Abstract
Crohn's disease (CD) is a chronic inflammatory disorder characterized by immune response dysregulation. Tumor necrosis factor-α (TNFα) is a key cytokine in the pathogenesis of CD, as indicated by the efficacy of anti-TNF-α therapy with infliximab (IFX). However, approximately 30–40% of CD patients fail to respond to IFX with still unclear underlying mechanisms. This study compares the inflammatory phenotype of monocytes from CD patients, who respond or non-respond to IFX. Under basal conditions, the mRNA for the cytokines TNFα, IL-23, IL-1β and the chemokines CXCL8/IL-8, CCL5/RANTES and CCL2/MCP-1 was up-regulated in monocytes from non-responders than responders. The expression of the same cytokines and CCL2/MCP-1 was higher in non-responders also upon LPS treatment. Moreover, higher secretion of TNFα, IL-1β, IFNγ and IL-2 proteins occurred in the supernatants of LPS-treated non-responders cells. Resistance to IFX in CD may result from a transcriptional dysregulation of circulating monocytes, leading to hyperactivation of pro-inflammatory pathways. Monocytes’ cytokine profile may thus represent a predictive marker of response to IFX. Monocytes were isolated from blood samples of 19 CD patients (11 responders, 8 non-responders) and incubated with or without LPS. Cytokine profiles were assessed by RT-qPCR and, in the supernatants, by ELISA assay.
Subject terms: Cell biology, Immunology, Gastroenterology
Introduction
Crohn’s disease (CD) is a chronic inflammatory disorder potentially affecting the whole gastrointestinal tract, with segmental and transmural involvement of the gastrointestinal mucosa. Although the precise etiology of the disease is still controversial, there is a general consensus on a multifactorial pathogenesis, including genetic predisposition, environmental factors, and dysregulation of both innate and adaptive immune system responses against intestinal microflora1.
The hallmark of CD is a chronic inflammation promoted and sustained by hyperactivated effector immune cells through an increased production of proinflammatory cytokines, mainly tumor necrosis factor (TNF)2. Lamina propria macrophages isolated from colonic biopsies of CD patients have been shown to spontaneously produce increased amounts of TNF, that correlate with the degree of tissue involvement and mucosal inflammation3, demonstrating their central role in the perpetuation of inflammation.
TNF is synthesized as a membrane-bound protein (mTNF) which is released as soluble form (sTNF) after cleavage of the extracellular domain by TNF-converting enzyme (TACE)4. The cytokine is endowed with different functions: by binding to the ubiquitous TNF receptor 1 (TNFR1), sTNF can alternatively activate caspase-dependent apoptosis, and promote proinflammatory cytokine expression through the activation of the transcription factor nuclear factor B (NF-κB)5; on the other hand, TNFR2, whose expression is restricted to immune cell system, mainly binds to mTNF to increase lymphocytes T regulators (Tregs) stability, expansion and functions6.
The critical relevance of the cytokine in CD is confirmed by the efficacy of anti-TNF antibodies-based therapies, which lead to a clinical response in up to 60% of patients and a long-term maintenance of remission in a large number of subjects (up to 63.45%)7,8. Among them, the monoclonal antibody Infliximab (IFX), known as Remicade (Janssen Biotech, Horsham, PA, USA), deserves particular attention, being the first biological response modifier approved by the US Food and Drug Administration (FDA) for the treatment of CD in 1998 and thereby becoming the first inhibitor of TNF employed in the clinical practice9. Although the exact mechanisms of action of TNF-targeting agents are still a matter of debate, there is a general consensus that one of their main therapeutic properties is mediated by binding to mTNF that finally leads to the indirect induction of intestinal T cells apoptosis by blocking the TNFR2-dependent signaling10,11.
Unfortunately, anti-TNF agents fails to show therapeutic efficacy in a relevant group of CD patients: approximately one-third of patients do not respond to initial anti-TNF therapy (primary nonresponse), while 30–50% of patients lose response along the treatment (secondary nonresponse). The reasons for this failure remain still poorly understood. Among the possible causes, insufficient serum drug levels and the development of neutralizing anti-drug antibodies have been proposed to explain the lack of response12,13. Furthermore, the existence of cytokine signaling pathways alternative to TNF has been suggested as a possible cause for therapeutic failure14; in this context, a recent study by Schmitt et al. describes an hyperproduction of IL-23 by CD macrophages that is expected to overcome the induction of apoptosis triggered by anti-TNF antibodies, by binding to IL-23R on TNFR2 expressing T cells, causing resistance to the therapy15. In this perspective, macrophages should be investigated as major players of resistance mechanisms.
The aim of the present study is to explore the inflammatory phenotype of immune cells isolated from CD patients responding (responders) or not responding (non-responders) to the therapy with IFX, as classified according to endoscopic and clinical criteria, in order to highlight possible differences between the two groups of patients. Since it has been ascertained that the population of mucosal macrophages is constantly replenished by circulating monocytes16, the expression of inflammatory markers has been investigated in blood isolated monocytes, so as to evaluate their potential as predictive biomarkers for the response to anti-TNF therapy.
Results
A total of 19 CD patients (mean age 39.2 years, range 19–78 years) under therapy with IFX were included. Among them, 11 were responders to anti-TNF therapy, and 8 were non-responders. Among non-responders, 2 were primary non-responders, 6 were secondary non-responders. The demographic and clinical features of the study sample are displayed in Table 1. The responder group was comparable to the non-responder group except for the location of CD, with the disease being only ileal for the majority of responders (72.7%) and ileo-colonic for the non-responders (62.5%) (p = 0.043). Only 2 out of 19 patients (10.5%) had comorbidities of hematologic origin; in particular, one patient among the responders presented with essential thrombocythemia, and one patient in the non-responders group presented with polycythemia vera in follow-up. Overall, 3 out of 19 patients (15.8%) were smokers of approximately 1 pack/year, equally distributed between responders and non-responders, 18.2% and 12.5%, respectively.
Table 1.
Clinical features of the CD patients enrolled.
| Total (n = 19) | Responders (n = 11) | Non-responders (n = 8) | p value | |
|---|---|---|---|---|
| Age [mean (range)] | 39.2 (19–78) | 40.9 (23–64) | 36.9 (19–78) | 0.272 |
| Sex [n (%)] | ||||
| M | 13 (68.4) | 9 (81.8) | 4 (50) | 0.319 |
| F | 6 (31.6) | 2 (18.2) | 4 (50) | |
| BMI [mean (range)] | 22.5 (16.6–28.4) | 22.5 (16.6–28.4) | 22.6 (19–23.6) | 1 |
| Comorbidities [n (%)] | ||||
| None | 17 (89.5) | 10 (90.9) | 7 (87.5) | 0.879 |
| Hematologic disorders | 2 (10.5) | 1 (9.1) | 1 (12.5) | |
| Ongoing therapy [n (%)] | ||||
| IFX | 14 (73.7) | 9 (81.8) | 5 (62.5) | 0.603 |
| IFX + AZA | 5 (26.3) | 2 (18.2) | 3 (37.5) | |
| Smoke [n (%)] | ||||
| Yes | 3 (15.8) | 2 (18.2) | 1 (12.5) | 1 |
| No | 16 (84.2) | 9 (81.8) | 7 (87.5) | |
| CD location (Montreal classification) [n (%)] | ||||
| Ileal | 10 (52.6) | 8 (72.7) | 2 (25) | 0.043 |
| Colonic | 3 (15.8) | 2 (18.2) | 1 (12.5) | |
| Ileo-colonic | 6 (31.6) | 1 (9.1) | 5 (62.5) | |
| SES-CD [n (%)] | ||||
| Remission | 10 (52.6) | 10 (90.9) | 0 (0) | < 0.0001 |
| Mild activity | 5 (26.3) | 1 (9.1) | 4 (50) | |
| Moderate activity | 4 (21.1) | 0 | 4 (50) | |
| Severe activity | 0 | 0 | 0 | |
| CDAI [n (%)] | ||||
| Asymptomatic remission | 10 (52.6) | 10 (90.9) | 0 (0) | < 0.0001 |
| Mildly to moderately active | 6 (31.6) | 1 (9.1) | 5 (62.5) | |
| Moderately to severely active | 3 (15.8) | 0 | 3 (37.5) | |
| Severely active to fulminant | 0 | 0 | 0 | |
| Serum IFX concentration, µg/ml [mean (range)] | 5.25 (1.4–12.9) | 5.15 (2.9–7) | 5.4 (1.4–12.9) | 0.596 |
| Anti-IFX antibodies [n (%)] | ||||
| Yes | 1(5.3) | 0 | 1(12.5) | 0.389 |
| No | 17 (89.4) | 11 (100) | 6 (75) | |
| n/a | 1 (5.3) | 0 | 1 (12.5) | |
The disease duration varied among patients both in “responders” and in “non-responders”. In particular, the mean duration of the disease was 6.59 years among “responders” (range 0.5–20 years) and 9.06 years among “non-responders” (range 0.5–22 years).
Most of the patients (73.7%) were treated with IFX alone, although some patients (37.5% non-responders vs. 18.2% responders) underwent a combined therapy with IFX and azathioprine, without significant differences between the two groups (p = 0.603). The overall duration of IFX therapy varied among patients in both groups. In particular, the mean duration of IFX therapy was 31 months among “responders” (range 2–87 months) and 26 months among “non-responders” (range 2–70 months).
In accordance with the SES-CD and CDAI indexes, none of the patients presented with severe disease, and, even among non-responders, CD presented with mild to moderate clinical activity in the majority of patients (62.5%). Infliximab serum levels were within the normal ranges in all patients, except for one non-responder, who was treated with the higher dose of IFX (i.e. 10 mg/kg), in accordance with international protocols17. Overall, only 1 out of 19 patients had positive anti-IFX antibodies; this patient was not excluded because the IFX serum dose was within the normal range (6.4 µg/ml, normal range 3–7 µg/ml), and the antibody concentration was 10.3 ng/ml, which was at the upper limit of normal (10 ng/ml).
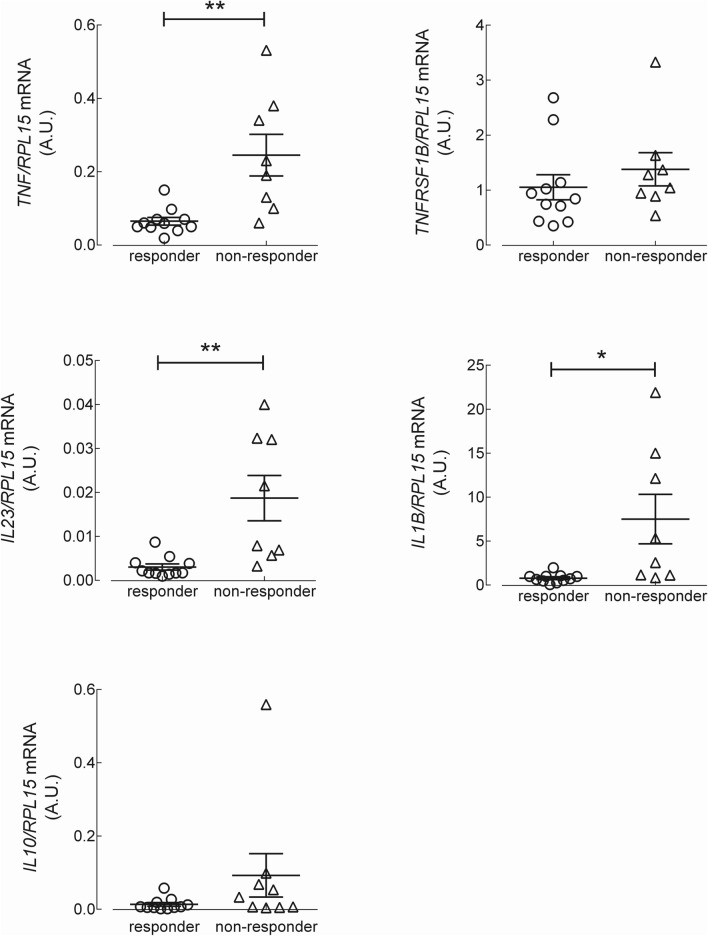
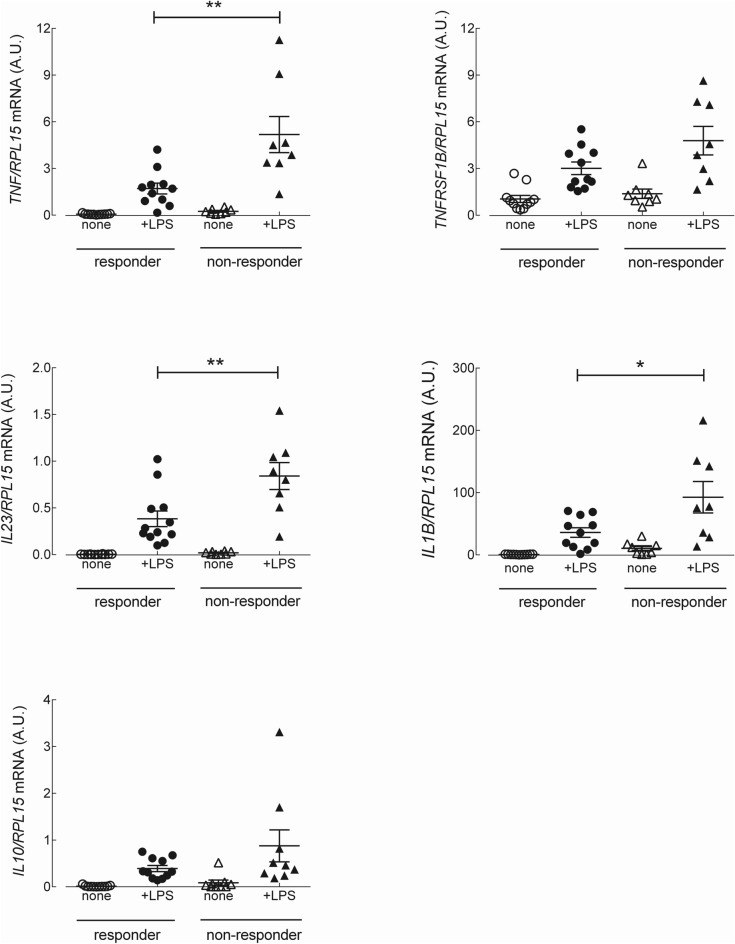
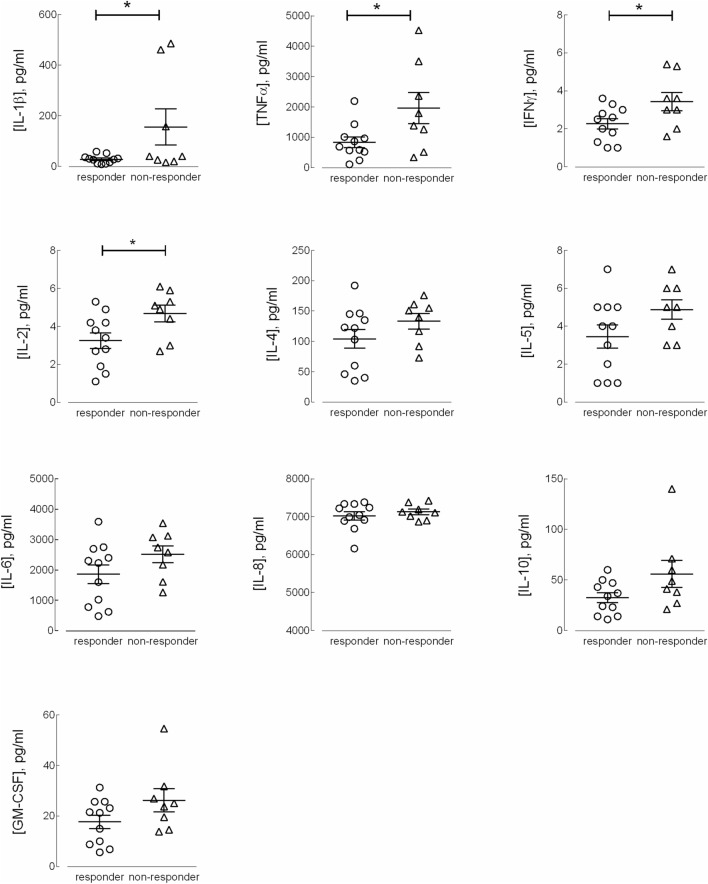
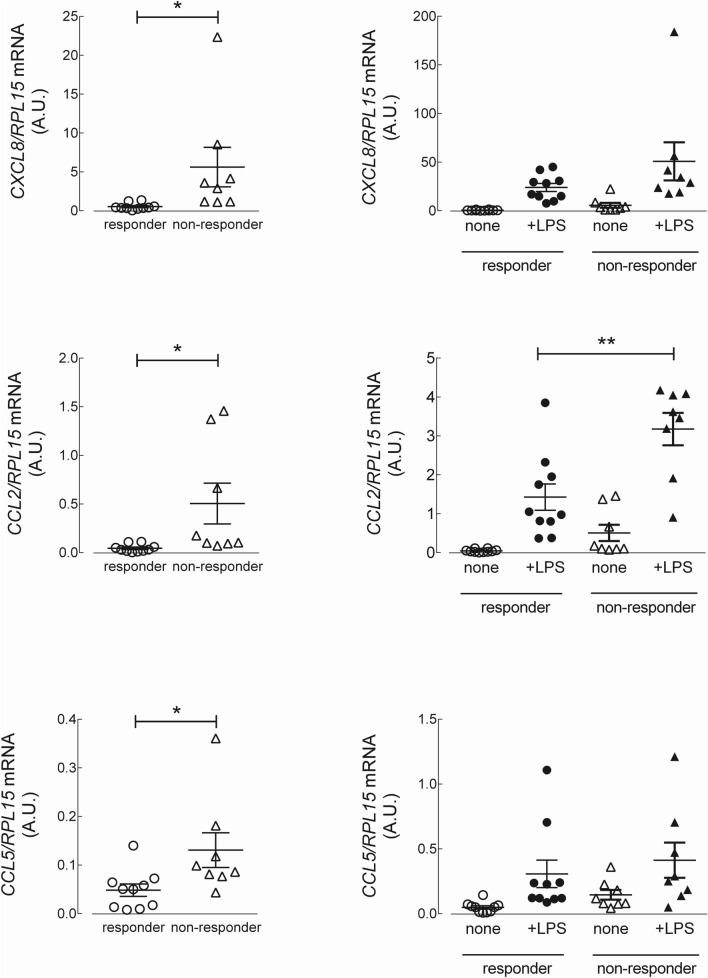
To explore the molecular mechanisms responsible for resistance to anti-TNF therapy in CD patients, we next analyzed the expression of inflammatory cytokines in monocytes, both under basal resting conditions and after priming with lipopolysaccharide (LPS). The data presented in Fig. 1 show that under basal resting conditions, the expression level of the mRNAs coding for TNF/TNF-α, IL23/IL-23 and IL1B/IL-1β were significantly higher in monocytes isolated from CD non-responders than in those from responders. The expression of TNFRSF1B/TNFR2 and of the anti-inflammatory cytokine IL10/IL-10 did not differ between the two groups of patients. The same analysis, performed on monocytes activated for 4 h with LPS (Fig. 2), demonstrated a marked induction of the tested genes in all CD patients, with TNF/TNF-α, IL23/IL-23 and IL1B/IL-1β more expressed in non-responders than in responders. Similar results were reached when assaying the supernatants collected from LPS-treated cells for the production of a panel of 10 cytokines (TNF-α, IL-8, IL-1β, IL-6, GM-CSF, IL-10, IL-2, IL-4, IL-5, and IFN-γ). The data obtained (Fig. 3) were consistent with the gene expression data for IL-1β and TNF-α, indicating that these cytokines were secreted at higher levels in non-responders than in responders; a similar pattern was observed for IFNγ and IL-2. The amount of the other cytokines tested (i.e., IL-4, IL-5, IL-6, IL-8, and GM-CSF) was, instead, comparable in the two groups. Finally, the expression of the chemokines CXCL8/IL-8, monocyte chemoattractant protein-1 (CCL2/MCP1) and CCL5/RANTES was tested. As shown in Fig. 4, under basal conditions, all these chemokines were more expressed in monocytes from non-responder than in responders; in LPS-treated cells, only CCL2 was differentially expressed in the two groups, reaching higher values in cells from non-responder patients.
Figure 1.
Analysis of gene expression in CD monocytes maintained under basal resting conditions. Cells, isolated from responders (n = 11) and nonresponders (n = 8) to the therapy with IFX, were cultured in complete medium for 4 h; the mRNA for the genes of interest was then analyzed as described in Material and Methods. The amount of the indicated genes, expressed as Arbitrary Unit (A.U.), was normalized for that of the housekeeping gene RPL15 using the formula 2ΔCt (see “Materials and methods”). Each symbol represents a single patient; the mean ± SEM is indicated as a line. *p < 0.05, **p < 0.01.
Figure 2.
Analysis of gene expression in LPS-stimulated CD monocytes. Cells, isolated as in Figure, were incubated for 4 h either in the absence (none) or in the presence of 0.1 µg/ml lipopolysaccharide (+ LPS). The amount of the indicated genes, expressed as Arbitrary Unit (A.U.), was normalized for that of the housekeeping gene RPL15 using the formula 2ΔCt (see “Materials and methods”).Each symbol represents a single patient; the mean ± SEM is indicated as a line. *p < 0.05, **p < 0.01.
Figure 3.
Cytokine secretion by LPS-stimulated CD monocytes. Supernatant from LPS-treated cells as in Figure were assayed for cytokine levels (see “Materials and methods”). Each symbol represents a single patient; the mean ± SEM is indicated as a line. *p < 0.05.
Figure 4.
mRNA levels of the chemokines IL-8, MCP-1 and RANTES in CD monocytes maintained under basal conditions or stimulated with LPS. Cells isolated from responders (n = 10) and non-responders (n = 8) to IFX were cultured for 4 h in complete medium, either in the absence (none) or in the presence of 0.1 µg/ml LPS (+ LPS). The amount of the indicated genes, expressed as Arbitrary Unit (A.U.), was normalized for that of the housekeeping gene RPL15 using the formula 2ΔCt (see “Materials and methods”). Each symbol represents a single patient; the mean ± SEM is indicated as a line. *p < 0.05, **p < 0.01.
Discussion
Despite the widespread use of anti-TNF therapies due to their proven efficacy in patients with CD, the key mechanisms of resistance to TNF-targeting drugs and the perpetuation of mucosal inflammation in CD patients are, thus far, only poorly understood; moreover, there is an unmet clinical need for prognostic tools or predictive biomarkers of resistance to anti-TNF agents12,14.
As there is consensus on the crucial role of intestinal macrophages in initiating and continuing inflammation in CD, we here present the results of a prospective study addressing the expression of inflammatory markers in immune cells from CD patients who respond to IFX therapy vs. patients who do not.
The clinical characterization of the two groups of patients, responders and non-responders to IFX, highlighted the expected difference in the distribution of SES-CD and CDAI scores. Interestingly, also the location of the disease described by Montreal classification was significantly different between the two groups: ileum was, indeed, involved in 16 out of 19 patients, but among them an isolated ileal localization was more represented among “responders”, while “non-responders” showed an extended ileo-colic disease in 62.5% of cases. The physiopathological mechanism leading to the development of the disease in the small bowel rather than only in the colon is scarcely explored, anyway literature data show that CD involving the small bowel typically manifests more severely, is more difficult to diagnose and to monitor during the follow-up and is more prone to develop complications (fistulas and strictures) than CD limited to the colon18.
Once identified the two groups of patients, we next investigated the inflammatory phenotype of blood circulating monocytes, to evaluate their potential as a diagnostic tool for therapy resistance, with an approach similar to that previously employed for celiac disease19,20. Results obtained suggest that the failure of therapy with IFX may derive from a deregulation of the cytokine profile of these cells, that associates with the activation of pro-inflammatory pathways. In particular, the expression of TNF-α, IL-1β, IL-23 appears higher in monocytes from non-responder patients than in responders, both under basal conditions and after priming with LPS. Conversely, the difference in expression of the anti-inflammatory cytokine IL-10 did not reach statistical significance. In addition, our study revealed an increased expression of the chemokines IL-8, MCP1 and RANTES in non-responders, with MCP1 differentially expressed in responders and resistant patients also upon LPS treatment. High levels of this chemokine have been described in pathological loci, such as the submucosa and muscularis of Crohn’s disease (CD)21, and the MCP1‐A2518G polymorphism is considered a protective factor for inflammatory bowel disease (IBD) in European populations22.
In our study, the exclusion of other clinical reasons for IFX failure, i.e. the presence of anti-IFX antibodies or low serum levels of the drug, strengthens the hypothesis of a role for the cytokine profile of circulating monocytes in the development of resistance to anti-TNF agents. For example, in agreement with the study by Schmitt et al., we demonstrate here a significant hyperexpression of IL-23 in innate immune cells, which is supposed to play a key role in the mechanism of resistance to anti-TNF therapy15. To this concern, the assessment of a high expression of the cytokine in circulating monocytes could be preventively employed to direct the therapy toward drugs alternative to anti-TNF agents, such as the anti-IL-23 ustekinumab, available in Europe since 2018.
Although this is a single-center prospective study including a limited number of patients, our findings suggest that monocyte analysis could be a reliable tool for the prediction of IFX efficacy; once identified which cytokines can be used as markers of resistance, monocyte typing could be performed in the early phases of induction of therapy, to spare useless and potentially detrimental ineffective infusions for resistant patients and limit the healthcare costs.
Conclusion
The present study suggests that resistance to IFX therapy in CD patients may result from an innate transcriptional dysregulation of monocytes, which leads to the excessive activation of pro-inflammatory pathways. The definition of a cytokine signature in blood monocytes could, hence, serve as a fingerprint for the early identification of IFX resistant patients, with the aim to optimize the therapeutic approach.
Materials and methods
Patient recruitment and monocyte isolation
CD patients were recruited at the Gastroenterology and Endoscopy Unit of the University Hospital of Parma, Italy, between September 2018 and June 2019 in occasion of their access to the hospital for treatment administration. Adult patients with a histologically confirmed diagnosis of CD of at least 6 months and under therapy with IFX were included in the study. Exclusion criteria included refusal to sign the informed consent form; diagnosis of UC or indetermined colitis; ongoing therapy with corticosteroids; presence of abscess; and contraindications for or reactions to IFX therapy. Demographic and clinical data were extracted from patient records and included body mass index (BMI), comorbidities, and ongoing medical therapies. CD was classified by using the Crohn’s Disease Activity Index (CDAI)23 and the Montreal classification for location of the disease24 clinically, whereas the Simple Endoscopic Score for Crohn’s Disease (SES-CD) was used to classify the disease endoscopically25. All patients underwent endoscopy within 2 months of inclusion in the present study. Patients were then classified as responders to anti-TNF therapy if they had a SES-CD score < 5 and/or CDAI score < 220. A diagram to report the inclusion of participants in the present study is included in Fig. 5. Peripheral blood samples of all patients (approximately 10–12 ml) were taken the day of the scheduled infliximab administration (every 8 weeks), before starting the infusion, and after clinical evaluation. Samples of serum from all the patients were employed for measuring the amount of circulating antibodies against IFX as well as IFX serum concentration by means of an ELISA commercial kit (Lisa Tracker, ALIFAX) according to the manufacturer’s instructions. IFX serum levels were considered in the normal range for values between 3 and 7 µg/ml, and anti-IFX antibodies were considered negative for values < 10 ng/ml26. In parallel, circulating monocytes were isolated from blood samples as previously described27,28. Briefly, heparinized blood, diluted 1:1 with PBS, was layered on Lympholyte-H gradient medium (EuroCone, Milan, Italy) and centrifuged at 800g for 20 min at 20 °C. Peripheral blood mononuclear cells (PBMCs) were collected from the interface and washed in RPMI medium with a centrifugation at 200g for 7 min at 20 °C. Cells were then suspended in RPMI containing 10% endotoxin-free fetal bovine serum (FBS) and seeded in 12-well plates. After a 60-min incubation at 37 °C in an atmosphere at 5% CO2, nonadherent cells were removed with vigorous washes in prewarmed RPMI medium. Adherent monocytes were cultured for 4 h in RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, and 1% penicillin/streptomycin in the absence or presence of 0.1 µg/ml lipopolysaccharides from E. coli 0111:B4 (LPS). After this period, the culture medium was collected and stored at − 20 °C for the determination of pro-inflammatory cytokines, while cells were employed for the analysis of gene expression upon RNA isolation. The viability of adherent monocytes was assessed by Trypan blue exclusion and was > 98% in all cases; CD14 expression was greater than 90%.
Figure 5.
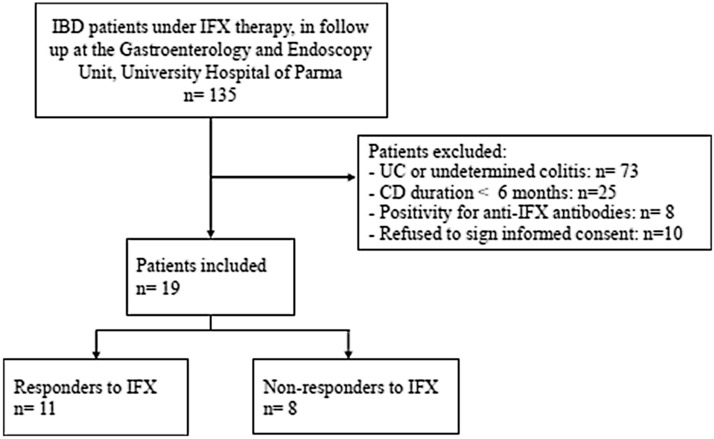
Diagram showing the flow of participants through the study.
RT-qPCR analysis
Total RNA was isolated with GeneJET RNA Purification Kit (Thermo Fisher Scientific), according to the manufacturer’s instructions, as previously described29. 1 µg of RNA was then reverse transcribed with a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific), and qPCR was performed on 20 ng of cDNA by employing a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). The amount of IL1B (NM_000576.3) and the amount of the reference gene RPL15 (ribosomal protein-like 15, NM_001253379.2) were monitored with specific TaqMan Gene Expression Assays (Thermo Fisher Scientific), while the expression of all other genes was measured using specific forward/reverse primer pairs (Table 2) and SYBR Green PCR Master Mix (Thermo Fisher Scientific). The amount of the genes of interest, expressed as Arbitrary Unit (A.U.), was calculated relative to that of the reference gene using the formula (where ).
Table 2.
Sequences of the primer pairs employed for RT-qPCR analysis.
| Gene/protein name NCBI Ref Seq identifier | Forward primer | Reverse primer |
|---|---|---|
| CXCL8/IL8 (NM_000584.4) | ACTGAGAGTGATTGAGAGTGGAC | AACCCTCTGCACCCAGTTTTC |
| IL10/IL-10 (NM_000572.3) | CAAGGCGCATGTGAACTCC | GATGTCAAACTCACTCATGGCT |
| IL23/IL-23 (NM_016584.3) | CTCAGGGACAACAGTCAGTTC | ACAGGGCTATCAGGGAGCA |
| CCL2/MCP-1 NM_002982.4 | CAGCCAGATGCAATCAATGCC | TGGAATCCTGAACCCACTTCT |
| CCL5/RANTES (NM_001278736.2) | CCAGCAGTCGTCTTTGTCAC | CTCTGGGTTGGCACACACTT |
| TNF/TNFα (NM_000594.4) | ATGAGCACTGAAAGCATGATCC | GAGGGCTGATTAGAGAGAGGTC |
| TNFRSF1B/TNFR2 (NM_001066.3) | TGAAACATCAGACGTGGTGTG | TGCAAATATCCGTGGATGAAGTC |
Cytokine measurement
Supernatants collected from monocyte cultures were assayed for secreted cytokines with the Bio-Plex System by Bio-Rad by employing the Cytokine Human Magnetic 10-Plex Panel (Thermo Fisher Scientific), which simultaneously measured the levels of TNF-α, IL-8, IL-1β, IL-6, GM-CSF, IL-10, IL-2, IL-4, IL-5, and IFN-γ in each sample. Briefly, all supernatants were clarified prior to the analysis by centrifugation (12,000 × g for 10 min at 4 °C); 50 µl of each medium were then added to the plate, previously prepared as required by the manufacturer. After 2 h incubation at room temperature in the dark, the plate was washed twice, then further incubated for 1 h with Biotinylated Detector Antibody. Finally, after 30 min in the presence of Streptavidin-RPE solution, the plate was washed again twice and inserted into the Bio-Plex System by Bio-Rad for the analysis. A standard curve was run in parallel, employing samples provided in the assay; the range of concentration analyzed for each cytokine in the assay employed was (in pg/ml) 6–5,190 for TNF-α, 11–8,150 for IL-8, 12–8,890 for IL-1β, 7–5,120 for IL-6, 7–5,210 for GM-CSF, 12–8,800 for IL-10, 14–10,390 for IL-2, 63–45,800 for IL-4, 10–7,380 for IL-5, and 6–4,450 for IFN-γ. The amount of cytokines in each sample was calculated from the standard curve using the software by Bio-Rad and expressed as pg/ml of incubation medium.
Materials
Fetal bovine serum and RPMI-1640 medium were purchased from EuroClone (Milano, Italy). Sigma-Aldrich (Milano, Italy) was the source of all other chemicals.
Statistics
GraphPad Prism 5.0 (GraphPad Software, USA) and SPSS (IBM, Statistics, USA) software were used for the statistical analyses. Clinical data are expressed as frequencies (n, %) or means (standard deviation). Group comparisons were carried out by using the chi-squared test and Mann–Whitney U test for categorical and continuous variables, respectively. Gene expression and cytokine production were analyzed with a two-tailed Student's t-test for unpaired data. Differences were considered statistically significant when p < 0.05.
Ethical considerations
The study protocol was approved by the local ethical committee (672/2018/OSS/UNIPR approved on 4 September 2018) and conducted in accordance with the principles of the Declaration of Helsinki (1964). Patients were screened for inclusion during follow-up visits for CD therapy administration. All the included patients provided written informed consent before the inclusion in the present study.
Acknowledgements
We thank Dr. Chiara Bonaguri, Laboratory of Clinical Chemistry and Hematology, University Hospital of Parma, for having provided the IFX serum levels and anti-IFX antibody levels for the recruited patients.
Author contributions
F.G.: Study design, patient recruitment, data collection and writing up of the first draft of the paper. B.M.R.: study design, monocytes isolation, cytokines measurement, writing up of the first draft of the paper. F.F., A.B., R.V.: monocytes isolation, cytokines measurement, statistical analysis. M.C.C., N.de’A.: statistical analysis, critical revision of study methods and draft. G.L.de’A., V.D.: supervision of the study design, methods and critical revision of the draft.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Federica Gaiani and Bianca Maria Rotoli.
References
- 1.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J. Clin. Investig. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neurath MF. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 3.Reinecker HC, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin. Exp. Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin AD, Wildenberg ME, van den Brink GR. Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J. Crohns Colitis. 2016;10:989–997. doi: 10.1093/ecco-jcc/jjw053. [DOI] [PubMed] [Google Scholar]
- 5.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 6.Yang S, Wang J, Brand DD, Zheng SG. Role of TNF–TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front. Immunol. 2018;9:784. doi: 10.3389/fimmu.2018.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berns M, Hommes DW. Anti-TNF-alpha therapies for the treatment of Crohn's disease: The past, present and future. Expert Opin. Investig. Drugs. 2016;25:129–143. doi: 10.1517/13543784.2016.1126247. [DOI] [PubMed] [Google Scholar]
- 8.Schnitzler F, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: Results from a single-centre cohort. Gut. 2009;58:492–500. doi: 10.1136/gut.2008.155812. [DOI] [PubMed] [Google Scholar]
- 9.Melsheimer R, Geldhof A, Apaolaza I, Schaible T. Remicade((R)) (infliximab): 20 Years of contributions to science and medicine. Biologics. 2019;13:139–178. doi: 10.2147/BTT.S207246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billmeier U, Dieterich W, Neurath MF, Atreya R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J. Gastroenterol. 2016;22:9300–9313. doi: 10.3748/wjg.v22.i42.9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adegbola SO, Sahnan K, Warusavitarne J, Hart A, Tozer P. Anti-TNF therapy in Crohn's disease. Int. J. Mol. Sci. 2018;19:289–294. doi: 10.3390/ijms19082244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: A review. Am. J. Gastroenterol. 2009;104:760–767. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 13.Steenholdt C, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: A randomised, controlled trial. Gut. 2014;63:919–927. doi: 10.1136/gutjnl-2013-305279. [DOI] [PubMed] [Google Scholar]
- 14.Steenholdt C, et al. Circulating cytokines and cytokine receptors in infliximab treatment failure due to TNF-alpha independent Crohn disease. Medicine (Baltimore) 2016;95:e3417. doi: 10.1097/MD.0000000000003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitt H, et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn's disease. Gut. 2019;68:814–828. doi: 10.1136/gutjnl-2017-315671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bain CC, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanauer SB, et al. Maintenance infliximab for Crohn's disease: The ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 18.Hall B, Holleran G, McNamara D. Small bowel Crohn's disease: An emerging disease phenotype? Dig. Dis. 2015;33:42–51. doi: 10.1159/000366047. [DOI] [PubMed] [Google Scholar]
- 19.Barilli A, et al. Gluten peptides drive healthy and celiac monocytes toward an M2-like polarization. J. Nutr. Biochem. 2018;54:11–17. doi: 10.1016/j.jnutbio.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Barilli A, Rotoli BM, Visigalli R, Dall'Asta V. Gliadin activates arginase pathway in RAW264.7 cells and in human monocytes. Biochim. Biophys. Acta. 2014;1842:1364–1371. doi: 10.1016/j.bbadis.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Grimm MC, Elsbury SK, Pavli P, Doe WF. Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J. Leukoc. Biol. 1996;59:804–812. doi: 10.1002/jlb.59.6.804. [DOI] [PubMed] [Google Scholar]
- 22.Li YW, et al. The -A2518G polymorphism in the MCP-1 gene and inflammatory bowel disease risk: A meta-analysis. J. Dig. Dis. 2015;16:177–185. doi: 10.1111/1751-2980.12232. [DOI] [PubMed] [Google Scholar]
- 23.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. doi: 10.1016/S0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 24.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daperno M, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: The SES-CD. Gastrointest. Endosc. 2004;60:505–512. doi: 10.1016/S0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 26.Steenholdt C, et al. Clinical implications of variations in anti-infliximab antibody levels in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2012;18:2209–2217. doi: 10.1002/ibd.22910. [DOI] [PubMed] [Google Scholar]
- 27.Ingoglia F, et al. Human macrophage differentiation induces OCTN2-mediated l-carnitine transport through stimulation of mTOR-STAT3 axis. J. Leukoc. Biol. 2017;101:665–674. doi: 10.1189/jlb.1A0616-254R. [DOI] [PubMed] [Google Scholar]
- 28.Rotoli BM, et al. Analysis of LPI-causing mutations on y+LAT1 function and localization. Orphanet. J. Rare Dis. 2019;14:63. doi: 10.1186/s13023-019-1028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotoli BM, et al. Functional analysis of OCTN2 and ATB0,+ in normal human airway epithelial cells. PLoS ONE. 2020;15:e0228568. doi: 10.1371/journal.pone.0228568. [DOI] [PMC free article] [PubMed] [Google Scholar]