]. In vitro bioactivity during release and in vivo efficacy in laser-induced CNV rodent model have been established [69, 70]. By controlling the amounts of microspheres suspended within the hydrogel, the total amount of drug delivered to the retina can be controlled without changing the volume and injectability of the system. By introducing hydrolytically degradable polymer poly (ethylene glycol)-co-(l-lactic acid) diacrylate to PNIPAAm-based hydrogels, they were able to make their hydrogel also biodegradable. It was found that this microsphere-hydrogel DDS was biocompatible and at the same time capable of release bioactive aflibercept for 6 months in vitro [70]. Currently, Kang-Mieler and colleagues are working on safety and treatment efficacy of their composite DDS in non-human primate models.
Fig. 1. Schematic of aflibercept-loaded microsphere-hydrogel composite DDS.
Aflibercept loaded PLGA microspheres are fabricated using Kang-Mieler modified double-emulsion, solvent evaporation technique. A poly(N-isopropylacrylamide) (PNIPAAm)-based thermoresponsive hydrogel precursors are mixed with poly(ethylene glycol)-co-(l-lactic acid) diacrylate (PEG-PLLA-DA) copolymer. The composite DDS is produced by suspending microspheres in hydrogel precursors and polymerisation is initiated by introducing ammonium persulfate (APS) and N,N,N′N′-tetramethylethylenediamine (TEMED).
Port delivery system (PDS)
ForSight VISION4, Inc. (Menlo Park, CA, USA) developed the PDS and licensed the technology to Genentech, Inc. (South San Francisco, CA, USA). The PDS is a non-degradable, refillable implant that is surgically inserted into the vitreous. A self-sealing septum in the centre of the implant allows refill of the drug reservoir without the need to remove the implant. Ranibizumab diffuses passively down a concentration gradient from the implant reservoir into the vitreous. The passive diffusion is controlled through a porous metal release control element specifically designed for ranibizumab [71]. One of the biggest advantages of PDS is that on-demand refills can be performed in an in-office procedure using a customised exchange needle. The replacement with new drug not only maintains drug potency, but also provides reproducible and predictable drug release after each refill. However, since the drug reservoir is non-biodegradable, both surgical implantation and removal are required which may increase risks of complications [72]. Recent Phase 2 data in the treatment of choroidal neovascularization secondary to AMD has shown that the median time to refill was 8.7, 13.0, and 15.0 months in the PDS 10 mg/ml, PDS 40 mg/ml, and PDS 100 mg/ml, respectively [71]. The data also demonstrated that the PDS 100 mg/ml arm had visual and anatomic outcomes comparable with monthly intravitreal ranibizumab injection group based on the adjusted mean BCVA [71]. The implant insertion and refill procedures were well tolerated by the patients. A phase 3 clinical trial in the realm of AMD has recruited its intended number of patients, and follow-up is currently ongoing. A more recent study looking at DMO has also been initiated.
Conclusion
Due to the ocular barriers, optimal drug delivery to the posterior segment of the eye still remains a big challenge. An ideal delivery system should maintain effective drug levels for the intended duration of treatment following a single application. Table 1 summarises a variety of drug delivery systems such as microneedles, microcatheters, the port delivery system, microspheres, nanoparticles, hydrogels, and composite applications. Each technology has its own advantages and limitations. The effectiveness will depend on the drug type and application.
Table 1.
Ocular drug delivery systems in clinical trials or in development.
| Delivery platform | Drug | Method of delivery | Developmental stage |
|---|---|---|---|
| Clearside Biomedical microneedle | Triamcinolone acetonide | Suprachoroidal injection |
P2(TYBEE) P3(PEACHTREE) |
| Microcannulation | Stem cell (CNTO 2476) | Suprachoroidal injection | P1/2 |
| Microcannulation | Iluvien® | Suprachoroidal injection | Ex-US P1 |
| Encapsulated cell technology | Ciliary neurotrophic factor (CNTF) | Intravitreal implant | P2 |
| Retiset® | Fluocinolone acetonide | Intravitreal implant | Launched |
| Iluvien® | Fluocinolone acetonide | Intravitreal implant | Launched |
| Ozurdex® | Dexamethasone | Intravitreal implant | Launched |
| PLGA nanoparticles | Bevacizumab | Intravitreal implant | Pre-clinical |
| PLGA microparticles | Bevacizumab | Intravitreal implant | Pre-clinical |
| Graybug vision GB-102 | Sunitinib malate | Intravitreal implant | P1/2a |
| Ocular therapeutix OTX-IVT | Aflibercept | Intravitreal implant | Pre-clinical |
| Ocular therapeutix OTX-TKI | Tyrosine kinase inhibitor | Intravitreal implant | Ex-US P1 |
| Thermoresponsive hydrogel | Bevacizumab or ranibizumab | Intravitreal implant | Pre-clinical |
| In situ hydrogel | Bevacizumab | Intravitreal implant | Pre-clinical |
| Microsphere-thermoresponsive composite system | Bevacizumab, ranibizumab, or aflibercept | Intravitreal implant | Pre-clinical |
| Port delivery system | Ranibizumab | Intravitreal implant | P2 |
Future improvements in drug delivery will evolve along two fronts: the development of new pharmacologic agents, and/or more effective delivery of currently available agents. Emphasis will most likely continue to be placed on less invasive, longer acting, and sustained-release formulations. With the recent progress in the field of biomaterials and nanotechnology, there is great promise and potential that these sustained ocular drug delivery systems will significantly impact the standard of care for a variety of clinical conditions in the near future.
Funding
We would like to acknowledge NIH Research Grants (EY025434 and EY029298). Jennifer J. Kang-Mieler: NIH Research Grants (EY025434; EY029298). Kayla M. Rudeen, Wenqiang Liu, William F. Mieler: None
Compliance with ethical standards
Conflict of interest
Jennifer J. Kang-Mieler: US Patents pending on “Microsphere-hydrogel drug delivery system”. Kayla M. Rudeen, Wenqiang Liu, William F. Mieler: None.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jennifer J. Kang-Mieler, Email: kang-mieler@iit.edu
William F. Mieler, Email: wmieler@uic.edu
References
- 1.Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21:S3–9. doi: 10.5301/EJO.2010.6049. [DOI] [PubMed] [Google Scholar]
- 2.Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. 2012;763:70–84. [PubMed] [Google Scholar]
- 3.Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv. 2004;4:371–88. doi: 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- 4.Yasukawa T, Ogura Y, Tabata Y, Kimuar H, Wiedemann P, Honda Y. Drug delivery systems for vitreoretinal diseases. Prog Retin Eye Res. 2004;23:253–81. doi: 10.1016/j.preteyeres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Kang-Mieler JJ, Osswald CR, Mieler WF. Advances in ocular drug delivery: emphasis on the posterior segment. Expert Opin Drug Deliv. 2014;11:1647–60. doi: 10.1517/17425247.2014.935338. [DOI] [PubMed] [Google Scholar]
- 6.Kang-Mieler JJ, Kiernan DF, Mieler WF. Duane’s ophthalmology: drug delivery to the posterior segment. Philadelphia: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 7.Yang C, Tirucherai GS, Mitra AK. Prodrug based optial drug delivery via membrane transporter/receptor. Expert Opin Biol Ther. 2001;1:159–75. doi: 10.1517/14712598.1.2.159. [DOI] [PubMed] [Google Scholar]
- 8.Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv. 2004;1:99–114. doi: 10.1517/17425247.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Lutz R, Wang N, Robinson M. Transport barriers in transscleral drug delivery for retinal diseases. Ophthal Res. 2007;39:244–54. doi: 10.1159/000108117. [DOI] [PubMed] [Google Scholar]
- 10.Yeh S. American Society of Retina Specialists 2018 Late-breaking Presentation. Suprachoroidally injected CLS-TA improves visual acuity and macular edema in noninfectious uveitis: results of the Phase 3 PEACHTREE study. 2018.
- 11.National Library of Medicine (US). Identifier NCT02595398, suprachoroidal injection of CLS-TA in subjects with macular edema associated with non-infectious uveitis (PEACHTREE). ClinicalTrials.gov [Internet], Bethesda, MD. 2015. https://clinicaltrials.gov/ct2/show/NCT02595398?term=PEACHTREE&draw=2&rank=1. Accessed 5 Nov 2019.
- 12.Ip MS, Nittala MG, Velaga S, Ciulla T, Sadda SR, on behalf of the TYBEE Study Group American Academy of Ophthalmology 2019 Presentation PO485. Suprachoroidal CLS-TA plus aflibercept compared with aflibercept monotherapy for DME: selected secondary results of the randomized phase 2 TYBEE trial. 2019.
- 13.REGENXBIO, Rockville, MD. https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-announces-exclusive-worldwide-option-and-license. Accessed 14 Nov 2019.
- 14.Lewis RA, von Wolff K, Tetz M, Koerber N, Kearney JR, Shingleton BJ, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults. Two-year interim clinical study results. J Cataract Refract Surg. 2009;35:814–24. doi: 10.1016/j.jcrs.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Kang SJ, Patel SR, Berezovsky DE, Zhang Q, Yang H, Grossniklaus HE. Suprachoroidal injection of microspheres with microcatheter in a rabbit model of uveal melanoma. Presented at the Association for Research in Vision and Ophthalmology Annual Meeting. Fort Lauderdale, FL: Investigative Ophthalmology & Visual Science; 2011.
- 16.Olsen TW, Feng X, Wabner K, Conston SR, Sierra DH, Folden DV, et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol. 2006;142:777–87. doi: 10.1016/j.ajo.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 17.Olsen TW, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron JD. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci. 2011;52:4749–56. doi: 10.1167/iovs.10-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzo S, Ebert FG, Bartolo ED, Barca F, Cresti F, Augustin C, et al. Suprachoroidal drug infusion for the treatment of severe subfoveal hard exudates. Retina. 2012;32:776–84. doi: 10.1097/IAE.0b013e3182278b0e. [DOI] [PubMed] [Google Scholar]
- 19.National Library of Medicine (US). Identifier NCT01226628, a study of the safety and efficacy of CNTO2476 in patients with age-related macular degeneration. ClinicalTrials.gov [Internet], 2010 October 22. Bethesda, MD. http://clinicaltrials.gov/ct2/show/NCT01226628?term=CNTO2476&rank=1. Accessed 14 Nov 2019.
- 20.Ho AC. Human adult umbilical stem cells: potential treatment for atrophic AMD. Retin Today. 2011;7:59–61. [Google Scholar]
- 21.El Rayes EN. Into the Suprachoridal space. Retin Today. 2018;13:28–32. [Google Scholar]
- 22.El Rayes EN. Suprachoroidal steroid delivery: enforcing our management for refractory macular edema. Retin Today. 2013;8:49–51. [Google Scholar]
- 23.Grzybowski A, Told R, Sacu S, Bandello F, Moisseiev E. Loewenstein A on behalf of the Euretina Board. 2018 update on intravitreal injections: euretina expert consensus recommendations. Ophthalmologica. 2018;239:181–93. doi: 10.1159/000486145. [DOI] [PubMed] [Google Scholar]
- 24.Shima C, Sakaguchi H, Gomi F, Kamei M, Ikuno Y, Oshima Y, et al. Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmol. 2008;86:372–6. doi: 10.1111/j.1600-0420.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 25.Bourges J, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, et al. Intraocular implants for extended drug delivery: therapeutic applications. Adv Drug Deliv Rev. 2006;58:1182–202. doi: 10.1016/j.addr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Lim JI, Wolitz RA, Dowling AH, Bloom HR, Irvine AR, Schwartz DM. Visual and anatomic outcomes associated with posterior segment complications after ganciclovir implant procedures in patients with AIDS and cytomegalovirus retinitis. Am J Ophthalmol. 1999;127:288–93. doi: 10.1016/s0002-9394(98)00443-7. [DOI] [PubMed] [Google Scholar]
- 27.Driot JY, Novack GD, Rittenhouse KD, Milazzo C, Pearson PA. Ocular pharmacokinetics of fluocinolone acetonide after RetisertTM intravitreal implantation in rabbits over a 1-year period. J Ocul Pharm Ther. 2004;20:269–75.. doi: 10.1089/1080768041223611. [DOI] [PubMed] [Google Scholar]
- 28.Ebrahim S, Peyman GA, Lee PJ. Applications of liposomes in ophthalmology. Surv Ophthalmol. 2005;50:167–82. doi: 10.1016/j.survophthal.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Emerich DF, Thanos C. NT-501: an ophthalmic implant of polymer-encapsulated ciliary neurotrophic factor-producing cells. Curr Opin Mol Ther. 2008;10:506–15. [PubMed] [Google Scholar]
- 30.Tao W. Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin Biol Ther. 2006;6:717–26.. doi: 10.1517/14712598.6.7.717. [DOI] [PubMed] [Google Scholar]
- 31.Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103:3896–901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kauper K, McGovern C, Sherman S, Heatherton P, Rapoza R, Stabilia P, et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative disease. Invest Ophthalmol Vis Sci. 2012;53:7484–91. doi: 10.1167/iovs.12-9970. [DOI] [PubMed] [Google Scholar]
- 33.National Library of Medicine (US). Identifier NCT02862938, study of NT-501 encapsulated cell therapy for glaucoma neuroprotection and vision restoration. ClinicalTrials.gov [Internet]. Bethesda, MD. 2016. https://clinicaltrials.gov/ct2/show/NCT02862938?cond=NT-501&rank=2. Accessed 4 Nov 2019.
- 34.National Library of Medicine (US). Identifier NCT03071965, Extension study of NT-501 ciliary neurotrophic factor (CNTF) implant for macular telangiectasia (MacTel). ClinicalTrials.gov [Internet]. Bethesda, MD. 2017. https://clinicaltrials.gov/ct2/show/NCT03071965?cond=NT-501&rank=1. Accessed 4 Nov 2019.
- 35.Chew EY, Clemons TE, Jaffe GJ, Johnson CA, Farsiu S, Lad EM, on behalf of the Macular Telangiectasia Type 2-Phase 2 CNTF Research Group. Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2: a randomized clinical trial. Ophthalmology. 2019;126:540–9. doi: 10.1016/j.ophtha.2018.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126:1191–201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 37.Haghjou N, Soheilian M, Abdekhodaie MJ. Sustained release intraocular drug delivery devices for treatment of uveitis. J Ophthalmic Vis Res. 2011;6:317–29. [PMC free article] [PubMed] [Google Scholar]
- 38.Jaffe GJ, McCallum RM, Branchaud B, Skalak C, Butuner Z, Ashton P. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology. 2005;112:1192–98. doi: 10.1016/j.ophtha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Retisert (fluocinolone acetonide intravitreal implant) 0.59 mg, Bausch & Lomb, Inc., Rochester, NY. 2014. https://www.bausch.com/ecp/our-products/rx-pharmaceuticals/rx-pharmaceuticals/retisert-fluocinolone-acetonide-intravitreal-implant-059-mg#.Xc2tIHv_pPY. Accessed 14 Nov 2019.
- 40.Kane FE, Burdan J, Cutino A, Green KE. Iluvien™: a new sustained delivery technology for posterior eye disease. Expert Opin Drug Deliv. 2008;5:1039–46. doi: 10.1517/17425247.5.9.1039. [DOI] [PubMed] [Google Scholar]
- 41.Kuppermann BD, Blumenkranz MS, Haller JA, Williams GA, Weinberg DV, Chou C, on behalf of the Dexamethasone DDS Phase II Study Group. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125:309–17. doi: 10.1001/archopht.125.3.309. [DOI] [PubMed] [Google Scholar]
- 42.Bucolo C, Drago F, Salomone S. Ocular drug delivery: a clue from nanotechnology. Front Pharmacol. 2012;3:188. doi: 10.3389/fphar.2012.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mi FL, Lin YM, Wu YB, Shyu SS, Tsai YH. Chitin/PLGA blend microspheres as a biodegradable drug-delivery system: phase-separation, degradation and release behavior. Biomaterials. 2002;23:3257–67. doi: 10.1016/s0142-9612(02)00084-4. [DOI] [PubMed] [Google Scholar]
- 44.Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6:319–27. [Google Scholar]
- 45.Herrero-Vanrell R, Bravo-Osuna I, Andres-Guerrero V, Vicario-de-la-Torre M, Molina-Martinez IT. The potential of using biodegradable microspheres in retinal diseases and other intraocular pathologies. Prog Retin Eye Res. 2014;42:27–43. doi: 10.1016/j.preteyeres.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Yeo Y, Park K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm Res. 2004;27:1–12. doi: 10.1007/BF02980037. [DOI] [PubMed] [Google Scholar]
- 47.Manoharan C, Singh J. Insulin loaded PLGA microspheres: effect of zinc salts on encapsulation, release, and stability. J Pharm Sci. 2009;98:529–42. doi: 10.1002/jps.21445. [DOI] [PubMed] [Google Scholar]
- 48.Varshochian R, Jeddi-Tehrani M, Mahmoudi AR, Khoshayand MR, Atyabi F, Sabzevari A, et al. The protective effect of albumin on bevacizumab activity and stability in PLGA nanoparticles intended for retinal and choroidal neovascularization treatments. Eur J Pharm Sci. 2013;50:341–52. doi: 10.1016/j.ejps.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Varshochian R, Riazi-Esfahani M, Jeddi-Tehrani M, Mahmoudi AR, Aghazadeh S, Mahbod M, et al. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J Biomed Mater Res A. 2015;103:3148–56. doi: 10.1002/jbm.a.35446. [DOI] [PubMed] [Google Scholar]
- 50.Ye Z, Ji Y-L, Ma X, Wen J-G, Wei W, Huang S-M. Pharmacokinetics and distributions of bevacizumab by intravitreal injection of bevacizumab-PLGA microspheres in rabbits. Int J Ophthalmol. 2015;8:653–8. doi: 10.3980/j.issn.2222-3959.2015.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.GB-102, Garybug Vision, Redwood City, CA. https://graybug.com. Accessed 14 Nov 2019.
- 52.National Library of Medicine (US). Identifier NCT03249740, a depot formulation of sunitinib malate (GB-102) in subjects with neovascular (wet) age-related macular degeneration. ClinicalTrials.gov [Internet], Bethesda, MD. 2017. https://clinicaltrials.gov/ct2/show/NCT03249740?term=graybug+vision&cntry=US&draw=2&rank=2. Accessed 14 Nov 2019.
- 53.National Library of Medicine (US). Identifier NCT03953079, a depot formulation of sunitinib malate (GB-102) compared to aflibercept in subjects with wet AMD (ALTISSIMO). ClinicalTrials.gov [Internet]. Bethesda, MD. 2019. https://clinicaltrials.gov/ct2/show/NCT03953079. Accessed 14 Nov 2019.
- 54.OTX-IVT (anti-VEGF antibody implant), Ocular Therapeutix, Bedford, MA. https://ocutx.com/research/otx-ivt/. Accessed 7 Nov 2019.
- 55.El-Hayek R, Jarrett T, Lattrell Z, Takach S, Jarret PK, McGrath M, et al. Efficacy of a 6 month sustained hydrogel delivery system for Tyrosine kinase inhibitors in a VEGF induced retinal leakage model. ARVO Abstract. Invest Ophthalmol Vis Sci. 2017;58:1968. [Google Scholar]
- 56.Ocular Therapeutix announces dosing of first patient in Phase 1 clinical trial for the treatment of wet AMD. Eyewire News. https://eyewire.news/articles/ocular-therapeutix-announces-dosing-of-first-patient-in-phase-1-clinical-trial-for-the-treatment-of-wet-amd/. Accessed 2 Feb 2019.
- 57.Agrawal AK, Das M, Jain S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin Drug Deliv. 2012;9:383–402. doi: 10.1517/17425247.2012.665367. [DOI] [PubMed] [Google Scholar]
- 58.Klouda L. Thermoresponsive hydrogels in biomedical applications a seven-year update. Eur J Pharm Biopharm. 2015;97:338–49. [DOI] [PubMed]
- 59.Drapala PW, Brey EM, Mieler WF, Venerus DC, Kang-Derwent JJ, Perez-Luna VH. Role of thermo-responsiveness and poly(ethylene glycol) diacrylate cross-link density on protein release from poly(N-isopropylacrylamide) hydrogels. J Biomater Sci Polym Ed. 2011;22:59–75. doi: 10.1163/092050609X12578498952315. [DOI] [PubMed] [Google Scholar]
- 60.Drapala PW, Jiang B, Chiu YC, Mieler WF, Brey EM, Kang-Mieler JJ, et al. The effect of glutathione as chain transfer agent in PNIPAAm-based thermo-responsive hydrogels for controlled release of proteins. Pharm Res. 2014;31:742–53. doi: 10.1007/s11095-013-1195-0. [DOI] [PubMed] [Google Scholar]
- 61.Turturro SB, Guthrie MJ, Appel AA, Drapala PW, Brey EM, Perez-Luna VH, et al. The effect of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials. 2011;32:3620–6. doi: 10.1016/j.biomaterials.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 62.Wang CH, Hwang YS, Chiang PR, Shen CR, Hong WH, Hsiue GH. Extended release of bevacizumab by thermosensitive biodegradable and biocompatible hydrogel. Biomacromolecules. 2012;13:40–48. doi: 10.1021/bm2009558. [DOI] [PubMed] [Google Scholar]
- 63.Yu Y, Lau LC, Lo AC, Chau Y. Injectable chemically crosslinked hydrogel for the controlled release of bevacizumab in vitreous: a 6-month in vivo study. Transl Vis Sci Technol. 2015;4:5. doi: 10.1167/tvst.4.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moritera T, Ogura Y, Hondo Y, Wadaf R, Hyoaf S-H, Ikadaf Y. Microspheres of biodegradable polymers as a drug-delivery system in the vitreous. Invest Ophthalmol Vis Sci. 1991;32:1785–90. [PubMed] [Google Scholar]
- 65.Kang-Mieler JJ, Dosmar E, Liu W, Mieler WF. Extended ocular drug delivery systems for the anterior and posterior segments: biomaterial options and applications. Expert Opin Drug Deliv. 2017;14:611–20. doi: 10.1080/17425247.2016.1227785. [DOI] [PubMed] [Google Scholar]
- 66.Osswald CR, Kang-Mieler JJ. Controlled and extended release of a model protein from a microsphere-hydrogel drug delivery system. Ann Biomed Eng. 2015;43:2609–17. doi: 10.1007/s10439-015-1314-7. [DOI] [PubMed] [Google Scholar]
- 67.Osswald CR, Kang-Mieler JJ. Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr Eye Res. 2016;41:1216–22. doi: 10.3109/02713683.2015.1101140. [DOI] [PubMed] [Google Scholar]
- 68.Liu W, Borrell MA, Venerus DC, Mieler WF, Kang-Mieler JJ. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl Vis Sci Technol. 2019;8:12. doi: 10.1167/tvst.8.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Osswald CR, Guthrie MJ, Avila A, Valio JA, Jr, Mieler WF, Kang-Mieler JJ. In vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Curr Eye Res. 2017;42:1293–301. doi: 10.1080/02713683.2017.1302590. [DOI] [PubMed] [Google Scholar]
- 70.Liu W, Lee BS, Mieler WF, Kang-Mieler WF. Biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of bioactive aflibercept in vitro. Curr Eye Res. 2019;44:264–74. doi: 10.1080/02713683.2018.1533983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Campochiaro PA, Marcus DM, Awh CC, Regillo C, Adamis AP, Bantseev V, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized Phase 2 Ladder clinical trial. Ophthalmology. 2019;126:1141–54. doi: 10.1016/j.ophtha.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 72.Rubio RG. Long-acting anti-VEGF delivery. Retin Today. 2014;8:78–80. [Google Scholar]