Abstract
Plant growth is often affected with hampered physiological and cellular functioning due to salinity and drought stress. To assess the effectiveness of plant bioregulators (PBRs) in mitigating abiotic stresses, a double spilt plot field study was conducted with three replications at ICAR-CSSRI, research farm, Nain, Panipat. The study comprised of three deficit irrigation regimes viz., 100, 80 and 60% of crop evapo-transpiration (ETc) (I1, I2 and I3), four levels of irrigation water salinity i.e. 2, 4, 8, 12 dS m−1 (S0, S1, S2 and S3) and two PBRs salicylic acid (SA; G1) and thiourea (TU; G2). Irrigations, as per regimes and salinity, were applied at identified critical stages of wheat and if needed in pearl millet. PBRs were applied as seed priming and foliar sprays at two sensitive stages of respective crops. The trend of plant height, and physiological and biochemical traits was similar under different treatments at both stages, but differed significantly only at reproductive stage. Water deficit caused significant reduction in pearl millet (5.1%) and wheat (6.7%) grain yields. The reduction in grain yield under 8 and 12 dS m−1 was 12.90 and 22.43% in pearl millet and 7.68 and 32.93% in wheat, respectively compared to 2 dS m−1. Application of either SA (G1) or TU (G2) significantly enhanced plant height and grain yield, but magnitude of the increment was higher with SA in pearl millet and with TU in wheat. Application of SA and TU increased grain yield by 14.42 and 12.98 in pearl millet, and 12.90 and 17.36% in wheat, respectively. The plant height, RWC, TC, MI, LP, proline, Fv/Fm and Na/K ratio significantly reduced by salinity stress in pearl millet and both water and salinity stress in wheat. Application of both PBRs proved beneficial to mitigate adverse effect of water deficit and salt stress by significantly improving physiological traits, biochemical traits and ultimately grain yield in both crops.
Keywords: Matric stress, Na+/K+, Osmotic stress, PBRs, Proline, RWC
Abbreviations: PBRs, Plant bioregulators; SA, Salicylic acid, TU, Thiourea, RWC, Relative water content; TC, Total chlorophyll; MI, Membrane Injury; LP, Lipid peroxidation; FW, Fresh weight; DW, Dry weight
1. Introduction
In changing climatic scenario, plants often face periods of soil and atmospheric water deficits and/or salinity stress because of scarcity of water availability and deteriorating quality across the globe. As per the available reports, 32–84% poor quality ground water is used for irrigation in different states of India (Minhas, 1996, Jangir and Yadav, 2011). Simultaneously, lack of precipitation, high rate of evapo-transpiration and un-sustainable use of water resources could also lead to drought and salinity problems in lesser productive arid and semi-arid regions. In response to abiotic stresses, plants use their in-built ability to adjust in stressful environment by altering various physiological, biochemical, cellular and molecular processes that eventually improve their growth and development.
Pearl millet [Pennisetum glaucum (L.) R. Br. emend. Stuntz.]-wheat (Triticum aestivum L.) is an important cropping system of the water scarce, salt affected, marginal lands underlain with salty groundwater in semi-arid regions. Moreover, pearl millet is highly vigorous, quick growing and relatively more drought and salinity tolerant major cereal crop after barley (Krishnamurthy et al., 2007). Whereas, wheat is second most important food crop, which is consumed approximately by 36% of the population and considered as semi tolerant crop with a salt tolerance limit of 6 dSm−1 (Chinnusamy et al., 2005, Shahzad et al., 2016). However, under deficit irrigation with saline water, the production losses owing to different environmental stresses is a major concern to cope with rising food needs (Shanker and Venkateswarlu, 2011). Application of plant bioregulators (PBRs) could play a crucial role in improving crop growth, development and productivity (Pasala et al., 2016). Among many PBRs examined to enhance the stress tolerance, salicylic acid (SA) and thiourea (TU) are widely used in different crops under variety of stresses controlled conditions (Srivastava et al., 2016). SA is an important phytohormone that regulates a wide range of metabolic and physiological reactions in plants like cell growth, germination, seedling establishment, respiration, responses to abiotic stresses (Karlidag et al., 2009), water stress in wheat (Anesheh et al., 2012) and salinity and water stresses in barley (Fayez and Bazaid, 2014). Whereas Thiourea (TU) is a synthetic PBR having 36% nitrogen and 42% sulfur that gained importance in plant stress tolerance. Therefore, the present study was conducted at field scale to evaluate the stress mitigation effect of salicylic acid and thiourea for increasing production of pearl millet-wheat cropping system.
2. Materials and methods
2.1. Experimental details
The field study on pearl millet-wheat was conducted during 2016–17 and 2017–18 at ICAR-CSSRI experimental farm located at Nain village, Panipat having inland salinity and poor quality water (Table 1). The study was conducted in double split plot design with three replications. It comprised of 36 treatment combinations i.e. three irrigation regimes based on crop evapo-transpiration (ETc; calculated using FAO Penman-Monteith method with already established crop coefficients) (Doorenbos and Pruitt, 1977) viz., irrigation water equivalent to 100% (I1), 80 (I2) and 60 (I3)of crop ET at critical growth stages in the main plots and four saline irrigation water treatments (by mixing available saline groundwater) having 2, 4, 8 and 12 dS m−1 electrical conductivity (EC) in sub plots and application of two plant bioregulators - viz., salicylic acid (SA) and thiourea (TU) along with control (without PBRs) in sub sub plots. Salicylic acid (SA) and Thiourea (TU) were applied through seed priming and twice in foliar spray. Seed priming was done with 1 mM salicylic acid (SA) and 500 ppm thiourea (TU) at sowing and as foliar sprays at specified critical growth stages in both crops (based on concentration standardized in laboratory germination test). In case of pearl millet, bioregulators were applied at vegetative (21 days after emergence) and flowering stages (55–60 DAS) and in wheat at booting (60–70 DAS) and milking stages (90–95 DAS) with equal amount of normal distilled water in control. All agronomic management practices i.e. fertilizer, irrigation, weed and insect pest were uniformly followed as per recommendation for the two crops in the area.
Table 1.
Physico-chemical properties of the experimental field soil and composition of irrigation water.
| Soil properties | ||||
|---|---|---|---|---|
| Soil depth (cm) | 0–15 | 15–30 | 30–60 | 60–90 |
| pHs | 7.43 ± 0.90 | 7.59 ± 1.11 | 7.62 ± 1.33 | 7.65 ± 0.57 |
| ECe (dS m-1) | 7.61 ± 2.32 | 9.28 ± 2.15 | 10.23 ± 2.26 | 10.03 ± 2.14 |
| Organic carbon (g kg−1) | 4.67 ± 0.62 | 2.67 ± 0.44 | 1.77 ± 0.30 | 1.58 ± 0.25 |
| Available Nitrogen (N) kg ha−1 | 127.81 ± 14.04 | 115.50 ± 10.25 | 107.46 ± 10.6 | 98.6 ± 6.57 |
| Available Phosphorus (P) kg ha−1 | 33.77 ± 4.28 | 27.80 ± 6.87 | 26.00 ± 4.71 | 23.90 ± 4.39 |
| Available Potassium (K) kg ha−1 | 209.40 ± 10.59 | 203.53 ± 9.01 | 201.35 ± 5.77 | 196.10 ± 8.11 |
| Composition of irrigation water | 2 dS m−1 | 4 dS m−1 | 8 dS m−1 | 12 dS m−1 |
| ECiw(dS m−1) | 1.91 | 4.10 | 8.10 | 12.10 |
| pHiw | 7.78 | 7.79 | 7.91 | 7.95 |
Data of soil properties are expressed as the means ± standard deviation of 108 experimental plots. Characters of pHs and pHiw- pH of soil saturation paste and irrigation water, respectively; ECe and ECiw- Electrical conductivity of soil saturation paste extract and irrigation water, respectively.
2.2. Plant material and observation recorded
Pearl millet variety-HHB-146 and wheat variety KRL 210 recommended for salinity and drought prone areas of north western plain zones (NWPZ) of India were used (Kumar et al., 2018). Plant height (cm) of randomly selected plants and grain yield (t ha−1) of net treatment plot were recorded at harvest. While for physiological and biochemical traits, the leaf sample from randomly selected and tagged plants, were collected after 14 days of foliar spray at reproductive stage of respective crops, quickly sealed in humified polythene bags, maintained at low temperature in ice filled boxes and transported to the laboratory. Relative water content (RWC%) was calculated by Weatherley method (1950), chlorophyll content (mg g−1 FW) was assessed using Hiscox & Israelstam method (1979) by dimethyl sulfoxide (DMSO) calculation according to Alan (1994). Membrane injury (MI %) estimated with Dionisio-Sese & Tobita procedure (1998), chlorophyll fluorescence (Fv/Fm) was recorded by dark adaptation method (Zhang et al., 2018) using fluorescence meter (Mini PAM, Walz) after adapting the leaf in dark for 25 min by using leaf clip on the tagged plant in the field. Bates et al. (1973) method was used for proline content (mg g−1 FW) estimation using 3% sulphosalicylic acid. Lipid peroxidation was recorded in terms of MDA (malondialdehyde) content using trichloroacetic acid and TBA (thiobarbituric acid) with the help of Heath and Packer method (1968). An extinction coefficient 155 mM−1 cm−1 was taken for MDA content (nmole g−1 FW) calculations.
2.3. Statistical analysis
All the recorded data were analysed using analysis of variance (ANOVA) technique (SAS) for split split plot design using SAS 9.2 software (SAS Institute, 2001) and pair wise comparisons were made using Tukey’s test at p ≤ 0.05.
3. Results
In pearl millet, significantly greater height was recorded during 2017 (202.08 cm) in comparison to 2016 (185.56 cm), but there were no significant differences due to various deficit irrigation regimes (Table 2). Plant height decreased significantly with increasing salinity stress except under S1 (4 dS m−1) which had acquired the maximum height among all the salinity levels (199.61 cm). Application of both SA (8.4%) and TU (7.2%) significantly increased plant height over control, but statistically equivalent to each other (Table 2). In case of wheat, the year 2016–17 recorded significantly taller plants (93.41 cm) than the year 2017–18 (91.22 cm). The plant height decreased significantly with increasing each level of drought (I1 to I3) and salinity (S0 to S3) stress (Table 2). There was significant increase in plant height with application of SA (10.8%) and TU (13.7%) over control, but magnitude of the increment was significantly higher in TU (97.07 cm) over SA. Interaction effect of deficit irrigation regimes and differential irrigation water salinity brought significant differences in height of both the crops. Integration of S1 + I3 recorded taller plant height (205.82 cm) than combinations of I1 with all salinity levels except S2 + I2, S2 + I3 and S3 with all the irrigation water regimes in pearl millet. In wheat, the association of G2 (TU) + S0 significantly increased plant height as compared to the most of remaining combinations of treatments. S0 with I3 resulted in significantly higher plant height as compared to rest other combinations except S1 + I3.
Table 2.
Effect of deficit irrigation regimes, differential irrigation water salinity and plant bioregulators on physiological traits.
| Pearl millet |
Wheat |
|||||||
|---|---|---|---|---|---|---|---|---|
| Treatments/ Studied traits | PH (cm) | RWC (%) | TC(mgg−1) | Fv/Fm | PH(cm) | RWC (%) | TC(mgg−1) | Fv/Fm |
| Years | ||||||||
| 2016/2016–17 | 185.56B | 77.48B | 1.61B | 0.605 | 93.41A | 72.87A | 2.15 | 0.65 |
| 2017/2017–2018 | 202.08A | 80.40A | 1.70A | 0.619 | 91.22B | 70.77B | 2.13 | 0.64 |
| SEd± | 2.33 | 0.56 | 0.04 | 0.019 | 0.78 | 0.12 | 0.06 | 0.01 |
| CD (P = 0.05) | 5.20 | 1.25 | 0.09 | NS | 1.74 | 0.27 | NS | NS |
| Deficit irrigation regimes | ||||||||
| I1 (100% ET) | 193.12 | 79.42 | 1.71 | 0.616 | 95.64A | 72.39A | 2.35A | 0.68A |
| I2 (80% ET) | 194.50 | 78.71 | 1.66 | 0.607 | 92.12B | 71.91B | 2.13B | 0.65B |
| I3 (60% ET) | 193.84 | 78.69 | 1.60 | 0.612 | 89.19C | 71.17C | 1.94C | 0.62C |
| SEd± | 2.86 | 0.69 | 0.05 | 0.016 | 0.96 | 0.15 | 0.07 | 0.01 |
| CD (P = 0.05) | NS | NS | NS | NS | 2.13 | 0.34 | 0.17 | 0.02 |
| Differential salinity levels | ||||||||
| S0 (2 dS m−1) | 198.27A | 84.89A | 1.77A | 0.666A | 102.50A | 75.18A | 2.36A | 0.69A |
| S1 (4 dS m−1) | 199.61A | 84.09A | 1.73A | 0.623B | 97.98B | 73.83AB | 2.28A | 0.67A |
| S2 (8 dS m−1) | 191.86B | 76.19B | 1.60B | 0.609B | 89.28C | 71.95B | 2.07B | 0.64B |
| S3 (12 dS m−1) | 185.53C | 70.59C | 1.52B | 0.549C | 79.51D | 66.32C | 1.85C | 0.60C |
| SEd± | 2.94 | 1.15 | 0.05 | 0.013 | 1.32 | 1.10 | 0.08 | 0.01 |
| CD (P = 0.05) | 5.81 | 2.27 | 0.09 | 0.027 | 2.62 | 2.17 | 0.15 | 0.02 |
| PBRs | ||||||||
| G0 (control) | 184.17B | 74.94B | 1.48B | 0.617 | 85.35C | 69.48B | 1.94B | 0.62B |
| G1 (1 mM SA) | 199.73A | 80.79A | 1.74A | 0.607 | 94.53B | 72.08A | 2.21A | 0.66A |
| G2(500 ppm TU) | 197.56A | 81.08A | 1.75A | 0.612 | 97.07A | 73.91A | 2.27A | 0.67A |
| SEd± | 2.54 | 0.99 | 0.04 | 0.014 | 1.14 | 0.95 | 0.07 | 0.01 |
| CD (P = 0.05) | 5.03 | 1.97 | 0.08 | NS | 2.26 | 1.88 | 0.13 | 0.02 |
Data presented are means ± S.E.M of three independent replications. Two-way ANOVA followed by Tukey’s test was used for analysis. Different letters denote significant differences at p < 0.05. PH – plant height; RWC – Relative water content; TC- Total chlorophyll; Fv/Fm- Fluorescence variable/ Fluorescence maximum.
RWC is a measurement of cellular and tissue hydration caused by cellular water deficit. Both the crops showed similar trends for decrease or increase in RWC except the study years i.e. pearl millet showed higher values during 2017 (80.40%), while wheat during 2016 (72.87%). In both crops, RWC decreased with increasing level of drought that was statistically significant in case of wheat. The RWC decreased significantly at 8 dSm−1 and further at 12 dSm−1 salinity stresses (Table 2). Significantly higher RWC was recorded i.e. 80.79 and 81.08% in pearl millet and 72.08 and 73.91% in wheat, respectively by the application of SA and TU (Table 2). Interaction effects of deficit irrigation regimes, irrigation water salinity and PBRs were found significant in both the crops. Chlorophyll content was also affected by study years, deficit irrigation regimes, varying levels of irrigation water salinity and PBRs. In case of pearl millet, deficit irrigation caused non-significant reduction in the chlorophyll content, whereas, it was significantly lower at ECiw 8 and 12 dS m-1salinity as compared to 4 dS m−1 and control (S0). Under salinity, the chlorophyll content was reduced by 9.6 and 14.12% at ECiw 8 and 12 dS m−1, respectively in comparison to control (Table 2). In case of wheat, similar trends of reduction in chlorophyll content were noted, but there was no significant effect of the study years, whereas each level of deficit irrigation significantly reduced the chlorophyll content. Both the applied PBRs significantly enhanced total chlorophyll content in both crops over control (Table 2). SA and TU were statistically equivalent with corresponding values of 1.74 and 1.75 mg g−1 in pearl millet and 2.21 and 2.27 mg g−1 in wheat, respectively. There was no significant effect of studied years, irrigation regimes and PBRs on Fv/Fm (chlorophyll fluorescence) in pearl millet, except for salinity stress, where the Fv/Fm decreased significantly with increasing irrigation water salinity. In case of wheat, the Fv/Fm values decreased significantly with deficit irrigation (0.68 at 100% and 0.62 at 60% ETc) and salinity (0.69 at ECiw ~ 2 dS m−1 and 0.60 at ECiw ~ 12 dS m−1). PBRs were found beneficial and attained significantly higher Fv/Fm values i.e. 0.66 with SA and 0.67 with TU as compared to without PBRs (0.62).
Different salinity levels, PBRs and years of study had significant effect on membrane injury in pearl millet leaves, but remained unaffected by deficit irrigation regimes (Table 3). Higher membrane injury was observed during the year 2016 (35.02%) as compared to (23.21%) in 2017. With increasing irrigation water salinity from S0 (2 dS m−1) to S3 (12 dS m−1), progressive increase in membrane injury (25% to 33.34% in pearl millet and 42.77% to 46.99% in wheat) was recorded. A significant reduction (~8% in pearl millet and 2–3% in wheat) in membrane injury were recorded under SA and TU application as compared to control (Table 3). Deficit irrigation did not cause any significant effect on proline accumulation in pearl millet while salinity resulted in significantly higher accumulation of proline i.e. 1.85 mg g−1 FW at ECiw ~ 4 dS m−1, 2.57 mg g−1 FW at ECiw ~ 8 dS m−1 and 3.07 mg g−1 FW at ECiw ~ 12 dS m−1 in comparison to control (1.63 mg g−1 FW at ECiw ~ 2 dS m−1). Both the PBRs had brought significant decrement in proline accumulation (20.97% reduction by SA and 28.99% reduction by TU) in comparison to untreated plots (Table 3). In case of wheat, study years didn‘t show any effect on proline accumulation. Wheat subjected to harsher environment under I3 (ETc – 60%) irrigated plots had attained significantly higher value of proline (2.68 mg g−1) as compared to I2 (2.44 mg g−1) and I1 (2.18 mg g−1). With each increasing salinity level, proline accumulation had significantly increased. The highest and lowest values were obtained under S3 (2.81 mg g−1) and S0 (2.11 mg g−1), respectively (Table 3). Both applied PBRs had resulted into lower values of proline. Further, TU (2.24 mg g−1) had significantly lower proline accumulation as compared to SA (2.41 mg g−1). Interactive effect of years × irrigation regimes × salinity levels × PBRs was found significant on proline accumulation. In pearl millet the maximum accumulation was got in 2016 with I2 + S3 (4.03 mg g−1) in comparison to all other combinations except same salinity under I1 (3.83 mg g−1) in the same year. In wheat combination of lowest salinity level with and no PBRs application maintained the lower values of proline as compared to others. Combination of 2017–18 + S3 (12 dS m−1) + G0 (Control) resulted into the highest value (3.46 mg g−1) as compared to rest others (Table 3).
Table 3.
Effect of deficit irrigation regimes, deferential irrigation water salinity and plant bioregulators on biochemical traits.
| Treatments/ Studied traits |
Pearl millet |
Wheat |
||||
|---|---|---|---|---|---|---|
| Years | MI (%) | Proline (mg g−1 FW) | LP (nmole g−1 FW | MI (%) | Proline (mg g−1 FW) | LP (nmole g−1 FW |
| 2016/2016–17 | 35.02A | 2.49A | 6.85A | 43.65B | 2.32 | 2.34B |
| 2017/2017–2018 | 23.21B | 2.08B | 5.57B | 45.58A | 2.55 | 2.46A |
| SEd± | 0.50 | 0.09 | 0.16 | 0.39 | 0.10 | 0.03 |
| CD (P = 0.05) | 1.11 | 0.19 | 0.37 | 0.88 | NS | 0.07 |
| Deficit irrigation regimes | ||||||
| I1 (100% ET) | 28.46 | 2.18 | 6.14 | 43.29B | 2.18C | 2.38 |
| I2 (80% ET) | 29.18 | 2.32 | 6.22 | 44.75A | 2.44B | 2.39 |
| I3 (60% ET) | 29.71 | 2.35 | 6.26 | 45.80A | 2.68A | 2.43 |
| SEd± | 0.61 | 0.10 | 0.20 | 0.48 | 0.12 | 0.04 |
| CD (P = 0.05) | NS | NS | NS | 1.07 | 0.28 | NS |
| Differential salinity levels | ||||||
| S0 (2 dS m−1) | 25.00D | 1.63D | 5.43C | 42.77C | 2.11D | 2.25B |
| S1 (4 dS m−1) | 27.79C | 1.85C | 5.57C | 43.82BC | 2.31C | 2.29B |
| S2 (8 dS m−1) | 30.33B | 2.57B | 6.72B | 44.87B | 2.51B | 2.45A |
| S3 (12 dS m−1) | 33.34A | 3.07A | 7.10A | 46.99A | 2.81A | 2.45A |
| SEd± | 0.84 | 0.10 | 0.19 | 0.64 | 0.07 | 0.06 |
| CD (P = 0.05) | 1.66 | 0.19 | 0.37 | 1.26 | 0.13 | 0.12 |
| PBRs | ||||||
| G0 (control) | 34.63A | 2.67A | 7.41A | 46.06A | 2.65A | 2.40 |
| G1 (1 mM SA) | 26.61B | 2.11B | 5.60B | 44.09B | 2.41B | 2.40 |
| G2(500 ppm TU) | 26.10B | 2.07B | 5.61B | 43.70B | 2.24C | 2.40 |
| SEd± | 0.73 | 0.08 | 0.16 | 0.55 | 0.06 | 0.05 |
| CD (P = 0.05) | 1.44 | 0.17 | 0.32 | 1.09 | 0.12 | NS |
Data presented are means ± S.E.M of three independent replications. Two-way ANOVA followed by Tukey’s test was used for analysis. Different letters denote significant differences at p < 0.05. MI – membrane injury; LP – Lipid peroxidation.
Lipid peroxidation (LP) did not show any significant effect of deficit irrigation in both the crops while salinity stress led to a significant increase in the content of MDA in both the crops i.e. 30.75% increase in MDA content in pearl millet and 8.88% in wheat at ECiw ~ 12 dS m−1 (Table 3). Application of PBRs (SA and TU) was found to reduce oxidative damage in pearl millet by reducing MDA content by 24% over control (no PBRs) but such effect was not observed in wheat (Table 3).
Na+/K+ and Na+/Ca2+ ratio are indicators of stress tolerance in plants. The study years did not cause any significant variation in Na:K ratio in pearl millet. Among varying irrigation regimes, higher Na:K ratio was obtained in I2 (80% ETc) (0.48) irrigation regime over other regimes (Table 4). The Na:K ratio was significantly increased by each progressive increase in salinity level and the uppermost value (0.57) was obtained under S3 (12 dS m−1). There was significant reduction in Na:K ratio by employing the PBRs (SA and TU; 0.40) as compared to control (0.60).
Table 4.
Effect of deficit irrigation regimes, deferential irrigation water salinity and plant bioregulators on Na:K, Na:Ca and grain yield.
| Treatments/ Studied traits | Pearl millet |
Wheat |
||||
|---|---|---|---|---|---|---|
| Na:K | Na:Ca | Grain Yield(t ha−1) | Na:K | Na:Ca | Grain Yield(t ha−1) | |
| Years | ||||||
| 2016/2016–17 | 0.46 | 2.95 A | 2.11B | 0.37 | 1.91 | 4.63A |
| 2017/2017–2018 | 0.46 | 2.60B | 2.43A | 0.39 | 2.00 | 4.25B |
| SEd± | 0.01 | 0.09 | 0.02 | 0.01 | 0.10 | 0.04 |
| CD (P = 0.05) | NS | 0.20 | 0.05 | NS | NS | 0.09 |
| Deficit irrigation regimes | ||||||
| I1 (100% ET) | 0.45 | 2.76 | 2.34A | 0.37 | 1.85 | 4.60A |
| I2 (80% ET) | 0.48 | 2.85 | 2.25B | 0.38 | 1.95 | 4.42B |
| I3 (60% ET) | 0.47 | 2.73 | 2.22B | 0.40 | 2.07 | 4.29C |
| SEd± | 0.01 | 0.11 | 0.03 | 0.01 | 0.12 | 0.05 |
| CD (P = 0.05) | NS | NS | 0.06 | NS | NS | 0.12 |
| Differential salinity levels | ||||||
| S0 (2 dS m−1) | 0.38D | 2.44C | 2.35A | 0.30D | 1.59C | 4.95A |
| S1 (4 dS m−1) | 0.43C | 2.57 BC | 2.37A | 0.33C | 1.72C | 4.92A |
| S2 (8 dS m−1) | 0.47B | 2.89B | 2.29B | 0.39B | 2.03B | 4.57B |
| S3 (12 dS m−1) | 0.57A | 3.30 A | 2.07C | 0.50A | 2.55 A | 3.32C |
| SEd± | 0.01 | 0.09 | 0.02 | 0.01 | 0.09 | 0.07 |
| CD (P = 0.05) | 0.02 | 0.18 | 0.05 | 0.02 | 0.19 | 0.14 |
| PBRs | ||||||
| G0 (control) | 0.60A | 3.80 A | 2.08B | 0.45A | 2.01 A | 4.03C |
| G1 (1 mM SA) | 0.40B | 2.37B | 2.38A | 0.36B | 1.74B | 4.55B |
| G2(500 ppm TU) | 0.40B | 2.38B | 2.35A | 0.33C | 1.66B | 4.73A |
| SEd± | 0.01 | 0.08 | 0.02 | 0.01 | 0.07 | 0.06 |
| CD (P = 0.05) | 0.02 | 0.16 | 0.04 | 0.02 | 0.15 | 0.12 |
Data presented are means ± S.E.M of three independent replications. Two-way ANOVA followed by Tukey’s test was used for analysis. Different letters denote significant differences at p < 0.05. Na:K- Sodium: Potassium; Na:Ca – Sodium:Calcium.
The study years and irrigation regimes had no significant effect on Na:K raio in wheat, although, the lowest and highest ratios were recorded under I3 (0.40) and I1 (0.37), respectively. Application of saline water irrigation resulted into significant increase in Na:K ratios with increase in each level of salinity (Table 4). Use of plant bioregulators significantly reduced the ratio over control (0.45), further, the reducing effect of TU (0.33) was significantly better over SA (0.36) Interaction effects of deficit irrigation, saline irrigation water and growth regulators were also found significant. In pearl millet significant interaction effect of deficit irrigation regimes and saline irrigation water was noticed on Na: K ratio of grain and the significantly higher ratio were obtained by S3 (12 dS m−1) under I2 and I3 regimes (respective value, 0.64 and 0.63). Combined effect of saline irrigation water and PBRs was noticed significant on Na: K ratio of grain and the maximum value was observed under S3 + G0 (0.79) which remained at par with S2 + G0 combination (0.68) only. In wheat, the group of S3 (12 dS m−1) + G0 (Control) + I1 (60% ET) [0.98] was recorded higher values as compared to other combinations of these treatments except S3 (12 dS m−1) + G1 (SA) + I2 (80% ET) [0.83] and S3 (12 dS m−1) + G0 (Control) + I2 (80% ET) [0.97].
In case of pearl millet, lower Na:Ca ratio (2.60) was recorded in year 2017 while for wheat in 2016–17 (1.91). Irrigation regimes had no significant effect on Na:Ca ratio in both the crops (Table 4). From ECiw 4 dSm−1 onwards, a significant increment was observed in Na:Ca ratio in both the crops and the extents of increase were 5.33, 18.44 and 35.25% in pearl millet and 8.18, 27.67 and 60.38% in wheat under S1, S2 and S3, respectively over S0 (Table 4). PBR's application significantly reduced the Na:Ca ratio by 37.6 and 37.4% in pearl millet and 13.43 and 17.41% in wheat by SA and TU, respectively (Table 4).
Pearl millet grain yield was significantly higher (15.16%) in year 2017 (2.43 t ha−1) as compared to 2016 (2.11 t ha−1). Under different irrigation regimes, the grain yield was significantly higher (2.34 t ha−1) in irrigation level I1 (100% ET) compared to deficit irrigations at I2 (80% ET) (2.25 t ha−1) and I3 (60% ET) (2.22 t ha−1). Among different salinity levels, grain yield declined significantly by 2.55% (2.29 t ha−1) at S2 (8 dS m−1) and further by 11.91% (2.07 t ha−1) under subsequent higher salinity S3 (12 dS m−1) level as compared to S0 (~2 dS m−1; 2.35 t ha−1) except S1 (4 dS m−1; 2.37 t ha−1) with at par yield (Table 4). Application of SA and TU resulted in significant increase in grain yield i.e. 14.42 (2.38 t ha−1) and 12.98% (2.35 t ha−1), respectively over the G0 (2.08 t ha−1). However there was no significant difference between both PBRs. In wheat (Table 4), significantly higher grain yield (4.63 t ha−1) was obtained in 2016–17 as compared to 2017–18 (4.25 t ha−1). Deficit irrigation regimes caused significant reduction in grain yield (p < 0.05) i.e. 3.91% under I2 (80% ETc) and 6.74% under I3 (60% ETc).
Among different irrigation water salinity levels, S0 (4.95 t ha−1) saline water has recorded the highest grain yield that was equivalent to S1. The grain yield was significantly reduced at 8 dS m−1 (S2; 4.57 t ha−1) and subsequently at S3 (3.32 t ha−1). The application of both PBRs (SA and TU) significantly improved the wheat grain yield over control. The application of TU (4.73 t ha−1) proved significantly better than SA (4.55 t ha−1) in increasing wheat grain yields.
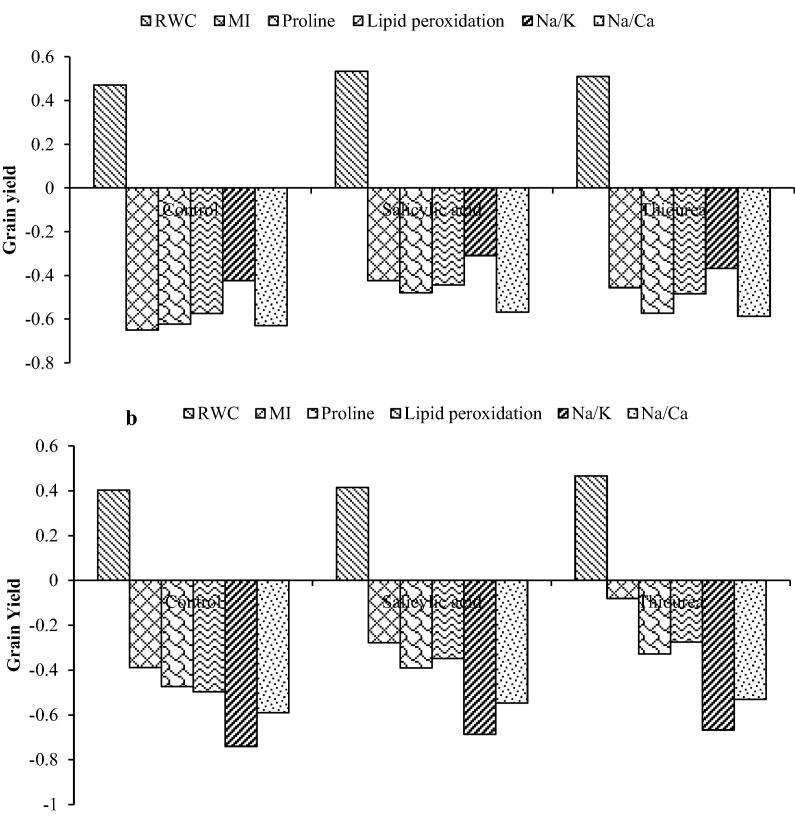
Co-relation analysis
The association of different traits with grain yield under the effect of deficit irrigation using different salinity water irrigation and use of PBRs was assessed by correlation analysis. There were significant changes in the studied traits with the application of both the PBRs in relation with grain yield (Fig. 1). Both the PBRs showed effectiveness in mitigating the adverse effect of stresses in the two crops, but SA was found more effective for pearl millet while TU proved relatively better for wheat.
Fig. 1.
Pearson’s correlation coefficients for association using SPSS v19 among different physiological and biochemical traits with grain yield in pearl millet and wheat.
4. Discussion
Pearl millet–wheat is an important cropping system covering around 2.26 million ha arid and semi-arid tracts in India having water deficit and osmotic stress conditions (Yadav and Subba Rao, 2002, Kumar et al., 2005). Survival of plants, in stressful environments by manipulating growth, physiological, biochemical and molecular responses; is an important indicator of their ability to overcome multiple and simultaneous stresses (Lichtenthaler, 1996).
Plant response to environmental stresses is fundamentally analysed by growth analysis. In both pearl millet and wheat, decline in plant height in response to salinity and drought, occurs mainly due to decrease in osmotic potential of the soil solution in presence of excess soluble salts. Decrease in osmotic potential of soil solution causes ion imbalance and toxicity by hindering water and nutrients uptake in plants (Kumar et al., 2016, Yadav and Dagar, 2016). Stress either due to drought or salinity is known to decrease plant water uptake ability that leads to reduced plant growth and impaired metabolic processes by the accumulation of toxic ions (Kumar et al., 2018). In this study, both the crops showed decrease in RWC under stress conditions. But the application of SA and TU maintained the hydration of cells up to an optimal level, under stress conditions, through accumulation of osmolytes, which sustained water uptake and increased RWC of tissues (Pooja and Sharma, 2016). Decrease in chlorophyll content under water deficit and salinity stress either in pearl millet or in wheat could be attributed to the rate of degradation of chlorophyll or due to reduction in activity of chlorophyll biosynthesis enzymes (Singh et al., 2016, Mann et al., 2019). Further aggravated stress symptoms have also been observed with greater reductions in chlorophyll concentration (Kumar et al., 2018). Chlorophyll is an important molecule associated with photosynthesis, and these plant bio-regulators by maintaining cellular osmoticum helps in improving the chl a and b contents (Burman et al., 2004, Seckin et al., 2009). Generally Fv/Fm reflects the maximum efficiency of the light absorbption and its conversion into chemical energy which is considered as the most powerful eco-physiological tool to study the photosynthetic process in plants (Maxwell and Johnson, 2000, Murchie and Lawson, 2013). In the present study, Fv/Fm declined under the effect of abiotic stresses either individual or combined, might be due to the disturbances in the integrity of membrane and function of thylakoids in chloroplasts (Wang et al., 2009). Increased gas exchange attributes due to application of salicylic acid and thiourea might be helpful in the improvement of the photosynthetic performance and chlorophyll florescence enabling plant to tolerate environmental stresses (Khan et al., 2003, Wang and Li, 2007, Pooja and Sharma, 2016).
Cell membrane stability is an established index to evaluate crop plants against abiotic stress tolerance (Kumar et al., 2016). Significant changes were noted on MI in both pearl millet and wheat under drought and salinity. Abiotic stresses particularly drought and salinity enhances the degree of membrane fatty acids saturation by changing the properties of proteins that ultimately increased permeability of plasma membrane (Chinnusamy and Zhu, 2003, Pooja et al., 2019, Pooja et al., 2020). Salicylic acid and thiourea have been found to mitigate the negative effect of stresses on MI through the activation of anti-oxidative defense machinery that is connected with the active oxygen species accumulation and plays a vital role in maintaining redox state of membrane proteins (Pooja and Kumar, 2012, Asthir et al., 2013).
Plants generally protect themselves against abiotic stresses by the osmolytes accumulation and their elevation is attributed to a stress tolerance mechanism (Sorahinobar et al., 2016). Proline an important stress indicator stabilizes and protects macromolecules against the severities of ROS by improving osmotic adjustments (Lata et al., 2017, Ahmad et al., 2012). Results of present study recorded higher proline content with increased intensity of stressesin both the crops. The results also indicated significant participation of SA and TU in maintaining cell turgor, boosting nitrogen assimilation and decline in proline oxidase activity as suggested earlier (Chen and Murata, 2011, Khan et al., 2013, Pooja and Sharma, 2016). Increased proline regulated water potential in plant cells enhanced the plant defense under various abiotic stresses (Lata et al., 2019, Sharma et al., 2019). Abiotic stress induced ROS caused alteration of cell membrane produced by oxidation of acids of the lipid bi-layer (Carrasco-Ríos et al., 2013). In this study also MDA content increased in pearl millet and wheat under saline conditions, whereas deficit irrigations had little effect. Higher MDA level might induce more leakage of electrolytes from cells and increased H2O2 accumulation (Kukreja et al., 2006), as a consequence of lipid per-oxidation has long been taken as an indicator of stress tolerance. The application of SA and TU might helped in maintenance of fatty acid breakdown, also led to enhanced activity of antioxidant enzymes and resulted in MDA content decrease (Abdelkader et al., 2012, Pooja and Sharma, 2016).
Stresses increased Na+/K+ in both the crops, that might ascribe to K+ and Na+ competition at the plasma membrane, K+ transport inhibition in xylem tissues and/or Na+ induced K+ efflux from the roots (Mann et al., 2015, Kumar et al., 2018). Salinity caused significant increase in the ratio of Na+/Ca2+ in both the crops. Reduced Ca2+ uptake under stress environments induced reduction in the binding of Ca2+ to the plasma membrane (Rengel, 1992, Singh et al., 2018), resulting in a change in membrane permeability that can be detected as leakage of K+ from the cell (Cramer et al., 1985). PBRs i.e. SA and TU were found effective in reducing the Na+/K+ and Na+/Ca2+ ratio in pearl millet and wheat. Besides accumulating compatible solutes like proline and sugars, these PBRs also helped in elevating the level of K+ (a major compatible inorganic solute) in plant cell that have an osmo-protectant function to protect plants during osmotic and/or ionic stresses (Pooja and Sharma, 2016, Sharma et al., 2019).
About 15-16% more grain yield of pearl millet was recorded in study year 2017 than 2016 may be due to 29 mm higher rainfall in 2017. In case of wheat, grain yield was found more in study year 2016–17 than 2017–18 (Table 4). During 2016–17 cropping season, rainfall received (93.30 mm) was 23.10 mm (32.9%) higher than same period in 2017–18 (70.2 mm). This helped to leach down more of the excess soluble salt added with saline water irrigation below root zone and also nullified the effect of moisture deficit treatments. In addition, temperature and moisture conditions during vegetative phase and relatively dryer weather conditions during grain development resulted in better growth and productivity may be another reason for higher yields in first year. Enhanced grain and biomass yields and yield attributes of crops due to congenial climatic conditions also reported earlier (Wakchaure et al., 2016). Imposed matric stress i.e. from I1 regime to I2 and further I3 decreased the pearl millet and wheat yield (3.91 and 6.74%) (Table 4) are in agreement with finding of Al-Ghobari and EL Marazky (2014), who reported 6.83 and 41.98% average reduction in wheat grain yield under 80 and 60% ETc based deficit irrigations. Imposed deficit irrigation (80 and 60% ETc) reduced wheat yield due to lowering of water potential, reduction in photosynthesis, decrease in leaf expansion, impaired photosynthetic machinery, premature leaf senescence as well as due to a reduction in the assimilate partitioning and activities of sucrose and starch synthesis enzymes (Wahid et al., 2005, Waraich et al., 2011). Under saline irrigation of 8 (S2) and 12 dS m−1 (S3), pearl millet and wheat yield declined by 2.55, 7.68 and 11.91, 32.93%, respectively, compared to 2 dS m−1 (S0) treatment. The decrease in yield under salinity might be due to high osmotic stress caused ruptured cell membrane, reduced osmotic potential, decreased photosynthesis, protein synthesis and translocation/mobilization of nutrient/food materials from plant parts to grain that ultimately reduced dry matter partitioning in source to sink and declined yield of pearl millet and wheat (Kumar et al., 2018). These facts also supported that plant under salinity stress faces many abnormalities in physiological, morphological and biochemical processes (Parida and Das, 2005), and lead to reduced yield. Reduction in grain yield due to increasing irrigation water salinity could be due to higher Na+ and lower K+ content in plant. Pearl millet and wheat yield was significantly enhanced by priming and spraying both of bioregulators (SA and TU) as compared to control (G0). The application of TU found superior in case of wheat that increased 3.96 and 17.36% grain yield as compared to SA and control, respectively. Azimi et al. (2013) found increased wheat grain yield by tune of 24.39% by spraying 0.75 mM SA. Mobilization of dry matter (reserves)/sucrose from leaves to grains via effects on phloem loading increased in wheat with TU spray at tiller stage. Salicylic acid, a phenolic phytohormone, regulates the growth and development of plants, photosynthesis, transpiration, ion absorption and transport (Khan et al., 2014), and thus its application has shown positive responses in mitigating drought (Chini et al., 2004) and osmotic stress (Borsani et al., 2001). Salicylic acid also has immense role in enhancing root system and thus can rectify the salt induced growth and production inhibition and greater biomass.
5. Conclusion
Plant height and grain yield reduction were recorded with increasing moisture and salinity stresses in both pearl millet and wheat. Abiotic stresses specially drought and salinity were mitigated by seed priming and foliar spray of salicylic acid and thiourea at critical growth stages of both the crops. The increasing counteractive effect of PRBs to salt stress was observed with increasing level of salinity. SA and TU use in both crops improved RWC and TC and recovered MI, proline, sodium concentration, lipid peroxidation. TU proved more effective in wheat but SA performed better for pearl millet that too in mild salinity. Thus, the two PBRs can be recommended, after the large scale field testing and standardization of their economic spray schedules, for improving the pearl millet-wheat crop performance under marginal quality irrigation conditions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
This research was supported by grants from the University Grants Commission Fellowship, India 2016 (F./201516/ NFO-2015-17OBC-RAJ-38714/(SAIII/Website). The authors are thankful to Director, NDRI and Director, CSSRI, Karnal for providing necessary facilities for the research work.
Author contribution
R.K.Y. conceived and developed the work. T. Y. conducted the experiments and analysed the results. A.K., R.K.Y. and R. K. participated to data discussion and critically revised the manuscript. T.Y., A.K., G.Y. and M.K. wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelkader A.F., Hassanein R.A., Ali H. Studies on effects of salicylic acid and thiourea on biochemical activities and yield production in wheat (Triticum aestivum var. Gimaza 9) plants grown under drought stress. African J. Biotechnol. 2012;11:12728–12739. doi: 10.5897/AJB11.3134. [DOI] [Google Scholar]
- Ahmad P., Kumar A., Ashraf M., Akram N.A. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.) Afr. J. Biotechnol. 2012;11(11):2694–2703. doi: 10.5897/AJB11.3203. [DOI] [Google Scholar]
- Alan R., W. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Plant Physiol. 1994;144:307–313. [Google Scholar]
- Al-Ghobari H.M., El Marazky M.S.A. Effect of smart sprinkler irrigation utilization on water use efficiency for wheat crops in arid regions. Int. J. Agric. Biol. Eng. 2014;7:26–35. [Google Scholar]
- Anesheh H.P., Emam Y., Ashraf M., Foolad M.R. Exogenous application of salicylic acid and chlormequat chloride alleviates negative effects of drought stress in wheat. Adv. Stud. Biol. 2012;4:501–520. [Google Scholar]
- Asthir B., Thapar R., Farooq M., Bains N.S. Exogenous Application of Thiourea Improves the Performance of Late Sown Wheat by Inducing Terminal Heat Resistance. Int. J. Agric. Biol. 2013;15(6):1337–1342. [Google Scholar]
- Azimi M.S., Daneshian J., Sayfzadeh S., Zare S. Evaluation of amino acid and salicylic acid application on yield and growth of wheat under water deficit. Int. J. Agric. Crop Sci. 2013;5:816. Available online at www.ijagcs.com. [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Borsani O., Valpuesta V., Botella M.A. Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant physiology. 2001;126(3):1024–1030. doi: 10.1104/pp.126.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman U., Garg B.K., Kathju S. Interactive effects of thiourea and phosphorus on clusterbean under water stress. Biol. Plant. 2004;48(1):61–65. doi: 10.1023/B:BIOP.0000024276.03834.8d. [DOI] [Google Scholar]
- Carrasco-Ríos L., Rojas C., Pinto M. Contrasting physiological responses to high salinity between two varieties of corn’Lluteño’(salt tolerant) and’Jubilee’(salt sensitive) Chilean J. Agric. Res. 2013;73(3):205–212. doi: 10.4067/S0718-58392013000300001. [DOI] [Google Scholar]
- Chen T.H., Murata N. Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant, Cell Environ. 2011;34(1):1–20. doi: 10.1111/j.1365-3040.2010.02232.x. [DOI] [PubMed] [Google Scholar]
- Chinnusamy V., Zhu J.K. Topics in Curr. Genet. Springer; Berlin, Heidelberg: 2003. Plant salt tolerance. In Plant responses to abiotic stress; pp. 241–270. 10.1007/978-3-540-39402-0_10. [Google Scholar]
- Chini A., Grant J.J., Seki M., Shinozaki K., Loake G.J. Drought tolerance established by enhanced expression of the CC–NBS–LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. The Plant Journal. 2000;38(5):810–822. doi: 10.1111/j.1365-313X.2004.02086.x. [DOI] [PubMed] [Google Scholar]
- Chinnusamy V., Jagendorf A., Zhu J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005;45(2):437–448. doi: 10.2135/cropsci2005.0437. [DOI] [Google Scholar]
- Cramer G.R., Läuchli A., Polito V.S. Displacement of Ca2+ by Na+ from the plasmalemma of root cells: A primary response to salt stress? Plant Physiol. 1985;79(1):207–211. doi: 10.1104/pp.79.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio-Sese M.L., Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135(1):1–9. doi: 10.1016/S0168-9452(98)00025-9. [DOI] [Google Scholar]
- Doorenbos J., Pruitt W.O. Background and development of methods to predict reference crop evapotranspiration (ETo). Appendix II in FAO-ID-24. Irrig. Drain. 1977;Pap:108–119. [Google Scholar]
- Fayez K.A., Bazaid S.A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci. 2014;13:45–55. doi: 10.1016/j.jssas.2013.01.001. [DOI] [Google Scholar]
- Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hiscox J.D., Israelstam G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979;57(12):1332–1334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- Jangir R.P., Yadav B.S. Management of saline irrigation water for enhancing crop productivity. J. Sci. Ind. Res. 2011;70:622–627. http://nopr.niscair.res.in/handle/123456789/12490 [Google Scholar]
- Karlidag H., Yildirim E., Turan M. Salicylic acid ameliorates the adverse effect of salt stress on strawberry. Scientia Agricola. 2009;66(2):180–187. doi: 10.1590/S0103-90162009000200006. [DOI] [Google Scholar]
- Khan M.I.R., Asgher M., Khan N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.) Plant Physiol. Biochem. 2014;80:67–74. doi: 10.1016/j.plaphy.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Khan M.I.R., Iqbal N., Masood A., Per T.S., Khan N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013;8(11) doi: 10.4161/psb.26374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W., Prithiviraj B., Smith D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003;160(5):485–492. doi: 10.1078/0176-1617-00865. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy L., Serraj R., Rai K.N., Hash C.T., Dakheel A.J. Identification of pearl millet (Penniseetum glaucum L. R. Br.) lines tolerant to soil salinity. Euphytica. 2007;158 (1–2), 179–88 10.1007/s10681-007-9441-3. [Google Scholar]
- Kukreja S., Nandwal A.S., Kumar N., Sharma S.K., Sharma S.K., Kundu B.S., Unvi V., Sharma P.K. Response of chickpea roots to short-term salinization and desalinization: Plant water status, ethylene evolution, antioxidant activity and membrane integrity. Physiol. Mol. Biol. Plants. 2006;12(1):67–73. [Google Scholar]
- Kumar A., Krishnamurthy S.L., Lata C., Kumar P., Devi R., Kulshrestha N., Yadav R.K., Sharma S.K. Salinity and drought induced changes in gas exchange attributes and chlorophyll fluorescence characteristics of rice (Oryza sativa) varieties. Ind. J. Agric. Sci. 2016;86(6):718–726. [Google Scholar]
- Kumar A., Sharma S.K., Lata C., Devi R., Kulshrestha N., Krishnamurthy S.L., Singh K., Yadav R.K. Impact of water deficit (salt and drought) stress on physiological, biochemical and yield attributes on wheat (Triticum aestivum) varieties. Ind. J. Agric. Sci. 2018;88(10):1624–1632. [Google Scholar]
- Kumar P., Nanwal R.K., Yadav S.K. Integrated nutrient management in pearl millet (Pennisetum glaucum)-wheat (Triticum aestivum) cropping system. Ind. J. Agric. Sci. 2005;75(10):640–643. [Google Scholar]
- Lata C., Kumar A., Sharma S.K., Singh J., Sheokand S., Mann A., Rani B. Tolerance to combined boron and salt stress in wheat varieties: Biochemical and molecular analyses. Ind. J. Expt. Biol. 2017;55:321–328. [Google Scholar]
- Lata, C., Soni, S., Kumar, N., Kumar, A., Pooja, Mann, A. and Rani, S., 2019. Adaptive mechanism of stress tolerance in Urochondra (grass halophyte) using roots study. Ind. J. Agric. Sci. 89, 1050-1053.
- Lichtenthaler H.K. Vegetation stress: an introduction to the stress concept in plants. J. Plant Physiol. 1996;148(1–2):4–14. doi: 10.1016/S0176-1617(96)80287-2. [DOI] [Google Scholar]
- Mann, A., Bishi, S.K., Mahatma, M.K. and Kumar, A., 2015. Metabolomics and salt stress tolerance in plants. In: Managing salt tolerance in plants: molecular and genomic perspectives. (Taylor and Francis Group/LLC), 251-266.
- Mann, A., Kaur, G., Kumar, A., Sanwal, S.K., Singh, J. and Sharma, P.C., 2019. Physiological response of chickpea (Cicer arietinum L.) at early seedling stage under salt stress conditions. Leg. Res. DOI: 10.18805/LR-4059.
- Maxwell K., Johnson G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000;51(345):659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- Minhas P.S. Saline water management for irrigation in India. Agric. Water Manag. 1996;30:l-24. doi: 10.1016/0378-3774(95)01211-7. [DOI] [Google Scholar]
- Murchie E.H., Lawson T. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J. Exp. Bot. 2013;64(13):3983–3998. doi: 10.1093/jxb/ert208. [DOI] [PubMed] [Google Scholar]
- Parida A.K., Das A.B. Ecotoxicol. Environ; Saf: 2005. Salt tolerance and salinity effects on plants; p. 60. [DOI] [PubMed] [Google Scholar]
- Pasala R.K., Khan M.I.R., Minhas P.S., Farooq M.A., Sultana R., Per T.S., Deokate P.P., Khan N.A., Rane J. Can plant bio-regulators minimize crop productivity losses caused by drought, heat and salinity stress? An integrated review. J. Appl. Bot. Food Qual. 2016;89:113–125. doi: 10.5073/JABFQ.2016.089.014. [DOI] [Google Scholar]
- Pooja and Sharma, K.D., 2016. Salicylic acid induced amelioration of salinity stress in mungbean. Scholar‘s Press, Omni Scriptum GmbH & Co. KG, Germany.
- Pooja, Nandwal, A.S., Chand, M., Kumari, A., Rani, B., Goel, V. and Kulshreshtha, N., 2020. Soil moisture deficit induced changes in antioxidative defense mechanism of four sugarcane varieties differing in their maturity. Ind.J. Agr.Sci. 90(3), 56–61.
- Pooja, Nandwal, A.S., Chand, M., Singh, K., Mishra, A.K., Kumar, A., Kumari, A. and Rani, B., 2019. Varietal variation in physiological and biochemical attributes of sugarcane varieties under different soil moisture regimes. Ind. J. Expt. Biol. 57 (10), 721-732.
- Pooja, Sharma, K.D. and Kumar, A., 2012. Improvement in plant water relation and photosynthetic activity of Mungbean (Vigna radiata) In response to salicylic acid under Salinity stress. Ind. J. Plant Physiol. 17 (3&4), 268-274.
- Rengel Z. The role of calcium in salt toxicity. Plant, Cell Environ. 1992;15:625–632. [Google Scholar]
- Sas, S.A.S., 2001. STAT User’s Guide for Personal Computers, Release 6.12. SAS Institute Inc. Cary, NC, USA.
- Seckin B., Sekmen A.H., Turkan I. An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J. Plant Growth Regul. 2009;28(1):12–20. doi: 10.1007/s00344-008-9068-1. [DOI] [Google Scholar]
- Shahzad M., Saqib Z.A., Hafeez F., Bilal M., Khan S.K., Asad S.A., Akhtar J. Growth-related changes in wheat (Triticum aestivum L.) genotypes grown under salinity stress. J. Plant Nutr. 2016;39:1257–1265. [Google Scholar]
- Shanker A.K., Venkateswarlu B. In Tech Publisher; Rijeka, Croatia: 2011. Abiotic stress in plants–mechanisms and adaptations; p. 428. [Google Scholar]
- Sharma A., Shahzad B., Kumar V., Kohli S.K., Sidhu G.P.S., Bali A.S., Handa N., Kapoor D., Bhardwaj R., Zheng B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):285. doi: 10.3390/biom9070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Kumar A., Datta A., Yadav R.K. Evaluation of guava (Psidium guajava) and bael (Aegle marmelos) under shallow saline watertable conditions. Ind. J. Agric. Sci. 2018;88(5):720–725. [Google Scholar]
- Singh A., Sharma P.C., Meena M.D., Kumar A., Mishra A.K., Kumar P., Chaudhari S.K., Sharma D.K. Effect of salinity on gas exchange parameters and ionic relations in bael (Aegle marmelos Correa) Ind. J. Hort. 2016;73:48–53. [Google Scholar]
- Sorahinobar M., Niknam V., Ebrahimzadeh H., Soltanloo H., Behmanesh M., Enferadi S.T. Central role of salicylic acid in resistance of wheat against Fusarium graminearum. J. Plant Growth Regul. 2016;35:477–491. doi: 10.1007/s00344-015-9554-1. [DOI] [Google Scholar]
- Srivastava A.K., Pasala R., Minhas P.S., Suprasanna P. Plant bioregulators for sustainable agriculture: integrating redox signaling as a possible unifying mechanism. Adv. Agron. 2016;137:237–278. [Google Scholar]
- Wahid, A., Rasul, E., Rao, R.A. and Iqbal, R.M., 2005. Photosynthesis in leaf, stem, flower and fruit. Handb. Photosynth. (2nd Ed.), 2, 479-497. CRC Press, Florida.
- Wakchaure G.C., Minhas P.S., Ratnakumar P., Choudhary R.L. Optimising supplemental irrigation for wheat (Triticum aestivum L.) and the impact of plant bio-regulators in a semi-arid region of Deccan Plateau in India. Agric. Water Manag. 2016;172:9–17. doi: 10.1016/j.agwat.2016.04.004. [DOI] [Google Scholar]
- Wang H., Liu R.L., Jin J.Y. Effects of zinc and soil moisture on photosynthetic rate and chlorophyll fluorescence parameters of maize. Biol. Plant. 2009;53:191–194. [Google Scholar]
- Wang L.J., Li S.H. The effects of salicylic acid on distribution of 14C-assimilation and photosynthesis in young grape plants under heat stress. Acta Hortic. 2007;738:779–785. [Google Scholar]
- Waraich E.A., Ahmad R., Ashraf M.Y. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 2011;5:764. [Google Scholar]
- Weatherley P. Studies in the water relations of the cotton plant: I. The field measurement of water deficits in leaves. New Phytol. 1950;49(1):81–97. [Google Scholar]
- Yadav R.L., Subba Rao A. Modipuram, Meerut, U. P; 2002. Atlas of cropping systems in India. PDCSR bulletin No. 2001–02. Project Directorate for cropping systems research. [Google Scholar]
- Yadav R. K. and Dagar J. C. 2016. Innovations in utilization of poor-quality water for sustainable agricultural production 219-261-. In: Innovative Saline Agriculture (eds. Dagar et al.) Springer-ISBN978-81-322-2770-0.
- Zhang C.F., Pan C.D., Chen H. Effects of leaf-to-fruit ratio on chlorophyll fluorescence parameters of walnut (Juglans regia L.) leaves. Photosynthetica. 2018;56(4):1429–1436. doi: 10.1007/s11099-018-0842-4. [DOI] [Google Scholar]