Graphical abstract
Keywords: Woodwardia unigemmata, Fern, GC-MS, Antioxidant activity, Antibacterial activity
Abstract
Present work elucidates the antioxidant and antibacterial activity of Woodwardia unigemmata (Makino) Nakai along with chemical characterization using its aqueous (AEW), methanol (MEW), and hexane (HEW) extracts. Chemical profile of different extracts was illustrated by using Gas chromatography and mass spectrometry (GC-MS) analysis. Antioxidant activities were tested using DPPH and FRAP assays, total phenolic and flavonoid content by Folin-Ciocalteu and aluminum chloride method, respectively. Further, antibacterial activity against six plant and four animal pathogenic bacteria was analyzed by employing the disc diffusion assay. GC-MS analysis revealed the presence of catechol (21.96%), glycerol (20.22%), n-pentadecanoic acid (6.95%), glyceryl monoacetate (6.35 %), ethyl acetimidate (5.39 %) and 3-hydroxy-2,3-dihydromaltol (5.36%) in AEW; β-sitosterol (17.39%), pentadecanoic acid (9.81%), vitamin E (7.82%) and glycerol (7.05%) in MEW; γ-sitosterol (33.45%), vitamin E (10.04%) and campesterol (7.32%) in HEW as major constituents. Maximum phenolics (873 ± 6.01 mgGAE/g dry extract) as well as flavonoids (151 ± 11.44 mgQE/g dry extract) content was found in MEW, which also showed remarkable antioxidant potential (IC50 6.07 ± 1.4 µg/ml for DPPH and 768 ± 10.4 mg AAE/g dry extract for FRAP assay. In antibacterial activity, maximum inhibition (15 ± 0.9 mm) was observed for HEW against R. solanacearum, followed by AEW against A. tumefaciens and X. phaseoli (11 ± 0.3 mm each). MEW was found positive only against A. tumefaciens. Significant minimum inhibitory concentration (MIC) value observed for AEW against L. monocytogenes (10 mg/ml). Polar extracts had remarkable antioxidant potential, while non-polar extract did show significant antibacterial activity. Further, GC- MS reports indicated that this traditionally useful fern species can be an excellent source of biologically active compounds.
1. Introduction
Woodwardia unigemmata (Makino) Nakai, commonly known as jewelled chain fern belongs to family Blechnaceae. So far, thirteen (Gasper et al., 2016) to fourteen (Kramer et al., 1990, Cranfill and Kato, 2003) species of the genus Woodwardia have been recognized all over the world, out of which W. unigemmata is the single species of this genus in India (Fraser-Jenkins et al., 2017). This species is predominantly distributed in the northern hemisphere, especially in eastern Asia (Kramer et al., 1990). It is evergreen fern with arching, bipinnately-divided fronds reaching up to 1.5 m in length. In its native habitat it grows as a forest understory plant. It is terrestrial and lithophytic, grows well in beds of hill ravines. It is widely distributed throughout Himalayan Region usually between 1200 and 2400 m altitude.
There is a vast literature on traditional and economic uses of this plant. The decoction of rhizome and fronds internally administrated in dysentery, dried rhizome used as purgative, fronds used in skin diseases, and infertility (Gaur and Bhatt, 1994, Nwosu, 2002). Starch extracted from rhizomes is used to prepare cake, noodles, and liquor in China (Dai et al., 2003, Yun et al., 2009, Liu et al., 2012). In combination with some other ferns, it is used for the preparation of Chinese herbal preparation (Blechni Rhizoma). This Rhizoma has been traditionally used to treat hepatitis, Human Immunodeficiency Virus (HIV), injury, swelling, fever, measles, and erysipelas (Tsai and Hwang, 1999).
Previously, various natural biologically active compounds have been discovered from different species of this genus (Gonzalez et al., 2000, Hanus et al., 2003, Hiroshi et al., 1992, Zhong et al., 2008) including W. unigemmata. Four stenones including ecdysternone, ponasteroside A, woodwardic acid, and two flavonoids were isolated from the ethanol extract of W. japonica, (Luan et al., 2002, Liu and Gao, 2011). Two cyclohexenone glycosides were isolated from American fern W. virginica (Rezanka et al., 2003). Recently, new glucoside, woodorien was isolated from W. orientalis extract, which was the most potent inhibitor of type I Herpes simplex virus (Xu et al., 1993).
Only a few reports are available on antioxidant activity Zhong et al. (2008) and phytochemical constituents Rui et al. (2017) of W. unigemmata. Plant-based chemical compounds have a wide range of industrial and pharmacological uses. These reports indicate that this genus restrains different biologically active compounds. With this view, the present study has been taken up for studying the phytochemical profile, antioxidant and antibacterial activity of different polar and non-polar solvent (aqueous, methanol, and hexane) extracts of jewelled chain fern.
2. Material and methods
2.1. Collection and extraction of plant material
The fresh aerial part of W. unigemmata (Makino) Nakai was collected from Central Himalaya, India (1725 m asl; 29.8378° N, 80.1861° E) following Jain and Rao (1977). Taxonomic identification of the specimen (KU-BOT-WU02) was made by plant taxonomists, Late Prof. Y.P.S. Pangtey and Prof. P.C. Pandey, Department of Botany, D.S.B. Campus, Kumaun University, Nainital, Uttarakhand, India, and further authenticated by C.R. Fraser Jenkins. Solvent extraction was done following Handa et al. (2008), with some modification: Freshly collected plant material was thoroughly washed and shade dried (25 ± 2 °C) under sterilized conditions. After complete the drying process, the dried parts were powdered using an electric grinder. Powdered samples (25 g) were then immediately soaked in 150 ml each of aqueous, methanol and hexane for 36hr in a shaker (120 rpm). Extracts were then filtered and concentrated to dryness using a rotary evaporator at 35°C, and stored in the dark at 4°C for the further analysis.
2.2. Chemical characterization via GC-MS analysis
Three extracts of W. unigemmata (MEW- methanol extract, AEW- aqueous extract, and HEW- hexane extract) were analyzed by GC/MS analysis using GC MS-QP Plus, equipped with an Rtx- 5 MS capillary column (0.25 mm film thickness, 0.25 mm internal diameter, and 30 m in length) (J&W Scientific, Agilent, Santa Clara, CA, USA) with helium as carrier gas: with a flow rate of 1.21 ml/min. The initial oven temperature was set at 100 °C for 2 min, and finally to 280 °C with a rate of 10 °C/min. The components were identified by comparing the spectral data of peaks with the corresponding standard mass spectra of NIST-MS and Wiley library.
2.3. Total phenolic content
Total phenolics were estimated by following Folin- Ciocalteu Regent (FCR) method of Singleton and Rossi (1965) with little modification. Different concentrations (10–100 μg/ml) of standard and test samples were prepared to form the stock solution. 0.5 ml of each concentration was mixed with 0.2 ml of FCR, 0.5 ml Na2CO3 (20%). After 30 min of incubation at room temperature, absorbance was recorded at 765 nm against the blank. The concentration of total phenolic content was calculated as mg Gallic acid equivalent/g of dry extract, from the calibration curve of the standard solution of Gallic acid (Y = 0.004x; R2 = 0.995).
2.4. Total flavonoid content
Total Flavonoids were determined using the AlCl3 method with some modifications (Willet, 2002). Different dilutions (10–100 μg/ml) of Quercetin (standard) and tests were prepared. Each sample (0.5 ml) was mixed with 0.1 ml Aluminium chloride (10%), 1 M Potassium acetate (0.1 ml), raising the final volume to 5 ml by adding distilled water. After incubation at room temperature for 30 min, the absorbance was recorded at 415 nm. Based on the Quercetin calibration curve (Y = 0.006x; R2 = 0.983), the flavonoid content was calculated and articulated in terms of mg Quercetin equivalents/ g of dry extract.
2.5. Antioxidant activity
Antioxidant potential of different extracts of W. unigemmata (HEW, MEW and AEW) were screened using DPPH (2,2- diphenyl- 1-picryl-hydrazyle) scavenging assay and FRAP (ferric reducing antioxidant power) assay.
2.6. DPPH assay
Scavenging of free radical (DPPH) by different extracts was determined by following Xie et al. (2010) with some modifications. 2 ml of fresh DPPH – methanol solution (0.1 mM) was mixed with 2 ml of the plant extract at different concentrations (10–100 μg/ ml). The reaction mixture was incubated in the dark for 30 min at room temperature and analyzed spectrophotometrically at 517 nm against blank. Ascorbic acid was used as the positive control. The free radical scavenging potential of plant extracts was estimated using the formula:
% Inhibition = [(Absorbance of Control - Absorbance of test Sample) / (Absorbance of Control)] × 100
IC50 value of test samples and standard was determined using the percentage inhibition versus concentration graph.
2.7. FRAP assay
Ferric Reducing Antioxidant Power (FRAP) assay was carried out following Benzie and Strain (1999) with slight revision. Extracted samples (100 µl were mixed with FRAP reagent (2.5 ml) in a test tube. Both samples and blank were incubated at 370C for 10 min, and absorbance of the sample was recorded against blank at 593 nm. A series of 10 to 100 µl were prepared using an aqueous solution of ascorbic acid as standard. On the basis of ascorbic acid calibration curve (Y = 0.022x; R2 = 0.992), the FRAP values were articulated as mg ascorbic acid equivalent / g of dry extract.
2.8. Microorganisms used
Four animal pathogenic (Klebsiella pneumoniae MTCC No.7028, Listeria monocytogenes MTCC No. 657, Pseudomonas aeruginosa MTCC No.3542, Proteous mirabilis MTCC No. 3310; from Microbial Type Culture Collection - IMTECH, Chandigarh, India), and six plant pathogenic (Agrobacterium tumefaciens MTCC No.609, Ralstonia solanacearum ITCC No. BH0007, Xanthomonas campestris ITCC No. BD0006, Xanthomonas oryzae ITCC No. PI0012; Xanthomonas phaseoli and Erwinia chrysanthemi from Indian Agriculture Research Institute, New Delhi, India, and, Plant Pathology Department, G. B. Pant University, Pantnagar, India) bacterial strains were used. Respective stock culture (1% v/v) was inoculated using nutrient agar broth and activated at 37°C for 18 h for further use (Andrews, 2005).
2.9. Screening for antibacterial activity
Disc diffusion method was deployed for antibacterial tests of selected microorganisms (Bauer et al., 1966). Nutrient agar plates (9 cm size) were prepared and cooled down to 20 ± 2 °C. Test bacterial inoculums 100 μl containing 106 CFU/ml of test bacteria were spread uniformly by bacterial spreader. Each organism (culture) was inoculated on three (3) plates (replicate). 20 μl of extract (50 mg/ml) was loaded onto the sterile discs (6 mm). These loaded discs were then placed on the surface of the inoculated agar plates at equidistance, and then these plates were incubated at 37 ± 1 °C for 24 h. Positive (Gentamicin −10 mcg, ampicillin −10 mcg) as well as negative controls (respective solvent) were also used. After incubation (24 h) of nutrient agar plates, a clear halo zone around the discs signified a positive antimicrobial activity, and the zone diameter expressed in millimeters including disc size (Vineela and Elizabeth, 2005). The mean of the halo zone diameter (±SD) was accounted for the antibacterial activity of the extracts.
2.10. Evaluation of minimum inhibitory concentrations (MIC’s)
The lowest concentration which is responsible for the visible inhibition of the microbial growth is defined as MIC. In present analysis, plant extracts which were found efficient at screening (50 mg/ml) were further analyzed using different dilutions (30, 20, 10, 0.5, and 0.25 mg/ml) following two fold serial dilution technique (Zaidi et al., 2009). The loaded filter paper discs with requisite concentrations were placed on agar plates, which in turn were then incubated at 35 °C (24 h). The halo zones were measured and noted with respect to the effective concentrations.
2.11. Statistical analysis
All experiments were done in triplicates, and results obtained were expressed as mean ± standard error (SE). Analysis of correlation coefficients of determination was calculated by using SPSS (version 16 for window).
3. Results
3.1. GC-MS analysis of different extracts of W. unigemmata
Outcome of the GC-MS results affirm the presence of different bioactive compounds in all three extracts. Different proportions of major constituents (>5%) highlights the influence of solvents on extractability. Investigation of AEW reveal the existence of 26 compounds, representing 88% of the total volume (Table 1) with catechol (21.96%), glycerol (20.22%), n-pentadecanoic acid (6.95%), glyceryl monoacetate (6.35%), ethyl acetimidate (5.39%), and 3-hydroxy-2,3-dihydromaltol (5.36%) as major components. As indicated in Table 2, β- sitosterol (17.39%), pentadecanoic acid (9.81%), vitamin E (7.82%), and glycerol (7.05%) are the major contributors of entire 24 constituents (representing 82% of total volume) reported for MEW. Similarly, HEW displayed a total of 15 compounds, comprising 72.42% of total fraction with γ-sitosterol (33.45%), vitamin E (10.04%), and campesterol (7.32%) as its prominent compounds (Table 3).
Table 1.
Chemical composition of aqueous extract of W. unigemmata (AEW).
| S.No. | Compound | Molecular Formula | Retention Time | Molecular Weight | Nature of Compound | % Contribution |
|---|---|---|---|---|---|---|
| 1 | methyl 2-ethylacetoacetate | C7H12O3 | 4.59 | 144 | Ester | 0.99 |
| 2 | ehtyl acetimidate | C4H9NO | 4.94 | 87 | Ester | 5.39 |
| 3 | 2-hydroxy-2-cyclopenten-1-one | C5H6O2 | 2.34 | 98 | Ketone | 2.34 |
| 4 | Glycerol | C3H8O3 | 7.25 | 92 | Alcohol | 20.22 |
| 5 | acetopropionic acid | C5H8O3 | 8.02 | 116 | fatty acid | 1.09 |
| 6 | propyl acetate | C5H10O2 | 8.19 | 102 | Ester | 0.70 |
| 7 | tetraacetic acid lactone | C8H8O4 | 8.83 | 168 | Ketone | 1.22 |
| 8 | α-methylacetoacetic ester | C7H12O3 | 9.92 | 144 | Ester | 0.83 |
| 9 | 3-hydroxy-2,3-dihydromaltol | C6H8O4 | 10.10 | 144 | Ketone | 5.36 |
| 10 | Catechol | C6H6O2 | 11.30 | 110 | Phenol | 21.96 |
| 11 | glyceryl monoacetate | C5H10O4 | 12.14 | 134 | Alcohol | 6.35 |
| 12 | acetopropyl acetate | C7H12O3 | 12.94 | 144 | Ester | 3.03 |
| 13 | 3-methoxyacetophenone | C9H10O2 | 13.23 | 150 | Ketone | 0.66 |
| 14 | pyrogallol | C6H6O3 | 14.92 | 126 | Phenol | 1.19 |
| 15 | 3-methylsalicylaldehyde | C8H8O2 | 15.70 | 136 | Aldehyde | 1.24 |
| 16 | 3-oxo-α-ionol | C13H20O2 | 18.49 | 208 | sesquiterpenoid | 0.59 |
| 17 | Loliolide | C11H16O3 | 19.93 | 196 | Benzofuran | 1.11 |
| 18 | tetradecanoic acid | C14H28O2 | 20.07 | 228 | fatty acid | 0.86 |
| 19 | neophytadiene | C20H38 | 21.08 | 278 | sesquiterpenoid | 0.57 |
| 20 | n-pentadecanoic acid | C15H30O2 | 22.66 | 242 | fatty acid | 6.95 |
| 21 | Methyl (7E)-7-hexadecenoate | C17H32O2 | 24.75 | 268 | Ester | 0.75 |
| 22 | 9-octadecenoic acid | C18H34O2 | 25.02 | 282 | fatty acid | 0.35 |
| 23 | glycerol, 2-palmitate | C19H38O4 | 29.14 | 330 | fatty acid | 0.58 |
| 24 | squalene | C30H50 | 33.80 | 410 | Hydrocarbon | 0.24 |
| 25 | vitamin E | C29H50O2 | 38.45 | 430 | Alcohol | 0.27 |
| 26 | stigmast-5-en-3-ol | C29H50O | 42.54 | 414 | Steroid | 3.26 |
| Total | 88.1 | |||||
Table 2.
Chemical composition of methanol extract of W. unigemmata (MEW)
| S.No. | Compound | Molecular Formula | Retention Time | Molecular Weight | Nature of Compound | % Contribution |
|---|---|---|---|---|---|---|
| 1 | ehtyl acetimidate | C4H9NO | 4.92 | 87 | Ester | 3.59 |
| 2 | 2,4 dihydroxy-2,5- dimethyl-3(2H)- furan-3-one | C6H8O4 | 6.65 | 144 | Ketone | 0.53 |
| 3 | 4-morpholineacetonitrile | C6H10N2O | 8.87 | 126 | Nitriles | 1.35 |
| 4 | isopentane | C5H12 | 9.08 | 72 | Hydrocarbon | 0.91 |
| 5 | 4H-pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl | C6H8O4 | 10.11 | 144 | Ketone | 2.77 |
| 6 | glycerol | C3H8O3 | 10.63 | 92 | Alcohol | 7.05 |
| 7 | dihydroxybenzol | C6H6O2 | 11.54 | 110 | Phenol | 1.02 |
| 8 | 5-hydroxymethylfurfural | C6H6O3 | 11.89 | 126 | Furfural | 0.93 |
| 9 | 2,4-diacetoxypentane | C9H16O4 | 12.10 | 188 | Hydrocarbon | 4.56 |
| 10 | 1-Methyl-3,8,9-trioxabicyclo[4.2.1]nonane | C7H12O3 | 13.04 | 144 | Hydrocarbon | 1.70 |
| 11 | cyclopentyl alcohol | C5H10O | 15.62 | 86 | Alcohol | 3.48 |
| 12 | dihydroactinidiolide | C11H16O2 | 16.88 | 180 | Terpene | 0.32 |
| 13 | loliolide | C11H16O3 | 20.32 | 196 | Benzofurans | 1.41 |
| 14 | neophytadiene | C20H38 | 21.08 | 278 | sesquiterpenoid | 3.00 |
| 15 | hexadecanoic acid, methyl ester | C17H34O2 | 22.20 | 270 | fatty acid | 0.92 |
| 16 | pentadecanoic acid | C15H30O2 | 22.68 | 270 | fatty acid | 9.81 |
| 17 | phytol | C20H40O | 24.43 | 296 | Diterpene | 3.54 |
| 18 | glycerol-α-monostearate | C21H42O4 | 29.13 | 358 | Alcohol | 2.58 |
| 19 | 1,2-benzenedicarboxylic acid | C24H38O4 | 29.41 | 390 | fatty acid | 4.87 |
| 20 | 9-hexadecenal | C16H30O | 32.39 | 238 | Hydrocarbon | 1.10 |
| 21 | squalene | C30H50 | 33.80 | 410 | Hydrocarbon | 0.36 |
| 22 | α-tocospiro | C29H50O4 | 34.39 | 462 | Alcohol | 1.01 |
| 23 | vitamin E | C29H50O2 | 38.45 | 416 | Alcohol | 7.82 |
| 24 | β-sitosterol | C29H50O | 42.56 | 414 | Steroid | 17.39 |
| Total | 82.02 | |||||
Table 3.
Chemical composition of hexane extract of W. unigemmata (HEW)
| S. No. | Compound | Molecular Formula | Retention Time | Molecular Weight | Nature of Compound | % Contribution |
|---|---|---|---|---|---|---|
| 1 | 4,6-dimethyldodecane | C14H30 | 12.52 | 198 | Hydrocarbon | 0.23 |
| 2 | dihydroactinidiolide | C11H16O2 | 16.86 | 180 | Terpene | 0.59 |
| 3 | neophytadiene | C20H38 | 21.08 | 278 | sesquiterpenoid | 2.49 |
| 4 | 2-hydroxycyclopentadecanone | C15H28O2 | 22.42 | 240 | Ketone | 1.13 |
| 5 | pentadecanoic acid | C15H30O2 | 22.79 | 242 | fatty acid | 3.90 |
| 6 | phytol | C20H40O | 24.43 | 296 | Diterpene | 4.38 |
| 7 | 4,8,12,16-tetramethylheptadecan-4-olide | C21H40O2 | 27.07 | 324 | Aldehyde | 1.21 |
| 8 | glycerol -α-monostearate | C21H42O4 | 29.14 | 358 | Alcohol | 0.74 |
| 9 | 6,9-cis-3,4-epoxy-nonadecadiene | C19H34O | 32.40 | 278 | Hydrocarbon | 0.60 |
| 10 | α-tocospiro | C29H50O4 | 34.39 | 462 | Alcohol | 1.60 |
| 11 | γ-tocopherol | C28H48O2 | 37.07 | 416 | Alcohol | 0.58 |
| 12 | vitamin E | C29H50O2 | 38.52 | 430 | Alcohol | 10.04 |
| 13 | campesterol | C28H48O | 40.50 | 400 | Steroid | 7.32 |
| 14 | γ-sitosterol | C29H50O | 42.84 | 414 | Steroid | 33.45 |
| 15 | cycloartenol | C30H50O | 44.82 | 426 | Alcohol | 4.06 |
| Total | 72.42 | |||||
3.2. Quantitative phytochemical analysis
Results for total polyphenolic compounds (total phenolic content –TPC; total flavonoid content -TFC) are summarized in Table 4. When comparing the TPC content in terms of mg GAE gm−1 dry extract, MEW was found to show a higher TPC value (873 ± 6.01) followed by AEW (363 ± 7.27) and HEW (31.67 ± 10.94). A similar trend was also noted for the TFC (in terms of mg QE gm−1 dry extract) among extracts, i.e., MEW (151 ± 11.24) has a higher TFC compared to AEW (146 ± 7.84) and HEW (43.89 ± 1.47).
Table 4.
Total Phenolic, Flavonoid Content and antioxidant activity (DPPH, FRAP) in different extracts of W. unigemmata.
| Plant extracts |
Phytochemicals |
Antioxidant activity |
||
|---|---|---|---|---|
| TPC (mg GAE gm−1 dry extract) | TFC (mg QE gm−1 dry extract) | FRAP values (mg AAE gm−1 dry extract) | DPPH assay (IC50 value, µg/ml) | |
| Aqueous extract | 363 ± 7.27 | 146 ± 7.84 | 605 ± 7.08 | 50.68 ± 2.5 |
| Methanol extract | 873 ± 6.01 | 151 ± 11.24 | 768 ± 10.4 | 6.07 ± 1.4 |
| Hexane extract | 31.67 ± 10.94 | 43.89 ± 1.47 | 85 ± 5.13 | 87.27 ± 7.3 |
| *Corr. with TPC | – | – | 0.915 | −0.998 |
| *Corr. with TFC | – | – | 0.982 | −0.858 |
Values are mean ± SE of three replicate, TPC- Total Phenolic Content: TFC- Total Flavonoid Content; GAE- Gallic Acid Equivalent; QE- Quercetin Equivalent; AAE- Ascorbic acid Equivalent; *Correlation is significant at the 0.05 level
3.3. Antioxidant activity
3.3.1. DPPH radical scavenging activity
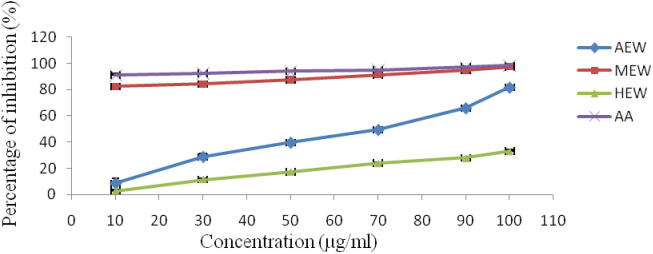
The free radical scavenging activity of different extracts is shown in Table 4 and Fig. 1. MEW exhibited most effective inhibition activity (97.26%) for DPPH radical at 100 µg/ml concentration with IC50, 6.07 ± 1.4 μg/ml. A moderate radical scavenging activity (81.65% at 100 µg/ml; IC50-50.68 ± 2.5 µg/ml) was observed by AEW, followed by HEW (32.98% at 100 µg/ml; IC50, 87.27 ± 7.3 µg/ml).
Fig. 1.
Antioxidant activity of W. unigemmata extracts against DPPH radical; AEW- aqueous extract; MEW- Methanol extract; HEW- Hexane extract; AA-Ascorbic Acid.
3.3.2. FRAP assay
The reducing activity (Fe+3 to Fe+2) of AEW, MEW, and HEW are presented in Table 4. Here also, MEW showed greater FRAP value as 768 ± 10.4 in terms of mg AAE gm−1 dry extract. AEW and HEW demonstrated a significant difference in comparison with MEW, with FRAP value of 605 ± 7.08 and 85 ± 5.13 mg AAE gm−1 dry extract, respectively.
3.4. Antibacterial activity of different extracts
In vitro antibacterial activity of W. unigemmata extracts were studied against animal and plant pathogenic bacterial strains, as presented in Table 5.
Table 5.
Antibacterial activity of different extracts of W. unigemmataTable 4.
| Bacterial strain | Aqueous Extract |
Methanol Extract |
Hexane Extract |
Gentamicin |
Ampicillin |
|||
|---|---|---|---|---|---|---|---|---|
| ZOI (mm) | MIC (mg ml−1) | ZOI (mm) | MIC (mg ml−1) | ZOI (mm) | MIC (mg ml−1) | ZOI (mm) at 10mcg/ml | ZOI at 10mcg/ml | |
| RS | 8 ± 2.7 | 20 | na | na | 15 ± 0.9 | 30 | 23 ± 2.3 | 15 ± 2.3 |
| EC | na | na | na | na | 8 ± 2.6 | 30 | 21 ± 1.8 | 7 ± 2.2 |
| XO | na | na | na | na | 10 ± 0.7 | 30 | 25 ± 1.1 | 21 ± 0.8 |
| XP | 11 ± 0.3 | 20 | na | na | 9 ± 0.3 | 20 | 22 ± 3.9 | 16 ± 2.5 |
| XC | 10 ± 00 | 20 | na | na | 7 ± 00 | 30 | 25 ± 1.3 | 22 ± 1.4 |
| AG | 11 ± 0.3 | 30 | 8 ± 00 | 30 | 8 ± 00 | 30 | 22 ± 1.7 | 16 ± 2.7 |
| KP | na | na | na | na | 7 ± 0.3 | 30 | 28 ± 1.2 | 18 ± 1.2 |
| LM | 10 ± 3.5 | 10 | na | na | 10 ± 3.9 | 10 | 21 ± 0.7 | na |
| PM | na | na | na | na | na | na | 22 ± 1.2 | na |
| PA | na | na | na | na | na | na | 22 ± 1.5 | 15 ± 5.9 |
RS- R. solanacearum; EC- E. chrysanthemi; XO- X. oryzae; XP- X. phaseoli; XC- X. compestris; AG- A. tumefaciens; KP- K. pneumoniae; LM- L. monocytogenes; PM- P. mirabilis; PA- P. aeruginosa; na- Not active, ZOI- zone of inhibition.
HEW displayed the broad-spectrum antibacterial activity against tested bacterial strains. Its highest inhibitory effect was observed against R. solanacearum (ZOI- 15 ± 0.9 mm, MIC-30 mg ml−1), moderate against X. oryzae (10 ± 0.7 mm, MIC-30 mg ml−1) and L. monocytogenes (10 ± 3.9 mm, MIC 10 mg ml−1) and comparatively less against X. phaseoli (9 ± 0.3 mm), A. tumefaciens (8 ± 0.0 mm), E. chrysanthemi (8 ± 2.6 mm), X. campestris (7 mm) and K. pneumoniae (7 mm). AEW was found moderately active against five tested bacterial strains (Table 5). Further, all tested bacterial strains except A. tumefaciens (ZOI, 8 mm) were found resistant to MEW.
4. Discussion
Analyzing PSMs, together with preliminary clinical traits, have rightfully gained dominant importance in validating their acclaimed health vitalizing effects. The present investigation supports previous findings for ferns having biologically active PSMs (Liu et al., 1986, Mishra and Verma, 2010, Laware and Limaye, 2012, Chowdhary et al., 2010, Francisco and Driver, 1984, Harada and Saiki, 1955, Hu et al., 2008, Li et al., 2014, Ma and Gang, 2004, Ma et al., 2003). Major components observed in this study are well documented in earlier reports. Catechol, individually, as an antioxidant agent, has significant activity against degenerative diseases (Berberian et al., 2007). Also, its antimicrobial properties, as well as contribution in various metabolic processes are well documented (Jeong et al., 2009, Robinson et al., 1992, Sigma-Aldrich., 2006). Glycerol, a most versatile and valuable chemical, also has application as an additive (Bonnardeaux et al., 2006), and as a substitute for propylene glycol (Pagliaro and Rossi, 2008).
Similarly, plant phytosterols (β and γ- sitosterols) are well established for their anti-diabetic and cholesterol-lowering properties through in vitro and in vivo models (Wang and Ng, 1999, Balamurugan et al., 2012, Hwang et al., 2008). Recently, Rui et al. (2017) reported one new kaempferol as well as six other compounds from methanol extract of W. unigemmata. However, their absence in all three extract tested in present study can be explained on the basis of chemical variation due to various ecological feature such as temperature, water stress, light condition as well as phenological development (Upadrasta et al., 2011;Abdelgawad et al., 2014). It also explains the substantial differences obtained from previous reports.
Antioxidant activity of phenolics is due to their reducing effect, as hydrogen donor and singlet oxygen quenchers (Chang et al., 2001, Rice-Evans et al., 1997). Polyphenols represent a heterogeneous group of PSMs, which includes flavonoids, phenolic acids, stilbenes, and lignans (Hussain et al., 2016). They are well-documented antioxidant (Hussain et al., 2016) and antibacterial agents (Okwu, 2004, Afolabi et al., 2007). The later been attributed to their iron deprivation or hydrogen bonding ability with microbial enzymes (Scalbert, 1991, Davidson and Naidu, 2000). Similarly, Cowan (1999) credited the complex-forming ability of flavonoids with the microbial cell wall and soluble proteins, which makes them responsible for antimicrobial activity.
The antioxidant activity and TPC in the three samples were in the order of MEW > AEW > HEW, which demonstrates the influence of solvents extractability. Several reports have shown that the nature of the solvent exerts a significant influence on the phenolic extraction of plants (Akowuah et al., 2004, Turkmen et al., 2006). Further, MEW and AEW maintained high antioxidant activity, which is comparable in both total DPPH and FRAP assays. This observation suggests that chemical/s responsible solitarily or synergistically can be extracted in polar solvents in much higher amount. Further, these chemical/s can function using two dissimilar machinery, for free radical scavenging activity, through a single electron transfer in FRAP (Ashby, 1988), and as hydrogen transfer reaction for DPPH activity (Prior et al., 2005, Mader et al., 2007, Shalaby and Shanab, 2013).
We observed a positive correlation between total phenolic content and antioxidant activities (Table 4) in a dose-dependent manner, which affirm several previous reports (Velioglu et al., 1998, Mei et al., 2009, Pandey et al., 2019). Previously, Zhong et al., (2008), also reported high DPPH scavenging activity (IC50-7.20 µg/ml) of ethanol extract of W. unigemmata. The proximity of polarity might be a possible explanation for similar findings between methanol and ethanol. Interestingly, HEW, which had low antioxidant activity, exhibited higher broad-spectrum antibacterial activity than AEW and MEW. This variation could be explained based on a high amount of γ-sitosterol (33%) alone or synergistically affecting microbial growth. Further, the docking capacity of certain molecules (γ-sitosterol, in this case) with target protein might be responsible for hindering biosynthetic pathways of microorganisms. These results are of enormous value, mainly in the view of growing drug resistance among bacteria.
These findings of the present study are promising and actively encouraging for further research, such as fractionation of extracts and confirmation of mechanism involved. To the best of the facts known and our understanding, this is the pioneer report on the chemical characterization together as well as the antioxidant and antibacterial activity of any fern species from the Indian Himalayan region.
5. Conclusion
The present study reports the presence of many medicinally valuable and industrially relevant compounds, including glycerol, catechol, tocopherols, phytosterols, etc. in W. unigemmata. Tested plant extracts showed remarkable antibacterial activity against plant as well animal pathogenic bacteria. In addition, they are recorded with rich polyphenolic compounds (TPC and TFC) with efficient antioxidant activity in both, DPPH, and FRAP assays. Based on these observations, W. unigemmata can be recommended as a source of many biologically active and industrially relevant compounds. Further, research on individual compound is required to assess their potential, especially in the pharmacology sector.
Funding
This research received no external funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors extend their appreciation to the researchers supporting project number (RSP-2020/56) King Saud University, Riyadh, Saudi Arabia. Authors are also thankful to C.R. Fraser Jenkins for authenticating the plant sample.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2020.06.006.
Contributor Information
Kapil Khulbe, Email: kapilkhulbebot@kunainital.ac.in.
Asad Syed, Email: assyed@ksu.edu.sa.
Abdallah M. Elgorban, Email: aelgorban@ksu.edu.sa.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abdelgawad H., Peshev D., Zinta G., Van den Ende W., Janssens I.A., Asard H. Climate Extreme Effects on the Chemical Composition of Temperate Grassland Species under Ambient and Elevated CO2: A Comparison of Fructan and Non-Fructan Accumulators. Plos ONE. 2014;9,(e92044) doi: 10.1371/journal.pone.0092044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afolabi C., Akinmoladun E.O., Dan-Ologe I.A. Phytochemical constituents and antioxidant properties of extracts from the leaves of Chromolaena odorata. Sci. Res. Essays. 2007;2:191–194. [Google Scholar]
- Akowuah A.G., Ismail Z., Norhayati I., Sadikun A., Khamsah S.M. Sinensitin, eupatorin, 30 -hydroxy-5, 6,7,40 -tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of Orthosiphon stamineus from Malaysia. Food Chem. 2004;87:569–666. [Google Scholar]
- Andrews J.M. BSAC standardized disc susceptibility testing method (version 4) J. Antimicrob. Chemother. 2005;56:60–76. doi: 10.1093/jac/dki124. [DOI] [PubMed] [Google Scholar]
- Ashby E.C. Single-electron transfer, a major reaction pathway in organic chemistry. An answer to recent criticisms. ACC Chem. Res. 1988;21:414–421. [Google Scholar]
- Balamurugan R., Stalin A., Ignacimuthu S. Molecular docking of γ-sitosterol with some target related to diabetes. Eur. J. Med. Chem. 2012;47:38–43. doi: 10.1016/j.ejmech.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Bauer A.W., Kirby W.M., Sherris J.C., Truck M. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- Benzie I.F.F., Strain J.J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fiuids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- Berberian V., Allen C.C.R., Sharma N.D., Boyd D.R., Hardacre C. A comparative study of the synthesis of 3-substituted catechols using an enzymatic and a chemoenzymatic method. Adv. Synth. Catal. 2007;349:727–739. [Google Scholar]
- Bonnardeaux, J., 2006. Glycerin Overview, Report for the Western Australia Department of Agriculture and Food (from Frost & Sullivan: ‘‘R&D creating new avenues for glycerol’’. Available online at the URL.
- Chang S.T., Wu J.H., Wang S.Y., Kang P.L., Yang N.S., Shyur L.F. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J. Agric. Food Chem. 2001;49:3420–3424. doi: 10.1021/jf0100907. [DOI] [PubMed] [Google Scholar]
- Chowdhary S., Verma D.L., Pande R. Antioxidative properties of flavonoids from Cheilanthes anceps Swartz. J. Am. Sci. 2010;6:203–207. [Google Scholar]
- Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranfill, R., Kato, M., 2003. Phylogenetics, biogeography, and classification of the woodwardioid ferns (Blechnaceae). 25-48 in: Chandra S, Srivastava M. (eds.), Pteridology in the New Millenium. Kluwer Academic Publishers, Dordrecht.
- Dai X.L., Li X.G., Wu S.F. List of Chinese edible pteridophytes. Quarterly of Forest By-Product and Speciality in China. 2003;4:5–6. [Google Scholar]
- Davidson P.M., Naidu A.S. Phyto-phenols. In: Naidu A.S., editor. Natural Food Antimicrobial Systems. CRC Press; Boca Raton, FL, USA: 2000. pp. 265–294. [Google Scholar]
- Francisco M.S., Driver G.C. Anti-microbial activity of phenolic acids in Pteridium aquilinum. Am. Fern. J. 1984;74:87–96. [Google Scholar]
- Fraser-Jenkins, C.R., Gandhi, K.N., Kholia, B.S., Benniamin, A., 2017. An annotated checklist of Indian pteridophytes part-1 (Lycopodiaceae to Thelypteridaceae). Dehradun, Messrs Bishen Singh Mahendra Pal Singh.
- Gasper A.L.D., Dittrich V.A.D.O., Smith A.R., Salino A. A classification for Blechnaceae (Polypodiales: Polypodiopsida): New genera, resurrected names, and combinations. Phytotaxa. 2016;275(3):191–227. [Google Scholar]
- Gaur R.D., Bhatt B.P. Folk Utilization of some pteridophytes of Deoprayag area in Garhwal Himalaya:India. Econ. Bot. 1994;48:146–151. [Google Scholar]
- Gonzalez G.A.M., Carmen A.C., Candelaria G.F., Catalina L.A., Jaime B.B. Triterpenoids from Woodwardia radicans. Biochem. Syst. Ecol. 2000;28:497–499. doi: 10.1016/s0305-1978(99)00085-x. [DOI] [PubMed] [Google Scholar]
- Handa, S.S., Khanuja, S.P.S., Longo, G., Rakesh, D.D., 2008. Extraction Technologies for Medicinal and Aromatic Plants, (1stedn), no. 66. Italy: United Nations Industrial Development Organization and the International Centre for Science and High Technology.
- Hanus L.O., Rezanka T., Dembitsky V.M. A trinorsesterterpene glycoside from the North American fern Woodwardia virginica (L.) Smith. Phytochem. 2003;63:869–875. doi: 10.1016/s0031-9422(03)00290-5. [DOI] [PubMed] [Google Scholar]
- Harada T., Saiki Y. Pharmaceutical studies on ferns. VIII. Distribution of flavonoids in ferns. Pharmaceut. Bull. 1955;3:469–472. doi: 10.1248/cpb1953.3.469. [DOI] [PubMed] [Google Scholar]
- Hiroshi W., Tsunchiro K., Nobutoshi T., Takao M., Yasuhisa S., Chiu- Ming C. Chemical and chemotaxonomial studies of ferns. LXXXI. Characteristic lignans of blechnaceaeous ferns. Chem. Pharm. Bull. 1992;40:2099–2101. [Google Scholar]
- Hu H.B., Cao H., Jian Y.F. Chemical constituents and antimicrobial activities of extracts from Pteris multifida. Chem. Nat. Comp. 2008;44:106–108. [Google Scholar]
- Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C., Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.L., Kim H.N., Jung H.H., Kim J.E., Choi D.K., Hur J.M. Beneficial effects of beta-sitosterol on glucose and lipid metabolism in L6 myotube cells are mediated by AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2008;377:1253–1258. doi: 10.1016/j.bbrc.2008.10.136. [DOI] [PubMed] [Google Scholar]
- Jain, S.K., Rao, R.R., 1977. A Handbook of Field and Herbarium Methods. New Delhi.
- Jeong E.Y., Jeon J.H., Lee C.H., Lee H.S. Antimicrobial activity of pyrocatechin isolated from Diospyros kaki Thunb. roots and its derivatives toward intestinal bacteria. Food Chem. 2009;115:1006–1010. [Google Scholar]
- Kramer, K.U., Chambers, T.C., Hennipman, E., 1990. Blechnaceae – Pp. 60 – 68 in: Kramer, K.U., Green, P.S. (ed.), The families and genera of vascular plants. Edited by K. Kubitzky. Volume I. Pteridophytes and gymnosperms.– Berlin: Springer.
- Laware, S., Limaye, A., 2012. Adiantum a medicinal herb: An Antidiabetic and Antioxidant Potentials of Adiantum. Paperback. Lap Lambert, UK: Academic Publishers, pp. 124.
- Li S., Zhao M., Li Y. Preparative isolation of six antitumour biflavonoids from Selaginella doederleinii Hieron by high-speed counter-current chromatography. Phytochem. Anal. 2014;25:127–133. doi: 10.1002/pca.2478. [DOI] [PubMed] [Google Scholar]
- Liu J.S., Zhu Y.L., Yu C.M., Zhou Y.Z., Han Y.Y., Wu F.W., Qi B.F. The structures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Canadian J. Chem. 1986;64:837–839. [Google Scholar]
- Liu T., Gao Z. Phytochemical investigation of Woodwardia japonica. Planta Med. 2011;77 doi: 10.1055/s-0031-1273584. [DOI] [Google Scholar]
- Liu Y., Wujisguleng W., Long C. Food uses of ferns in China: a review. Acta Societatis Acta Soc. Bot. Pol. 2012;81:263–270. [Google Scholar]
- Luan X., Wang H., Wen Y.Y. Studies on the chemical constituents of Woodwardia japonica. J. Trop. Subtrop. Bot. 2002;10:361–365. [Google Scholar]
- Ma L.Y., Ma S.C., Wei F. Uncinoside A and B, two new antiviral chromone glycosides from Selaginella uncinata. Chem. Pharm. Bull. 2003;51:1264–1267. doi: 10.1248/cpb.51.1264. [DOI] [PubMed] [Google Scholar]
- Ma X., Gang D.R. The Lycopodium alkaloids. Nat. Prod. Rep. 2004;21:752–772. doi: 10.1039/b409720n. [DOI] [PubMed] [Google Scholar]
- Mader E.A., Davidson E.R., Mayer J.M. Large ground-state entropy changes for hydrogen atom transfer reactions of iron complexes. J. Am. Chem. Soc. 2007;129:5153–5166. doi: 10.1021/ja0686918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei R.Q., Wang Y.H., Du G.H., Liu G.M., Zhang L., Cheng Y.X. Antioxidant lignans from the fruits of Broussonetia papyrifera. J. Nat. Prod. 2009;72:621–625. doi: 10.1021/np800488p. [DOI] [PubMed] [Google Scholar]
- Mishra R., Verma D.L. Flavonoids from Cheilanthes anceps (Blanford) New York Sci. J. 2010;3:22–26. [Google Scholar]
- Nwosu M.O. Ethnobotanical studies on some pteridophytes of Southern Nigeria. Econ. Bot. 2002;56:255–259. [Google Scholar]
- Okwu D.E. Phytochemicals and vitamin content of indigenous spices of Southeastern Nigeria. J. Sustain. Agric. Environ. 2004;6:30–37. [Google Scholar]
- Pagliaro M., Rossi M. The future of glycerol. New usages for a versatile raw material. Chem. Sus. Chem. 2008;1 653-653. [Google Scholar]
- Pandey A., Belwal T., Tamta S., Bhatt I.D., Rawal R.S. Phenolic compounds, antioxidant capacity and antimutagenic activity in different growth stages of in vitro raised plants of Origanum vulgare L. Mol. Biol. Rep. 2019;46:2231–2241. doi: 10.1007/s11033-019-04678-x. [DOI] [PubMed] [Google Scholar]
- Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Rezanka T., Dembitsky V.M., Hanus L.O. Two cyclohexenone glycosides from the North American fern Woodwardia virginica (L.) Smith. Phytochem. 2003;63:931–937. doi: 10.1016/s0031-9422(03)00358-3. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C.A., Miller N.J., Paganga G. Antioxidant properties of phenolic compounds. Trends Pl. Sci. 1997;4:304–309. [Google Scholar]
- Robinson G.K., Stephens G.M., Dalton H., Geary P.J. The production of catechols from benzene and toluene by Pseudomonas putida in glucose fedbatch culture. Biocatal. Biotransfor. 1992;6:81–100. [Google Scholar]
- Rui Ma., Hong Pan, Shen T., Peng Li, Yanan C., Zhenyu L., Xiaxia D., Shuqi W. Interaction of Flavonoids from Woodwardia unigemmata with Bovine Serum Albumin (BSA): application of spectroscopic techniques and molecular modeling methods. Molecules. 2017;22:13–17. doi: 10.3390/molecules22081317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–3883. [Google Scholar]
- Shalaby E.A., Shanab S.M.M. Antioxidant compounds, assays of determination and mode of action. Afr. J. Pharm. Pharmacol. 2013;7:528–539. [Google Scholar]
- Sigma–Aldrich., 2006. Material Safety Data Sheet, Toxicological Information, Section 11. Sigma–Aldrich Korea, Ltd., Yongin, South Korea.
- Singleton V.I., Rossi J.A. Colorimetry of total phenolics with phosphomolibdic-phosphotungstic acid reagent. Am. J. Enol. Viticult. 1965;16:144–158. [Google Scholar]
- Tsai, H.H., Hwang, S.M., 1999. Compositions of matter useful in the treatment of viral infections derived from plant extracts. United States Patent, Patent Number, 5, 989, 556.
- Turkmen N., Sari F., Polat G., Velioglu Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006;99:835–841. [Google Scholar]
- Upadrasta, L., Mukhopadhyay, M., Banerjee, R., 2011. Tannins: Chemistry, biological properties and biodegradation. In: Sabu A., Roussos S., Aguilar, C.N. Chemistry and Biotechnology of Polypherwls. India: Cibet Publishing House, 5–32.
- Velioglu Y.S., Mazza G., Gao Y.L., Oomah B.D. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food Chem. 1998;46:4113–4117. [Google Scholar]
- Vineela C.H., Elizabeth K.M. Antimicrobial activity of marine algae of Visakhapatnam city, Andhra Pradesh. Asian J. Microbial. Biotech. Environ. Sci. 2005;7:209–212. [Google Scholar]
- Wang H.X., Ng T.B. Natural products with hypoglycemic hypotensive, hypocholesterolemic, antiatherosclerotic and antithrombotic activities. Life Sci. 1999;65:2663–2677. doi: 10.1016/s0024-3205(99)00253-2. [DOI] [PubMed] [Google Scholar]
- Willet W.C. Balancing life-style and genomics research for diseases prevention. Science. 2002;296:695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- Xie J.H., Xie M.Y., Nie S.P., Shen M.Y., Wang Y.X., Li C. Isolation, chemical composition and antioxidant activities of a water soluble polysaccharide from Cyclocarya paliurus (Batal.) lljinskaja. Food Chem. 2010;119:1626–1632. [Google Scholar]
- Xu H.X., Kadota S., Kurokawa M., Shiraki K., Matsumoto T., Namba T. Isolation and Structure of Woodorien, a New Glucoside Having Antiviral Activity, from Woodwardia orientalis. Chem. Pharm. Bull. 1993;41:1803–1806. doi: 10.1248/cpb.41.1803. [DOI] [PubMed] [Google Scholar]
- Yun X.L., Zhao N.W., Pan L.T., Zhao J.H. Resource distribution and exploitation of edible ferns in Guizhou (II) J. Anhui. Agri. Sci. 2009;37:16317–16319. [Google Scholar]
- Zaidi S.F.H., Kazuki Y., Makoto K., Khan U., Toshiro S. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori. J. Ethnopharmacol. 2009;121:286–291. doi: 10.1016/j.jep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Zhong T.D., Yun S.F., Zhi G.T., Ming H.Y., Xu Y.Q., Li F., Cao Q.E. Phenolic content and radical scavenging capacity of 31 species of ferns. Fitoterapia. 2008;79:581–583. doi: 10.1016/j.fitote.2008.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.